Abstract
Background
Although opioids play a critical role in the management of cancer pain, the ongoing opioid epidemic has raised concerns regarding their persistent use and abuse. We lack data-driven tools in oncology to understand the risk of adverse opioid-related outcomes. This project seeks to identify clinical risk factors and create a risk score to help identify patients at risk of persistent opioid use and abuse.
Methods
Within a cohort of 106 732 military veteran cancer survivors diagnosed between 2000 and 2015, we determined rates of persistent posttreatment opioid use, diagnoses of opioid abuse or dependence, and admissions for opioid toxicity. A multivariable logistic regression model was used to identify patient, cancer, and treatment risk factors associated with adverse opioid-related outcomes. Predictive risk models were developed and validated using a least absolute shrinkage and selection operator regression technique.
Results
The rate of persistent opioid use in cancer survivors was 8.3% (95% CI = 8.1% to 8.4%); the rate of opioid abuse or dependence was 2.9% (95% CI = 2.8% to 3.0%); and the rate of opioid-related admissions was 2.1% (95% CI = 2.0% to 2.2%). On multivariable analysis, several patient, demographic, and cancer and treatment factors were associated with risk of persistent opioid use. Predictive models showed a high level of discrimination when identifying individuals at risk of adverse opioid-related outcomes including persistent opioid use (area under the curve [AUC] = 0.85), future diagnoses of opioid abuse or dependence (AUC = 0.87), and admission for opioid abuse or toxicity (AUC = 0.78).
Conclusion
This study demonstrates the potential to predict adverse opioid-related outcomes among cancer survivors. With further validation, personalized risk-stratification approaches could guide management when prescribing opioids in cancer patients.
Pain remains one of the most feared and burdensome symptoms associated with cancer, and its curative therapies (1). More than half of cancer patients undergoing curative treatment experience pain rated as moderate to severe, warranting opioid use (2,3). Despite the accepted role of opioid analgesics in acute pain relief, the utility of opioid use in chronic pain (ie, pain lasting longer 3 to 6 months) remains controversial (4,5). Chronic opioid use can lead to diminishing analgesic efficacy with the possibility of toxicity including depression, sedation, loss of concentration, hyperalgesia, and hypogonadism (4,6,7). Additional known risks with prolonged opioid use include dependence, misuse, abuse, drug diversion, and unintentional overdosing (8). Furthermore, the ongoing opioid epidemic has raised concerns among patients and oncology providers regarding addiction and misuse (1). With an estimated 16.9 million cancer survivors in the United States and two-thirds of newly diagnosed cancer patients living more than 5 years, a better understanding of persistent opioid use, abuse and toxicity in oncology patients is imperative (6,9).
Optimal pain management with opioids requires a patient-specific assessment of benefits and risks (7,8). Along these lines, the American Society of Clinical Oncology (ASCO) recommends a risk-stratified approach to pain management and prescribing opioids (6). Specific risk-mitigation strategies include adherence monitoring, drug screening, alternative pain management strategies, judicious opioid use, and referral to pain specialists (6). Current guidelines for risk stratification, however, are based on expert opinion or instruments validated in nononcology cohorts that may omit risk factors relevant to cancer patients (10–13). An evidence-based risk-stratification approach could help clinicians better identify those at risk of adverse opioid-related events who might benefit from proactive adherence monitoring and mitigation. The purpose of this study was to determine rates and factors associated with persistent opioid use, diagnoses of opioid abuse, and admissions for opioid toxicity among a large cohort of cancer survivors who received curative intent cancer therapy. Additionally, we created and validated predictive models to help provide a clinically applicable approach to identifying patients at risk.
Methods
Data Source
Patients were selected from the Veterans Affairs (VA) Informatics and Computing Infrastructure (VINCI) database (14). VINCI is a comprehensive nationwide database that contains detailed electronic health record information on all veterans within the VA healthcare system. VINCI contains information on patient demographics, past medical history, medications, procedures, diagnoses, emergency room visits, clinic visits, and hospitalizations (14). Among cancer patients, additional data are collected by trained cancer registrars regarding stage at diagnosis, treatment, and recurrence in accordance with standardized protocols from the American College of Surgeons (15).
