Abstract
Background
Some breast tumors expressing greater than 1% and less than 10% estrogen receptor (ER) positivity (ER-borderline) are clinically aggressive; others exhibit luminal biology. Prior ER-borderline studies included few black participants.
Methods
Using the Carolina Breast Cancer Study (phase I: 1993–1996; 2: 1996–2001; 3: 2008–2013), a population-based study that oversampled black women, we compared ER-borderline (n = 217) to ER-positive (n = 1885) and ER-negative (n = 757) tumors. PAM50 subtype and risk of recurrence score (ROR-PT, incorporates subtype, proliferation, tumor size) were measured. Relative frequency differences (RFD) were estimated using multivariable linear regression. Disease-free interval (DFI) was evaluated by ER category and endocrine therapy receipt, overall and by race, using Kaplan Meier and Cox models. Statistical tests were two-sided.
Results
ER-borderlines were more frequently basal-like (RFD = +37.7%, 95% confidence interval [CI] = 27.1% to 48.4%) and high ROR-PT (RFD = +52.4%, 95% CI = 36.8% to 68.0%) relative to ER-positives. Having a high ROR-PT ER-borderline tumor was statistically significantly associated with black race (RFD = +26.2%, 95% CI = 9.0% to 43.3%). Compared to ER-positives, DFI of ER-borderlines treated with endocrine therapy was poorer but not statistically significantly different (hazard ratio [HR] = 2.03, 95% CI = 0.89% to 4.65%), whereas DFI was statistically significantly worse for ER-borderlines without endocrine therapy (HR = 3.33, 95% CI = 1.84% to 6.02%). However, black women with ER-borderline had worse DFI compared to ER-positives, even when treated with endocrine therapy (HR = 2.77, 95% CI = 1.09% to 7.04%).
Conclusions
ER-borderline tumors were genomically heterogeneous, with survival outcomes that differed by endocrine therapy receipt and race. Black race predicted high-risk ER-borderlines and may be associated with poorer endocrine therapy response.
Estrogen receptor (ER) positivity is a prognostic indicator and a powerful predictor of endocrine therapy response in breast cancer (1–4). ER status is typically assessed using immunohistochemical (IHC) stains from which percentage and intensity of positively stained tumor cells can be quantified. In most tumors, ER is either entirely absent or clearly expressed (5,6). However, a small subset of breast tumors exhibits weak (≥1 to <10%) ER-positivity. Many of these so-called borderline tumors have pathological features of ER-negative tumors, with survival outcomes intermediate between ER-negative and ER-positive cases (7–15). As a result, identifying ER-borderline tumors that are responsive to endocrine therapy has been a persistent clinical challenge. In 2010, the American Society of Clinical Oncology and College of American Pathologists issued clinical guidelines classifying ER-positive breast cancers as those with greater than or equal to 1% staining; in these tumors, endocrine therapy is recommended (16). Prior to these guidelines, many clinicians and investigators used greater than or equal to 10% ER positivity to determine eligibility for endocrine therapy (17–19). The evidence supporting the 2010 change in ER threshold was largely derived from clinical trials with low representation of minority women (3,18,19).
The Carolina Breast Cancer Study (CBCS) phase III enrolled black and white women with invasive breast cancer from 2008 to 2013, spanning the 2010 change in clinical guidelines for ER-positivity. This allows a unique opportunity to evaluate ER-borderline outcomes comparing women with guideline-concordant care excluding and including endocrine therapy. Moreover, the study is well positioned to evaluate the role of both race and genomics in ER-borderline breast cancer. The study oversampled black women and conducted genomic profiling to measure intrinsic subtype and risk of recurrence scores.
In this study, we compared the genomic features of ER-borderline tumors to ER-positive and ER-negative tumors in phases I, II, and III of the CBCS and analyzed the disease-free interval (DFI) of women enrolled in phase III to evaluate outcomes of ER-borderline tumors treated with and without endocrine therapy. This work identifies demographic and clinical characteristics associated with poor-prognosis molecular features and assesses whether ER-borderline recurrence rates differ by race or initiation of endocrine therapy.
Methods
Study Population
The CBCS is a population-based study conducted in North Carolina (phase I: 1993–1996; phase II: 1996–2001; and phase III: 2008–2013); study details have been described previously (20,21). Supplementary Figure 1 (available online) depicts study population inclusion. Briefly, women age 20–74 years diagnosed with a first primary invasive breast cancer were enrolled using rapid case ascertainment, oversampling black and younger women (age <50 years). Health history was collected during in-home interviews (22,23). Race was self-reported and categorized as white or black. Less than 2% of nonblack participants self-identified as multiracial, Hispanic, or other race and/or ethnicities and were grouped with white case patients for statistical analyses. The study was approved by the Office of Human Research Ethics at the University of North Carolina at Chapel Hill (UNC). Informed consent was obtained from each participant.
Tumor Characteristics
Tumor size, node status, and stage were abstracted from medical records and pathology reports. Combined grade was centrally assigned by a single breast cancer pathologist (JG) using the Nottingham breast cancer grading system (24); grade was missing for phase II. Progesterone receptor (PR) status was determined from medical records in 80% of cases from phases I and II, and from IHC staining at UNC for remaining cases. HER2 status was determined by IHC staining at UNC for phases I and II and pathology reports for phase III (23).
ER Categories
Quantitative ER data were available for 2859 cases. ER expression was abstracted from medical and pathology records for 496 cases (17%) from phases I and II. Quality assurance studies in CBCS found high accuracy in clinical data ascertainment (23). For remaining cases, formalin-fixed paraffin-embedded (FFPE) tumor tissue blocks were previously sectioned and IHC stained at the Translational Pathology Laboratory at UNC (25). For 1685 cases, multiple tissue microarray cores per case were assessed using automated algorithms (Aperio Technologies, Vista, CA). A core-to-case collapsing method was applied to determine ER percent positivity using a tumor cellularity-weighted approach. Agreement with clinical record was 93% (25). For 679 cases, whole slides were assessed by automated algorithm. Agreement with clinical record was 81% for phases I and II and found to be 89% for phase III (23). For all cases, ER category was defined by percentage of cells staining positively for ER at any intensity, with less than 1% negative, greater than or equal to 1% to less than 10%, and greater than or equal to 10% positive.
Genomic Assessment
The details of RNA isolation and quantification have been published previously (26). Briefly, at UNC central laboratory, RNA was isolated from FFPE tumor blocks using Qiagen FFPE RNeasy kit (Germantown, MD, USA) and quantified using NanoString assay (27). Data that passed quality control using NanoString nSolver software (Seattle, WA, USA) were normalized following nCounter protocol, including background subtraction, positive control normalization, and reference gene normalization. Normalized data were log2 transformed, standardized across samples, and median centered across genes. Genomic analysis was performed for all participants with tumor blocks available. Relative to participants without genomic data, those with genomic data had larger tumors, higher tumor stage, and higher grade and were more likely to be lymph node positive. There was no statistically significant difference in age at diagnosis.
P53 status was assessed using a previously validated 52-gene TP53-dependent signature (28). Mutant-like vs normal-like class was determined by similarity-to-centroid approach using distance-weighted discrimination (29). ESR1 expression was measured using an ESR1-specific probe and NanoString RNA counting methods.
As described previously, PAM50 predictor was used to categorize tumors as luminal A, luminal B, HER2-enriched, basal-like, and normal-like and to calculate risk of recurrence score (ROR-PT), which incorporates subtype with additional weighting by a proliferation gene signature and tumor size (26,30). The ROR-PT score predicts individual risk of distant recurrence (31,32) Normal-like samples were excluded because of insufficient tumor cellularity (n = 50).
Breast Cancer Recurrence
Recurrence data were available only for phase III (n = 2157). DFI was defined as time from diagnosis to subsequent recurrent breast cancer (local, regional, or distant) (33). Breast cancer recurrence, including date, was verified using the medical records of women who reported having a recurrence during telephone follow-up contact, which occurred at 9, 18, 38, 66, 80, 92, and 104 months from enrollment. Recurrence data in this analysis are complete through September 2018. We excluded women with stage IV disease at first diagnosis (n = 65), ER-positive women who did not receive endocrine therapy (n = 100), and ER-negative women who received endocrine therapy (n = 56). HER2-positive (n = 297) and missing HER2 status (n = 2) were excluded. ER-borderlines were grouped by endocrine therapy receipt (n = 36 received endocrine therapy, n = 58 did not). Chemotherapy receipt was assessed. We performed a sensitivity analyses excluding 30 women who experienced a recurrence within 1 year of their first primary breast cancer diagnosis.
Statistical Analyses
Linear binomial regression was used to calculate relative frequency differences (RFD), interpretable as the percentage difference between index and referent groups, and 95% confidence intervals (CI). P values were from two-sided χ2 tests (α = 0.05).
Finite mixture modeling was used to dichotomize tumors as ESR1 high and ESR1 low (34,35). Using the expectation-maximization algorithm described by Do and Batzoglou (36), we estimated parameters that minimized model deviance and assigned a cutoff as the value at which the probability of belonging to the low-ESR1 peak was equivalent to a type-I error at 0.05. Cutoff selection was performed using R version 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria). Median ESR1 expression levels were compared using Kruskal-Wallis test.
We used multiple linear regression to test whether receipt of endocrine therapy among ER-borderlines was associated with clinical variables. Kaplan-Meier curves of DFI were compared using log-rank test with Bonferroni correction for multiple comparisons. P values comparing 5-year recurrence rates were calculated using z-test, assuming normal distribution using the difference of the two rates and standard errors (37). Multivariable Cox proportional hazards models were used to estimate associations between DFI (outcome) and four categories of exposure: ER-positive, ER-borderline with endocrine therapy, ER-borderline without endocrine therapy, and ER-negative. Model 1 adjusted for age and stage. Model 2 additionally adjusted for chemotherapy receipt. Proportional hazards assumptions were assessed visually and found to be valid. The addition of PR status did not substantially change effect estimates (data not shown). We tested for statistical interaction between race and exposure category using likelihood ratio test (α = 0.10). Statistical analyses were conducted using SAS version 9.4 (SAS Institute Inc, Cary, NC).
Results
Clinical, Demographic, and Genomic Features of ER-Borderline Tumors
We first examined clinical and demographic features by ER category in all three phases of CBCS. The 2859 eligible participants included 1509 (52.8%) white and 1350 (47.2%) black women; 7.6% (n = 217) of case patients were ER-borderline, 26.5% (n = 757) were ER-negative, and 65.9% (n = 1885) were ER-positive. ER-negatives and ER-borderlines both exhibited a statistically significantly younger age at diagnosis and were more common among premenopausal women compared to ER-positives. Frequency of ER-borderline tumors did not vary by race. ER-negatives and ER-borderlines both were more frequently high grade (Table 1).
Table 1.
Demographic and clinical characteristics of case patients in Carolina Breast Cancer Study phases I, II, and III by immunohistochemical-defined estrogen receptor category
| Characteristic | ER expression |
||
|---|---|---|---|
| <1% (n = 757; 26.5%) | ≥1–<10% (n = 217; 7.6%) | ≥10% (n = 1885; 65.9%) | |
| Study phase, No. (%) | |||
| 1 | 66 (8.7) | 60 (27.7) | 133 (7.1) |
| 2 | 150 (19.8) | 17 (7.8) | 276 (14.6) |
| 3 | 541 (71.5) | 140 (64.5) | 1476 (78.3) |
| Age at diagnosis, No. (%), y | |||
| <50 (referent) | 432 (57.1) | 127 (58.5) | 857 (45.5) |
| ≥50 | 325 (42.9) | 90 (41.5) | 1028 (54.5) |
| RFD* (95% CI), % | −9.6 (−12.9 to −6.3) | −4.9 (−7.7 to −2.3) | Referent |
| P† | <.001 | <.001 | |
| Menopausal status, No. (%) | |||
| Premenopausal (referent) | 373 (49.3) | 115 (53.0) | 785 (41.6) |
| Postmenopausal | 384 (50.7) | 102 (47.0) | 1100 (58.4) |
| RFD* (95% CI), % | −6.5 (−9.9 to −3.2) | −4.3 (−6.9 to −1.6) | Referent |
| P† | <.001 | .002 | |
| Race, No. (%) | |||
| White (referent) | 288 (38.0) | 118 (54.4) | 1103 (58.5) |
| AA/Black | 469 (62.0) | 99 (46.6) | 782 (41.5) |
| RFD* (95% CI), % | 16.1 (12.6 to 19.4) | 1.4 (−1.1 to 3.9) | Referent |
| P† | <.001 | .26 | |
| PR status, No. (%)‡ | |||
| Positive (referent) | 86 (11) | 96 (44) | 1613 (86) |
| Negative | 665 (89) | 121 (56) | 264 (14) |
| RFD* (95% CI), % | 65.7 (62.5 to 68.9) | 25.6 (20.8 to 30.4) | Referent |
| P† | <.001 | <.001 | |
| HER2 status, No. (%)§ | |||
| Negative | 595 (78.7) | 141 (65.0) | 1427 (75.8) |
| Positive | 123 (16.3) | 47 (21.7) | 273 (14.5) |
| Missing | 38 (5.0) | 29 (13.3) | 183 (9.7) |
| Combined grade, No. (%)|| | |||
| 1 | 13 (2.2) | 30 (18.1) | 448 (29.7) |
| 2 | 84 (14.4) | 45 (27.1) | 711 (47.2) |
| 3 | 485 (83.3) | 91 (54.8) | 349 (23.1) |
| RFD, grade 3 vs grade 1/2* (95% CI), % | 48.6 (44.8 to 52.5) | 13.6 (9.5 to 17.7) | Referent |
| P† | <.001 | <.001 | |
| Tumor size, No. (%), cm|| | |||
| ≤2 | 318 (42.8) | 99 (46.7) | 1130 (60.7) |
| >2–≤5 | 337 (45.4) | 93 (43.9) | 583 (31.3) |
| >5 | 88 (11.8) | 20 (9.4) | 149 (8.0) |
| RFD, >2 vs ≤2 (95% CI), %* | 5.7 (−0.7 to 12.2) | 0.0 (−5.1 to 5.1) | Referent |
| P† | .08 | .99 | |
| Node status, No. (%)|| | |||
| Negative (referent) | 448 (59.4) | 134 (62.0) | 1187 (63.2) |
| Positive | 306 (40.6) | 82 (38.0) | 691 (36.8) |
| RFD* (95% CI), % | 0.8 (−2.6 to 4.2) | −0.3 (−2.9 to 2.3) | Referent |
| P† | .64 | .82 | |
| Stage, No. (%)|| | |||
| I | 232 (31.0) | 88 (41.3) | 904 (48.5) |
| II | 386 (52.5) | 85 (39.9) | 712 (38.2) |
| III | 112 (15.0) | 34 (16.0) | 198 (10.6) |
| IV | 19 (2.5) | 6 (2.8) | 49 (2.6) |
| RFD, III/IV vs I/II* (95% CI), % | 4.9 (0 to 9.9) | 3.3 (−0.9 to 7.4) | Referent |
| P† | .05 | .13 | |
Relative frequency differences (RFD) adjusted for age and race (except for race models, which were adjusted for age only, and age and menopausal status models, which were adjusted for race only). AA = African American; CI = confidence interval; ER = estrogen receptor; PR = progesterone receptor.
Two-sided χ2 test.
Fourteen case patients had missing PR status.
RFD not calculated because of uneven missingness across ER category.
A total of 603 case patients had missing grade, 42 had missing size, 11 had missing node status, and 34 had missing stage.
We next analyzed gene expression profiles of tumors from phases I to III to compare molecular features by ER category. Genomic data were available for 1508 case patients. Intrinsic subtype distribution among ER-borderlines was mixed. ER-negatives and ER-borderlines had a statistically significantly higher frequency of HER2-enriched (17.7% ER-negative, RFD = +67.5%, 95% CI = 58.6 to 76.4; P < .001; 14.3% ER-borderline, RFD = +27.2%, 95% CI = 12.2 to 42.2; P < .001; two-sided χ2 test ) and basal-like subtypes (71.7% ER-negative, RFD = +80.3%, 95% CI = 76.1 to 84.4; P < .001; 41.8% ER-borderline, RFD = +37.7%, 95% CI = 27.1 to 48.4; P < .001; two-sided χ2 test) relative to ER-positive (2.7% HER2-enriched, 5.1% basal-like) (Figure 1). The relative frequency of high ROR-PT score was statistically significantly higher both for ER-negatives (RFD = 67.9%, 95% CI = 61.9 to 73.8; P < .001) and ER-borderlines (RFD = +52.4%, 95% CI = 36.8 to 68.0; P < .001) vs ER-positives (Table 2).
Figure 1.
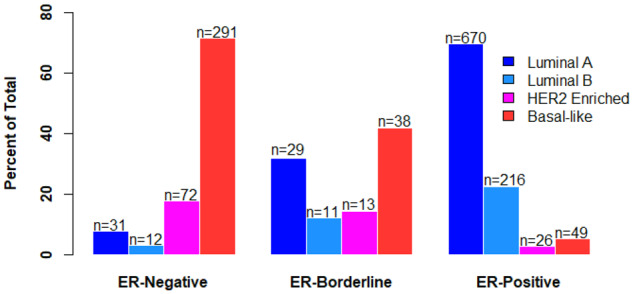
Intrinsic subtype distribution by estrogen receptor (ER) category in Carolina Breast Cancer Study phases I, II, and III. ER-negatives and ER-borderlines had a statistically significantly higher frequency of HER2-enriched (17.7% ER-negative, relative frequency difference [RFD] = +67.5%, 95% confidence interval [CI] = 58.6 to 76.4, P < .001; 14.3% ER-borderline, RFD = +27.2%, 95% CI = 12.2 to 42.2, P <.001; two-sided χ2 test) and basal-like subtypes (71.7% ER-negative, RFD = +80.3%, 95% CI = 76.1 to 84.4, P <.001; 41.8% ER-borderline, RFD = +37.7%, 95% CI = 27.1 to 48.4, P <.001; two-sided χ2 test) relative to ER-positive (2.7% HER2-enriched, 5.1% basal-like).
Table 2.
Distribution of genomic tumor characteristics by immunohistochemical-defined estrogen receptor category in Carolina Breast Cancer Study phases I, II, and III
| Characteristic | ER expression |
||
|---|---|---|---|
| <1% (n = 422; 28%) | ≥1 to <10% (n = 96; 6%) | ≥10% (n = 990; 66%) | |
| ROR-PT* | |||
| Mean score (SD) | 60.24 (20.72) | 48.61 (21.61) | 32.31 (22.30) |
| Low, No. (%) | 19 (4.6) | 8 (8.5) | 277 (28.3) |
| Medium, No. (%) | 217 (52.0) | 62 (66.0) | 622 (63.5) |
| High, No. (%) | 181 (43.4) | 24 (25.5) | 81 (8.3) |
| RFD, high vs low or medium (95% CI), %† | 67.9 (61.9 to 73.8) | 52.4 (36.8 to 68.0) | Referent |
| P‡ | <.001 | <.001 | |
| p53 status | |||
| WT, No. (%) | 155 (36.7) | 49 (51.0) | 722 (72.9) |
| Mutant-like, No. (%) | 267 (63.3) | 47 (49.0) | 268 (27.1) |
| RFD, mutant-like vs WT (95% CI), %† | 29.8 (24.7 to 34.8) | 8.0 (3.8 to 12.2) | Referent |
| P‡ | <.001 | <.001 | |
| ESR1 expression | |||
| ESR1-low, No. (%)§ | 372 (88.2) | 68 (70.8) | 158 (15.9) |
| ESR1-high, No. (%)§ | 50 (11.9) | 28 (29.2) | 836 (84.1) |
| RFD, low vs high (95% CI), %† | 63.2 (58.8 to 67.6) | 26.3 (20.2 to 32.5) | Referent |
| P‡ | <.001 | <.001 | |
Fifty case patients had missing PAM50 subtype, 17 case patients had missing ROR-PT. CI = confidence interval; ER = estrogen receptor; RFD = relative frequency difference; ROR-PT = risk of recurrence, proliferation and tumor size weighted; WT = wild type.
Adjusted for age and race.
Two-sided χ2 test.
ER-negative defined as ESR1 messenger RNA (mRNA) less than 8.77, ER-positive defined as ESR1 mRNA greater than or equal to 8.77.
ER-borderlines also varied with respect to p53 status and quantitative ESR1. ER-negative and ER-borderline tumors both were more likely to be p53 mutant-like and ESR1-low than ER-positive tumors. Median ESR1 expression level of ER-borderline was statistically significantly different from ER-negative (P < .001, Kruskal-Wallis test) and ER-positive (P < .001, Kruskal-Wallis test) with highest expression among ER-positives (median = 10.3, interquartile range [IQR] = 9.3–11.3) followed by ER-borderlines (median = 7.1, IQR = 4.9–9.3) then ER-negatives (median = 5.1, IQR = 4.0–6.6) (Supplementary Figure 2, available online).
We next assessed whether demographic and clinical variables could identify ER-borderline tumors with poor-prognosis genomic features in phases I–III. Black race was statistically significantly associated with high ROR-PT among borderline tumors (38.8% black vs 12.5% white women, RFD = +26.2%, 95% CI = 9.0 to 43.3) (Table 3). High tumor grade was statistically significantly associated with ER-borderlines of basal-like subtype (55% high vs 15% low or medium grade, RFD = +41.9%, 95% CI = 17.7 to 66.0).
Table 3.
Associations of patient and clinical characteristics with high-risk genomic features among estrogen receptor–borderline tumors in Carolina Breast Cancer Study phases I, II, and III
| Characteristic | Intrinsic subtype |
ROR-PT score |
||||||
|---|---|---|---|---|---|---|---|---|
| Nonbasaln = 53 | Basal-like n = 38 | Relative frequency difference* (95% CI), % | P † | Low/ medium ROR-PT n = 65 | High ROR-PTn = 24 | Relative frequency difference* (95% CI), % | P † | |
| Race | ||||||||
| White, No. (%) | 24 (60.0) | 16 (40.0) | Referent | .77 | 35 (87.5) | 5 (12.5) | Referent | .005 |
| Black, No. (%) | 29 (56.9) | 22 (43.1) | +3.1 (−17.1 to 23.3) | 30 (61.2) | 19 (38.8) | 26.2 (9.0 to 43.3) | ||
| Age, y | ||||||||
| <50, No. (%) | 34 (65.4) | 18 (34.6) | Referent | .11 | 37 (72.6) | 14 (27.5) | Referent | .79 |
| ≥50, No. (%) | 19 (48.7) | 20 (51.3) | +16.6 (−3.7 to 37.0) | 28 (73.7) | 10 (26.3) | −1.2 (−17.7 to 15.2) | ||
| Menopausal status | ||||||||
| Premenopausal, No. (%) | 31 (66.0) | 16 (34.0) | Referent | 0.12 | 33 (71.7) | 13 (28.3) | Referent | .57 |
| Postmenopausal | 22 (50.0) | 22 (50.0) | +20.6 (−10.8 to 51.9) | 32 (74.4) | 11 (25.6) | −17.8 (−52.2 to 16.7) | ||
| Grade‡ | ||||||||
| Low or intermediate, No. (%) | 22 (84.6) | 4 (15.4) | Referent | <.001 | 22 (88.0) | 3 (12.0) | Referent | .09 |
| High, No. (%) | 24 (45.3) | 29 (54.7) | +41.9 (17.7 to 66.0) | 32 (61.5) | 20 (38.5) | +23.8 (−5.8 to 53.5) | ||
Adjusted for age and/or race. CI = confidence interval; ER = estrogen receptor; ROR-PT = risk of recurrence, proliferation and tumor size weighted.
Two-sided χ2 test.
Twelve participants had missing grade.
ER-Borderline Tumor Association With Recurrence Outcomes
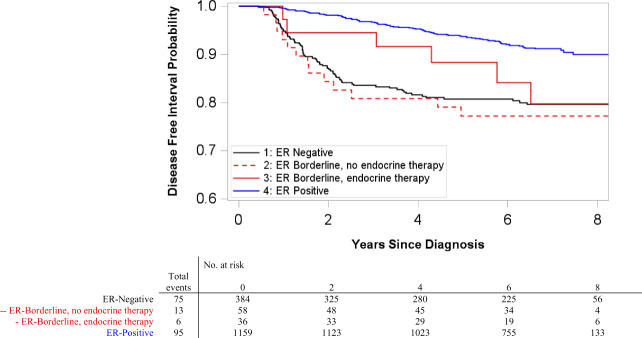
Phase III enrollment spanned 2008–2013 and included women with HER2-negative ER-borderline tumors who received treatment with and without endocrine therapy (16). The recurrence rates for these tumors were compared to those of ER-negatives treated without endocrine therapy and ER-positives treated with endocrine therapy. A total of 189 recurrences occurred over median follow-up of 6.69 years (range = 0.40–9.96). Chemotherapy use was 82.8% (n = 48) among ER-borderlines without endocrine therapy, 69.4% (n = 25) among ER-borderlines with endocrine therapy, 91% (n = 348) among ER-negatives, and 47% (n = 548) among ER-positives. Receipt of endocrine therapy among borderlines was statistically significantly associated with PR-positive status, but not with race, age, year of diagnosis, menopausal status, or stage. ER-negatives and ER-borderlines without endocrine therapy had statistically significantly worse DFI relative to ER-positives, but DFI was not statistically significantly different between ER-positives and ER-borderlines who initiated endocrine therapy (Figure 2). Five-year DFI probability was 93.7% (95% CI = 92.2 to 95.1) for ER-positives, 88.2% (95% CI = 77.3 to 99.1; P = .32, compared to ER-positive, z-test) for ER-borderlines with endocrine therapy, 77.3% for ER-borderlines without endocrine therapy (95% CI = 66.4 to 88.1; P = .002, compared to ER-positive, z-test), and 80.7% for ER-negatives (95% CI = 76.7 to 84.7; P = < .001, compared to ER-positive, z test). Compared to ER-positives, hazard of recurrence for ER-borderlines with endocrine therapy was poorer but not statistically significantly different (hazard ratio [HR] = 2.03, 95% CI = 0.89 to 4.65), whereas ER-orderlines without endocrine therapy fared statistically significantly worse (HR = 3.33, 95% CI = 1.84 to 6.02) (Table 4).
Figure 2.
Kaplan-Meier disease-free interval (DFI) curves among HER2-negative case patients by estrogen receptor (ER) category in Carolina Breast Cancer Study phase III. Included ER-positive case patients who received endocrine therapy (ET) and ER-negative case patients who did not receive ET. Pairwise two-sided log-rank tests were performed with Bonferroni correction for multiple comparisons. ER-negative and ER-positive case patients had statistically significantly different DFI (P < .001). Similarly, ER-borderline case patients not receiving endocrine therapy and ER-positive case patients had statistically significantly different DFI (P < .001). All other pairwise comparisons were not statistically significant.
Table 4.
Adjusted hazard ratios for disease-free interval among HER2-negative case patients by estrogen receptor category and endocrine therapy receipt in Carolina Breast Cancer Study phase III
| Group | No. (No. of recurrences) | All |
White |
Black |
|||
|---|---|---|---|---|---|---|---|
| Reduced model* | Full model† | Reduced model* | Full model† | Reduced model* | Full model† | ||
| ER-positive‡ | |||||||
| HR (95% CI) | 1064 (95) | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| P§ | — | — | — | — | — | — | — |
| ER-borderline, no endocrine therapy | |||||||
| HR (95% CI) | 45 (13) | 3.27 (1.83 to 5.86) | 3.33 (1.84 to 6.02) | 4.78 (2.03 to 11.29) | 4.22 (1.75 to 10.23) | 2.29 (1.03 to 5.11) | 2.53 (1.13 to 5.70) |
| P§ | — | <.001 | <.001 | <.001 | .001 | .04 | .02 |
| ER-borderline, endocrine therapy | |||||||
| HR (95% CI) | 30 (6) | 2.01 (0.88 to 4.60) | 2.03 (0.89 to 4.65) | 1.00 (0.14 to 7.29) | 0.99 (0.14 to 7.19) | 2.55 (1.01 to 6.44) | 2.77 (1.09 to 7.04) |
| P§ | — | .10 | .09 | .99 | .99 | .048 | .03 |
| ER-negative‡ | |||||||
| HR (95% CI) | 309 (75) | 2.41 (1.77 to 3.28) | 2.45 (1.77 to 3.41) | 2.63 (1.59 to 4.35) | 2.36 (1.39 to 4.02) | 2.14 (1.43 to 3.21) | 2.42 (1.57 to 3.74) |
| P§ | — | <.001 | <.001 | <.001 | .002 | <.001 | <.001 |
Adjusted for age, stage. CI = confidence interval; ER = estrogen receptor; HR = hazard ratio.
Adjusted for age, stage, and chemotherapy receipt.
Included ER-positive case patients who received endocrine therapy, included ER-negative case patients who did not receive endocrine therapy.
Two-sided χ2 test.
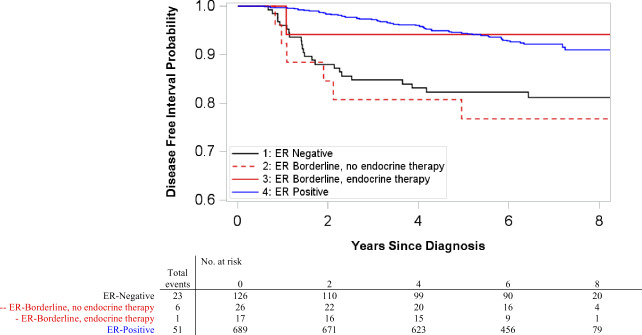
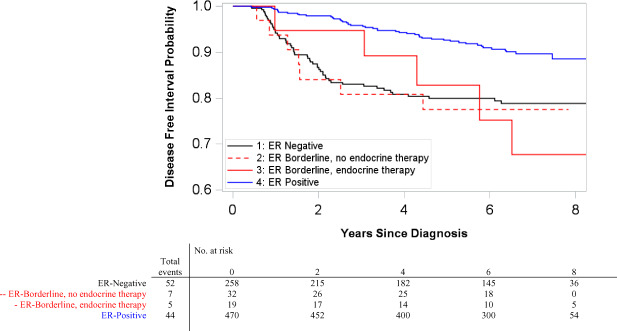
DFI was also examined cross-classified on ER category and race, with results suggesting possible interaction between the two (likelihood ratio test P = .11). Among white women, ER-borderlines with endocrine therapy had recurrence risk similar to ER-positives (HR = 0.99, 95% CI = 0.14 to 7.19, adjusted for age, stage, and chemotherapy receipt), whereas ER-borderlines without endocrine therapy had statistically significantly worse DFI (HR = 4.22, 95% CI = 1.75 to 10.23, adjusted for age, stage, and chemotherapy receipt) (Figure 3). Black women with ER-borderline, however, experienced worse DFI regardless of endocrine therapy receipt (Figure 4). Black women with ER-borderline receiving endocrine therapy had a hazard ratio of 2.77 (95% CI = 1.09 to 7.04, adjusted for age, stage, and chemotherapy receipt); black women not receiving endocrine therapy had a hazard ratio of 2.53 (95% CI = 1.13 to 5.70, adjusted for age, stage, and chemotherapy receipt) (Table 4). Sensitivity analysis excluding 30 recurrences that occurred within 1 year of first primary diagnosis did not change the direction or statistical significance of effect estimates.
Figure 3.
Kaplan-Meier disease-free interval (DFI) curves among HER2-negative white women by estrogen receptor (ER) category in Carolina Breast Cancer Study phase III. Included ER-positive case patients who received endocrine therapy (ET) and ER-negative case patients who did not receive ET. Pairwise two-sided log-rank tests were performed with Bonferroni correction for multiple comparisons. ER-negative and ER-positive case patients had statistically significantly different DFI (P < .001). All other pairwise comparisons were not statistically significant.
Figure 4.
Kaplan-Meier disease-free interval (DFI) curves among HER2-negative black women by estrogen receptor (ER) category in Carolina Breast Cancer Study phase III. Included ER-positive case patients who received endocrine therapy (ET) and ER-negative case patients who did not receive ET. Pairwise two-sided log-rank tests were performed with Bonferroni correction for multiple comparisons. ER-negative and ER-positive case patients had statistically significantly different DFI (P < .001). All other pairwise comparisons were not statistically significant.
Discussion
This study evaluated clinical and genomic features of 217 breast tumors classified as greater than or equal to 1% to less than 10% ER-positive in a large, racially diverse population-based cohort. Relative to ER-positive tumors, ER-borderlines shared many demographic and clinical characteristics with ER-negative tumors, including a higher frequency of younger and premenopausal women, high-grade, high ROR-PT score, and p53 mutant-like status. However, genomic analyses revealed that ER-borderline tumors are more heterogeneous with regard to intrinsic subtype than either ER-negative or ER-positive. DFI in ER-borderlines treated with endocrine therapy was intermediate between ER-negatives and ER-positives, whereas DFI in ER-borderlines without endocrine therapy was statistically significantly worse than ER-positives and statistically indistinguishable from ER-negatives. In race-stratified analyses, the benefit of endocrine therapy was evident for white women, but not for black women, who experienced statistically significantly worse DFI when diagnosed with ER-borderline, regardless of endocrine therapy receipt.
Our finding that ER-borderline tumors have genomic characteristics distinct from ER-positives is in line with earlier studies of mostly white women. Multigene RNA-based genomic characteristics have been reported in several cohorts of ER-borderline cases from clinical trials or academic referral centers (7,8,14,15). Our proportion of ER-borderlines, representing only 8% of breast cancers, is consistent with those in earlier reports, which have found the frequency of ER-borderlines to range from 3% to 13%; Supplementary Table 1 (available online) summarizes prior studies for comparison (7–9,14,38,39). The distribution of intrinsic subtypes observed among ER-borderlines resembles a mixture of that seen in the other two ER categories. Here, we report 44% luminal, with the remainder composed of HER2-enriched and basal-like subtype. This closely aligns with Cheang et al. (8), who found 60% of ER 1–9% positive tumors (n = 65) were basal-like and HER2-enriched subtype. In contrast, Iwamoto et al. (7) reported 92% basal-like and HER2-enriched subtype among 1–9% ER-positive cases (n = 25), which may be due to the higher proportion of advanced tumors in their study (50% of ER 1–9% were >5 cm in size vs 9% in this study). Iwamoto et al. also found that 1–9% ER tumors had ESR1 expression values similar to ER-negatives, whereas we found that ER-borderline ESR1 expression is statistically distinct both from ER-negative and ER-positive tumors. This suggests a mixed nature of the intrinsic subtype distribution among ER-borderlines.
Because clinical guidelines for ER-positivity changed during the enrollment period of CBCS phase III (2008–2013), our study allowed for analysis of ER-borderline outcomes with and without endocrine therapy initiation. Consistent with prior studies, we found that among all women, ER-borderlines who were treated with endocrine therapy exhibited recurrence rates that were not statistically significantly different from ER-positive tumors, whereas ER-borderlines who did not receive endocrine therapy had recurrence rates similar to ER-negatives and statistically significantly different from ER-positives. This finding may reflect a true association between endocrine therapy and ER-borderline outcomes, but it is important to recognize that patients in this observational study were not randomly assigned treatment. Patients not receiving endocrine therapy may have been undertreated in other modalities, which were not accounted for in our analysis.
The genomic features and survival outcomes of ER-borderline tumors among black and white women have not been previously examined. Although our race-stratified analyses were limited in precision because of small numbers of participants, the lack of endocrine therapy benefit among black women with ER-borderline tumors may indicate an important disparity. This disparity may reflect differences in tumor biology as indicated by higher ROR-PT scores among ER-borderline black women but may also reflect different rates of endocrine therapy adherence between black and white women. In previous work in CBCS, black women undergoing endocrine therapy experienced a larger burden of side effects and were more likely to report nonadherence than white women (40). Understanding the factors contributing to endocrine therapy nonadherence will be critical to optimizing treatment delivery for all women.
This study has several strengths, including its large cohort of nearly 50% black and white women, enabling a population-based estimate of the prevalence of ER-borderline breast cancer. We also had detailed treatment and follow-up data and centralized genomic analyses. However, these findings are subject to some limitations. First, there are likely factors not accounted for in this analysis that may influence the association between ER-borderline status and risk of recurrence, such as additional treatment modalities, adherence to endocrine therapy, duration of therapy, and time from diagnosis to treatment. Second, our follow-up data are not yet mature enough to include overall or breast cancer-specific survival, or late recurrences. Thus, this analysis reflects patterns within the early window following diagnosis. Finally, ER-borderline is a relatively uncommon breast cancer phenotype. Consequently, although our study is larger than previous studies of ER-borderline tumor genomics, the small number of ER-borderline tumors precluded a more detailed stratification of recurrence by genomic subtype and resulted in low precision in race-stratified analyses. Nonetheless, this study contributes valuable insight to the small subset of breast cancer cases with low percent positivity of ER.
In summary, we found that although many ER-borderline tumors share similarities with ER-negative tumors, these tumors are, as a group, heterogeneous. Black race and high grade at diagnosis predicted high-risk tumor characteristics among ER-borderline cases. Furthermore, we found that whereas white women with ER-borderline who received endocrine therapy had recurrence risk similar to ER-positives, black women with ER-borderline experienced higher recurrence risk regardless of endocrine therapy receipt. Further work is needed to understand how biology and treatment adherence interact to produce disparities in outcomes for black women.
Funding
This work was supported by a grant from the UNC Lineberger Comprehensive Cancer Center funded by the University Cancer Research Fund (LCCC2017T204), Susan G. Komen Foundation, the National Cancer Institute of the National Institutes of Health (P50-CA58223, U01-CA179715 to MAT and CMP, T32-CA057726 to HCB, F30-CA236199 to HCB), and the National Institute of Environmental Health Sciences of the National Institutes of Health (P30-ES010126 to MAT). HCB is a recipient of the Gertrude B. Elion Mentored Medical Student Research Award of Triangle Community Foundation.
Notes
Affiliations of authors: Department of Epidemiology, Gillings School of Global Public Health (HCB, MAT, XS), Department of Genetics (CMP), Department of Medical Oncology (KERH, LAC), and Department of Pathology and Laboratory Medicine (BCC), University of North Carolina at Chapel Hill, Chapel Hill, NC; Centre for Cancer Research and Cell Biology, Queen’s University Belfast, Belfast, Northern Ireland, UK (EHA); City of Hope National Medical Center, Department of Population Sciences, Duarte, CA (JG).
Prior presentation: Presented at the 2018 San Antonio Breast Cancer Symposium, San Antonio, TX, December 4–8, 2018.
Disclaimers: CMP is an equity stock holder, consultant, and board of directors member of BioClassifier LLC and GeneCentric Diagnostics. CMP is also listed as an inventor on patent applications on the Breast PAM50 assay.
Role of the funder: This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funder had no role in study design, data collection, analysis or interpretation, or writing of the manuscript.
Supplementary Material
References
- 1. Elledge R, Allred D.. Clinical aspects of estrogen and progesterone receptors In: Diseases of the Breast. 3rd ed Philadelphia, PA: Lippincott Williams and Wilkins; 2004:602–617. [Google Scholar]
- 2. Bartlett JM, Brookes CL, Robson T, et al. Estrogen receptor and progesterone receptor as predictive biomarkers of response to endocrine therapy: a prospectively powered pathology study in the Tamoxifen and Exemestane Adjuvant Multinational trial. J Clin Oncol. 2011;29(12):1531–1538. doi: 10.1200/JCO.2010.30.3677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Harvey JM, Clark GM, Osborne CK, Allred DC.. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17(5):1474–1481. [DOI] [PubMed] [Google Scholar]
- 4. Pertschuk LP, Kim DS, Nayer K, et al. Immunocytochemical estrogen and progestin receptor assays in breast cancer with monoclonal antibodies. Histopathologic, demographic, and biochemical correlations and relationship to endocrine response and survival. Cancer. 1990;66(8):1663–1670. [DOI] [PubMed] [Google Scholar]
- 5. Collins LC, Botero ML, Schnitt SJ.. Bimodal frequency distribution of estrogen receptor immunohistochemical staining results in breast cancer: an analysis of 825 cases. Am J Clin Pathol. 2005;123(1):16–20. [DOI] [PubMed] [Google Scholar]
- 6. Nadji M, Gomez-Fernandez C, Ganjei-Azar P, Morales AR.. Immunohistochemistry of estrogen and progesterone receptors reconsidered: experience with 5,993 breast cancers. Am J Clin Pathol. 2005;123(1):21–27. [DOI] [PubMed] [Google Scholar]
- 7. Iwamoto T, Booser D, Valero V, et al. Estrogen receptor (ER) mRNA and ER-related gene expression in breast cancers that are 1% to 10% ER-positive by immunohistochemistry. J Clin Oncol. 2012;30(7):729–734. [DOI] [PubMed] [Google Scholar]
- 8. Cheang MCU, Martin M, Nielsen TO, et al. Defining breast cancer intrinsic subtypes by quantitative receptor expression. Oncologist. 2015;20(5):474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yi M, Huo L, Koenig KB, et al. Which threshold for ER positivity? A retrospective study based on 9639 patients. Ann Oncol. 2014;25(5):1004–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fujii T, Kogawa T, Dong W, et al. Revisiting the definition of estrogen receptor positivity in HER2-negative primary breast cancer. Ann Oncol. 2017;28(10):2420–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Raghav KPS, Hernandez-Aya LF, Lei X, et al. Impact of low estrogen/progesterone receptor expression on survival outcomes in breast cancers previously classified as triple negative breast cancers. Cancer. 2012;118(6):1498–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Landmann A, Farrugia DJ, Zhu L, et al. Low estrogen receptor (ER)–positive breast cancer and neoadjuvant systemic chemotherapy. Am J Clin Pathol. 2018;150(1):34–42. [DOI] [PubMed] [Google Scholar]
- 13. Balduzzi A, Bagnardi V, Rotmensz N, et al. Survival outcomes in breast cancer patients with low estrogen/progesterone receptor expression. Clin Breast Cancer. 2014;14(4):258–264. [DOI] [PubMed] [Google Scholar]
- 14. Deyarmin B, Kane JL, Valente AL, et al. Effect of ASCO/CAP guidelines for determining ER status on molecular subtype. Ann Surg Oncol. 2013;20(1):87–93. [DOI] [PubMed] [Google Scholar]
- 15. Sheffield BS, Kos Z, Asleh-Aburaya K, et al. Molecular subtype profiling of invasive breast cancers weakly positive for estrogen receptor. Breast Cancer Res Treat. 2016;155(3):483–490. [DOI] [PubMed] [Google Scholar]
- 16. Hammond MEH, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Immunohistochemical Testing of Estrogen and Progesterone Receptors in Breast Cancer. J Clin Oncol. 2010;28(16):2784–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Elledge RM, Green S, Pugh R, et al. Estrogen receptor (ER) and progesterone receptor (PgR), by ligand-binding assay compared with ER, PgR and pS2, by immuno-histochemistry in predicting response to tamoxifen in metastatic breast cancer: a Southwest Oncology Group Study. Int J Cancer. 2000;89(2):111–117. [PubMed] [Google Scholar]
- 18. Regan MM, Viale G, Mastropasqua MG, et al. Re-evaluating adjuvant breast cancer trials: assessing hormone receptor status by immunohistochemical versus extraction assays. J Natl Cancer Inst. 2006;98(21):1571–1581. [DOI] [PubMed] [Google Scholar]
- 19. Dowsett M, Allred C, Knox J, et al. Relationship between quantitative estrogen and progesterone receptor expression and human epidermal growth factor receptor 2 (HER-2) status with recurrence in the Arimidex, tamoxifen, alone or in combination trial. J Clin Oncol. 2008;26(7):1059–1065. [DOI] [PubMed] [Google Scholar]
- 20. Newman B, Moorman PG, Millikan R, et al. The Carolina Breast Cancer Study: integrating population-based epidemiology and molecular biology. Breast Cancer Res Treat. 1995;35(1):51–60. [DOI] [PubMed] [Google Scholar]
- 21. Hair BY, Hayes S, Tse C-K, Bell MB, Olshan AF.. Racial differences in physical activity among breast cancer survivors: implications for breast cancer care. Cancer. 2014;120(14):2174–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Millikan RC, Newman B, Tse C-K, et al. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat. 2008;109(1):123–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295(21):2492.. [DOI] [PubMed] [Google Scholar]
- 24. Elston CW, Ellis IO.. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19(5):403–410. [DOI] [PubMed] [Google Scholar]
- 25. Allott EH, Cohen SM, Geradts J, et al. Performance of three-biomarker immunohistochemistry for intrinsic breast cancer subtyping in the AMBER Consortium. Cancer Epidemiol Biomarkers Prev. 2016;25(3):470–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Troester MA, Sun X, Allott EH, et al. Racial differences in PAM50 subtypes in the Carolina Breast Cancer Study. J Natl Cancer Inst. 2018;110(2):176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Geiss GK, Bumgarner RE, Birditt B, et al. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol. 2008;26(3):317–325. [DOI] [PubMed] [Google Scholar]
- 28. Troester MA, Herschkowitz JI, Oh DS, et al. Gene expression patterns associated with p53 status in breast cancer. BMC Cancer. 2006;6(1):276.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marron JS, Todd MJ. Distance weighted discrimination. http://www.optimization-online.org/DB_HTML/2002/07/513.html. Accessed January 10, 2018.
- 30. Parker JS, Mullins M, Cheang MCU, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27(8):1160–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dowsett M, Sestak I, Lopez-Knowles E, et al. Comparison of PAM50 risk of recurrence score with oncotype DX and IHC4 for predicting risk of distant recurrence after endocrine therapy. J Clin Oncol. 2013;31(22):2783–2790. [DOI] [PubMed] [Google Scholar]
- 32. Gnant M, Filipits M, Greil R, et al. Predicting distant recurrence in receptor-positive breast cancer patients with limited clinicopathological risk: using the PAM50 risk of recurrence score in 1478 postmenopausal patients of the ABCSG-8 trial treated with adjuvant endocrine therapy alone. Ann Oncol. 2014;25(2):339–345. [DOI] [PubMed] [Google Scholar]
- 33. Hudis CA, Barlow WE, Costantino JP, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol. 2007;25(15):2127–2132. [DOI] [PubMed] [Google Scholar]
- 34. Schlattmann P. Medical Applications of Finite Mixture Models. Berlin, Heidelberg: Springer; 2009. [Google Scholar]
- 35. Trang NV, Choisy M, Nakagomi T, et al. Determination of cut-off cycle threshold values in routine RT–PCR assays to assist differential diagnosis of norovirus in children hospitalized for acute gastroenteritis. Epidemiol Infect. 2015;143(15):3292–3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Do CB, Batzoglou S.. What is the expectation maximization algorithm? Nat Biotechnol. 2008;26(8):897–899. [DOI] [PubMed] [Google Scholar]
- 37. Parkin DM, Hakulinen T.. Cancer registration: principles and methods. Analysis of survival. IARC Sci Publ. 1991;95:159–176. [PubMed] [Google Scholar]
- 38. Ogawa Y, Moriya T, Kato Y, et al. Immunohistochemical assessment for estrogen receptor and progesterone receptor status in breast cancer: analysis for a cut-off point as the predictor for endocrine therapy. Breast Cancer. 2004;11(3):267–275. [DOI] [PubMed] [Google Scholar]
- 39. Chen T, Zhang N, Moran MS, Su P, Haffty BG, Yang Q.. Borderline ER-positive primary breast cancer gains no significant survival benefit from endocrine therapy: a systematic review and meta-analysis. Clin Breast Cancer. 2018;18(1):1–8. [DOI] [PubMed] [Google Scholar]
- 40. Wheeler SB, Spencer J, Pinheiro LC, et al. Endocrine therapy nonadherence and discontinuation in black and white women. J Natl Cancer Inst. 2019;111(5):498–508. doi: 10.1093/jnci/djy136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.