Abstract
Background
Adjuvant bisphosphonates, when given in a low-estrogen environment, can decrease breast cancer recurrence and death. Treatment guidelines include recommendations for adjuvant bisphosphonates in postmenopausal patients. SWOG/Alliance/Canadian Cancer Trials Group/ECOG-ACRIN/NRG Oncology study S0307 compared the efficacy of three bisphosphonates in early-stage breast cancer.
Methods
Patients with stage I–III breast cancer were randomly assigned to 3 years of intravenous zoledronic acid, oral clodronate, or oral ibandronate. The primary endpoint was disease-free survival (DFS) with overall survival as a secondary outcome. All statistical tests were two-sided.
Results
A total of 6097 patients enrolled. Median age was 52.7 years. Prior to being randomly assigned, 73.2% patients indicated preference for oral vs intravenous formulation. DFS did not differ across arms in a log-rank test (P = .49); 5-year DFS was 88.3% (zoledronic acid: 95% confidence interval [CI] = 86.9% to 89.6%), 87.6% (clodronate: 95% CI = 86.1% to 88.9%), and 87.4% (ibandronate: 95% CI = 85.6% to 88.9%). Additionally, 5-year overall survival did not differ between arms (log rank P = .50) and was 92.6% (zoledronic acid: 95% CI = 91.4% to 93.6%), 92.4% (clodronate: 95% CI = 91.2% to 93.5%), and 92.9% (ibandronate: 95% CI = 91.5% to 94.1%). Bone as first site of recurrence did not differ between arms (P = .93). Analyses based on age and tumor subtypes showed no treatment differences. Grade 3/4 toxicity was 8.8% (zoledronic acid), 8.3% (clodronate), and 10.5% (ibandronate). Osteonecrosis of the jaw was highest for zoledronic acid (1.26%) compared with clodronate (0.36%) and ibandronate (0.77%).
Conclusions
We found no evidence of differences in efficacy by type of bisphosphonate, either in overall analysis or subgroups. Despite an increased rate of osteonecrosis of the jaw with zoledronic acid, overall toxicity grade differed little across arms. Given that patients expressed preference for oral formulation, efforts to make oral agents available in the United States should be considered.
Bone is a common site of metastasis in breast cancer and a frequent initial site of distant recurrence (1,2). Circulating breast cancer cells are attracted to surfaces of bone resorption, where they harness the bone microenvironment to influence their survival (3,4). Cancer cells produce cytokines that uncouple the balance between osteoclasts and osteoblasts, leading to an increase in bone resorption (5). Resorbing bone releases cytokines promoting survival of malignant cells, establishing the “vicious cycle” of bone metastases (6).
Bisphosphonates are bone-targeting drugs that bind to sites of bone resorption and become internalized by osteoclasts, inhibiting their function. They are rapidly cleared from the circulation but have a half-life in bone that is measurable in years. Bisphosphonates are effective in treating conditions with excessive bone resorption and osteoclast activity, including osteoporosis. In early-stage breast cancer, bisphosphonates impede bone mineral density loss associated with aromatase inhibitors and ovarian suppression (7–10). In bone metastases, bisphosphonates reduce skeletal-related events and improve quality of life (11–14).
There is evidence for possible direct and indirect effects of bisphosphonates on cancer cells, including immunomodulatory activity and synergy with anticancer agents (15). Aminobisphosphonates, distinguished by the addition of a nitrogen atom, have been shown to inhibit tumor cell proliferation, induce apoptosis, and reduce skeletal tumor burden in models of bone metastasis (16–18).
The oral bisphosphonate clodronate is approved in many countries (although not in the United States) to treat bone metastases. Ibandronate is approved in Europe in intravenous (IV) and oral form for bone metastases; however, it is available only in an osteoporosis-strength in the United States. Zoledronic acid is widely available as an IV infusion, with dose and interval dependent on indication.
Bisphosphonates are generally well tolerated, with a relatively low risk of serious adverse effects. Gastrointestinal side effects are common with oral formulations. IV bisphosphonates are associated with renal toxicity, electrolyte imbalances, and first-infusion acute-phase reactions. Osteonecrosis of the jaw (ONJ) is an uncommon but serious complication of bisphosphonates (19). Concerns have been raised about femur fragility fractures with long-term bisphosphonate use (20).
Clinical trials have evaluated whether adjuvant bisphosphonates can prevent breast cancer metastases. Some reported improvements in recurrences and deaths, whereas others showed no benefit (9,10,21–27). Subset analyses led to the hypothesis that adjuvant bisphosphonates may be effective only in women with a “low estrogen state.” (15) A meta-analysis of 26 randomized adjuvant bisphosphonate trials was performed by the Early Breast Cancer Collaborative Trialists’ Collaborative Group (EBCTCG) (28). In a preplanned subgroup analysis, bisphosphonate use in postmenopausal women was associated with a 28% reduction in bone metastasis and an 18% reduction in breast cancer deaths. The meta-analysis did not find statistically significant differences between agents, dose, or frequency of dosing, although no trial directly compared agents, doses, or schedules. The meta-analysis concluded that in a low-estrogen environment, adjuvant bisphosphonates decrease breast cancer recurrences and deaths.
SWOG S0307 was designed to compare three bisphosphonates in early-stage breast cancer. The nonaminobisphosphonate clodronate served as a reference control against which two aminobisphosphonates, zoledronic acid and ibandronate, were compared. S0307 hypothesized that aminobisphosphonates might more effectively prevent metastases compared with clodronate.
Methods
Patients
S0307 (ClinicalTrials.gov identifier: NCT00127205) enrolled female patients with pathologic stage I–III breast cancer following surgery (29). Patients were recruited from sites across the National Clinical Trials Network. Patients must have received, or have been planning to receive, systemic adjuvant therapy. Neoadjuvant therapy was permitted if enrolled after surgery. Radiation therapy was allowed at any time. Patients with previous bisphosphonate treatment for bone density were eligible if discontinued at registration. Other requirements were age 18 years and older, SWOG Performance Status 0–2, adequate local therapy, serum creatinine no more than 2 Institutional Upper Limit of Normal and calculated creatinine clearance no less than 30 ml/min, dental examination within 6 months of study initiation, and negative pregnancy test in women with reproductive potential. Patients with renal failure or history of prior malignancy (other than specified in situ cancers or other cancers from which they were disease free for ≥ 5 years) were excluded from participation. The protocol was approved by the National Cancer Institute (NCI) Central Institutional Review Board and institutional review boards of participating institutions. Patients provided written informed consent for participation, and patient safety was monitored by an independent data safety monitoring committee.