This study cohort included patients diagnosed with one of the 12 most-common noncutaneous, nonhematologic malignancies in VA patients (bladder, breast, colon, esophagus, stomach, head and neck, kidney, liver, lung, pancreas, prostate, or rectal cancer) from 2000 to 2015, treated with definitive local therapy (surgery, radiation therapy [RT] or both) and alive without recurrence 2 years after the initiation of treatment (Supplementary Figure 1 available online). Patients with metastatic disease or unknown stage at diagnosis were excluded. This study was reviewed and approved by the VA Health Care System. Waivers of consent and authorization were granted by the institutional review board (IRB) and the Research and Development Committee of the VA Health Care System (IRB Protocol Number 150169).
Covariates
Baseline patient, demographic, and cancer data were extracted from tumor registry data (16). Patient zip codes were used to obtain regional high school graduation rates, median household income level, and population density (urban or rural) (17,18). International Classification of Disease (ICD) codes (9th or 10th edition) in the year before the start of cancer treatment were used to define the NCI-adapted Charlson Comorbidity Index (CCI), which excludes cancer-related comorbidities. Similarly, ICD-9 or ICD-10 codes were used to capture precancer diagnoses of depression, alcohol abuse, nonopioid drug abuse, or opioid abuse (19–21). Additionally, we identified “high-risk” psychiatric conditions before cancer diagnosis, which included bipolar disorder, schizophrenia, obsessive compulsive disease (OCD), and attention deficit disorder (ADD), as defined by Webster and colleagues (10,22). Body mass index (BMI) at the time of cancer diagnosis was classified as healthy weight (18.5–25 kg/m2), underweight (< 18.5 kg/m2), or overweight (> 25 kg/m2) (23).
Opioid Use
Opioid use was determined from dispensed medication data in the VA outpatient pharmacy database. Similar to prior studies, patients were defined as opioid naive if no prescriptions were filled from 1 to 12 months before their first day of treatment (24–26). Prior chronic opioid use was defined as having filled equal to or more than 120 days’ supply of opioids between 1 to 12 months before treatment, or three opioid prescriptions from 3 to 6 months before treatment (24,25,27). Intermittent opioid use was defined as any opioid use from 1 to 12 months before treatment that did not meet criteria for chronic opioid use (24). Opioid use in the diagnosis and treatment period included any use extending from 1 month before the first day of treatment to 3 months after treatment (24,25).
Endpoints
The primary endpoint of persistent opioid use was defined with the previously published threshold of having filled 120 days’ or greater supply or 10 or more opioid prescriptions from 1 to 2 years after the start of curative treatment (28). This interval was selected as a time when patients should have completed primary and adjuvant cancer therapy and recovered from acute toxicity. Secondary endpoints included diagnoses of opioid abuse or dependence identified from ICD-9 and ICD-10 diagnosis codes, and admissions for opioid abuse, dependence or toxicity identified from inpatient admissions after the diagnosis date. Diagnoses of opioid abuse and dependence were analyzed together for the purposes of this study and approximate mild and moderate or severe opioid use disorder, respectively, as defined by the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) (29,30).
Statistical Analysis
Baseline covariates were compared between patients that became persistent opioid users and those that did not, using a chi-square test for categorical variables and a Student t-test for continuous variables. We used standard multivariable logistic regression models to identify associations between our study endpoints and predictive variables. We chose variables for the multivariable models a priori, which included patient-, demographic-, clinical-, and treatment-related variables. Because toxicities for surgery, radiation, and chemotherapy vary by cancer type and stage, we suspected a potential interaction between these factors. Accordingly, we tested for interaction terms between cancer type and stage and treatment factors in our regression models. Statistical analyses and modeling were performed using R version 3.5.1 (https://cran.r-project.org/). All tests were two-sided and a P value of less than .05 was considered statistically significant.
Predictive Modeling
For the predictive models only, imputation of missing variables for predictive modeling was accomplished via a multivariable imputation by chained equations (MICE) approach (31). Covariates were assumed to be missing at random, and distribution of missing data was evaluated using the MICE R package. In total, imputation replaced missing data for alcohol use (14.5% missing), tobacco history (14.4% missing), BMI category (3.5% missing), median income (2.3% missing), high school graduation rate (1.9% missing), and rural status (0.3% missing). A sensitivity analysis was also performed by including only complete cases for the logistic regression and predictive modeling, which generated similar findings (results not shown). The cohort was randomly divided 1:1 into a training and test (validation) data set. Covariates and interaction terms described above were selected as potential predictor variables in least absolute shrinkage and selection operator (LASSO) logistic regression models for each study endpoint (32,33). LASSO regression was selected as a robust supervised-learning approach that would facilitate variable selection for this high-dimensional dataset. We optimized the weighted penalty term (ƛ) by using 10-fold cross validation and selecting a final ƛ that was one standard error greater than the best-performing ƛ, as per standard practice (34). We also explored simpler and more parsimonious models by increasing ƛ until five characteristic covariates remained. The predictive models were created with the training data set, and discriminative ability of the risk score was assessed in the test data set using a receiver operator characteristic curve (ROC). An area under the curve (AUC) of 0.5 indicates the model was no better than random chance, and an AUC of 1.0 indicates perfect discrimination. A predictive risk score was developed using the linear predictors from the LASSO logistic regression model. For the persistent opioid use prediction model only, we categorized patients into predicted risk groups (low: ≤5% vs intermediate: > 5 and ≤ 25% vs. high >25%) based on cutoffs determined to be clinically relevant a priori. The simpler predictive models had similar discriminative ability as the complete models; therefore, we present the simpler models for each endpoint in the results section and included the more complex models in the Supplementary Table 2 (available online) and Supplementary Figure 2 (available online).
Results
Rates of Adverse Opioid Events
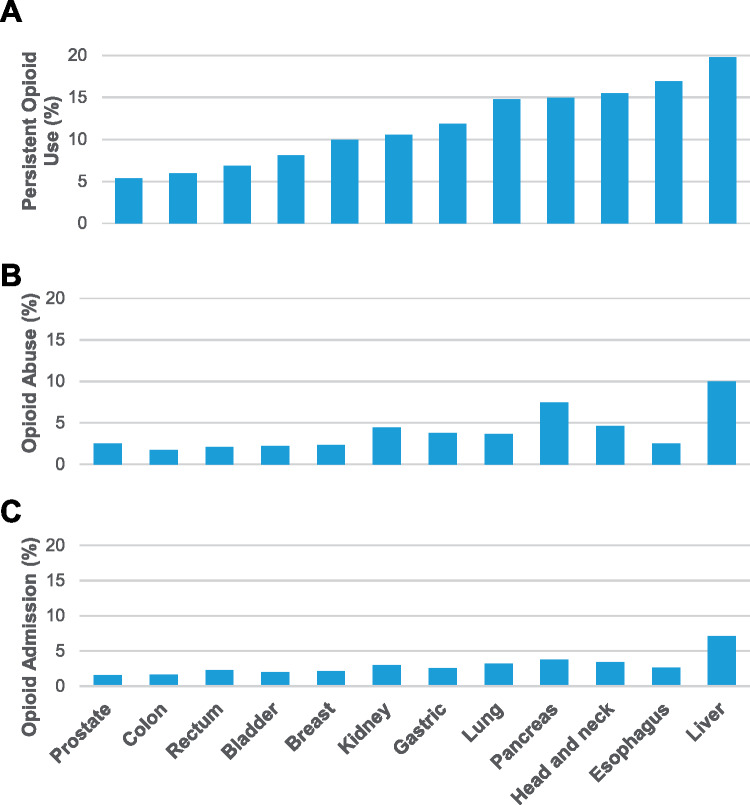
Among the 106 732 cancer survivors in this study, the overall incidence of persistent posttreatment opioid use was 8.3% (95% CI = 8.1% to 8.4%), which varied by cancer type, ranging from a low of 5.3% (95% CI = 5.1% to 5.5%) in prostate cancer patients to a high of 19.8% (95% CI = 17.2% to 22.5%) in liver cancer patients (Figure 1 and Table 1.). The rates of persistent opioid use after treatment varied substantially by a patient’s history of opioid use before their cancer diagnosis. The persistent posttreatment opioid use rates were lowest for opioid naïve patients (3.5% [95% CI = 3.3% to 3.6%]) followed by prior intermittent users (15.0% [95% CI = 14.4% to 5.6%]), and prior chronic users (72.2% [95% CI = 70.9% to 73.4%]). Among naïve patients, the rates of opioid use varied by whether patient’s received opioids during the diagnostic and treatment period. Those prescribed an opioid during the diagnostic and treatment period had rates of persistent posttreatment use of 6.2% (95% CI = 6.0% to 6.5%), compared with 1.5% (95% CI = 1.4% to 1.6%) of those that did not receive a prescription. The rate of posttreatment diagnoses of opioid abuse or dependence was 2.9% (95% CI = 2.8% to 3.0%), and opioid-related admissions occurred in 2.1% (95% CI = 2.0% to 2.2%) of patients.
Figure 1.
Rates of adverse opioid events among cancer survivors. Rates of persistent opioid use (top), new diagnosis of opioid abuse or dependence (middle), and admission for opioid abuse, dependence, or toxicity (bottom) by cancer type. HNC = head and neck cancer.
Table 1.
Patient, cancer, and treatment characteristics of patients stratified by the primary outcome of persistent opioid 1 year after treatment*
| Covariate | No persistent opioid use (n = 97 923) | Persistent opioid use (n = 8808) |
|---|---|---|
| Age (mean [SD]), y | 65.02 (8.59) | 62.07 (8.05) |
| Male (%) | 94 850 (96.9) | 8400 (95.4) |
| Race (%) | ||
| Black | 22 076 (22.5) | 1712 (19.4) |
| Other | 2770 (2.8) | 221 (2.5) |
| White | 73 077 (74.6) | 6875 (78.1) |
| Employed (%) | 12 145 (12.4) | 651 (7.4) |
| Married (%) | 49 416 (50.5) | 3971 (45.1) |
| Zip code metrics | ||
| Rural (%) | 26 211 (26.8) | 2355 (26.8) |
| % with HS diploma (mean [SD]) | 85.6 (8.3) | 85.7 (7.8) |
| Median income (mean $10k [SD]) | 50.1 (1.85) | 48.9 (1.69) |
| Tobacco use (%) | ||
| Current | 30 143 (30.8) | 4054 (46.0) |
| Never | 20 531 (21.0) | 1083 (12.3) |
| Past | 32 940 (33.6) | 2595 (29.5) |
| Unknown | 14 309 (14.6) | 1076 (12.2) |
| Alcohol use (%) | ||
| Current | 37 476 (38.3) | 3211 (36.5) |
| None | 29 975 (30.6) | 2472 (28.1) |
| Past | 16 049 (16.4) | 2030 (23.0) |
| Unknown | 14 423 (14.7) | 1095 (12.4) |
| BMI (%) | ||
| Healthy weight | 25 932 (27.4) | 2638 (30.8) |
| Overweight | 64 845 (68.6) | 5302 (61.9) |
| Underweight | 3704 (3.9) | 623 (7.3) |
| Prior diagnoses | ||
| Alcohol abuse (%) | 14 959 (15.3) | 2473 (28.1) |
| Depression (%) | 19 202 (19.6) | 3566 (40.5) |
| High-risk psychiatric condition (%) | 5078 (5.2) | 776 (8.8) |
| Nonopioid drug abuse (%) | 6810 (7.0) | 1407 (16.0) |
| Opioid abuse (%) | 1384 (1.4) | 558 (6.3) |
| CCI (%) | ||
| 0 | 41 292 (42.2) | 2857 (32.4) |
| 1 | 18 793 (19.2) | 1845 (20.9) |
| 2 | 17 342 (17.7) | 1552 (17.6) |
| 3+ | 20 496 (20.9) | 2554 (29.0) |
| Prior opioid use (%) | ||
| Opioid naive-new prescription | 33 383 (34.1) | 2216 (25.2) |
| Opioid naive-no prescription | 50 115 (51.2) | 779 (8.8) |
| Prior chronic use | 1354 (1.4) | 3509 (39.8) |
| Prior intermittent use | 13 071 (13.3) | 2304 (26.2) |
| Primary cancer (%) | ||
| Bladder | 4946 (5.1) | 434 (4.9) |
| Breast | 2456 (2.5) | 270 (3.1) |
| Colon | 10 007 (10.2) | 630 (7.2) |
| Esophagus | 806 (0.8) | 164 (1.9) |
| Gastric | 586 (0.6) | 79 (0.9) |
| Head and neck | 9315 (9.5) | 1701 (19.3) |
| Kidney | 7142 (7.3) | 842 (9.6) |
| Liver | 739 (0.8) | 182 (2.1) |
| Lung | 8132 (8.3) | 1409 (16.0) |
| Pancreas | 251 (0.3) | 44 (0.5) |
| Prostate | 51 361 (52.5) | 2894 (32.9) |
| Rectum | 2182 (2.2) | 159 (1.8) |
| AJCC 7th ed. stage (%) | ||
| I | 30 206 (30.8) | 3226 (36.6) |
| II | 52 384 (53.5) | 3531 (40.1) |
| III | 11 026 (11.3) | 1216 (13.8) |
| IV | 4307 (4.4) | 835 (9.5) |
| Local treatment (%) | ||
| RT | 36 160 (36.9) | 3118 (35.4) |
| Surgery | 57 255 (58.5) | 5017 (57.0) |
| Surgery + RT | 4508 (4.6) | 673 (7.6) |
| Chemotherapy (%) | 8416 (8.6) | 1534 (17.4) |
The groups significantly differed for all covariates (P < .01) except for rural status and rates of high school graduation. P values were calculated with a two-sided chi-square test for categorical variables and a two-sided t-test for continuous variables.
HS = high school; BMI = body mass index; CCI = Charlson Comorbidity Index; AJCC = American Joint Committee on Cancer; ed. = edition; RT = radiation therapy.
Factors Associated with Opioid-Related Endpoints
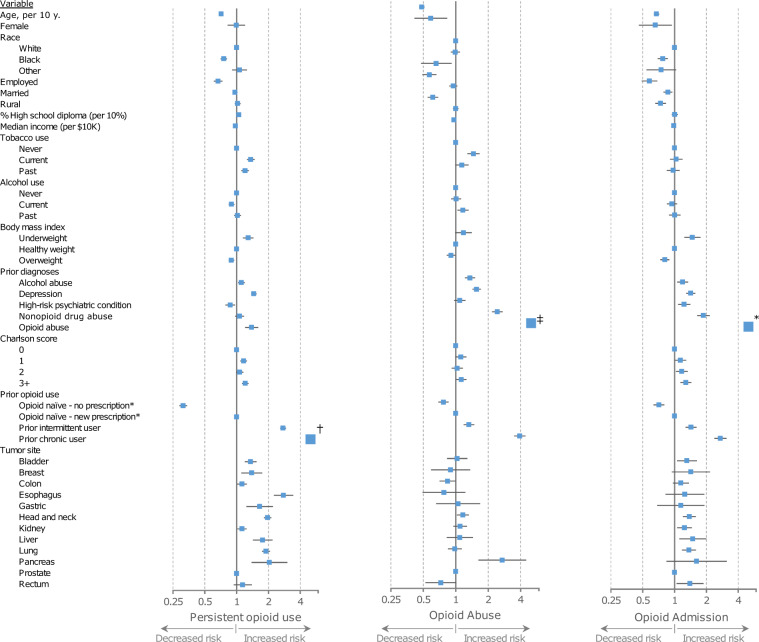
On multivariable analysis, several factors were associated with the risk of persistent opioid use (Figure 2). Younger age, white race, unemployment at the time of cancer diagnosis, lower median income, increased comorbidity, and current or prior tobacco use were all associated with increased adjusted odds of persistent opioid use. Prior diagnoses of alcohol abuse, nonopioid drug abuse, opioid abuse, and depression were associated with increased odds. Prior history of chronic opioid use and prior intermittent use were associated with substantially increased odds of persistent opioid use. Among opioid-naive patients, those without an opioid prescription during the diagnostic or treatment period had a lower risk persistent opioid use compared with those who received an opioid prescription. Bladder, breast, esophagus, stomach, head and neck, liver, lung, and pancreas cancer were associated with higher odds compared with prostate cancer.
Figure 2.
Association of covariates with adverse opioid events. Forest plot showing multivariable adjusted odds ratios (ORs) of covariates for persistent opioid use (left), a future diagnosis of opioid abuse or dependence (middle), or inpatient admission related to opioid toxicity (right). Error bars represent 95% confidence intervals. †Not to scale, OR = 35.42 (32.71–38.36); ‡Not to scale, OR = 13.52 (11.99–15.25); *Not to scale OR = 7.22 (6.29–8.28).
Stratified analyses evaluating the influence of American Joint Committee on Cancer stage, local treatment, and chemotherapy on the risk of persistent opioid use is presented in Supplementary Table 1 (available online). In general, stage, local treatment, and chemotherapy use were not associated with persistent opioid use outside of a few disease-site specific scenarios. Higher stage colon, lung, and head and neck cancer patients had an increased odds of persistent opioid use compared with lower stage patients. Definitive RT was associated with increased odds of persistent opioid use compared with definitive surgery in prostate and lung cancer patients. Kidney cancer patients receiving chemotherapy had an increased odds of persistent opioid use compared with those who did not receive chemotherapy.
Factors associated with the risk of future opioid abuse or dependence and opioid-related admissions are presented in Figure 2.
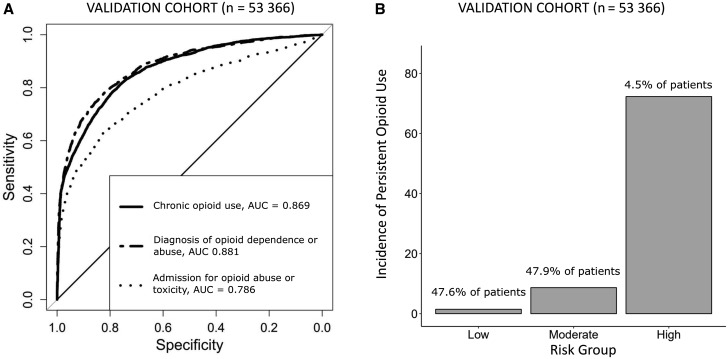
Risk Score to Predict Adverse Opioid-Related Endpoints
Our LASSO regression to create predictive risks scores identified patient, tumor, and treatment-related factors associated with the risk of the three opioid-related endpoints. Predictive covariates varied across the three different models (see Table 2 for predictive factors). Use of chemotherapy was a risk factor associated with an increased adjusted risk of all three opioid-related outcomes. Other factors associated with an increased risk varied by model, though included history of depression, prior opioid use, prior opioid abuse, alcohol abuse, and nonopioid drug abuse. Age was associated with a decreased risk of adverse outcomes. The individual models demonstrated a relatively high level of discrimination in predicting persistent opioid use (AUC = 0.85), opioid abuse or dependence (0.87), and opioid-related admission (0.78). The predictive models for persistent opioid use effectively stratified patients into low-, intermediate-, and high-risk groups (Figure 3b). The full predictive model demonstrated minimally improved discrimination for persistent opioid use (AUC = 0.87), opioid abuse or dependence (0.88), and opioid-related admissions (0.79) (see Supplementary Table 2 [available online] for predictive factors). We developed an online risk tool for these predictive models (www.CancerOpioidRisk.org) to assist with clinical implementation.
Table 2.
LASSO logistic regression predictive model covariates by outcome*
| Variable | Chronic opioid use | Opioid abuse | Opioid toxicity |
|---|---|---|---|
| Intercept | −2.670 | −2.833 | −3.966 |
| Age, per 10 y | – | −0.018 | −0.002 |
| Depression | 0.109 | – | 0.026 |
| Alcohol abuse | – | 0.018 | – |
| Nonopioid drug abuse | – | 1.095 | 0.625 |
| Past opioid drug abuse | – | 2.616 | 2.186 |
| Prior opioid use | |||
| Opioid naïve – new prescription (Referent) | – | – | – |
| Opioid naïve – no prescription | −0.726 | – | −0.045 |
| Prior chronic use | 3.209 | – | – |
| Prior intermittent use | 0.554 | – | – |
| Chemotherapy | 0.078 | 0.877 | 0.677 |
Table of log odds covariates from final predictive models, omitting covariates not predictive in any model. LASSO = least absolute shrinkage and selection operator; RT = radiation therapy; AJCC 7 = American Joint Committee on Cancer staging system 7th edition; Referent = reference group.
Figure 3.
Validation of parsimonious model. A) Receiver operating characteristic curve showing discrimination of LASSO model in predicting persistent opioid use, a future diagnosis of opioid abuse or dependence and future admissions for opioid abuse, dependence, or toxicity. B) Bar plot showing incidence of persistent opioid use for the predicted risk groups. AUC = area under the curve; LASSO = least absolute shrinkage and selection operator.
Discussion
Opioids are an effective and often irreplaceable analgesic for acute pain in cancer patients (1,6). Opioid use in chronic cancer is, however, complex, and providers and patients must consider the risks of treatment. Rates of persistent opioid use after curative cancer treatment have been estimated to be between 10.4% to 33.3%, although definitions of persistent use vary between studies (24,35,36). An additional study showed that cancer survivors had increased rates of chronic opioid use when compared with noncancer controls; however, by 6 years after diagnosis, the rates did not differ (37). Optimally managing cancer patients with opioids requires effective risk-stratification methods to identify individuals at higher risk of poor outcomes (6). Similar to cancer stage informing the management of antineoplastic therapy, an accurate prediction of future opioid-related morbidity can be used to personalize pain management and mitigate adverse outcomes. Current guidelines suggest strategies including establishing a signed treatment agreement, periodic urine drug testing, patient and caregiver education, referrals to palliative medicine or a pain specialist, avoidance of high-risk formulations, and minimizing total daily dose for patients at increased risk of adverse opioid-related outcomes (6,38–41).
This study identified multiple patient, cancer, and treatment factors statistically significantly associated with risk for persistent opioid use in cancer patients. Cancers with more intensive, multimodal therapies had the highest adjusted risk for persistent opioid use including esophagus, pancreas, liver, head and neck, and lung cancer. Prior opioid use was highly associated with future chronic use. The rate of persistent use was 72.2% among prior chronic users compared to 1.5% of opioid-naive patients who did not receive a prescription during treatment. Our results also support prior research demonstrating increased risk for opioid use among younger patients, the unemployed, current or former smokers, and those with a prior diagnosis of depression or drug abuse (10,26,42–44). Other factors associated with opioid risk identified in this study, such as race, median income, nonabusive alcohol use, comorbidity, BMI, and cancer type, have not been previously reported (42,45,46). We found no association between sex and persistent opioid use, which differs from other studies (10,26,28,43), though one must consider the skewed sex distribution of our study population within the VA health-care system. Many patient-, cancer-, and treatment-related factors were consistently predictive of the three opioid-related study endpoints, which likely stems from persistent opioid use being a mediator for downstream adverse opioids events.
The data-driven predictive models developed in this project differ from existing opioid risk-prediction tools. The Opioid Risk Tool represents a commonly used screening tool developed by expert opinion to predict aberrant behavior in noncancer patients (10). Select risk factors for persistent use in our models agreed with predictors used in the Opioid Risk Tool, including age, history of drug or alcohol abuse, and depression. In contrast with the Opioid Risk Tool, having a high-risk psychiatric condition (ADD, OCD, or schizophrenia) in our predictive model was not associated with increased adjusted risk for persistent use among cancer survivors, which could be a response to more rigid monitoring or prevention strategies in these patients. Additional screening tools include the Screener and Opioid Assessment for Patients with Pain-Revised (SOAPP), its revised edition (SOAPP-R), the Brief Risk Questionnaire, and the current opioid misuse measure (COMM)—all of which are self-reported questionnaires that assess psychologic and behavior patterns identified by experts to be associated with opioid misuse (11,47–49). It should be noted that all of these previously developed tools were explicitly developed and validated in noncancer populations. Additionally, one must consider that the predictive ability of self-reported questionnaires can be limited by their dependence on accurate reporting of potentially incriminating behaviors (48). The domains covered by the psychometric questionnaires are largely independent of the factors used in this population-based study. These inherent differences make direct comparisons between risk-prediction models difficult; however, one could hypothesize that the two approaches may be complementary.
This study has several limitations worth considering. Most notably, one must consider whether the results from a cohort of predominantly male military veterans will generalize to a nonmilitary population. In addition to sex differences, veterans are more likely to have health insurance coverage and less likely to live below the poverty line, as compared with the general population (50). Furthermore, combat veterans have higher rates of exposure to mental and physical trauma that could increase their risk for substance abuse or dependence (51). Validation in a non-VA cohort of cancer patients is required to help understand the generalizability of our findings and determine the predictive ability for the general population.
The retrospective nature of this analysis raises questions surrounding the accuracy of ascertaining opioid use, abuse, or dependence from electronic health records. Our observed rates of adverse opioid-related events were similar to other studies (24–26,36,52), although, overall, these events may be underreported in cancer patients, especially when using claims-based data (52). It is also possible that there was a misclassification of recurrence status and that some patients included in this cohort had disease progression and underwent additional salvage therapy. The observational population-based nature of this study also precludes the ability to evaluate known predictive factors such as prior trauma, family history, or focused patient-directed questions that have been previously shown to be associated with opioid abuse (10,12,13). The primary endpoint of persistent opioid use is limited to opioid prescriptions prescribed within the VA system. There are also limitations in our definition of opioid abuse or dependence, which typically requires the observation a problematic pattern of opioid use leading to clinical impairment or distress (29).
Despite these limitations, the current study represents one of the largest comprehensive evaluations of persistent opioid use and abuse in cancer survivors, and the first to construct a predictive model in oncology patients (24,35,43). The absolute rate of persistent opioid use, abuse, and dependence was relatively low among this cohort of cancer survivors, especially among those without prior opioid use. Improved risk stratification will allow for personalized risk assessment and improve the safety of pain management in cancer survivors. Future work is needed to externally validate these models, ideally in a prospective setting.
Funding
This study was supported by an ASCO Conquer Cancer Foundation Young Investigator Award (LKV).
Notes
Affiliations of authors: Department of Radiation Medicine and Applied Sciences (LKV, PR, PS, VN, RD, LKM, BR, JDM), Center for Precision Radiation Medicine (LKV, LKM, BR, JDM), and Department of Family Medicine and Public Health and Division of Pain Medicine, Department of Internal Medicine (TF), University of California San Diego, La Jolla, CA.
The funder had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
JDM receives compensation for consulting from Boston Consulting Group. PR receives salary support from Peptide Logic, LLC. The other authors have no disclosures.
Portions of this manuscript were presented in abstract form at the American Society for Radiation Oncology in Chicago, Illinois, September 16, 2019.
Supplementary Material
References
- 1. Paice JA, Von Roenn JH.. Under- or overtreatment of pain in the patient with cancer: how to achieve proper balance. J Clin Oncol. 2014;32(16):1721–1726. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Cancer Pain Relief: With a Guide to Opioid Availability. Geneva: World Health Organization; 1996. http://www.who.int/iris/handle/10665/37896. Accessed May 31, 2019. [Google Scholar]
- 3. van den Beuken-van Everdingen MHJ, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, Patijn J.. High prevalence of pain in patients with cancer in a large population-based study in The Netherlands. Pain. 2007;132(3):312–320. [DOI] [PubMed] [Google Scholar]
- 4. Ballantyne JC, Shin NS.. Efficacy of opioids for chronic pain. Clin J Pain. 2008;24(6):469–478. [DOI] [PubMed] [Google Scholar]
- 5. Treede R-D, Rief W, Barke A, et al. A classification of chronic pain for ICD-11. Pain. 2015;156(6):1003–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Paice JA, Portenoy R, Lacchetti C, et al. Management of chronic pain in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016;34(27):3325–3345. [DOI] [PubMed] [Google Scholar]
- 7. Del Fabbro E. Assessment and management of chemical coping in patients with cancer. J Clin Oncol. 2014;32(16):1734–1738. [DOI] [PubMed] [Google Scholar]
- 8. Portenoy RK, Ahmed E.. Principles of opioid use in cancer pain. J Clin Oncol. 2014;32(16):1662–1670. [DOI] [PubMed] [Google Scholar]
- 9.American Cancer Society. Cancer Treatment & Survivorship Facts & Figures 2019–2021. Atlanta: American Cancer Society; 2019. Accessed December 22, 2019. [Google Scholar]
- 10. Webster LR, Webster RM.. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the opioid risk tool. Pain Med. 2005;6(6):432–442. [DOI] [PubMed] [Google Scholar]
- 11. Akbik H, Butler SF, Budman SH, Fernandez K, Katz NP, