Abstract
Background
Despite increasing incidence of hepatocellular carcinoma (HCC) among HIV-infected patients, it remains unclear if HIV-related factors contribute to development of HCC. We examined if higher or prolonged HIV viremia and lower CD4+ cell percentage were associated with HCC.
Methods
We conducted a cohort study of HIV-infected individuals who had HIV RNA, CD4+, and CD8+ cell counts and percentages assessed in the Veterans Aging Cohort Study (1999–2015). HCC was ascertained using Veterans Health Administration cancer registries and electronic records. Cox regression was used to determine hazard ratios (HR, 95% confidence interval [CI]) of HCC associated with higher current HIV RNA, longer duration of detectable HIV viremia (≥500 copies/mL), and current CD4+ cell percentage less than 14%, adjusting for traditional HCC risk factors. Analyses were stratified by previously validated diagnoses of cirrhosis prior to start of follow-up.
Results
Among 35 659 HIV-infected patients, 302 (0.8%) developed HCC over 281 441 person-years (incidence rate = 107.3 per 100 000 person-years). Among patients without baseline cirrhosis, higher HIV RNA (HR = 1.25, 95% CI = 1.12 to 1.40, per 1.0 log10 copies/mL) and 12 or more months of detectable HIV (HR = 1.47, 95% CI = 1.02 to 2.11) were independently associated with higher risk of HCC. CD4+ percentage less than 14% was not associated with HCC in any model. Hepatitis C coinfection was a statistically significant predictor of HCC regardless of baseline cirrhosis status.
Conclusion
Among HIV-infected patients without baseline cirrhosis, higher HIV RNA and longer duration of HIV viremia increased risk of HCC, independent of traditional HCC risk factors. This is the strongest evidence to date that HIV viremia contributes to risk of HCC in this group.
Hepatocellular carcinoma (HCC) is a growing cause of cancer death among people living with HIV infection (1). Driven largely by hepatitis C virus (HCV) coinfection, hepatitis B virus (HBV) coinfection, and alcoholic liver disease, the incidence of HCC among HIV-infected persons in North America has risen more than fourfold from 1995 to 2009 (2). Moreover, HIV-infected individuals have a fourfold higher risk of HCC than uninfected persons (3).
Despite the rising incidence of HCC among HIV-infected individuals, the determinants of this malignancy remain largely unknown in this group (4). Three prior studies found no association between HIV suppression and risk of HCC (5–7), but these studies did not evaluate the effects of longer durations or higher levels of HIV viremia, nor did they account for cirrhosis. Moreover, previous studies among predominantly HIV and HCV-coinfected patients reported that lower absolute CD4+ cell counts increased the risk of HCC (6,8–12). However, absolute CD4+ cell count may decrease during cirrhosis because of portal hypertension-induced splenic sequestration (13). Thus, studies evaluating associations between absolute CD4+ cell count and HCC risk cannot determine whether observed associations are driven by HIV-related immunosuppression or progression of liver disease. Consequently, it remains unclear if higher HIV RNA levels, longer duration of detectable HIV, and HIV-related immunosuppression contribute to development of HCC independent of traditional determinants. Identifying such factors could help define the mechanisms for the high rate of HCC among HIV-infected persons.
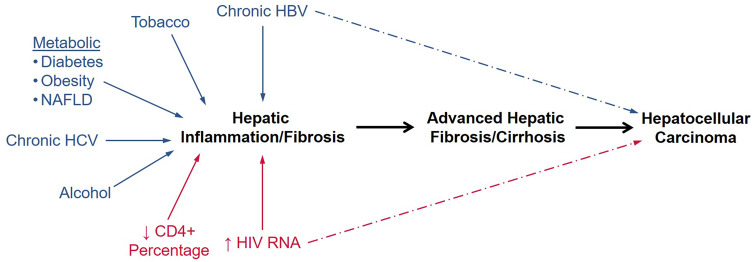
Cirrhosis, which represents the late stage of progressive hepatic fibrosis due to chronic liver disease, is an important step in the causal pathway toward HCC (Figure 1) (14). Cirrhosis promotes HCC through telomere dysfunction and alterations of the liver milieu (eg, increased production of toxic hepatic metabolites, cytokines, growth factors, and products of oxidative stress) (15). Because cirrhosis increases the risk of HCC substantially, studies evaluating the factors associated with HCC must account for baseline cirrhosis status.
Figure 1.
Pathway to development of hepatocellular carcinoma (HCC). The figure shows the contributions of the key modifiable determinants of HCC, including traditional risk factors (blue) and the hypothesized HIV-related determinants in this study (red). Both chronic hepatitis B virus (HBV) infection and HIV viremia could increase the risk of HCC by inducing hepatic fibrosis and cirrhosis or by directly promoting development of HCC outside of the liver fibrosis pathway. NAFLD = non-alcoholic fatty liver disease; RNA = ribonucleic acid.
We evaluated HIV-related and traditional risk factors for HCC among HIV-infected patients. We hypothesized that higher HIV RNA levels, longer duration of HIV viremia, and lower CD4+ cell percentage, which is not affected by liver disease progression (13), were statistically significant determinants of HCC. We also examined the risk of HCC with traditional risk factors in the general population, including older age, black race, overweight or obesity, diabetes mellitus, alcohol dependence or abuse, tobacco use, and HBV and HCV coinfection (16). To account for the role of cirrhosis in the development of HCC and the possibility that risk factors for HCC might vary by presence of cirrhosis, we stratified our analysis by baseline cirrhosis status.
Methods
Study Design and Data Source
We conducted a retrospective cohort study among HIV-infected individuals in the Veterans Aging Cohort Study (VACS) between October 1, 1999, and September 30, 2015 (17). The VACS consists of electronic medical record data from HIV-infected patients receiving care at Veterans Health Administration (VA) facilities across the United States. Data include demographics, hospital and outpatient diagnoses (recorded using International Classification of Diseases, Ninth Revision [ICD-9] codes), procedures, laboratory results, and dispensed medications. Death date was determined from the VA Vital Status File. The study was approved by the institutional review boards of the University of Pennsylvania, Corporal Michael J. Crescenz VA Medical Center in Philadelphia, VA Connecticut Healthcare System, and Yale University.
Study Patients
HIV-infected patients were included if they had HIV RNA, CD4+ cell count and percentage, and CD8+ cell count and percentage simultaneously assessed (which occurs routinely as part of HIV care in the VA system) between October 1, 1999, and September 30, 2015, and had at least 180 days of observation after determination of these laboratory results. We defined the start of follow-up as 180 days after the date that HIV RNA, CD4+, and CD8+ results were assessed. The 180 days prior to start of follow-up represented the baseline period, during which baseline comorbidities and laboratory results were collected. Patients were excluded if they had HCC diagnosed prior to start of follow-up. Follow-up continued until HCC, death, or last VA visit before September 30, 2015.
