Abstract
Objective
To test the hypothesis that aerobic exercise is associated with improvements in cognition and cerebrovascular regulation, we enrolled 206 healthy low-active middle-aged and older adults (mean ± SD age 65.9 ± 6.4 years) in a supervised 6-month aerobic exercise intervention and assessed them before and after the intervention.
Methods
The study is a quasi-experimental single group pre/postintervention study. Neuropsychological tests were used to assess cognition before and after the intervention. Transcranial Doppler ultrasound was used to measure cerebral blood flow velocity. Cerebrovascular regulation was assessed at rest, during euoxic hypercapnia, and in response to submaximal exercise. Multiple linear regression was used to examine the association between changes in cognition and changes in cerebrovascular function.
Results
The intervention was associated with improvements in some cognitive domains, cardiorespiratory fitness, and cerebrovascular regulation. Changes in executive functions were negatively associated with changes in cerebrovascular resistance index (CVRi) during submaximal exercise (β = −0.205, p = 0.013), while fluency improvements were positively associated with changes in CVRi during hypercapnia (β = 0.106, p = 0.03).
Conclusion
The 6-month aerobic exercise intervention was associated with improvements in some cognitive domains and cerebrovascular regulation. Secondary analyses showed a novel association between changes in cognition and changes in cerebrovascular regulation during euoxic hypercapnia and in response to submaximal exercise.
Normal aging is typically associated with both physical and cognitive decline. With increasing age, both maximal heart rate and cardiorespiratory fitness decline due to increased arterial stiffness and fibrosis, among other factors.1 Arterial stiffness is due in part to endothelial dysfunction, which alters the ability of blood vessels to dilate in response to nitric oxide, resulting in reduced blood flow.2 Consequential cognitive decline is typically seen in memory, executive functioning, and processing speed.3
Exercise has been shown to have numerous beneficial effects, including on brain health.4–7 A recent systematic review6 reported that 9 of 10 studies found beneficial effects,8 including improvements in memory,9 attention, executive functioning,10,11 and cerebrovascular perfusion.12
Several studies have analyzed the relationship between exercise and cerebral blood flow (CBF).12–14 Many used arterial spin labeling, a technique that examines changes in blood flow in the brain with a high level of spatial but poor temporal resolution. Fewer have used transcranial Doppler ultrasound (TCD),15 a noninvasive and relatively cheaper technique with great temporal resolution.16
To date, no study has investigated the effects of a 6-month aerobic exercise intervention on cognition and cerebrovascular regulation measured with TCD, in response to both euoxic hypercapnia and submaximal exercise. Here, we hypothesized that 6 months of aerobic exercise training would be associated with improved cognitive functioning and cerebrovascular function. A secondary objective was to explore the association between the observed changes in cognition and changes in cerebrovascular regulation.
Methods
Participants
Brain in Motion (BIM) participants were community-dwelling, generally healthy (i.e., no active uncontrolled cardiovascular illnesses), but low-active middle-aged and older adults with intact global cognition (i.e., score ≥24 on the Montreal Cognitive Assessment [MoCA]),17 recruited through word of mouth, social media, posters, and newspaper advertisements in the city of Calgary, Alberta, Canada.
Previously published18 inclusion criteria for the BIM study were obtained by telephone interview and included (1) body mass index <35 kg/m2; (2) able to walk up and down ≥20 stairs independently; (3) no previous diagnosis of cardiovascular or cerebrovascular disease, type I diabetes mellitus, respiratory disease, neurologic disease, or cognitive impairments; (4) nonsmoker for ≥12 months; (5) no major surgery or trauma; and (6) permission from participants' attending health care professional for participation in study. After the telephone interview, participants were invited for onsite screening, which included medical history assessment conducted by a study physician and achieving a score ≥24 on the MoCA.17
Physical activity inclusion criteria
At the screening appointment, participants were asked the following questions for each physical activity that they completed weekly: (1) How many days per week do you exercise? (2) What is your total amount of time (minutes) of exercise per day? (3) On a scale of 1 to 10, please rate the intensity of your exercise (perceived scale: 1–4 = low intensity, 5–6 = moderate, 7–10 = vigorous). (4) How many days per week do you exercise? The study inclusion criteria for physical activity were based on the American College of Sports Medicine guidelines.19 To be eligible for the study, potential participants had to exercise no more than 4 d/wk at a moderate intensity for ≤30 min/d or no more than 2 d/wk at a vigorous intensity for ≤20 min/d.
Information on the metabolic equivalents for the physical activities that participants completed in the year preceding baseline testing has been reported and previously published.20
Standard protocol approvals, registrations, and patient consents
All participants provided informed written consent before enrollment. The University of Calgary Conjoint Health Research Ethics Board provided ethics approval (CHREB:REB 14-2284).
Study design
The BIM study is a quasi-experimental single-group pre/postintervention study. Specific details of the study design and methods have been published.18 Participants completed a total of 5 testing sessions (figure 1). At each session, participants underwent a full cognitive assessment, cerebrovascular function testing, and a fitness assessment (V̇O2max) during 3 separate visits to the laboratory. In this study, the scores obtained in the 2 baseline testing sessions conducted before the start of the intervention (month 0 vs 6) were compared to estimate practice effects on the cognitive tests administered. Assessments obtained in the second preintervention baseline (month 6) and after intervention (month 12) were used to evaluate changes in cognition, cardiorespiratory fitness, and cerebrovascular regulation over the course of the exercise intervention.
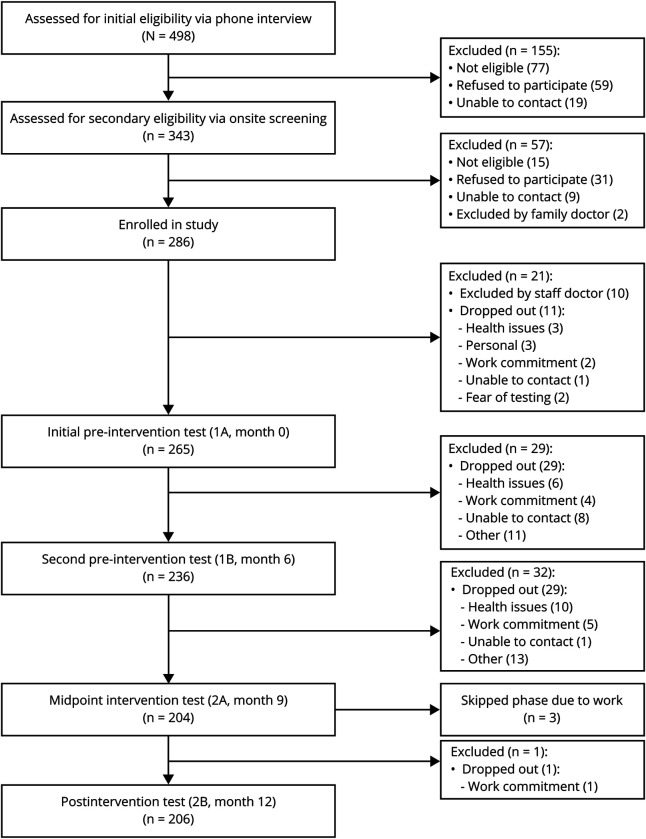
Figure 1. Flowchart of Brain in Motion study participants.
Six-month aerobic exercise intervention
Participants were enrolled in a supervised aerobic exercise program held 3 d/wk at the University of Calgary. Each session included a 5-minute warmup, aerobic exercise, and a 5-minute cooldown with stretching. Exercise prescriptions followed the American College of Sports Medicine guidelines.21 As participants progressed through the program, the duration of exercise increased from 20 to 40 minutes. The aerobic exercise intensity was determined by individual V̇O2max and increased over the course of the sessions from 30% to 45% up to 60% to 70% of participants' heart rate reserve. In addition to the structured intervention, participants were asked to complete an unsupervised exercise session on their own once a week. A logbook kept by participants recorded whether these requested unsupervised sessions and any other independent exercise sessions were completed by them. Further details are provided elsewhere.18
Cognitive assessment
The cognitive assessment consisted of a broad neuropsychological battery measuring processing speed (Symbol-Digit Modalities Test22), executive functions (Cart Sorting Test and Color-Word Inference Test from the Delis-Kaplan Executive Function system23 battery), verbal memory (Buschke Selective Reminding Test24), figural memory (Medical College of Georgia Complex Figure25), fluency (Verbal Fluency Test from the Delis-Kaplan Executive Function system23 battery), and attention (Auditory Consonant Trigram Test26). The choice of tests was based on prior literature on the relationship between exercise and cognition.6 Total testing time was ≈2 hours, and 7 different tests were administered with a total of 20 different outcomes. A detailed description of the tests is available from PRISM Dataverse (doi.org/10.5683/SP2/R5ATDC).
Maximal aerobic capacity
Maximal aerobic capacity (as measured by V̇O2max) and anthropometric measurements were assessed at each testing phase. Exercise testing was completed in the Clinical and Translational Exercise Physiology Laboratory, Cumming School of Medicine, University of Calgary by Certified Exercise Physiologists (Canadian Society of Exercise Physiology). A full description of the assessment is given in the published study protocol.18
In short, the V̇O2max test was conducted on a motorized treadmill that followed a modified Bruce protocol.27 Participants were instructed to exercise until volitional exhaustion or they reached optimal criteria for stopping the test (following recommendations from the American College of Sports Medicine Guidelines for Exercise Testing and Prescription, 10th edition, 2018).21 The criteria for determining whether maximum peak oxygen consumption value was measured included a plateau in uptake of oxygen with increasing work rate (<2.0 mL/kg/min), a respiratory exchange ratio ≥1.15, and achievement of age-predicted maximal heart rate or greater: 220 − (age × 0.65). Outcome measures from this test were maximal oxygen uptake (milliliters per kilogram per minute) and heart rate (beats per minute) and were collected as mean values from the final 15 seconds of the test. These outcomes were used to determine workload for the submaximal exercise portion of the cerebrovascular assessment and to calculate heart rate prescriptions for the exercise intervention.
Cerebrovascular function
Cerebrovascular function testing was conducted at each testing phase within the week after the V̇O2max assessment but on a separate day. The protocol for this test has been previously developed and validated28 and is detailed in the published study methods.18 Cerebrovascular function was assessed at rest and in response to 2 stimuli: euoxic hypercapnia and submaximal exercise. On the day of the test, participants refrained from exercise, eating, and drinking caffeine-containing drinks for at least 4 hours before the test. While fitted with all physiologic testing equipment (3-lead ECG, finger pulse photoplethysmography, and finger pulse oximetry), participants sat quietly in a chair. TCD ultrasound (2-MHz probe; Toc Neurovision, Multigon Industries, Inc, Yonkers, NY) was used to estimate CBF from the middle cerebral artery (MCA). The TCD probe was placed over the temporal region above the zygomatic process, and the MCA was located with the use of previously described techniques.29 Using specialized and dedicated software (Chamber, University Laboratory of Physiology, Oxford, UK), we recorded baseline resting end-tidal respiratory values (Pco2 and Po2). With their nose occluded, participants breathed room air for 10 minutes through a mouthpiece connected to a fine capillary attached to a mass spectrometer (AMIS 2000, Innovision, Odense, Denmark). These averaged end-tidal values were used to determine the desired stages of CO2 levels in the euoxic hypercapnia test.
The euoxic hypercapnia test lasted 17 minutes and consisted of 1 minute of room air breathing, followed by a 5-minute stage holding end-tidal Pco2 (Petco2) constant at +1 mm Hg above the participant's resting Petco2 value, and 2 three-minute step-like changes to +5 mm Hg and then to +8 mm Hg above the participant's resting Petco2 value, with a final recovery period in which the Petco2 was returned to +1 mm Hg above the participant's resting Petco2 value in a stepwise manner and held at that level for 5 minutes. Throughout, the Peto2 was held constant at near-resting values (i.e., 88 mm Hg). To accurately control these desired levels of Peto2 and Petco2 at each stage, the technique of dynamic end-tidal forcing and dedicated software (BreatheM version 2.40; University Laboratory of Physiology, Oxford, UK) were used.30,31
Participants sat quietly for 20 to 30 minutes before submaximal exercise testing. For this 30-minute test, participants breathed room air only through the mouthpiece while the nose was kept closed with a nose clip. The test consisted of a 5-minute rest, 2 six-minute submaximal exercise workloads (relative workload of 40% V̇O2max and absolute workload of 65 W) on a cycle ergometer separated by a 6-minute rest, and a 6-minute recovery after the second workload. For both of the workloads, participants were instructed and coached to maintain 50 to 60 rpm. The relative workload of 40% V̇O2max was determined from each participant's individual V̇O2max at the testing phase. The 65 W workload was maintained through both testing phases. This workload was chosen because it approximates an oxygen consumption of 1.0 L/min and represents an oxygen demand for many activities of daily living.32
The main outcomes of the euoxic hypercapnia and submaximal exercise tests were mean arterial pressure (MAP), mean peak blood flow velocity through the MCA averaged over the cardiac cycle (V̅P), cerebrovascular conductance index (CVCi), and cerebrovascular resistance index (CVRi). Data for these measures were collected every 10 milliseconds (instantaneous value) and then averaged over each cardiac cycle.33 For each stage of hypercapnia and submaximal exercise, the data were again averaged over the final 30 seconds of each stage. CVCi is a measure of cerebrovascular tone (vasodilation) during times of constant pressure and is described as the change in V̅P for a given change in MAP. CVRi is a measure of cerebrovascular tone (vasoconstriction) during times of constant flow and is described as the change in MAP for a given change in V̅P.34,35 V̅P reactivity and CVC reactivity were calculated with a linear regression to describe the slope of the change in V̅P (or CVC) divided by the slope of the change in Petco2 across the 3 stages of the hypercapnia test.
Statistical analysis
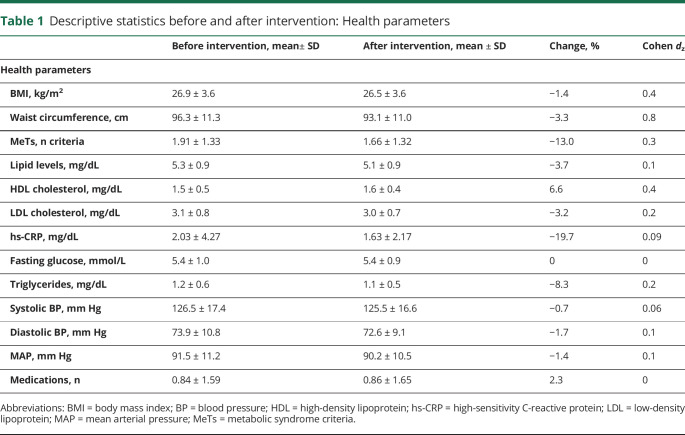
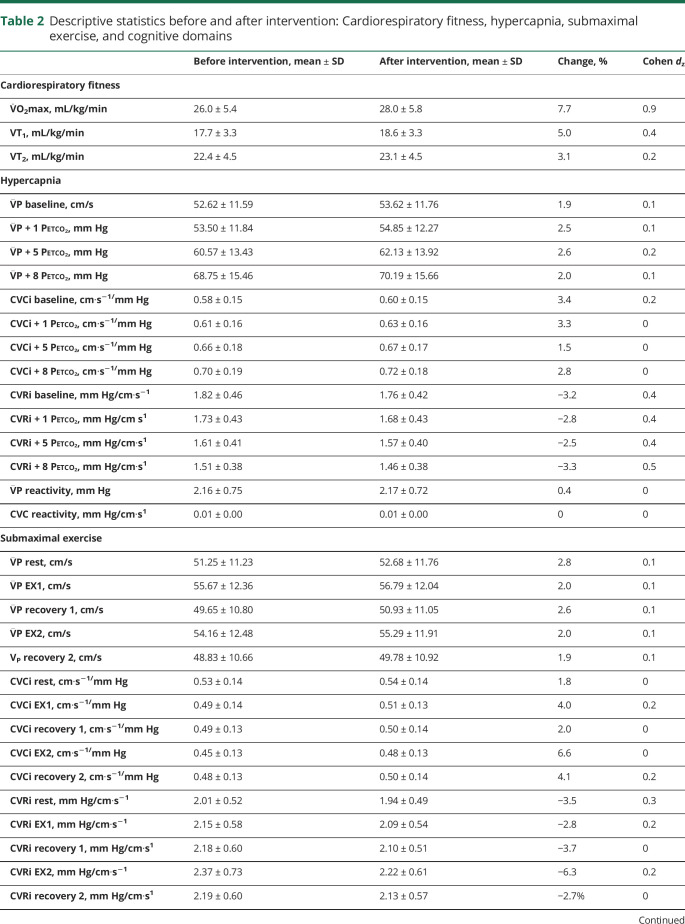
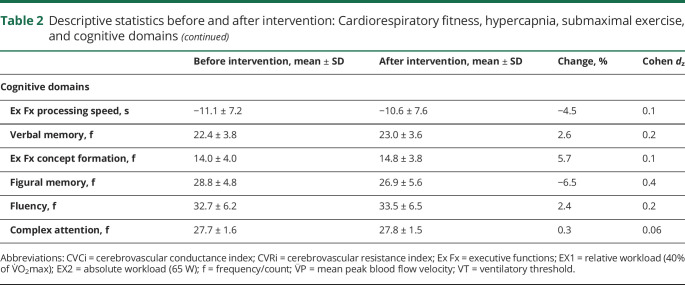
Data were analyzed with IBM SPSS Statistics for Mac, version 24.0 (IBM, Armonk, NY). All analyses were 2 tailed, and significance was set at p < 0.05. Descriptive statistics are reported in tables 1 and 2.
Table 1.
Descriptive statistics before and after intervention: Health parameters
Table 2.
Descriptive statistics before and after intervention: Cardiorespiratory fitness, hypercapnia, submaximal exercise, and cognitive domains
A power analysis conducted before participant recruitment showed that a sample size of 250 participants with an anticipated 15% dropout rate was needed to achieve a 90% power in showing a significant impact on our primary outcome measure. Details are available elsewhere.18
Principal component analysis on cognitive outcomes
To reduce the number of cognitive outcomes analyzed, a principal component analysis (PCA) was used. Principal components were generated from a correlation matrix of the original outcomes collected on the first preintervention baseline assessment (month 0). We used the Kaiser criterion, retaining only components with an eigenvalue >1, and compared these with the ones from a Monte Carlo simulation to further determine the appropriate number of components to retain.36 Factor axes were then subjected to a Varimax rotation.37 Scores on the individual tests from the other test phases were then summed in the corresponding domains using the factor structure identified for the first preintervention baseline assessment.
Cognitive practice effects (month 0 vs 6)
Paired-sample t tests were used to assess for evidence of practice effects from repeated administration of the cognitive battery38 by comparing scores obtained at the initial preintervention testing (month 0) with scores obtained at the time of the second preintervention testing session 6 months later (month 6). No testing sessions or interventions took place during the 6 months between these 2 assessments.
Main outcome: Changes in cognitive function with the exercise intervention (month 6 vs 12)
The main goal of this study was to examine changes in cognitive function over the course of the exercise intervention. Paired-sample t tests were used to assess changes in the cognitive domains between the second preintervention baseline (month 6) and the postintervention assessment (month 12).
Paired-sample t tests were also used to assess changes pre/postintervention (month 6 vs 12) in cardiorespiratory fitness (V̇O2max), resting measure of CBF (V̅P, CVCi, CVRi), and V̅P and CVCi reactivity. Comparisons of CBF results obtained at initial (month 0) and second (month 6) preintervention testing have been published39 and showed no significant changes between these 2 time points.
Independent-samples t tests were used instead to test sex differences in all outcomes.
Secondary outcome: Exploratory analysis of the relationship between changes in cognition and changes in cerebrovascular function
A secondary goal of this study was to explore the relationship between cognitive changes over the course of the exercise intervention and changes in cerebrovascular regulation as measured by outcomes of the euoxic hypercapnia and submaximal exercise tests. This exploratory analysis was conducted only on the cognitive domains that did not show practice effects.
A PCA was used to reduce the number of outcomes from the hypercapnia and submaximal exercise tests. Factors were generated from a correlation matrix of the original change scores for all the CBF outcomes (V̅P, CVCi, CVRi, V̅P reactivity, CVCi reactivity) derived from each step of the hypercapnia and submaximal exercise tests (see above). Change scores were computed by subtracting preintervention scores from postintervention scores (Δ = postintervention − preintervention) and then entered into the PCA. We used the Kaiser criterion and extracted only components with an eigenvalue >1. Results were then compared to the ones from a Monte Carlo simulation.36 Factor axes were subjected to a Varimax rotation37 to ensure independence between the generated factors.
Finally, multiple linear regressions were used to test for associations between changes in cognition and changes in CBF. We entered the postintervention scores for the cognitive domains as a dependent variable in the model. We then entered preintervention cognitive scores and age as forced confounding variables in block 1 and the generated ΔCBF factors in block 2 as independent predictors.
Data availability
The data from this study, including anonymized participant data, study protocol, and statistical analyses, will be shared with qualified researchers on request to the principal investigator (M.J.P., poulin@ucalgary.ca).
Results
Participants
Two hundred six individuals (105 [51%] female; mean ± SD age 65.9 ± 6.4 years; mean ± SD years of formal education 15.9 ± 2.5; mean± SD MoCA score 27.5 ± 1.4) completed the exercise intervention. At the preintervention baseline assessment (month 6), 63 participants reported being on antihypertension medications, 6 on antihyperglycemics, and 42 on lipid-lowering medications. After the intervention (month 12), 65 participants reported to be on antihypertension medications, 6 on antihyperglycemics, and 41 on lipid-lowering medications. A flow diagram of study participants is provided in figure 1, including a detailed breakdown of reasons for exclusions and dropouts from initial screening.
PCA on cognitive outcomes
The Kaiser-Meyer-Olkin40 measure of sampling adequacy in the PCA analysis on the cognitive outcomes was 0.73, above the minimum recommended value of 0.6. The Bartlett test of sphericity was significant (χ2 [190] = 2,400.1, p < 0.001), indicating that the cognitive outcomes were appropriate for the reduction analysis. The main outcome of the PCA was the generation of 6 factors that explained 72.5% of the total variance as suggested by both the Kaiser criterion and the Monte Carlo simulation. Rotated component loadings are available on PRISM Dataverse in table e-1, doi.org/10.5683/SP2/R5ATDC.
Cognitive practice effects (month 0 vs 6)
Comparisons between the initial preintervention (month 0) and second preintervention testing at 6 months (month 6) showed evidence of practice effects in the executive functions/processing speed (month 0 mean ± SD = −12.5 ± 7.5 vs month 6 mean ± SD = −11.0 ± 7.2, t200 = −8.066, p < 0.001, Cohen dZ = 0.5), verbal memory (21.5 ± 3.5 vs 22.4 ± 3.8, t201 = −4.914, p < 0.001, Cohen dZ = 0.3), figural memory (27.9 ± 5.6 vs 28.8 ± 4.8, t204 = −2.825, p = 0.005, Cohen dZ = 0.2), and complex attention (27.4 ± 1.6 vs 27.7 ± 1.6, t202 = −2.819, p = 0.005, Cohen dZ = 0.2) domains. No statistically significant practice effects were present for either the executive functions/concept formation (p = 0.178) or fluency (p = 0.686) domain.
Main outcome: Changes in cognitive function with the exercise intervention (month 6 vs 12)
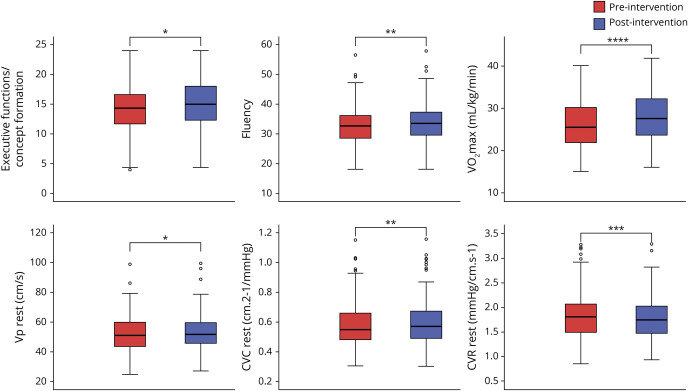
Figure 2 depicts changes in cognitive domains, cardiorespiratory fitness, and cerebrovascular outcomes over the course of the exercise intervention.
Figure 2. Boxplots of changes before and after 6 months of aerobic exercise intervention in executive functions/concept formation and fluency, V̇o2max, and resting baseline CBF outcomes.
CBF = cerebral blood flow; CVC = cerebrovascular conductance; CVR = cerebrovascular resistance. ****p < 0.0001, ***p < 0.001, **p < 0.01, *p < 0.05.
Positive changes before and after intervention in the executive functions/processing speed (t201 = −2.203, p = 0.029, Cohen dZ = 0.1), executive functions/concept formation (t204 = −2.349, p = 0.020, Cohen dZ = 0.1), verbal memory (t200 = −3.331, p = 0.001, Cohen dZ = 0.2), and fluency (t203 = −2.907, p = 0.004, Cohen dZ = 0.2) domains were observed. The figural memory domain showed a negative change (t204 = 6.438, p < 0.001, Cohen dZ = 0.4), possibly due to differences in difficulty between the versions administered before and after intervention. No changes were found in complex attention.
We observed a statistically significant decrease in cardiorespiratory fitness (V̇O2max; t204 = 2.056, p = 0.041) between the initial preintervention testing (V̇O2max ± SD = 26.3 ± 5.5 mL/kg/min, month 0) and the second preintervention testing at 6 months (V̇O2max ± SD = 26.0 ± 5.4 mL/kg/min, month 6), which confirmed that participants did not engage in sufficient aerobic exercise to have a training effect before starting the intervention. In contrast, with the intervention, there was a positive change in cardiorespiratory fitness (V̇O2max; t204 = −13.426, p < 0.001, Cohen dZ = 0.9), confirming that the aerobic exercise intervention was effective in improving the cardiorespiratory fitness of participants.
Resting baseline V̅P (t202 = −2.492, p = 0.014, Cohen dZ = 0.1) and resting baseline CVCi (t202 = −2.809, p = 0.005, Cohen dZ = 0.1) determinations increased. A decrease in resting baseline CVRi was also found (t202 = 3.378, p = 0.001, Cohen dZ = 0.2). No differences were found in V̅P reactivity (p = 0.788) and CVCi reactivity (p = 0.254) before and after intervention. No sex differences were found in the change pre/postintervention in all outcomes.
Secondary outcome: Exploratory analysis of the relationship between changes in cognition and changes in cerebrovascular function
The Kaiser-Meyer-Olkin40 measure of sampling adequacy in the PCA on the change in CBF (ΔCBF) outcomes was 0.75. The Bartlett test of sphericity was significant (χ2 [406] = 5,884.9, p < 0.001). The main outcome of the PCA was the generation of 6 factors. The Monte Carlo simulation was not consistent with the Kaiser criterion and suggested the retention of 4 factors. However, the 6 generated factors were consistent with the physiologic theoretical model and were therefore used. Final rotated component loadings are available on PRISM Dataverse in table e-2, doi.org/10.5683/SP2/R5ATDC.
We investigated the relationship between cognitive changes before and after intervention and changes in cerebrovascular regulation only for the executive functions/concept formation and fluency domains, which were the only domains that did not show changes between the 2 preintervention baselines.
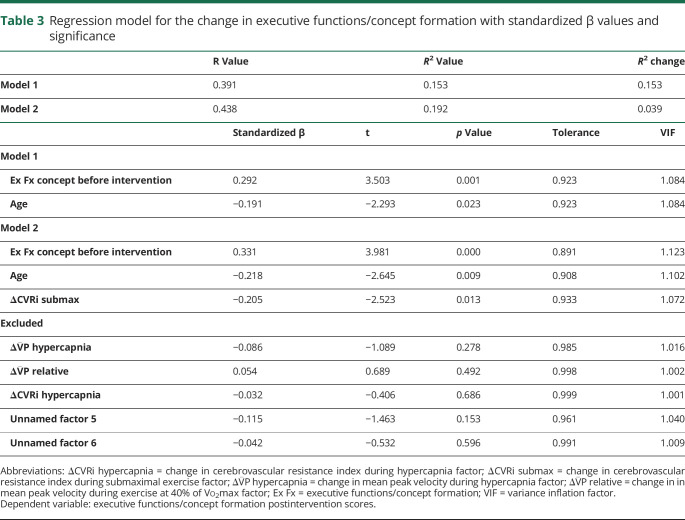
Multiple linear regression on the change in executive functions/concept formation domain showed that after accounting for preintervention test scores (β = 0.331, t133 = 3.981, p < 0.001) and age (β = −0.218, t133 = −2.645, p = 0.009), there was a negative association between the magnitude of the change before and after intervention in CVRi during submaximal exercise and the magnitude of the change before and after intervention in the executive functions/concept formation domain (β = −0.205, t133 = −2.523, p = 0.013, R = 0.438, R2 change = 0.039). Table 3 details results for the models.
Table 3.
Regression model for the change in executive functions/concept formation with standardized β values and significance
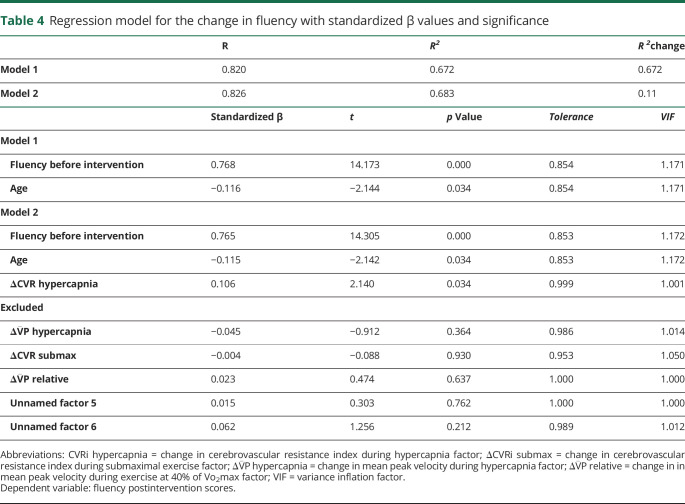
The multiple linear regression on the fluency domain showed that after accounting for preintervention test scores (β = 0.765, t132 = 14.305, p < 0.001) and age (β = −0.115, t132 = −2.142, p = 0.034), the magnitude of the change before and after intervention in CVRi during euoxic hypercapnia was positively associated with the magnitude of the change before and after intervention in the fluency domain (β = 0.106, t133 = 2.140, p = 0.034, R = 0.826, R2 change = 0.011). Table 4 details results for the models.
Table 4.
Regression model for the change in fluency with standardized β values and significance
Discussion
This study examined the effects of a 6-month aerobic exercise intervention on cognition in generally healthy low-active middle-aged and older adults in the BIM study. Furthermore, the study explored the extent to which the changes seen in cognitive performance were related to changes in cerebrovascular regulation. The intervention was associated with improvements in participants' performance on a number of cognitive domains and with improvements in participants' cardiorespiratory fitness and resting measures of cerebrovascular function. The exploratory analysis showed a negative association between the magnitude of the change before and after intervention in the executive functions/concept formation domain and the magnitude of the CVRi change before and after intervention during submaximal exercise after accounting for age. On the other hand, we found a positive association between the magnitude of the change before and after intervention in the fluency domain and the change before and after intervention in CVRi during euoxic hypercapnia after accounting for age. These results indicate that 6 months of aerobic exercise is associated with improvements in some aspects of cognition and that changes in the executive function and fluency domains were differentially associated with changes in cerebrovascular function in healthy, low-active older adults.
Aging is associated with decreased cerebrovascular reserve (i.e., the ability of the cerebrovasculature to dilate in response to a stimulus such as hypercapnia and submaximal exercise).41 This can lead to hypoperfusion and may contribute to an increased risk of ischemic injury and Alzheimer disease.41–43 In the current study, the 6-month aerobic exercise intervention was associated with improved resting CBF, as observed by increased V̅P at rest, and improvements in other measures of cerebrovascular function (i.e., increases in CVCi, and decreases CVRi) with arterial euoxic hypercapnia and submaximal exercise. Euoxic hypercapnia generates predominantly a local (i.e., humoral) stimulus to the pial (i.e., resistance) vessels of the brain. The cerebrovasculature is exquisitely sensitive to subtle changes in the arterial partial pressure of carbon dioxide. With an increase in carbon dioxide (i.e., hypercapnia), a rapid increase in CBF ensues as the vasculature dilates in response to the stimulus. Therefore, CVRi decreases.44 Conversely, with acute exercise, there is a significant and rapid increase in blood pressure to ensure oxygen delivery to working muscles with much smaller perturbations in CBF.45,46 In this instance, CVRi increases. We found that 6 months of aerobic exercise training was associated with decreased CVRi in response to the same preintervention hypercapnia but that CVRi decreased also during submaximal exercise. Thus, training enhanced the vasodilatory effect of CO2 on the cerebral vasculature and blunted the increase in cerebrovascular resistance during submaximal exercise observed before the intervention, suggesting an improvement in cerebrovascular reserve. This is a potentially important finding when we consider recent literature reporting that decreases in CBF precede the accumulation of markers of neurodegenerative disease (e.g., amyloid, tau) and predict conversion from mild cognitive impairment to Alzheimer disease.42
Our results concerning the improvements in cognitive function with training align with results of the noted recent systematic review.6 A recent study47 found that increases in cardiorespiratory fitness over 2 years were associated with improved executive functions and processing speed but not memory. Our results are in line with these findings. A novel finding of this study is that measures of cerebrovascular function during euoxic hypercapnia and submaximal exercise appear to be differentially associated with specific cognitive domains. Specifically, we found positive associations between the magnitude of the change in fluency and the change in CVRi during euoxic hypercapnia and a negative association between the magnitude of the change in executive functions/concept formation and CVRi during submaximal exercise. Findings in the literature on how brain perfusion changes during hypercapnia in areas associated with verbal fluency (inferior frontal gyrus, Broadmann areas 44, 45, and 4748) are controversial. Some studies49 report hypoperfusion in these areas; others50 report hyperperfusion. A recent study14 found that a 12-week aerobic exercise intervention improved working memory and verbal fluency in both healthy controls and individuals with mild cognitive impairment and promoted positive changes in CBF, depending on participants' cognitive status. Similarly, our study suggests that aerobic exercise training may be beneficial for verbal fluency performance through increased CBF and decreased CVRi perhaps in brain areas selectively involved in verbal fluency tasks. Further studies using techniques with greater spatial resolution are needed to confirm this hypothesis.
A recent review45 examined regional distribution of brain blood flow during moderate to exhaustive exercise using near-infrared spectroscopy in the prefrontal cortex, an area commonly associated with executive functions (specifically dorsolateral prefrontal cortex51). The authors report increased CBF in the prefrontal cortex during submaximal exercise (≥30% to <60% of VO2peak) and reduced CBF during exhaustive exercise (≥60% to VO2peak). In our submaximal exercise protocol, individuals have a first bout of exercise at 40% of their V̇O2max and a second bout at 65 W, which would be considered vigorous-intensity exercise according to this systematic review. We could therefore expect no change or even decreases in perfusion in the cortex. Previous studies have described this decrease in CBF during exhaustive exercise as driven by decreases in Pco2 resulting from respiratory compensation for metabolic acidosis.52
This study has several limitations. The lack of a control group does not allow us to exclude the possibility that other factors (i.e., social or cognitive stimulation from the exercise intervention) may have played a role in the observed cognitive improvements. Participants were asked to complete an additional unsupervised exercise session once a week and to record these and other workouts done on their own in a logbook. These additional sessions were not accounted for in our analyses. It is possible that there were significant differences between individuals in how much additional exercise they were doing and that these differences affected our measures of cardiorespiratory fitness, cognition, and cerebrovascular regulation. Moreover, participants were white, well educated, and generally healthy with no evidence of active cardiovascular disease or significant cognitive impairment. Selection bias may have affected the direction and magnitude of the observed changes. Repeated neuropsychological assessments were associated with small but significant practice effects in several cognitive domains. Practice effects from the repeated administration of cognitive tests in longitudinal studies are an important problem.53 How to best minimize their impact, however, remains controversial. Studies suggest that practice effects are greatest when testing is repeated at a 2-week interval, but they persist for up to 3 months. They then tend to decrease with the passage of additional time.38 Bartels et al.38 reported that using an interval of 6 months between cognitive testing sessions could be an effective approach to eliminating practice effects. However, we opted for a more conservative strategy. While we report changes before and intervention for all cognitive domains, we restricted our analysis of the associations between changes in cognition and cerebrovascular function to those domains that did not show practice effects during the 6-month preintervention phase. We acknowledge that this strategy may have led us to exclude cognitive domains in which benefits from exercise occurred. Finally, due to the different versions of the tests used to assess figural memory, we attributed the decrease in figural memory scores after the intervention to the increased difficulty of the tests. However, we cannot exclude the possibility that memory declined during the 6 months between the preintervention and postintervention assessments.
Future studies should include individuals with clinically overt cardiovascular disease or impaired cognition because the benefits found in this healthy cohort may be greater in those with vascular risk factors that are known to be associated with a greater risk of developing dementia or preexisting cognitive deficits. They should also include individuals from diverse ethnic backgrounds and socioeconomic status. A randomized controlled trial design would allow researchers to draw stronger conclusions on the specific effects that aerobic exercise has on cognition compared to other types of exercise such as resistance training. Finally, further research on the differential effects of exercise type, dose, and timing could have an important impact on clinical practice by fostering the use of personalized exercise prescriptions that take into account the baseline health status and functional abilities of the individual.
We found that in a generally healthy low-active middle-aged to older adult cohort, changes in cognition were differentially associated with changes in cerebrovascular regulation. These findings add to what is known about the mechanisms by which aerobic exercise may counteract the cognitive decline seen with normal aging. Results from this study provide suggestive evidence that aerobic exercise has the potential to improve cognitive performance and cerebrovascular regulation in middle-aged and older adults, but future appropriately powered randomized controlled trials are needed to test the specific effects of aerobic exercise on cognition.
Acknowledgment
The authors acknowledge the support from all the staff members and research assistants from the BIM team and all participants in the study. They also acknowledge the work of the Certified Exercise Physiologists (Canadian Society of Exercise Physiology) in the Clinical and Translational Exercise Physiology Laboratory, Cumming School of Medicine, University of Calgary in completing the exercise testing required for the study.
Glossary
- BIM
Brain in Motion
- CBF
cerebral blood flow
- CVCi
cerebrovascular conductance index
- CVRi
cerebrovascular resistance index
- MCA
middle cerebral artery
- MAP
mean arterial pressure
- MoCA
Montreal Cognitive Assessment
- PCA
principal component analysis
- TCD
transcranial Doppler
Appendix. Authors
Footnotes
CME Course: NPub.org/cmelist
Study funding
The BIM study is funded by Canadian Institutes of Health Research (MOP_142470) and The Brenda Strafford Foundation Chair in Alzheimer Research. V.G. was supported by The Brenda Strafford Centre on Aging, within the O'Brien Institute for Public Health, and by the Alzheimer Research Society of Canada.
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Fontana L. Interventions to promote cardiometabolic health and slow cardiovascular ageing. Nat Rev Cardiol 2018;15:566–577. [DOI] [PubMed] [Google Scholar]
- 2.Ungvari Z, Tarantini S, Kiss T, et al. Endothelial dysfunction and angiogenesis impairment in the ageing vasculature. Nat Rev Cardiol 2018;15:555–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reuter-Lorenz PA, Park DC. How does it STAC up? Revisiting the scaffolding theory of aging and cognition. Neuropsychol Rev 2014;24:355–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tyndall AV, Clark CM, Anderson TJ, et al. Protective effects of exercise on cognition and brain health in older adults. Exerc Sport Sci Rev 2018;46:215–223. [DOI] [PubMed] [Google Scholar]
- 5.van Praag H. Exercise and the brain: something to chew on. Trends Neurosci 2009;32:283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joubert C, Chainay H. Aging brain: the effect of combined cognitive and physical training on cognition as compared to cognitive and physical training alone - a systematic review. Clin Interv Aging 2018;13:1267–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cabral DF, Rice J, Morris TP, Rundek T, Pascual-Leone A, Gomes-Osman J. Exercise for brain health: an investigation into the underlying mechanisms guided by dose. Neurotherapeutics 2019;16:580–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sink KM, Espeland MA, Castro CM, et al. Effect of a 24-month physical activity intervention vs health education on cognitive outcomes in sedentary older adults: the LIFE randomized trial. JAMA 2015;314:781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voss MW, Heo S, Prakash RS, et al. The influence of aerobic fitness on cerebral white matter integrity and cognitive function in older adults: results of a one-year exercise intervention. Hum Brain Mapp 2013;34:2972–2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu-Ambrose T, Nagamatsu LS, Graf P, Beattie BL, Ashe MC, Handy TC. Resistance training and executive functions: a 12-month randomized controlled trial. Arch Intern Med 2010;170:170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colcombe SJ, Kramer AF, Erickson KI, et al. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci USA 2004;101:3316–3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chapman SB, Aslan S, Spence JS, et al. Shorter term aerobic exercise improves brain, cognition, and cardiovascular fitness in aging. Front Aging Neurosci 2013;5:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith KJ, Ainslie PN. Regulation of cerebral blood flow and metabolism during exercise. Exp Physiol 2017;102:1356–1371. [DOI] [PubMed] [Google Scholar]
- 14.Alfini AJ, Weiss LR, Nielson KA, Verber MD, Smith JC. Resting cerebral blood flow after exercise training in mild cognitive impairment. J Alzheimers Dis 2019;67:671–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murrell CJ, Cotter JD, Thomas KN, Lucas SJ, Williams MJ, Ainslie PN. Cerebral blood flow and cerebrovascular reactivity at rest and during sub-maximal exercise: effect of age and 12-week exercise training. Age 2013;35:905–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sorond FA, Hollenberg NK, Panych LP, Fisher ND. Brain blood flow and velocity: correlations between magnetic resonance imaging and transcranial Doppler sonography. J Ultrasound Med 2010;29:1017–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rossetti HC, Lacritz LH, Cullum CM, Weiner MF. Normative data for the Montreal Cognitive Assessment (MoCA) in a population-based sample. Neurology 2011;77:1272–1275. [DOI] [PubMed] [Google Scholar]
- 18.Tyndall AV, Davenport MH, Wilson BJ, et al. The brain-in-motion study: effect of a 6-month aerobic exercise intervention on cerebrovascular regulation and cognitive function in older adults. BMC Geriatr 2013;13:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American College of Sports Medicine. ACSM's Guidelines for Exercise Testing and Prescription: Philadelphia: Lippincott Williams & Wilkins; 2013. [DOI] [PubMed] [Google Scholar]
- 20.Gill SJ, Friedenreich CM, Sajobi TT, et al. Association between lifetime physical activity and cognitive functioning in middle-aged and older community dwelling adults: results from the brain in motion study. J Int Neuropsychological Soc 2015;21:816–830. [DOI] [PubMed] [Google Scholar]
- 21.Thompson W, Gordon N, Pescatello L. American College of Sports Medicine's Guidelines for Exercise Testing and Prescription. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 22.Smith A. Symbol Digit Modalities Test. Los Angeles: Western Psychological Services; 1982. [Google Scholar]
- 23.Delis D. Delis-Kaplan Executive Function Scale (D-KEFS). San Antonio: The Psychological Corp; 2001. [Google Scholar]
- 24.Buschke H. Selective reminding for analysis of memory and learning. J Verbal Learn Verbal Behav 1973;12:543–550. [Google Scholar]
- 25.Ingram F, Soukup VM, Ingram P. The Medical College of Georgia Complex Figures: reliability and preliminary normative data using an intentional learning paradigm in older adults. Neuropsychiatry Neuropsychol Behav Neurol 1997;10:144–146. [PubMed] [Google Scholar]
- 26.Shura RD, Rowland JA, Miskey HM. Auditory consonant trigrams: a psychometric update. Arch Clin Neuropsychol 2016;31:47–57. [DOI] [PubMed] [Google Scholar]
- 27.McInnis KJ, Balady GJ. Comparison of submaximal exercise responses using the Bruce vs modified Bruce protocols. Med Sci Sports Exerc 1994;26:103–107. [PubMed] [Google Scholar]
- 28.Brown AD, McMorris CA, Longman RS, et al. Effects of cardiorespiratory fitness and cerebral blood flow on cognitive outcomes in older women. Neurobiol Aging 2010;31:2047–2057. [DOI] [PubMed] [Google Scholar]
- 29.Aaslid R, Markwalder TM, Nornes H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg 1982;57:769–774. [DOI] [PubMed] [Google Scholar]
- 30.Lambertsen CJ. Invited editorial on “Fast and slow components of cerebral blood flow response to step decreases in end-tidal PCO2 in humans”. J Appl Physiol 1998;85:386–387. [DOI] [PubMed] [Google Scholar]
- 31.Poulin MJ, Liang PJ, Robbins PA. Dynamics of the cerebral blood flow response to step changes in end-tidal PCO2 and PO2 in humans. J Appl Physiol 1996;81:1084–1095. [DOI] [PubMed] [Google Scholar]
- 32.Passmore R, Durnin JV. Human energy expenditure. Physiol Rev 1955;35:801–840. [DOI] [PubMed] [Google Scholar]
- 33.Poulin MJ, Liang PJ, Robbins PA. Fast and slow components of cerebral blood flow response to step decreases in end-tidal PCO2 in humans. J Appl Physiol 1998;85:388–397. [DOI] [PubMed] [Google Scholar]
- 34.Claassen JA, Zhang R, Fu Q, Witkowski S, Levine BD. Transcranial Doppler estimation of cerebral blood flow and cerebrovascular conductance during modified rebreathing. J Appl Physiol 2007;102:870–877. [DOI] [PubMed] [Google Scholar]
- 35.Lautt WW. Resistance or conductance for expression of arterial vascular tone. Microvasc Res 1989;37:230–236. [DOI] [PubMed] [Google Scholar]
- 36.Longman RS, Cota AA, Holden RR, Fekken GC. A regression equation for the parallel analysis criterion in principal components analysis: mean and 95th percentile eigenvalues. Multivariate Behav Res 1989;24:59–69. [DOI] [PubMed] [Google Scholar]
- 37.Osborne JW, Costello AB, Kellow JT. Best Practices in Exploratory Factor Analysis. Louisville:CreateSpace Independent Publishing Platform; 2014. [Google Scholar]
- 38.Bartels C, Wegrzyn M, Wiedl A, Ackermann V, Ehrenreich H. Practice effects in healthy adults: a longitudinal study on frequent repetitive cognitive testing. BMC Neurosci 2010;11:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spencer MD, Tyndall AV, Davenport MH, et al. Cerebrovascular responsiveness to hypercapnia is stable over six months in older adults. PLoS One 2015;10:e0143059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dziuban CD, Shirkey EC. When is a correlation matrix appropriate for factor analysis? Some decision rules. Psychol Bull 1974;81:358. [Google Scholar]
- 41.Davenport MH, Hogan DB, Eskes GA, Longman RS, Poulin MJ. Cerebrovascular reserve: the link between fitness and cognitive function? Exerc Sport Sci Rev 2012;40:153–158. [DOI] [PubMed] [Google Scholar]
- 42.Sweeney MD, Kisler K, Montagne A, Toga AW, Zlokovic BV. The role of brain vasculature in neurodegenerative disorders. Nat Neurosci 2018;21:1318–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marston KJ, Brown BM, Rainey-Smith SR, Peiffer JJ. Resistance exercise-induced responses in physiological factors linked with cognitive health. J Alzheimers Dis 2019;68:39–64. [DOI] [PubMed] [Google Scholar]
- 44.Ainslie PN, Poulin MJ. Ventilatory, cerebrovascular, and cardiovascular interactions in acute hypoxia: regulation by carbon dioxide. J Appl Physiol 2004;97:149–159. [DOI] [PubMed] [Google Scholar]
- 45.Rooks CR, Thom NJ, McCully KK, Dishman RK. Effects of incremental exercise on cerebral oxygenation measured by near-infrared spectroscopy: a systematic review. Prog Neurobiol 2010;92:134–150. [DOI] [PubMed] [Google Scholar]
- 46.Ogoh S, Ainslie PN. Cerebral blood flow during exercise: mechanisms of regulation. J Appl Physiol 2009;107:1370–1380. [DOI] [PubMed] [Google Scholar]
- 47.Pentikainen H, Savonen K, Ngandu T, et al. Cardiorespiratory fitness and cognition: longitudinal associations in the FINGER study. J Alzheimers Dis 2019;68:961–968. [DOI] [PubMed] [Google Scholar]
- 48.Wagner S, Sebastian A, Lieb K, Tuscher O, Tadic A. A coordinate-based ALE functional MRI meta-analysis of brain activation during verbal fluency tasks in healthy control subjects. BMC Neurosci 2014;15:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brannan S, Liotti M, Egan G, et al. Neuroimaging of cerebral activations and deactivations associated with hypercapnia and hunger for air. Proc Natl Acad Sci USA 2001;98:2029–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McKay LC, Adams L, Frackowiak RS, Corfield DR. A bilateral cortico-bulbar network associated with breath holding in humans, determined by functional magnetic resonance imaging. Neuroimage 2008;40:1824–1832. [DOI] [PubMed] [Google Scholar]
- 51.Lie CH, Specht K, Marshall JC, Fink GR. Using fMRI to decompose the neural processes underlying the Wisconsin Card Sorting Test. Neuroimage 2006;30:1038–1049. [DOI] [PubMed] [Google Scholar]
- 52.Poulin MJ, Syed RJ, Robbins PA. Assessments of flow by transcranial Doppler ultrasound in the middle cerebral artery during exercise in humans. J Appl Physiol 1999;86:1632–1637. [DOI] [PubMed] [Google Scholar]
- 53.Jones RN. Practice and retest effects in longitudinal studies of cognitive functioning. Alzheimers Demen (Amst) 2015;1:101–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data from this study, including anonymized participant data, study protocol, and statistical analyses, will be shared with qualified researchers on request to the principal investigator (M.J.P., poulin@ucalgary.ca).