Study Procedures
The study was an open-label randomized design with equal probability of receiving one of three bisphosphonate treatments for 3 years: (arm 1: ZA) intravenous zoledronic acid (monthly for 6 months, then every 3 months); (arm 2: CLOD) oral clodronate (1600 mg daily); or (arm 3: IBAN) oral ibandronate (50 mg daily). Standard dosing of zoledronic acid was 4 mg, with graduated reduction to 3 mg for renal impairment. Patients were instructed to take supplemental calcium and vitamin D.
Random assignments were generated by computer when a patient was enrolled by the treating clinic using the Oncology Patient Enrollment Network system maintained by NCI. Stratification factors were not used at randomization because of the large size of the trial.
Toxicity was graded according to Common Terminology Criteria for Adverse Events (version 4.0). If grade 3 or higher toxicity occurred that was due to bisphosphonate, treatment was held until toxicity resolved. Protocol treatment could be held for any reason up to 3 months. If treatment was held more than 3 months, the patient was removed from protocol therapy, but followed for outcomes. The 3-year treatment window was not extended if treatment was held.
Baseline dental examination was required prior to or concurrent with treatment initiation. If anticipating oral surgery, the patient was discouraged from enrolling in the trial. Information from patients experiencing symptoms consistent with ONJ was collected and reviewed by an oral medicine expert. On-treatment data collection forms specifically queried whether ONJ, fracture, or renal toxicity had occurred during the reporting period.
Statistical Methods
The original accrual goal was 6000 patients over 4 years. Because of slow early accrual, NCI policy mandated a reassessment, which changed the goal to 4500 patients. Then because of a rapid increase in accrual leading to shorter follow-up, the accrual goal was changed for the final time to 5400 patients distributed as follows: 2000 zoledronic acid, 2000 clodronate, and 1400 ibandronate. After the trial began, plans to market ibandronate in North America at the dose used in this trial were abandoned, so accrual was concentrated in the remaining two arms for the remainder of the trial. Nonetheless, ibandronate remained of interest because it might provide a highly active oral agent. The final accrual of 5400 was to occur over 4 years with an additional 5 years of follow-up planned before final analysis. All patients continue to be followed for 10 years from random assignment.
The primary outcome was disease-free survival (DFS), defined as time from registration to first occurrence of disease recurrence (local, regional, distant), new breast primary, or death from any cause. A secondary outcome was overall survival (OS), defined as time from registration to death from any cause. Patients not experiencing DFS or OS events were censored at date of last contact. Cumulative incidence of first recurrence to bone was computed with censoring at time of other recurrence or at last contact if no recurrence. For the purpose of this publication, follow-up for survival was locked on August 11, 2017.
The study was powered to find a statistically significant difference among the three arms at two-sided α = .05. This assumed that the worst treatment would have a 5-year DFS of 80% and that the best treatment compared to the worst treatment would have a hazard ratio (HR) of 0.80. Power to detect a difference among the three arms varied from 86% to 93% depending on DFS in the middle arm. Annual interim analyses began when 31% of the 1314 expected events had occurred. P values for the interim analyses were based on the Lan-DeMets spending function. Survival analysis methods include Kaplan-Meier plots, log-rank tests, and Cox regression analyses. The proportional hazards assumption was not tested formally here because the Kaplan-Meier curves show no separation among the curves at any time point, thus the assumption is satisfied graphically—difference among treatments does not depend on the time frame.
A post hoc landmarked log-rank test analysis was conducted among patients who did not have any DFS event by 3 years and remained on study. Women were classified as completers if they completed all 3 years of bisphosphonate therapy, and noncompleters if they did not. DFS (starting at 3 years) was compared across treatment arms and whether the women had completed therapy or not.
Results
Patient Characteristics
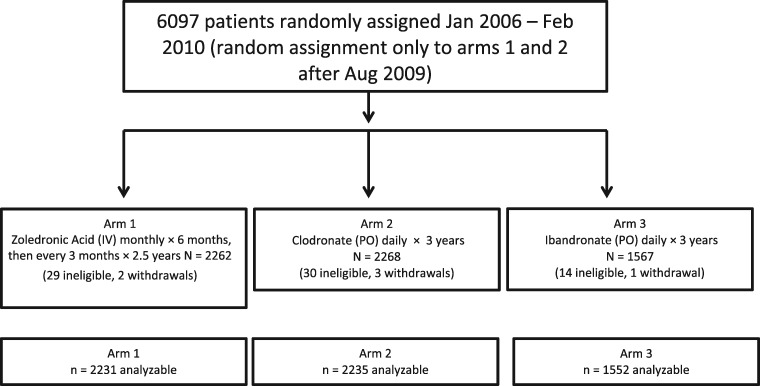
A total of 6097 patients were randomly assigned from January 2006 to February 2010 (CONSORT [Consolidated Standards of Reporting Trials] diagram, Figure 1). Random assignments to ibandronate stopped in August 2009. The trial overenrolled because 731 patients were enrolled in the last month of the trial, after the closure date was announced.
Figure 1.
CONSORT diagram for SWOG trial S0307. Random assignment was to three arms from January 2006 to August 2009. Pharmaceutical support for arm 3 ended early because of business decisions so random assignment was only to arms 1 and 2 during September 2009 to February 2010. Of the patients, 73 were ineligible after review of clinical characteristics recorded prior to random assignment. Another 6 patients withdrew their consent for inclusion of any data. A total of 6018 of the 6097 randomly assigned patients (98.7%) were included in the analysis. CONSORT = Consolidated Standards of Reporting Trials; IV = intravenous; PO = orally.
Table 1 shows that patient and tumor characteristics were balanced across treatment arms. Median age was 52.7 years. Most (78.5%) tumors were hormone receptor (HR) positive (estrogen receptor [ER] or progesterone receptor [PR] positive), 18.8% were HER2 positive and/or equivocal, 15.8% were triple negative (ER/PR/HER2 negative), and 49.0% were node positive. Chemotherapy was used or planned in 79.6% of women enrolled, and endocrine therapy was used or planned in 75.2%.
Table 1.
Baseline characteristics for all treatment arms*
| Baseline characteristics | Arm 1: ZA | Arm 2: CLOD | Arm 3: IBAN | Total |
|---|---|---|---|---|
| Randomly assigned | 2262 | 2268 | 1567 | 6097 |
| Ineligible/withdrew consent | 31 | 33 | 15 | 79 |
| Analyzed | 2231 | 2235 | 1552 | 6018 |
| Age, y | ||||
| Median (range) | 53.0 (24.8–85.7) | 52.6 (21.2–86.1) | 52.7 (26.1–85.8) | 52.7 (21.2–86.1) |
| <55, No. (%) | 1270 (56.9) | 1304 (58.3) | 894 (57.6) | 3468 (57.6) |
| ≥55, No. (%) | 961 (43.1) | 931 (41.7) | 658 (42.4) | 2550 (42.4) |
| Nodal status (n = 41 unknown), No. (%) | ||||
| Negative | 1089 (48.8) | 1152 (51.5) | 784 (50.5) | 3025 (50.3) |
| 1–3 | 722 (32.4) | 685 (30.6) | 489 (31.5) | 1896 (31.5) |
| ≥4 | 404 (18.1) | 385 (17.2) | 267 (17.2) | 1056 (17.5) |
| ER/PR (n = 7 unknown), No. (%) | ||||
| Negative, ER−, PR− | 480 (21.5) | 487 (21.8) | 319 (20.6) | 1286 (21.4) |
| Positive, ER+ or PR+ | 1747 (78.3) | 1747 (78.2) | 1231 (79.3) | 4725 (78.5) |
| HER2 status (n = 65 unknown), No. (%) | ||||
| HER2 negative | 1787 (80.1) | 1786 (79.9) | 1247 (80.3) | 4820 (80.1) |
| HER2 positive/equivocal | 418 (18.7) | 427 (19.1) | 288 (18.6) | 1133 (18.8) |
| Breast disease subtype (n = 65 unknown) | ||||
| ER+ or PR+, HER2− | 1437 (64.4) | 1419 (63.5) | 1015 (65.4) | 3871 (64.3) |
| ER+ or PR+, HER2+ | 292 (13.1) | 312 (14.0) | 205 (13.2) | 809 (13.4) |
| ER/PR−, HER2−, triple negative | 350 (15.7) | 367 (16.4) | 232 (14.9) | 949 (15.8) |
| ER/PR-, HER2+ | 126 (5.6) | 115 (5.1) | 83 (5.3) | 324 (5.4) |
| Stage (n = 142 unknown), No. (%) | ||||
| Stage I | 721 (32.3) | 770 (34.5) | 509 (32.8) | 2000 (33.2) |
| Stage II | 992 (44.5) | 954 (42.7) | 694 (44.7) | 2640 (43.9) |
| Stage III | 472 (21.2) | 460 (20.6) | 304 (19.6) | 1236 (20.5) |
| Chemotherapy (n = 10 unknown), No. (%) | ||||
| Not given or planned | 446 (20.0) | 454 (20.3) | 319 (20.6) | 1219 (20.3) |
| Given or planned | 1779 (79.7) | 1778 (79.6) | 1232 (79.3) | 4789 (79.6) |
| Hormonal therapy (n = 48 unknown), No. (%) | ||||
| Not given or planned | 542 (24.3) | 535 (23.9) | 367 (23.6) | 1444 (24.0) |
| Given or planned | 1670 (74.9) | 1686 (75.4) | 1170 (75.4) | 4526 (75.2) |
CLOD = clodronate; ER = estrogen receptors; IBAN = ibandronate; PR = progesterone receptors; ZA = zoledronic acid.
Treatment Completion
The percentage of patients completing 3 years of therapy was 60% overall, with only small differences by therapy: zoledronic acid (63.2%), clodronate (57.1%), and ibandronate (60.8%) (see Table 4). For those stating incompletion was due to toxicity or serious adverse events, the overall percentage was 14.5%, lower in the zoledronic acid arm (10.0%) vs 17.0% and 17.2% for the two oral agents. Prior to random assignment, most women indicated preference for oral bisphosphonates (73.3%) vs IV bisphosphonates (23.2%), with 3.6% unknown.
Table 4.
Selected toxicities (Common Terminology Criteria for Adverse Events v. 4), fractures, osteonecrosis, and treatment discontinuation by treatment arm*
| Adverse event | ZA (n = 2124) |
CLOD (n = 2185) |
IBAN (n = 1527) |
|||
|---|---|---|---|---|---|---|
| Grade |
Grade |
Grade |
||||
| 3 | 4 | 3 | 4 | 3 | 4 | |
| All grade 3, 4 adverse events (%) | 178 (8.4) | 9 (0.4) | 171 (7.8) | 10 (0.5) | 154 (10.1) | 6 (0.4) |
| Constitutional symptoms, No. | 13 | 1 | 17 | 0 | 8 | 0 |
| Gastrointestinal, No. | 10 | 0 | 51 | 0 | 34 | 0 |
| Metabolic/Laboratory, No. | 27 | 2 | 18 | 4 | 18 | 1 |
| Creatinine | 2 | 0 | 1 | 0 | 3 | 1 |
| Hypocalcemia | 1 | 2 | 1 | 1 | 1 | 0 |
| Musculoskeletal/Soft tissue, No. | 11 | 0 | 8 | 0 | 8 | 1 |
| Pain, No. | 90 | 3 | 60 | 0 | 70 | 3 |
| Renal/Genitourinary, No. | 3 | 0 | 2 | 0 | 7 | 0 |
| No. of patients with fractures (% of all patients) | 159 (7.1) | 207 (9.3) | 115 (7.4) | |||
| No. of patients with traumatic fractures (% of all patients) | 42 (1.9) | 45 (2.0) | 27 (1.7) | |||
| No. of patients with osteonecrosis (% of all patients) | 28 (1.26) | 8 (0.36) | 12 (0.77) | |||
| Off treatment because of AEs/side effects (%) | 224/2231 (10.0) | 381/2235 (17.0) | 267/1552 (17.2) | |||
| Completed all therapy (%) | 1410/2231 (63.2) | 1276/2235 (57.1) | 943/1552 (60.8) | |||
*AEs = adverse events;: CLOD = clodronate; DFS = disease-free survival; IBAN = ibandronate; ZA = zoledronic acid.
Outcome
The fourth interim analysis was conducted in September 2014, with 56% of 1314 expected events. DFS did not differ across the three arms in a log-rank test (P = .71) (data not shown). All arms had 5-year DFS between 87% and 88%, although the expectation at the start of the trial was an 80% 5-year DFS rate for at least one arm. The data safety monitoring committee concluded that there was no chance of a statistically significant difference between arms and recommended early reporting of trial outcomes (30).
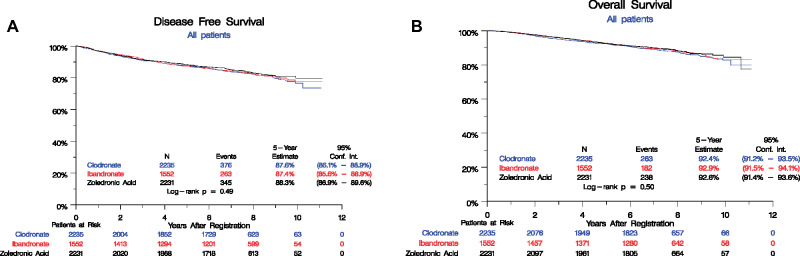
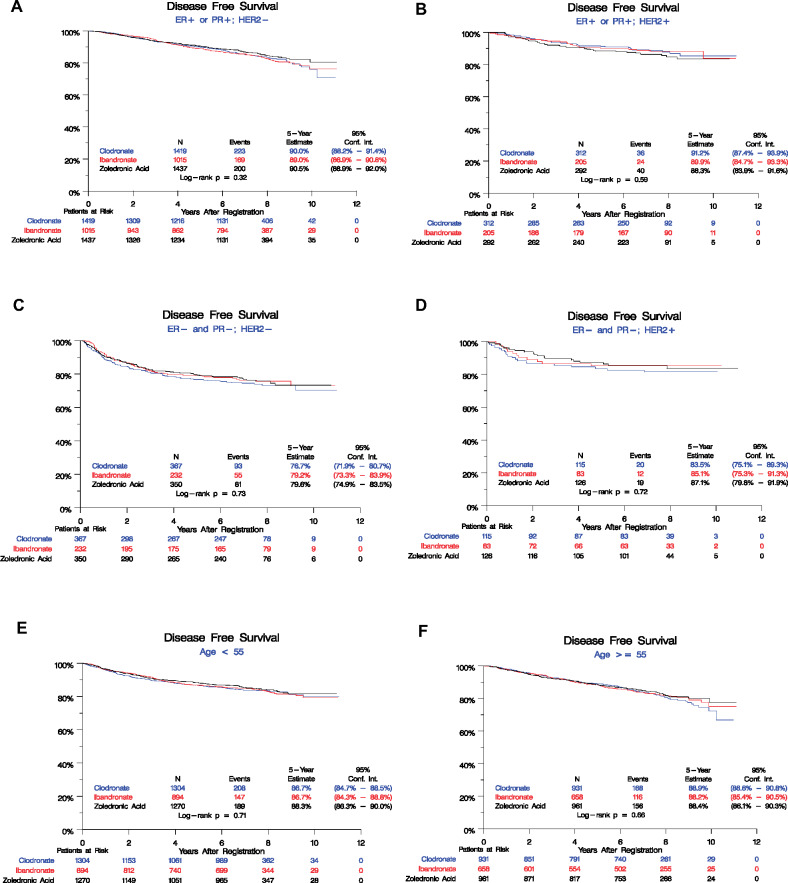
Since then, we have extended follow-up of DFS and OS and assessed site of first recurrence. A total of 984 DFS events have been observed (Figure 2). The overall log-rank test shows no difference among the arms (log-rank P = .49), with 5-year event rates overall 87.8% (95% confidence interval [CI] = 86.9% to 88.7%): zoledronic acid 88.3% (95% CI = 86.9% to 89.6%), clodronate 87.6% (95% CI = 86.1% to 88.9%), and ibandronate 87.4% (95% CI = 85.6% to 88.9%). A univariate Cox model also showed no statistically significant differences among the arms (P = .49), yielding the following pairwise hazard ratios: clodronate vs zoledronic acid (HR = 1.09, 95% CI = 0.94 to 1.26), ibandronate vs zoledronic acid (HR = 1.06, 95% CI = 0.90 to 1.24). There was no statistically significant difference between bisphosphonate agents by ER, PR or HER2 status, breast cancer subtype, age, stage, nodal status, or systemic treatment (Table 2, Figure 3).
Figure 2.
Kaplan-Meier estimates of disease-free survival (DFS) and overall survival (OS) in the adjuvant breast cancer trial S0307 by randomized treatment arms. A) DFS and (B) OS are shown among three arms: (1) zoledronic acid (black), (2) clodronate (blue), and (3) ibandronate (red). Numbers at risk at the beginning of each 2-year period are shown along the bottom by the treatment arm.
Table 2.
Five-year disease-free survival by treatment group and overall for baseline factors*
| Subgroup | ZA, % | CLOD, % | IBAN, % | Overall, % | P for treatment† |
|---|---|---|---|---|---|
| Overall | 88.3 | 87.6 | 87.4 | 87.8 | .49 |
| Age, y | |||||
| <55 | 88.3 | 86.7 | 86.7 | 87.3 | .71 |
| ≥55 | 88.4 | 88.9 | 88.2 | 88.5 | .66 |
| Nodal status | |||||
| Negative | 92.8 | 92.7 | 92.1 | 92.6 | .50 |
| 1–3 | 88.7 | 88.9 | 88.7 | 88.8 | .98 |
| ≥4 | 75.9 | 70.3 | 72.0 | 72.8 | .25 |
| Breast disease subtype | |||||
| ER+ or PR+, HER2- | 90.5 | 90.0 | 89.0 | 89.9 | .32 |
| ER+ or PR+, HER2+ | 88.3 | 91.2 | 89.9 | 89.8 | .59 |
| ER/PR-, HER2- (triple negative) | 79.6 | 76.7 | 79.2 | 78.4 | .73 |
| ER/PR-, HER2+ | 87.1 | 83.5 | 85.1 | 85.3 | .72 |
| HER2 status | |||||
| HER2 negative | 88.4 | 87.2 | 87.2 | 87.6 | .32 |
| HER2 positive or equivocal | 88.0 | 89.2 | 88.5 | 88.5 | .78 |
| ER/PR status | |||||
| Negative | 81.8 | 78.5 | 80.7 | 80.3 | .48 |
| Positive | 90.2 | 90.2 | 89.1 | 89.9 | .65 |
| Stage | |||||
| Stage I | 94.7 | 95.0 | 94.0 | 94.6 | .43 |
| Stage II | 89.2 | 88.5 | 89.0 | 88.9 | .82 |
| Stage III | 76.7 | 73.3 | 72.6 | 74.4 | .45 |
| Chemotherapy | |||||
| Not given or planned | 93.8 | 95.3 | 94.7 | 94.6 | .31 |
| Given or planned | 87.0 | 85.7 | 85.5 | 86.1 | .72 |
| Hormonal therapy | |||||
| Not given or planned | 83.6 | 79.2 | 82.1 | 81.6 | .39 |
| Given or planned | 89.9 | 90.5 | 89.1 | 89.9 | .81 |
CLOD = clodronate; ER = estrogen receptors; IBAN = ibandronate; PR = progesterone receptors; ZA = zoledronic acid.
Two-sided log-rank test was used to calculate P values.
Figure 3.
Kaplan-Meier estimates of disease-free survival (DFS) by randomized treatment arms in subgroups of SWOG trial S0307. DFS among three arms in subgroups of patients by different disease type and age are shown in (A) ER+ or PR+; HER2− subgroup, (B) ER+ or PR+; HER2+ subgroup, (C) ER− and PR−; HER2− subgroup, (D) ER− and PR−; HER2+ subgroup, (E) age < 55 years subgroup, (F) age ≥ 55 years subgroup. Numbers at risk at the beginning of each 2-year period are shown along the bottom by the treatment arm.
A total of 683 deaths have been observed with no difference in survival rates by treatment (log-rank P = .50) (Figure 2). Five-year OS is 92.6% (95% CI = 91.9% to 93.3%) overall: zoledronic acid 92.6% (95% CI = 91.4% to 93.6%), clodronate 92.4% (95% CI = 91.2% to 93.5%), and ibandronate 92.9% (95% CI = 91.5% to 94.1%).
In a landmarked analysis beginning at 3 years, there was no difference in DFS among the three treatment arms for those who completed therapy (P = .43) or those who did not (P = .38) using log-rank testing. However, completers of all 3 years of bisphosphonate therapy were less likely than noncompleters to have a DFS event after 3 years (HR = 0.68, 95% CI = 0.56 to 0.81; P < .001) adjusting for treatment.
Sites of first recurrence are shown in Table 3 by treatment arm. No difference in bone recurrence was observed among the arms (log-rank P = .93), with 52.5% of distant recurrences involving bone and 27.0% bone only.
Table 3.
Site of first recurrence*
| Category | Arm 1: ZA | Arm 2: CLOD | Arm 3: IBAN | Total No. (%) |
|---|---|---|---|---|
| Total No. of analyzable patients | 2231 | 2235 | 1552 | 6018 (100.0) |
| Number of patients with a DFS event | 345 | 376 | 263 | 984 (16.3) |
| Number of patients who died without recurrence | 59 | 81 | 54 | 194 (3.2) |
| Number of patients with recurrence | 286 | 295 | 209 | 790 (13.1) |
| Local/Regional only, No. | 41 | 55 | 36 | 132 |
| % of recurrences | 14.3 | 18.6 | 17.2 | 16.7 |
| % of all patients | 1.8 | 2.5 | 2.3 | 2.2 |
| Contralateral only, No. | 17 | 18 | 17 | 52 |
| % of recurrences | 5.9 | 6.1 | 8.1 | 6.6 |
| % of all patients | 0.8 | 0.8 | 1.1 | 0.9 |
| Distant recurrence, No. | 218 | 207 | 146 | 571 |
| % of recurrences | 76.2 | 70.2 | 69.9 | 72.3 |
| % of all patients | 9.8 | 9.3 | 9.4 | 9.5 |
| Unknown location of recurrence, No. (% of recurrences) | 10 (3.5) | 15 (5.1) | 10 (4.8) | 35 (4.4) |
| Bone as first site of distant recurrence, No. | 110 | 108 | 82 | 300 |
| % of all recurrences | 38.5 | 36.6 | 39.2 | 38.0 |
| % of all distant recurrences | 50.5 | 52.2 | 56.2 | 52.5 |
| Bone only, No. (% of distant recurrences) | 62 (28.4) | 48 (23.2) | 44 (30.1) | 154 (27.0) |
| Bone and nodes only, No. (% of distant recurrences) | 2 (0.9) | 2 (1.0) | 2 (1.4) | 6 (1.0) |
| Bone and other distant sites, beside nodes, No. (% of distant recurrences) | 46 (21.1) | 58 (28.0) | 36 (24.7) | 140 (24.5) |
| Liver/lung/other visceral without bone as first site of distant recurrence, No. (% of distant recurrences) | 57 (26.1) | 67 (32.4) | 40 (27.4) | 165 (28.9) |
| Brain/other CNS , ± any other site, No. (% of distant recurrences) | 31 (14.2) | 24 (11.6) | 22 (15.1) | 78 (13.7) |
CLOD = clodronate; CNS = central nervous system; DFS = disease-free survival; IBAN = ibandronate; ZA = zoledronic acid.
Toxicity
Table 4 shows selected toxicity. Slightly more patients had grades 3 or 4 events with ibandronate (10.5%) than zoledronic acid (8.8%) or clodronate (8.3%). There were no grade 5 toxicities. The predominant toxicity in all arms was pain, with grades 3 or 4 rates for ibandronate (4.8%), zoledronic acid (4.3%), and lowest for clodronate (2.7%). The oral agents had higher rates of gastrointestinal toxicity compared with zoledronic acid. Electrolyte imbalances and renal toxicity were low. Rates of ONJ were highest for zoledronic acid (1.26%), then ibandronate (0.77%), followed by clodronate (0.36%), which was statistically significant (exact Fisher P = .003). Fracture rates were higher for clodronate (9.3%) compared with ibandronate (7.4%) and zoledronic acid (7.1%) (exact Fisher P = .02), with differences mostly in the spine. Traumatic fracture differences were not statistically significant (clodronate 2.0%, zoledronic acid 1.9%, ibandronate 1.7%; exact Fisher P = .83).
Discussion
S0307 showed no statistically significant difference in efficacy by type of bisphosphonate, including subgroups defined by age and tumor subtype. The 87.8% 5-year DFS far exceeded the original assumption that at least one arm would have a 5-year DFS of 80%, thus power was lower than planned. HR-positive subtypes had 89.9% overall 5-year DFS, irrespective of HER2 status, whereas HER2+ and HR− and triple-negative subtypes had lower 5-year DFS rates (85.3% and 78.4%, respectively). Despite use of bisphosphonates in all arms, a high number of patients with distant recurrence had bone involvement (52.5%).
There was no difference in efficacy by type of bisphosphonate in those 55 years or older or younger than 55 years. Although evidence supports a low-estrogen state as essential for adjuvant bisphosphonate benefit, S0307 was unable to show a difference between agents even in patients 55 years and older.
Toxicity was low and similar across arms. There was greater gastrointestinal toxicity with oral bisphosphonates and higher reported pain with zoledronic acid and ibandronate. ONJ was greatest with zoledronic acid and lowest with clodronate, which was a statistically significant finding, although rates were low. Fractures were slightly higher in the clodronate arm. Prior studies have shown that bisphosphonates and Receptor activator of nuclear factor kappa-β (RANK) ligand inhibitors decrease fracture rates in breast cancer patients receiving adjuvant systemic therapy (8–10,26,31) .Treatment adherence was moderate, with 60.3% of patients completing all 3 years of therapy. Adherence did not appear to differ across treatments, but direct comparison is complicated by the fact that IV zoledronic acid adherence can be directly measured, whereas the oral agents may not always be taken as prescribed.
Because S0307 showed a lack of evidence for efficacy difference between bisphosphonates and had no placebo or no-treatment arm, this trial does not allow assessment of the degree of benefit bisphosphonates offer, if any, in early-stage breast cancer. The finding that completers of all assigned therapy had improved DFS relative to noncompleters at 3 years suggests indirectly a possible benefit to continuing therapy, although a direct comparison to a control arm would be preferable. In its early development, S0307 did have a no-treatment control arm, but with reporting of a positive adjuvant clodronate trial prior to activation and availability of newer aminobisphosphonates, it was decided that clodronate would serve as the baseline (23). We hypothesized that the nitrogen-containing bisphosphonates might add benefit beyond clodronate. Alternatively, greater possible toxicity with these agents might have resulted in early termination of treatment leading to poorer DFS, so our design allowed for this possibility as well.
The EBCTCG meta-analysis led to the inclusion of bisphosphonates as adjuvant therapy for postmenopausal patients in recent guidelines (32–35). The Cancer Care Ontario/American Society of Clinical Oncology clinical practice guideline advises consideration be given to zoledronic acid 4 mg IV every 6 months or clodronate 1600 mg orally daily as adjuvant therapy for postmenopausal breast cancer patients (31). S0307, the only trial that directly compares bisphosphonate agents in the adjuvant breast cancer setting, provides reassurance that clodronate is not inferior to zoledronic acid or ibandronate.
S0307 did not test the less-intensive 6-monthly dosing interval of zoledronic acid included in recent guidelines. The S0307 dosing schedule for zoledronic acid was an intensified, modified “bone metastasis” treatment dose starting with monthly dosing. In metastatic breast cancer, a less intensified every 12-week dosing interval has recently been shown to be similar in reducing skeletal-related events to dosing every 4 weeks (36–38). Although the optimal adjuvant zoledronic acid dosing interval has not been defined, monthly dosing regimens such as given in S0307 are not currently supported.
The majority of patients on S0307 indicated a preference for oral bisphosphonate formulations. IV zoledronic acid is approved as a 4 mg dose in treating bone metastases and as a 5 mg dose for managing low bone mass in the United States and elsewhere, but is not Food and Drug Administration–labeled as an adjuvant breast cancer therapy. Clodronate, readily available outside the United States, is not available in the United States in any form. Ibandronate is not available in the United States at the dose used in S0307. Given that oral bisphosphonates are preferred by patients, efforts to make them available in the United States should be considered.
The optimal duration of adjuvant bisphosphonates in early-stage breast cancer is unknown. S0307 chose 3 years of treatment, and trials included in the EBCTCG meta-analysis ranged from 2 to 5 years’ duration (28). The recently reported SUCCESS A trial compared 5 vs 2 years of zoledronic acid, with no difference between the two durations at early follow-up (39). Clinical practice guidelines do not define an optimal duration of adjuvant bisphosphonates.
Whether RANK ligand inhibitors can be substituted for bisphosphonates in the adjuvant breast cancer setting is unknown. Two recent studies evaluated denosumab in early-stage breast cancer with conflicting results (40,41). The Cancer Care Ontario/American Society of Clinical Oncology guideline states that data for adjuvant denosumab look promising but are currently insufficient to make any recommendation (32).
The majority of women diagnosed with early-stage breast cancer will be cured, but the risk of distant recurrence and death still exists. The low event rate in S0307 is good news for breast cancer patients. The body of evidence confirms the benefit of adjuvant bisphosphonates in reducing recurrences and deaths in early-stage, postmenopausal patients. S0307 provides the first comparison of three different bisphosphonates, suggesting no differences in efficacy, minor differences in toxicities, and low rates of ONJ. The risks associated with adjuvant bisphosphonates are small in the context of benefits gained. Research is needed to determine whether biomarkers, such as MAF, could allow for selective use of bisphosphonates in those likely to benefit most, avoiding side effects and cost in patients unlikely to benefit (42). There is work to be done in optimizing use of adjuvant bone-targeting agents, including choice of drug, dose and dosing interval, duration of therapy, and definition of patients and tumors deriving most benefit. Nonetheless, bisphosphonates should be included in the management of postmenopausal, early-stage breast cancer patients.
Funding
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award numbers CA180888, CA180819, CA180820, CA180821, CA180868, CA180863, CA189952, CA189953, CA180834, CA189858, CA190002, CA189808, CA180801, CA189821, CA180846, CA189856, CA180830, CA189972, CA180835, CA180858, CA189817, CA180818, CA189872, CA189804, CA189853, CA189860, CA189954, CA189830, CA189822, CA189971, CA180828, CA189957, CA189997, CA189854, CA189809, CA189829, and CA189873; legacy grants CA13612, CA46282, CA45450, CA11083, CA35119, CA35128, CA46368, CA52654, CA74647, CA63850, CA12644, CA46113, CA04919, CA73590, CA22433, CA68183, CA58723, CA37981, CA76447, CA58416, and CA76448; and in part by the Breast Cancer Research Foundation, Susan G. Komen, Berlex Pharmaceuticals (Bayer HealthCare Pharmaceuticals Inc), Roche/Genentech (Roche Holding AG), and Novartis Pharmaceuticals, Inc.
Notes
Affiliations of authors: University of Washington, Seattle, WA (JRG, KZD); SWOG Statistical Center, Seattle, WA (WEB, JLM, DLL); Tom Baker Cancer Centre, University of Calgary, Calgary, Alberta, Canada (AHGP); Stony Brook Cancer Center, Stony Brook University Cancer Center, Stony Brook, NY (ATS); University of Michigan, Ann Arbor, MI (DFH, CHVP); Columbia University, New York, NY (DLH); Fred Hutchinson Cancer Research Center, Seattle, WA (MMS); Ottawa Hospital Research Institute, University of Ottawa, Ottawa, Ontario, Canada (MC); University of North Carolina, Chapel Hill, NC (ECD); Mayo Clinic, Rochester, MN (JNI); University of Alabama, Birmingham, AL (CIF); University of Colorado, Denver, CO (ADE); Cancer Care of WNC, Greenville, NC (MJM); Beaumont NCORP/William Beaumont Hospital, Royal Oak, MI (JHM); Wichita NCORP, Wichita, KS (SRD); University of California at Davis, Sacramento, CA (HKC); Cancer Therapy and Evaluation Program, National Cancer Institute, Bethesda, MD (JSA); University of Arizona, Tucson, AZ (RBL); The University of Texas MD Anderson Cancer Center, Houston, TX (GNH).
†Deceased.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication. Berlex Pharmaceuticals (Bayer), Roche/Genentech, and Novartis provided study drug and drug distribution support.
JRG has received fees for consulting or advising from Genentech/Roche, Genomic Health, Novartis, Pfizer, Merck, Immunomedics, Astra Zeneca, Puma, Inbiomotion, Radius, and Sandoz/Hexal AG. WEB has received research support to his institution from Merck and Astra Zeneca. AHGP has received research support from Amgen, Novartis, Pfizer; honoraria from Pfizer, Novartis, and Amgen; and fees for consulting or advising from Pfizer, Novartis, and Amgen. ATS has received fees for consulting or advising from Amgen, Novartis, Biothera, Pfizer, and Astra Zeneca and has received research support to her institution from Amgen and Seattle Genetics. DFH has received fees for consulting or advising from Cepheid, Freenome, Cellworkis, CVS Caremark Breast Panel, Agendia, Merrimack, Eli Lilly, Menarini Silon Biosystems, Puma, and Astra Zeneca and has stock options with ONcimmune and InBiomotion. MMS has received fees for consulting or advising from Amgen. CHVP has received research support from Bayer and reports patents, royalties, or other intellectual property from Now UpToDate. ECD has received fees for consulting or advising from STRAT and Novartis (spouse) and research support from Merck, Cerulean, Pfizer, Novartis, Lilly, and Bayer. CIF has received an honorarium from Biotheranostics, fees for consulting or advising from Biotheranostics, travel support from Biotheranostics, and research support from Novartis, Eli Lilly, Oncothyreon, Genentech/Roche, and Pfizer. ADE has stock or other ownership interest in Allergan, Abbott, Celgene, Biomarin, Abbvie, Agilent, Alexion, Lilly, Bristol-Myers Squibb, Merck, Amgen, Incyte, Pfizer, Gilead, and Tesaro and has received research funding from Eisai, Immune Design, Genentech, Lilly, Innocrin, and Astrellas. JHM has employment, leadership role, and stock or ownership to report for Michigan Healthcare Professionals. GNH has received fees for consulting or advising from Novartis and has received research funding from Novartis. All other authors declare no competing interests.
The authors would like to express thanks to all the patients who enrolled in the S0307 clinical trial, as well as the staff and investigators at the participating study sites.
The clinical data used in this report will be available from the Cancer Therapy Evaluation Program in the National Cancer Institute 6 months after publication.
This work was previously presented at the San Antonio Breast Cancer Symposium 2014 and 2018 and the Annual Meeting of the American Society of Clinical Oncology in 2015 and 2019.
References
- 1. Body JJ, Quinn G, Talbot S, et al. Systematic review and meta-analysis on the proportion of patients with breast cancer who develop bone metastases. Crit Rev Oncol Hematol. 2017;115:67–80. [DOI] [PubMed] [Google Scholar]
- 2. Berman AT, Thukral AD, Hwang WT, et al. Incidence and patterns of distant metastases for patients with early-stage breast cancer after breast conservation treatment. Clin Breast Cancer. 2013;13(2):88–94. [DOI] [PubMed] [Google Scholar]
- 3. Weilbaecher KN, Guise TA, McCauley LK.. Cancer to bone: a fatal attraction. Nat Rev Cancer. 2011;11(6):411–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wilson C, Holen I, Coleman R, et al. Seed, soil and secreted hormones: potential interactions of breast cancer cells with their endocrine/paracrine microenvironment and implications for treatment with bisphosphonates. Cancer Treat Rev. 2012;38(7):877–889. [DOI] [PubMed] [Google Scholar]
- 5. Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350(16):1655–1664. [DOI] [PubMed] [Google Scholar]
- 6. Mundy GR. Bone resorption and turnover in health and disease. Bone. 1987;8:S9–S16. [PubMed] [Google Scholar]
- 7. Hadji P, Aapro MS, Body JJ, et al. Management of aromatase inhibitor–associated bone loss in postmenopausal women with breast cancer: practical guidance for prevention and treatment. Ann Oncol. 2011;22(12):2546–2555. [DOI] [PubMed] [Google Scholar]
- 8. Gnant M, Mlineritsch B, Luschin-Ebengreuth G, et al. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 5-year follow-up of the ABCSG-12 bone-mineral density substudy. Lancet Oncol. 2008;9(9):840–849. [DOI] [PubMed] [Google Scholar]
- 9. Brufsky AM, Harker WG, Beck JT, et al. Final 5-year results of Z-FAST trial: adjuvant zoledronic acid maintains bone mass in postmenopausal breast cancer patients receiving letrozole. Cancer. 2012;118(5):1192–1120. [DOI] [PubMed] [Google Scholar]
- 10. Eidtmann H, de Boer R, Bundred N, et al. Efficacy of zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: 36-month results of the ZO-FAST Study. Ann Oncol. 2010;21(11):2188–2194. [DOI] [PubMed] [Google Scholar]
- 11. Paterson AH, Powles TJ, Kanis JA, et al. Double-blind controlled trial of oral clodronate in patients with bone metastases from breast cancer. J Clin Oncol. 1993;11(1):59–65. [DOI] [PubMed] [Google Scholar]
- 12. Body JJ, Diel IJ, Lichinitzer M, et al. Oral ibandronate reduces the risk of skeletal complications in breast cancer patients with metastatic bone disease: results from two randomised, placebo-controlled phase III studies. Br J Cancer. 2004;90(6):1133–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lipton A, Theriault RL, Hortobagyi GN, et al. Pamidronate prevents skeletal complications and is effective treatment in women with breast carcinoma and osteolytic bone metastases. Cancer. 2000;88(5):1082–1090. [DOI] [PubMed] [Google Scholar]
- 14. Rosen LS, Gordon D, Kaminski M, et al. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: a randomized, doubleblind, multicenter, comparative trial. Cancer. 2003;98(8):1735–1744. [DOI] [PubMed] [Google Scholar]
- 15. Coleman RE. Adjuvant bone-targeted therapy to prevent metastasis: lessons from the AZURE study. Curr Opin Support Palliat Care. 2012;6(3):322–329. [DOI] [PubMed] [Google Scholar]
- 16. Guo RT, Cao R, Liang PH, et al. Bisphosphonates target multiple sites in both cis- and trans-prenyltransferases. Proc Natl Acad Sci U S A. 2007;104(24):10022–10027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fromigue O, Lagneaux L, Body JJ.. Bisphosphonates induce breast cancer cell death in vitro. J Bone Miner Res. 2000;15(11):2211–2221. [DOI] [PubMed] [Google Scholar]
- 18. Clezardin P. Bisphosphonates antitumor activity: an unrevealed side of a multifaceted drug class. Bone. 2011;48(1):71–79. [DOI] [PubMed] [Google Scholar]
- 19. Migliorati CA. Bisphosphonates and oral cavity avascular bone necrosis. J Clin Oncol. 2003;21(22):4253–4254. [DOI] [PubMed] [Google Scholar]
- 20. Kharwadkar N, Mayne B, Lawrence JE, et al. Bisphosphonates and atypical subtrochanteric fractures of the femur. Bone Joint Res. 2017;6(3):144–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Diel IJ, Jaschke A, Solomayer EF, et al. Adjuvant oral clodronate improves the overall survival of primary breast cancer patients with micrometastases to the bone marrow: a long-term follow-up. Ann Oncol. 2008;19(12):2007–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Saarto T, Vehmanen L, Virkkunen P, et al. Ten-year follow-up of a randomized controlled trial of adjuvant clodronate treatment in node-positive breast cancer patients. Acta Oncol. 2004;43(7):650–656. [DOI] [PubMed] [Google Scholar]
- 23. Powles T, Paterson A, McCloskey E, et al. Reduction in bone relapse and improved survival with oral clodronate for adjuvant treatment of operable breast cancer. Breast Cancer Res. 2006;8(2):R13.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Paterson AHG, Anderson SJ, Lembersky BC, et al. Oral clodronate for adjuvant treatment of operable breast cancer (National Surgical Adjuvant Breast and Bowel Project protocol B-34): a multicentre, placebo-controlled, randomised trial. Lancet Oncol. 2012;13(7):734–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. von Minckwitz G, Möbus V, Schneeweiss A, et al. German adjuvant intergroup node-positive study: a phase III trial to compare oral ibandronate versus observation in patients with high-risk early breast cancer. J Clin Oncol. 2013;31(28):3531–3539. [DOI] [PubMed] [Google Scholar]
- 26. Coleman R, Cameron D, Dodwell D, et al. Breast-cancer adjuvant therapy with Zoledronic Acid. N Engl J Med. 2011;365(15):1396–1405. [DOI] [PubMed] [Google Scholar]
- 27. Gnant M, Mlineritsch B, Stoeger H, et al. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 62-month follow-up from the ABCSG-12 randomized trial. Lancet Oncol. 2011;12(7):631–641. [DOI] [PubMed] [Google Scholar]
- 28.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet. 2015;386(10001):1353–1361. [DOI] [PubMed] [Google Scholar]
- 29.Clinicaltrials.gov. S0307 Phase III Trial of Bisphosphonates as Adjuvant Therapy for Primary Breast Cancer. https://clinicaltrials.gov/ct2/show/NCT00127205. Accessed November 27, 2019. [PubMed]
- 30. Gralow J, Barlow WE, Paterson AHG, et al. Phase III trial of bisphosphonates as adjuvant therapy in primary breast cancer: SWOG/Alliance/ECOG-ACRIN/NCIC Clinical Trials Group/NRG Oncology Study S0307. J Clin Oncol. 2015;33(suppl; abstr):503. [Google Scholar]
- 31. Gnant M, Pfeiler G, Dubsky PC, et al. Adjuvant denosumab in breast cancer (ABCSG-18): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2015;386(9992):433–443. [DOI] [PubMed] [Google Scholar]
- 32. Dhesy-Thind S, Fletcher GG, Blanchette PS, et al. Use of adjuvant bisphosphonates and other bone-modifying agents in breast cancer: a Cancer Care Ontario and American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2017;35(18):2062–2081. [DOI] [PubMed] [Google Scholar]
- 33. Hadji P, Coleman RE, Wilson C, et al. Adjuvant bisphosphonates in early breast cancer: consensus guidance for clinical practice from a European panel. Ann Oncol. 2016;27(3):379–390. [DOI] [PubMed] [Google Scholar]
- 34. Senkus E, Kyriakides S, Ohno S, et al. Primary breast cancer: ESMO clinical practice guidelines. Ann Oncol. 2015;26(suppl 5):v8–v30. [DOI] [PubMed] [Google Scholar]
- 35. Curigliano G, Burstein HJ, Winer E, et al. De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann Oncol. 2017;28(8):1700–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hortobagyi GN, Van Poznak C, Harker WG, et al. Continued treatment effect of zoledronic acid dosing every 12 vs 4 weeks in women with breast cancer metastatic to bone: the OPTIMIZE-2 randomized clinical trial. JAMA Oncol. 2017;3(7):906–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Himelstein AL, Foster JC, Khatcheressian JL, et al. Effect of longer-interval vs standard dosing of zoledronic acid on skeletal events in patients with bone metastases: a randomized clinical trial. JAMA. 2017;317(1):48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Van Poznak C, Somerfield MR, Barlow WE, et al. Role of bone-modifying agents in metastatic breast cancer: an American Society of Clinical Oncology-Cancer Care Ontario Focused Guideline Update. J Clin Oncol. 2017;35(35):3978–3986. [DOI] [PubMed] [Google Scholar]
- 39. Janni W, Friedl TWP, Fehm T, et al. Extended adjuvant bisphosphonate treatment over five years in early breast cancer does not improve disease-free and overall survival compared to two years of treatment: phase III data from the SUCCESS A study. San Antonio, TX: San Antonio Breast Cancer Symposium; December 2017. Abstract GS1-06.
- 40. Gnant M, Pfeiler G, Steger GG, et al. Adjuvant denosumab in postmenopausal patients with hormone receptor-positive breast cancer (ABCSG-18): disease-free survival results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(3):339–351. [DOI] [PubMed] [Google Scholar]
- 41. Coleman RE, Finkelstein D, Barrios CH, et al. Adjuvant denosumab in early breast cancer: first results from the international multicenter randomized phase III placebo controlled D-CARE study. J Clin Oncol. 2018;36(suppl):501. [Google Scholar]
- 42. Coleman R, Hall A, Albanell J, et al. Effect of MAF amplification on treatment outcomes with adjuvant zoledronic acid in early breast cancer: a secondary analysis of the international, open-label, randomised, controlled, phase 3 AZURE (BIG 01/04) trial. Lancet Oncol. 2017;18(11):1543–1552. [DOI] [PubMed] [Google Scholar]