Main Study Outcomes
The primary outcome was incident HCC diagnosis. HCC diagnoses were determined from the VA national cancer registry by topography code C22.0 (liver) and morphology codes 8170–8180 (HCC) from the International Classification of Diseases for Oncology, Third Edition (ICD-O-3) (18), consistent with Surveillance, Epidemiology, and End Results coding algorithms (19). The VA cancer registry records cancers diagnosed and/or treated within the VA (7). ICD-O-3 codes validly identify cancer diagnoses, including HCC (20). To account for lags in reporting diagnoses in the cancer registry and minimize the likelihood of missing HCC events, we supplemented HCC case finding with ICD-9 diagnoses for HCC (155.0, 155.1, and 155.2) recorded in the VA electronic medical record. Prior research has shown that use of both VA cancer registry records and ICD-9 diagnoses have 90% sensitivity for incident cancer diagnosis when compared to chart review; however, positive predictive value varied (96% for VA cancer registry; 63% for ICD-9 diagnosis) (20,21). Consequently, HCC diagnoses from the registry and claims were confirmed by medical record review by trained adjudicators. For all confirmed HCC diagnoses, we determined the presence of cirrhosis by review of medical records within 1 year prior to HCC diagnosis. Details on cirrhosis adjudication appear in the Supplementary Methods (available online).
Data Collection
Baseline data included age, sex, race and/or ethnicity, body mass index, diabetes [defined by random glucose ≥200 mg/dL, hemoglobin A1c ≥6.5%, or antidiabetic drug use (22)], alcohol dependence or abuse, injection and/or noninjection drug use, tobacco use (ever), HBV coinfection (ever positive HBV surface antigen), HCV coinfection (ever detectable HCV RNA or genotype), cirrhosis, HIV RNA, absolute CD4+ and CD8+ counts and percentages, and antiretroviral therapy (ART) use. Cirrhosis was defined by a hospital discharge diagnosis or outpatient diagnosis for cirrhosis or hepatic decompensation (Supplementary Table 1 available online). Prior studies validated this determination within the VA system, with no less than 90% of these diagnoses confirmed by medical records (23,24). Alanine aminotransferase (ALT), aspartate aminotransferase (AST), and platelet count were collected from dates closest, but within 360 days prior, to start of follow-up. FIB-4, a noninvasive measure of hepatic fibrosis, was calculated by (age [years] x AST [U/L])/(platelet count [109/L]) x (ALT [U/L])1/2) (25).
Time-varying variables were assessed on a monthly basis and included HIV RNA, CD4+ cell count or percentage, CD8+ cell count or percentage, and diabetes. When multiple results of the same test were measured within a month, to be most conservative, we used the highest HIV RNA and CD8+ results and lowest CD4+ result. When a test was not updated during a month, we carried forward the value from the previous month until the next available result. HIV RNA, CD4+, and CD8+ results were updated at the beginning of each 30-day interval from baseline but were lagged by 180 days [approximate mean doubling time of HCC tumors <5 centimeters in length (26)] to reduce the possibility that the presence of HCC influenced these variables (ie, reverse causality).
Statistical Analysis
We determined unadjusted incidence rates (IR) of HCC (events per 100 000 person-years), overall and by HBV and HCV status. We used multivariable Cox regression to determine adjusted hazard ratios (HR; 95% confidence interval [CI]) of HCC for risk factors of interest. HIV-related factors included higher time-updated HIV RNA level, longer duration of detectable HIV (≥500 copies/mL), and lower time-updated CD4+ percentage. Traditional HCC risk factors examined included older age, black race, overweight or obesity, diabetes, alcohol dependence or abuse, ever use of tobacco, HBV coinfection, and HCV coinfection (16). Given the importance of cirrhosis on development of HCC, analyses were stratified by baseline cirrhosis status.
To evaluate the effects of HIV viremia on HCC risk, we created four separate models that examined HIV as a time-updated: continuous variable by 1.0 log10 increments (model #1); categorical variable defined by detectable HIV (≥500 copies/mL; model #2); categorical variable classified by increasing categories of HIV RNA (<500 copies/mL; 500–9999 copies/mL; ≥10 000 copies/mL; model #3); and categorical variable classified by increasing consecutive months of detectable HIV (compared to those with undetectable HIV; model #4). For model #4, detectable HIV was evaluated as a monthly time-updated variable. Once a patient was classified with HIV viremia, consecutive months were counted until a viral load less than 500 copies/mL was identified. If detectable HIV recurred, the count of consecutive months of viremia was restarted at one month. We determined hazard ratios of HCC associated with increasing consecutive months of detectable HIV (1–11 months; ≥12 months) compared to persons whose HIV RNA were suppressed throughout follow-up, adjusting for all other risk factors.
We performed five sensitivity analyses to assess the robustness of our results. First, we repeated the analysis accounting for competing risk of death (27). Second, we repeated analyses, lagging HIV RNA and CD4+ cell percentages by 360 and 540 days. Third, we evaluated risk factors for HCC separately among persons with HBV coinfection, HCV coinfection, and without viral hepatitis. Fourth, to explore the potential impact of hepatic steatosis on HCC risk, we classified patients with possible fatty liver disease prior to start of follow-up based on the presence of both obesity (body mass index ≥30 kg/m2) and diabetes mellitus, because these act synergistically to promote steatosis (28), and examined associations with HCC. Fifth, because duration of HIV-related immunosuppression might affect HCC risk, we determined whether longer consecutive months with CD4+ percentage less than 14% increased risk of HCC. Finally, in an exploratory analysis, we examined the risk of HCC with lower time-updated CD4+ to CD8+ ratio, which indicates dysfunctional immune activation (29), by cirrhosis status.
Proportionality of hazards was assessed by log-log plots and Schoenfeld residuals (30). To address the potential bias of missing data among covariates, we implemented multiple imputation using chained equations by means of 10 imputations using all variables in Table 1 (31). Results across the 10 datasets were combined to arrive at confidence intervals that accounted for within- and across-dataset variances. Data were analyzed using SAS 9.4 (SAS Institute Inc., Cary, NC). All P values were two-sided. Statistical significance was declared if the 95% confidence interval did not cross 1.00.
Table 1.
Baseline characteristics of study patients, stratified by cirrhosis diagnosis
| Characteristic* | Overall No. (%) (n = 35 659) | Baseline no cirrhosis† No. (%) (n = 34 886) | Baseline cirrhosis† No. (%) (n = 733) |
|---|---|---|---|
| Median age (IQR), y | 46 (39–53) | 46 (39–53) | 49 (44–55) |
| Male sex | 34 792 (97.6) | 34 032 (97.6) | 760 (98.3) |
| Race/ethnicity | |||
| Black | 17 069 (47.9) | 16 750 (48.0) | 319 (41.3) |
| Caucasian | 13 859 (38.9) | 13 522 (38.8) | 337 (43.6) |
| Hispanic | 2720 (7.6) | 2632 (7.5) | 88 (11.4) |
| Other/Unknown | 2011 (5.6) | 1982 (5.7) | 29 (3.8) |
| Body mass index | |||
| Underweight, <18.50 kg/m2 | 837 (2.3) | 819 (2.3) | 18 (2.3) |
| Normal, 18.50–24.99 kg/m2 | 14 083 (39.5) | 13 768 (39.5) | 315 (40.8) |
| Overweight, 25.00–29.99 kg/m2 | 11 415 (32.0) | 11 184 (32.1) | 231 (29.9) |
| Obesity, 30.00–34.99 kg/m2 | 3882 (10.9) | 3789 (10.9) | 93 (12.0) |
| Morbid obesity, ≥35.00 kg/m2 | 1323 (3.7) | 1299 (3.7) | 24 (3.1) |
| Missing weight and/or height | 4119 (11.6) | 4027 (11.5) | 92 (11.9) |
| Diabetes mellitus | 3308 (9.3) | 3150 (9.0) | 158 (20.4) |
| History of alcohol dependence/abuse | 10 538 (29.6) | 10 064 (28.8) | 474 (61.3) |
| History of injection/noninjection drug use | 16 235 (45.5) | 15 781 (45.2) | 454 (58.7) |
| Tobacco use | |||
| Never | 9533 (26.7) | 9393 (26.9) | 140 (18.1) |
| Ever‡ | 24 707 (69.3) | 24 158 (69.2) | 549 (71.0) |
| Unknown | 1419 (4.0) | 1335 (3.8) | 84 (10.9) |
| Hepatitis C virus coinfection§ | |||
| Detectable HCV RNA or genotype | 11 392 (31.9) | 10 940 (31.4) | 452 (58.5) |
| Ever treated with HCV antiviral | 1354 (11.9) | 1304 (11.9) | 50 (11.1) |
| HCV antibody+/HCV RNA- | 1055 (3.0) | 1020 (2.9) | 35 (4.5) |
| HCV antibody- | 21 472 (60.2) | 21 247 (60.9) | 225 (29.1) |
| Never tested | 1740 (4.9) | 1679 (4.8) | 61 (7.9) |
| Hepatitis B virus coinfection‖ | |||
| HBsAg+ | 1981 (5.6) | 1873 (5.4) | 108 (14.0) |
| Ever treated with HBV-active antiretroviral | 1840 (92.9) | 1748 (93.3) | 92 (85.2) |
| HBsAg- | 31 712 (88.9) | 31 096 (89.1) | 616 (79.7) |
| Never tested | 1966 (5.5) | 1917 (5.5) | 49 (6.3) |
| HIV RNA | |||
| Median (IQR), log10 cells/mm3 | 3.2 (1.7–4.6) | 3.2 (1.7–4.6) | 3.0 (1.7–4.6) |
| ≥500 copies/mL | 20 216 (56.7) | 19 791 (56.7) | 425 (55.0) |
| CD4+ cell percentage | |||
| Median (IQR) | 22 (14–31) | 22 (14–31) | 22 (14–31) |
| ≥28% | 11 776 (33.0) | 11 530 (33.1) | 246 (31.8) |
| 14–27.99% | 14 798 (41.5) | 14 456 (41.4) | 342 (44.2) |
| <14% | 8513 (23.9) | 8337 (23.9) | 176 (22.8) |
| Unknown | 572 (1.6) | 563 (1.6) | 9 (1.2) |
| CD4+/CD8+ ratio | |||
| Median (IQR) | 0.40 (0.21–0.69) | 0.40 (0.21–0.69) | 0.42 (0.21–0.70) |
| <1.0 | 31 553 (88.5) | 30 879 (88.5) | 674 (87.2) |
| Median alanine aminotransferase (IQR), U/L | 31 (21–47) | 30 (21–47) | 41 (27–71) |
| Not assessed at baseline | 2362 (6.6) | 2338 (6.7) | 24 (3.1) |
| Median aspartate aminotransferase (IQR), U/L | 30 (23–44) | 29 (22–43) | 55 (32–94) |
| Not assessed at baseline | 1987 (5.6) | 1964 (5.6) | 23 (3.0) |
| Platelet count, x 106/L | |||
| ≥150 000 | 30 570 (85.7) | 30 194 (86.6) | 376 (48.6) |
| <150 000 | 4812 (13.5) | 4420 (12.7) | 392 (50.7) |
| Not assessed at baseline | 277 (0.8) | 272 (0.8) | 5 (0.6) |
| Median baseline FIB-4 (IQR) | 1.18 (0.81–1.78) | 1.17 (0.81–1.74) | 3.00 (1.58–5.82) |
| Unable to be calculated at baseline | 3325 (9.3) | 3290 (9.4) | 35 (4.5) |
| On antiretroviral therapy | 24 506 (68.7) | 23 980 (68.7) | 526 (68.0) |
| Most common baseline antiretroviral regimens¶ | |||
| Efavirenz/tenofovir/emtricitabine | 3639 (14.8) | 3565 (14.9) | 74 (14.1) |
| Efavirenz/zidovudine/lamivudine | 1764 (7.2) | 1738 (7.2) | 26 (4.9) |
| Nelfinavir/zidovudine/lamivudine | 1177 (4.8) | 1156 (4.8) | 21 (4.0) |
| Indinavir/zidovudine/lamivudine | 1033 (4.2) | 1015 (4.2) | 18 (3.4) |
| Atazanavir/tenofovir/emtricitabine | 965 (3.9) | 950 (4.0) | 15 (2.9) |
| Nelfinavir/stavudine/lamivudine | 888 (3.6) | 863 (3.6) | 25 (4.8) |
| Indinavir/stavudine/lamivudine | 725 (3.0) | 708 (3.0) | 17 (3.2) |
| Nevirapine/zidovudine/lamivudine | 663 (2.7) | 652 (2.7) | 11 (2.1) |
| Efavirenz/stavudine/lamivudine | 611 (2.5) | 590 (2.5) | 21 (4.0) |
HBsAg = hepatitis B surface antigen; HBV = hepatitis B virus; HCV = hepatitis C virus; HIV = human immunodeficiency virus; IQR = interquartile range; RNA = ribonucleic acid.
Results reported as n (%) unless otherwise specified.
Cirrhosis defined as any diagnosis of compensated or decompensated cirrhosis prior to the start of follow-up.
Ever tobacco use includes current and prior tobacco use.
Hepatitis C virus coinfection defined as positive quantitative HCV RNA (absolute value determinable or not), positive qualitative HCV RNA, or quantifiable HCV genotype at baseline or during follow-up.
Hepatitis B virus coinfection defined as positive HBV surface antigen at baseline or during follow-up.
Percentages represent proportion of antiretroviral use among patients prescribed therapy.
Results
Patient Characteristics
We identified 37 946 HIV-infected individuals in the VACS who had HIV RNA, CD4+, and CD8+ results measured simultaneously between October 1, 1999, and September 30, 2015. After exclusions, 35 659 remained in the final sample.
Patients in the cohort had a median age of 46 years at baseline and were predominantly male, black, and overweight or obese (Table 1). Tobacco use, alcohol dependence or abuse, and injection and/or noninjection drug use were common. At the start of follow-up, 56.7% had HIV RNA level of 500 or more copies/mL, 23.9% had CD4+ percentage less than 14%, and 88.5% had a CD4+ to CD8+ ratio less than 1.0. A total of 31.9% had HCV coinfection, and 5.6% were HBV-coinfected. Only 11.9% of HCV-coinfected persons received anti-HCV therapy at baseline or during follow-up.
Overall, 68.7% received ART during the baseline period. ART regimens prescribed reflected the antiretrovirals used at the time of study entry (Table 1). Among 1981 HBV-coinfected individuals, 1211 (61.1%) were on ART at baseline. Of these, 689 (56.9%) received HBV-active ART with lamivudine or emtricitabine alone, 374 (30.9%) with tenofovir plus emtricitabine or lamivudine, and 27 (2.2%) with tenofovir alone; 121 (10.0%) were on ART without an HBV-active antiretroviral. By the end of follow-up, 1840 (92.9%) received HBV-active ART.
A total of 773 (2.2%) patients had a baseline diagnosis of cirrhosis. These patients were older and more commonly had diabetes, alcohol dependence or abuse, and HCV or HBV coinfection compared to those without cirrhosis (Table 1).
Incidence Rates of HCC
Overall, 302 (0.8%) medical record-confirmed HCC diagnoses were identified over 281 441 person-years (IR = 107.3/100 000 person-years) with median duration of follow-up of 7.4 (interquartile range = 3.3–12.8) years. Rates were particularly high among the 1981 HBV-coinfected (IR = 350.4/100 000 person-years) and 11 392 HCV-coinfected (IR = 224.6/100 000 person-years) individuals.
Patients diagnosed with HCC had a high prevalence of HCV coinfection (82.8%), alcohol dependence or abuse (63.2%), overweight or obesity (47.7%), and HBV coinfection (18.9%; Table 2). Notably, 99 (32.8%) did not have cirrhosis at HCC diagnosis (Table 2).
Table 2.
Characteristics of patients with incident hepatocellular carcinoma, stratified by cirrhosis as determined by review of medical records within one year prior to diagnosis
| Characteristic* | Overall incident HCC (n = 302) | No evidence of cirrhosis (n = 99) | Evidence of cirrhosis (n = 203) |
|---|---|---|---|
| Median age at diagnosis (IQR), y | 56.4 (51.3–61.1) | 57.5 (51.3–61.8) | 56.0 (51.3–60.9) |
| Median time to diagnosis (IQR), y | 7.2 (3.9–10.6) | 6.3 (3.5–10.3) | 7.4 (4.2–11.1) |
| Male sex | 299 (99.0) | 99 (100.0) | 200 (98.5) |
| Race/ethnicity | |||
| Black | 159 (52.6) | 62 (62.6) | 97 (47.8) |
| Caucasian | 97 (32.1) | 24 (24.2) | 73 (36.0) |
| Hispanic | 37 (12.3) | 10 (10.1) | 27 (13.3) |
| Other/Unknown | 9 (3.0) | 3 (3.0) | 6 (3.0) |
| Body mass index | |||
| Underweight, <18.50 kg/m2 | 25 (8.3) | 16 (16.2) | 9 (4.4) |
| Normal, 18.50–24.99 kg/m2 | 128 (42.4) | 47 (47.5) | 81 (39.9) |
| Overweight, 25.00–29.99 kg/m2 | 102 (33.8) | 25 (25.3) | 77 (37.9) |
| Obesity, 30.00–34.99 kg/m2 | 34 (11.3) | 9 (9.1) | 25 (12.3) |
| Morbid obesity, ≥35.00 kg/m2 | 8 (2.6) | 2 (2.0) | 6 (3.0) |
| Missing weight and/or height | 5 (1.7) | 0 (0.0) | 5 (2.5) |
| Diabetes mellitus | 94 (31.1) | 24 (24.2) | 70 (34.5) |
| History of alcohol dependence/abuse | 191 (63.2) | 61 (61.6) | 130 (64.0) |
| History of injection/noninjection drug use | 246 (81.5) | 81 (81.8) | 165 (81.3) |
| Tobacco use | |||
| Never | 40 (13.2) | 10 (10.1) | 30 (14.8) |
| Ever† | 262 (86.8) | 89 (89.9) | 173 (85.2) |
| Hepatitis C virus coinfection‡ | |||
| Detectable HCV RNA or genotype | 250 (82.8) | 79 (79.8) | 171 (84.2) |
| HCV antibody+/HCV RNA- | 5 (1.7) | 1 (1.0) | 4 (2.0) |
| HCV antibody- | 39 (12.9) | 15 (15.2) | 24 (11.8) |
| Never tested | 8 (2.6) | 4 (4.0) | 4 (2.0) |
| Hepatitis B virus coinfection§ | |||
| HBsAg+ | 57 (18.9) | 17 (17.2) | 40 (19.7) |
| HBsAg- | 236 (78.1) | 79 (79.8) | 157 (77.3) |
| Never tested | 9 (3.0) | 3 (3.0) | 6 (3.0) |
| Median HIV RNA (IQR), log10 copies/mL | 1.7 (1.7–2.6) | 1.7 (1.7–2.6) | 1.7 (1.7–2.7) |
| CD4 percentage | |||
| Median (IQR), % | 26 (17–35) | 26 (14–35) | 26 (18–35) |
| ≥28% | 135 (44.7) | 42 (42.4) | 93 (45.8) |
| 14–27.99% | 120 (39.7) | 35 (35.4) | 85 (41.9) |
| <14% | 47 (15.6) | 22 (22.2) | 25 (12.3) |
| CD4: CD8 ratio | |||
| Median (IQR) | 0.57 (0.31–0.93) | 0.52 (0.26–0.98) | 0.59 (0.33–0.91) |
| <1.0 | 239 (79.1) | 77 (77.8) | 162 (79.8) |
| Median alanine aminotransferase (IQR), U/L | 54 (36–79) | 52 (35–74) | 57 (36–79) |
| Not assessed within 360 days prior to HCC diagnosis | 2 (0.7) | 0 (0.0) | 2 (1.0) |
| Median aspartate aminotransferase (IQR), U/L | 68 (44–101) | 61 (38–95) | 73 (47–104) |
| Not assessed within 360 days prior to HCC diagnosis | 1 (0.3) | 0 (0.0) | 1 (0.5) |
| Platelet count <150 000 x 106/L | 172 (57.0) | 42 (42.4) | 130 (64.0) |
| FIB-4‖ | |||
| <1.45 | 27 (8.9) | 18 (18.2) | 9 (4.4) |
| 1.45–3.25 | 99 (32.8) | 39 (39.4) | 60 (29.6) |
| >3.25 | 173 (57.3) | 42 (42.4) | 131 (64.5) |
| Insufficient data to calculate FIB-4 | 3 (1.0) | 0 (0.0) | 3 (1.5) |
| On antiretroviral therapy | 229 (75.8) | 77 (77.8) | 152 (74.9) |
| Ever dideoxynucleoside analogue use¶ | 207 (68.5) | 74 (74.7) | 133 (65.5) |
HBsAg = hepatitis B surface antigen; HCC = hepatocellular carcinoma; HCV = hepatitis C virus; IQR = interquartile range; NRTI = nucleoside reverse transcriptase inhibitors; RNA = ribonucleic acid.
Results reported as n (%) unless otherwise specified.
Ever tobacco use includes current and prior tobacco use.
Hepatitis C virus coinfection defined as positive quantitative HCV RNA (absolute value determinable or not), positive qualitative HCV RNA, or quantifiable HCV genotype at baseline or during follow-up.
Hepatitis B virus coinfection defined as positive HBV surface antigen at baseline or during follow-up.
FIB-4 was calculated using current age and most recent alanine aminotransferase, aspartate aminotransferase, and platelet count within 360 days prior to HCC diagnosis.
Included didanosine, stavudine, zalcitabine, and zidovudine.
Determinants of HCC, by Baseline Cirrhosis Status
Among individuals without baseline cirrhosis, higher HIV RNA in model #1 (HR = 1.25, 95% CI = 1.12 to 1.40, per 1.0 log10 copies/mL increase), detectable HIV in model #2 (HR 1.46, 95% CI = 1.07 to 1.99), HIV RNA of 10 000 or more copies/mL in model #3 (HR =1.63, 95% CI = 1.11 to 2.40), and 12 or more months of detectable HIV viremia in model #4 (HR = 1.47, 95% CI = 1.02 to 2.11) increased the risk of HCC (Table 3). Absolute CD4+ counts less than 200 cells/mm3 increased HCC risk (Supplementary Table 2, available online); however, lower CD4+ percentage was not associated with an increased risk of HCC (Table 3). Additionally, in all models, older age, diabetes, HBV coinfection, HCV coinfection, history of alcohol dependence or abuse, and ever use of tobacco increased the risk of HCC (Table 3). Among individuals with baseline cirrhosis, only HCV coinfection was associated with an increased risk of HCC (Supplementary Table 3, available online).
Table 3.
Factors associated with incident hepatocellular carcinoma among HIV-infected patients in the Veterans Aging Cohort Study (October 1, 1999–September 30, 2015) without a baseline diagnosis of cirrhosis (n = 34 886; 270 hepatocellular carcinoma events)
| Characteristic | Unadjusted HR (95% CI) | Model #1* Adj. HR (95% CI) | Model #2† Adj. HR (95% CI) | Model #3‡ Adj. HR (95% CI) | Model #4§ Adj. HR (95% CI) |
|---|---|---|---|---|---|
| Age, per 10 y | 1.33 (1.17 to 1.51) | 1.48 (1.26 to 1.73) | 1.44 (1.23 to 1.69) | 1.45 (1.23 to 1.70) | 1.44 (1.23 to 1.69) |
| Male sex | 2.30 (0.74 to 7.19) | 1.38 (0.44 to 4.31) | 1.37 (0.44 to 4.30) | 1.37 (0.44 to 4.29) | 1.37 (0.44 to 4.30) |
| Race | |||||
| White | Reference | Reference | Reference | Reference | Reference |
| Black | 1.50 (1.15 to 1.97) | 0.97 (0.73 to 1.28) | 0.97 (0.74 to 1.29) | 0.97 (0.74 to 1.29) | 0.97 (0.74 to 1.29) |
| Hispanic | 1.77 (1.17 to 2.68) | 1.24 (0.82 to 1.89) | 1.23 (0.81 to 1.87) | 1.23 (0.81 to 1.88) | 1.23 (0.81 to 1.87) |
| Other | 1.40 (0.68 to 2.89) | 1.72 (0.83 to 3.58) | 1.72 (0.83 to 3.56) | 1.71 (0.83 to 3.55) | 1.72 (0.83 to 3.56) |
| Baseline body mass index | |||||
| Underweight, <18.50 kg/m2 | 0.63 (0.18 to 2.15) | 0.59 (0.17 to 2.08) | 0.59 (0.17 to 2.09) | 0.59 (0.17 to 2.09) | 0.59 (0.17 to 2.09) |
| Normal, 18.50–24.99 kg/m2 | Reference | Reference | Reference | Reference | Reference |
| Overweight, 25.00–29.9 kg/m2 | 0.88 (0.67 to 1.17) | 0.96 (0.72 to 1.27) | 0.96 (0.72 to 1.27) | 0.96 (0.72 to 1.27) | 0.96 (0.72 to 1.27) |
| Obesity, 30.00–34.9 kg/m2 | 0.92 (0.61 to 1.40) | 1.01 (0.65 to 1.56) | 1.01 (0.65 to 1.56) | 1.01 (0.65 to 1.56) | 1.01 (0.65 to 1.56) |
| Morbid obesity, ≥35.00 kg/m2 | 0.84 (0.39 to 1.80) | 0.98 (0.45 to 2.14) | 0.98 (0.45 to 2.13) | 0.98 (0.45 to 2.13) | 0.98 (0.45 to 2.13) |
| Time-updated diabetes mellitus | 1.70 (1.32 to 2.20) | 1.46 (1.12 to 1.91) | 1.45 (1.11 to 1.90) | 1.45 (1.11 to 1.90) | 1.45 (1.11 to 1.90) |
| Hepatitis B virus coinfection | |||||
| HBsAg- | Reference | Reference | Reference | Reference | Reference |
| HBsAg+ | 3.65 (2.68 to 4.97) | 3.92 (2.87 to 5.35) | 3.91 (2.86 to 5.34) | 3.91 (2.86 to 5.35) | 3.91 (2.86 to 5.34) |
| Never tested | 1.01 (0.48 to 2.15) | 1.12 (0.52 to 2.42) | 1.13 (0.52 to 2.44) | 1.13 (0.52 to 2.44) | 1.13 (0.52 to 2.44) |
| Hepatitis C virus coinfection | |||||
| HCV antibody- | Reference | Reference | Reference | Reference | Reference |
| Detectable HCV RNA or genotype | 9.25 (6.53 to 13.11) | 7.65 (5.35 to 10.94) | 7.68 (5.36 to 10.98) | 7.68 (5.37 to 11.00) | 7.68 (5.36 to 10.98) |
| HCV antibody+/HCV RNA- | 4.83 (1.90 to 12.28) | 3.73 (1.46 to 9.52) | 3.81 (1.49 to 9.72) | 3.80 (1.49 to 9.71) | 3.81 (1.49 to 9.72) |
| Never tested | 4.85 (2.16 to 10.88) | 4.64 (2.04 to 10.55) | 4.68 (2.06 to 10.65) | 4.68 (2.06 to 10.64) | 4.68 (2.06 to 10.65) |
| Time-updated CD4+ cell percentage | |||||
| ≥28% | Reference | Reference | Reference | Reference | Reference |
| 14–27.99% | 1.05 (0.81 to 1.37) | 0.86 (0.66 to 1.12) | 0.89 (0.68 to 1.16) | 0.88 (0.68 to 1.16) | 0.89 (0.68 to 1.16) |
| <14% | 1.52 (1.08 to 2.14) | 0.97 (0.66 to 1.43) | 1.12 (0.78 to 1.62) | 1.09 (0.75 to 1.59) | 1.12 (0.78 to 1.62) |
| History of alcohol abuse | 2.38 (1.85 to 3.05) | 1.45 (1.11 to 1.89) | 1.46 (1.12 to 1.90) | 1.46 (1.12 to 1.90) | 1.46 (1.12 to 1.90) |
| Tobacco use | |||||
| Never | Reference | Reference | Reference | Reference | Reference |
| Ever‖ | 2.58 (1.81 to 3.67) | 1.65 (1.14 to 2.38) | 1.66 (1.15 to 2.39) | 1.66 (1.15 to 2.40) | 1.66 (1.15 to 2.39) |
| Time-updated HIV RNA, per 1.0 log10 copies/mL | 1.26 (1.14 to 1.39) | 1.25 (1.12 to 1.40) | — | — | — |
| Current HIV RNA ≥500 copies/mL | 1.58 (1.19 to 2.10) | — | 1.46 (1.07 to 1.99) | — | |
| Current HIV RNA categories | |||||
| <500 copies/mL | Reference | Reference | |||
| 500–9999 copies/mL | 1.41 (0.94 to 2.11) | — | — | 1.31 (0.87 to 1.97)¶ | — |
| ≥10 000 copies/mL | 1.73 (1.22 to 2.47) | — | — | 1.63 (1.11 to 2.40)¶ | — |
| Consecutive months of HIV RNA ≥500 copies/mL | |||||
| HIV RNA always <500 copies/mL | Reference | — | — | — | Reference |
| 1–11 months of HIV RNA ≥500 copies/mL | 1.63 (1.07 to 2.49) | — | — | — | 1.46 (0.94 to 2.26) |
| ≥12 months of HIV RNA ≥500 copies/mL | 1.55 (1.10 to 2.18) | — | — | — | 1.47 (1.02 to 2.11) |
CI = confidence interval; HBsAg = hepatitis B surface antigen; HBV = hepatitis B virus; HCV = hepatitis C virus; HR = hazard ratio; RNA = ribonucleic acid.
Model #1 includes continuous values of HIV RNA as log10 copies/mL.
Model #2 includes current HIV RNA ≥500 copies/mL.
Model #3 includes current categories of HIV RNA ≥500 copies/mL.
Model #4 includes consecutive months of HIV RNA ≥500 copies/mL.
Ever tobacco use includes current and prior tobacco use.
Two-sided Ptrend = .01, based on multivariable Cox regression analysis.
Results were similar in analyses accounting for the competing risk of death (data not shown) and using 360-day and 540-day lags for HIV RNA and CD4+ cell percentage (Supplementary Tables 4–5, available online). Similar findings were observed when analyses were restricted to HBV-coinfected (Supplementary Table 6, available online) and HCV-coinfected (Supplementary Table 7, available online) individuals, although results for some risk factors did not achieve statistical significance given the smaller sample sizes in these groups. There were too few HCC events (n = 17) among HIV-infected patients without viral hepatitis to permit analysis. When possible fatty liver disease was evaluated, patients who had both baseline obesity and diabetes had a higher risk of HCC than those who did not (HR = 2.32, 95% CI = 1.19 to 4.52; Supplementary Table 8, available online). Longer consecutive months with CD4+ percentage less than 14% did not increase HCC risk (≥12 months: HR = 1.02, 95% CI = 0.69 to 1.51; 1–11 months: HR = 1.15, 95% CI = 0.65 to 2.05) compared to those with no less than 14% throughout follow-up.
In exploratory analyses, after adjustment for HIV RNA and traditional risk factors, the risk of HCC was not increased with lower time-updated CD4+ to CD8+ ratio among either individuals with baseline cirrhosis (HR = 1.000, 95% CI = 0.999 to 1.001, per 0.1 unit decrease) or without cirrhosis (HR = 0.991, 95% CI = 0.981 to 1.002, per 0.1 unit decrease).
Discussion
To our knowledge, this is the largest study to evaluate HIV-related and traditional risk factors for HCC among HIV-infected patients and the first to examine such determinants by baseline cirrhosis status. We stratified our analyses by baseline cirrhosis status to account for the possibility that risk factors for HCC might vary by the presence of cirrhosis. Among patients without baseline cirrhosis, time-updated detectable HIV (≥500 copies/mL), higher HIV RNA (particularly ≥10 000 copies/mL), and no less than 12 months of detectable HIV increased risk of HCC, independent of traditional risk factors. Older age, HBV, HCV, diabetes, alcohol dependence or abuse, and tobacco use, which are traditional risk factors for HCC, also increased HCC risk in this group. The risk of HCC was particularly high in those with both obesity and diabetes. Among patients with baseline cirrhosis, only HCV coinfection remained associated with HCC. Notably, lower CD4+ cell percentage was not associated with increased risk of HCC regardless of cirrhosis status.
Our study is the first to find that higher level and longer duration of HIV viremia contribute to HCC risk. HIV viremia could contribute to HCC by accelerating hepatic fibrosis progression to cirrhosis or by directly promoting hepatocarcinogenesis via immune dysregulation, oxidative stress, hepatocyte apoptosis, and/or depletion of CD4+ cells in the gastrointestinal tract with resultant microbial translocation (32–34). We have previously shown that suppression of HIV viremia can delay onset of cirrhosis (21). Our findings suggest that achieving and maintaining HIV suppression could mitigate the risk of HCC.
Three prior studies found no association between HIV RNA and risk of HCC, but none stratified analyses by baseline cirrhosis status. One study of 31 576 HIV-infected patients in the VA HIV Clinical Case Registry from 1985 to 2010 found that longer percentage of time with undetectable HIV RNA (<500 copies/mL) did not decrease HCC risk (5). A follow-up study among 8563 HIV and HCV-coinfected patients in this registry similarly found no association between duration of undetectable HIV RNA and HCC (6). However, both analyses included cirrhosis as a covariate in multivariable models. Because cirrhosis is in the causal pathway to HCC, controlling for cirrhosis could have adjusted away associations between HIV suppression and HCC. A third study among 42 441 HIV-infected patients in the VACS from 1999 to 2015 found that neither early (≤2 years) nor long-term (>2 years) HIV suppression decreased rates of HCC compared to 104 712 demographically similar uninfected persons (7). However, this analysis did not stratify results by baseline cirrhosis status.
Contrary to previous studies (6,8–12), we found that HIV-related immunosuppression, as measured by CD4+ cell percentage, was not associated with an increased risk of HCC. Notably, those prior studies evaluated the risk of HCC associated with lower absolute CD4+ count. Indeed, when we evaluated associations between absolute CD4+ count and HCC in our cohort, CD4+ counts less than 200 cells/mm3 increased HCC risk. However, absolute CD4+ count may decrease during cirrhosis as a result of portal hypertension-induced splenic sequestration, but CD4+ percentage remains unchanged during cirrhosis (13). Our results suggest that HIV-related immunosuppression is not an important contributor to HCC risk and that the findings of prior analyses likely reflected the effect of liver fibrosis progression on absolute CD4+ count.
Interestingly, 32.8% of patients with HCC in our study did not have evidence of cirrhosis based on review of medical records within one year prior to cancer diagnosis. HCC can develop in the absence of advanced hepatic fibrosis in chronic HBV infection or nonalcoholic fatty liver disease (35,36). Further research is needed to determine how frequently HCC occurs in the absence of cirrhosis in HIV and if this differs from uninfected persons.
The study has several potential limitations. First, we might have underestimated cirrhosis, because this condition is clinically silent. However, cirrhosis was identified using validated diagnoses, and the negative predictive value of this definition exceeded 99% (23,24). Second, we were unable to determine fatty liver disease, because this diagnosis requires liver imaging or biopsy to confirm. We classified possible fatty liver disease by baseline presence of both obesity and diabetes. Future studies should evaluate the effect of fatty liver disease on HCC in HIV. Third, we did not evaluate the risk of HCC with antiretroviral drugs, particularly those associated with hepatotoxicity (37), or viral hepatitis treatments. Additional research should evaluate the effects of these medications on incidence of HCC. Finally, our sample was predominantly comprised of male US veterans, but the study included 867 HIV-infected women.
In conclusion, among HIV-infected patients without cirrhosis, higher HIV RNA and longer duration of HIV viremia, in addition to HBV and HCV coinfection, were important determinants of HCC, independent of traditional risk factors. HIV-related immunosuppression, determined by CD4+ cell percentage, was not associated with increased risk of HCC. This study provides the strongest evidence to date that HIV viremia contributes to the risk of HCC in this group.
Funding
This study was funded, in part, by the National Cancer Institute (R01CA206465) and the National Institutes on Alcohol Abuse and Alcoholism (U01AA013566).
Notes
Affiliations of authors: Division of Infectious Diseases, Department of Medicine (JT, VLR), and Department of Biostatistics, Epidemiology, and Informatics, Center for Clinical Epidemiology and Biostatistics, Center for Pharmacoepidemiology Research and Training (JT, MJK, DMC, VLR), Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA; Center for Population Health Sciences, Stanford University School of Medicine, Stanford, CA (LSP); VA Connecticut Healthcare System, West Haven, CT (RLM, KD, JPT, JKL, THT, ACJ); Yale University School of Medicine, New Haven, CT (RLM, KD, JPT, JKL, THT, ACJ); VA Greater Los Angeles Healthcare System and David Geffen School of Medicine at UCLA, Los Angeles, CA (MBG); Infectious Diseases Section, Michael E. DeBakey VA Medical Center and Department of Medicine, Baylor College of Medicine, Houston, TX (MCR); Washington DC VA Medical Center and George Washington University Medical Center, Washington, DC (CLG); James J. Peters VA Medical Center, Bronx, NY, and Icahn School of Medicine at Mount Sinai, New York, NY (NB, STB); Department of Biostatistics, Rutgers University School of Public Health, New Brunswick, NJ (JAR).
The funding agencies had no involvement in the design of the study; the collection of data, analysis or interpretation of results; the development of this manuscript; or the decision to submit the manuscript for publication.
The authors have no conflicts of interest to disclose.
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the U.S. Government.
Supplementary Material
References
- 1. Engels EA, Yanik EL, Wheeler W, et al. Cancer-attributable mortality among people with treated human immunodeficiency virus infection in North America. Clin Infect Dis. 2017;65(4):636–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Silverberg MJ, Lau B, Achenbach CJ, et al. Cumulative incidence of cancer among persons with HIV in North America: a cohort study. Ann Intern Med. 2015;163(7):507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sahasrabuddhe VV, Shiels MS, McGlynn KA, Engels EA.. The risk of hepatocellular carcinoma among individuals with acquired immunodeficiency syndrome in the United States. Cancer. 2012;118(24):6226–6233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pinato DJ, Allara E, Chen TY, et al. Influence of HIV infection on the natural history of hepatocellular carcinoma: results from a global multicohort study. J Clin Oncol. 2019;37(4):296–304. [DOI] [PubMed] [Google Scholar]
- 5. Kowalkowski MA, Day RS, Du XL, Chan W, Chiao EY.. Cumulative HIV viremia and non-AIDS-defining malignancies among a sample of HIV-infected male veterans. J Acquir Immune Defic Syndr. 2014;67(2):204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kramer JR, Kowalkowski MA, Duan Z, Chiao EY.. The effect of HIV viral control on the incidence of hepatocellular carcinoma in veterans with hepatitis C and HIV coinfection. J Acquir Immune Defic Syndr. 2015;68(4):456–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Park LS, Tate JP, Sigel K, et al. Association of viral suppression with lower AIDS-defining and non-AIDS-defining cancer incidence in HIV-infected veterans: a prospective cohort study. Ann Intern Med. 2018;169(2):87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clifford GM, Rickenbach M, Polesel J, et al. Influence of HIV-related immunodeficiency on the risk of hepatocellular carcinoma. AIDS. 2008;22(16):2135–2141. [DOI] [PubMed] [Google Scholar]
- 9. Bruyand M, Dabis F, Vandenhende MA, et al. HIV-induced immune deficiency is associated with a higher risk of hepatocarcinoma, ANRS CO3 Aquitaine Cohort, France, 1998-2008. J Hepatol. 2011;55(5):1058–1062. [DOI] [PubMed] [Google Scholar]
- 10. Ioannou GN, Bryson CL, Weiss NS, Miller R, Scott JD, Boyko EJ.. The prevalence of cirrhosis and hepatocellular carcinoma in patients with human immunodeficiency virus infection. Hepatology. 2013;57(1):249–257. [DOI] [PubMed] [Google Scholar]
- 11. Merchante N, Merino E, Lopez-Aldeguer J, et al. Increasing incidence of hepatocellular carcinoma in HIV-infected patients in Spain. Clin Infect Dis. 2013;56(1):143–150. [DOI] [PubMed] [Google Scholar]
- 12. Gjærde LI, Shepherd L, Jablonowska E, et al. Trends in incidences and risk factors for hepatocellular carcinoma and other liver events in HIV and hepatitis C virus-coinfected individuals from 2001 to 2014: a multicohort study. Clin Infect Dis. 2016;63(6):821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McGovern BH, Golan Y, Lopez M, et al. The impact of cirrhosis on CD4+ T cell counts in HIV-seronegative patients. Clin Infect Dis. 2007;44(3):431–437. [DOI] [PubMed] [Google Scholar]
- 14. Forner A, Llovet JM, Bruix J.. Hepatocellular carcinoma. Lancet. 2012;379(9822):1245–1255. [DOI] [PubMed] [Google Scholar]
- 15. El-Serag HB, Rudolph KL.. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132(7):2557–2576. [DOI] [PubMed] [Google Scholar]
- 16. El-Serag HB, Kanwal F.. Epidemiology of hepatocellular carcinoma in the United States: Where are we? Where do we go? Hepatology. 2014;60(5):1767–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fultz SL, Skanderson M, Mole LA, et al. Development and verification of a “virtual” cohort using the National VA Health Information System. Med Care. 2006;44(8)(suppl 2):S25–30. [DOI] [PubMed] [Google Scholar]
- 18. Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, et al. International Classification of Diseases for Oncology. 3rd ed Geneva: World Health Organization; 2000. [Google Scholar]
- 19.National Cancer Institute. Surveillance, Epidemiology, and End Results Program: Site Recode ICD-O-3/WHO 2008 Definition. http://seer.cancer.gov/siterecode/icdo3_dwhoheme/index.html. Accessed August 16, 2018.
- 20. Park LS, Tate JP, Rodriguez-Barradas MC, et al. Cancer incidence in HIV-infected versus uninfected veterans: comparison of Cancer Registry and ICD-9 Code Diagnoses. J AIDS Clin Res. 2014;5(7):1000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lo Re V III, Kallan MJ, Tate JP, et al. Hepatic decompensation in antiretroviral-treated patients co-infected with HIV and hepatitis C virus compared with hepatitis C virus-monoinfected patients: a cohort study. Ann Intern Med. 2014;160(6):369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Butt AA, McGinnis K, Rodriguez-Barradas MC, et al. HIV infection and the risk of diabetes mellitus. AIDS. 2009;23(10):1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kramer JR, Davila JA, Miller ED, Richardson P, Giordano TP, El-Serag HB.. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther. 2007;27(3):274–282. [DOI] [PubMed] [Google Scholar]
- 24. Lo Re V III, Lim JK, Goetz MB, et al. Validity of diagnostic codes and liver-related laboratory abnormalities to identify hepatic decompensation events in the Veterans Aging Cohort Study. Pharmacoepidemiol Drug Saf. 2011;20(7):689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43(6):1317–1325. [DOI] [PubMed] [Google Scholar]
- 26. Barbara L, Benzi G, Gaiani S, et al. Natural history of small untreated hepatocellular carcinoma in cirrhosis: a multivariate analysis of prognostic factors of tumor growth rate and patient survival. Hepatology. 1992;16(1):132–137. [DOI] [PubMed] [Google Scholar]
- 27. Fine J, Gray RJ.. A proportional hazards model for the subdistribution of competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 28. Stefan N, Haring HU, Cusi K.. Non-alcoholic fatty liver disease: causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol. 2019;7(4):313–324. [DOI] [PubMed] [Google Scholar]
- 29. Serrano-Villar S, Gutierrez C, Vallejo A, et al. The CD4/CD8 ratio in HIV-infected subjects is independently associated with T-cell activation despite long-term viral suppression. J Infect. 2013;66(1):57–66. [DOI] [PubMed] [Google Scholar]
- 30. Hosmer DW, Lemeshow S.. Applied Survival Analysis: Regression Modeling of Time-to-Event Data. New York, NY: John Wiley & Sons, Inc; 1999. [Google Scholar]
- 31. Royston P. Multiple imputation of missing values: update. STATA J. 2005;5(2):188–201. [Google Scholar]
- 32. Balagopal A, Philp FH, Astemborski J, et al. Human immunodeficiency virus-related microbial translocation and progression of hepatitis C. Gastroenterology. 2008;135(1):226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Armah KA, McGinnis K, Baker J, et al. HIV status, burden of comorbid disease, and biomarkers of inflammation, altered coagulation, and monocyte activation. Clin Infect Dis. 2012;55(1):126–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brau N, Salvatore M, Rios-Bedoya CF, et al. Slower fibrosis progression in HIV/HCV-coinfected patients with successful HIV suppression using antiretroviral therapy. J Hepatol. 2006;44(1):47–55. [DOI] [PubMed] [Google Scholar]
- 35. Mittal S, El-Serag HB, Sada YH, et al. Hepatocellular carcinoma in the absence of cirrhosis in United States veterans is associated with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2016;14(1):124–131 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chayanupatkul M, Omino R, Mittal S, et al. Hepatocellular carcinoma in the absence of cirrhosis in patients with chronic hepatitis B virus infection. J Hepatol. 2017;66(2):355–362. [DOI] [PubMed] [Google Scholar]
- 37. Ryom L, Lundgren JD, De Wit S, et al. Use of antiretroviral therapy and risk of end-stage liver disease and hepatocellular carcinoma in HIV-positive persons. AIDS. 2016;30(11):1731–1743. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.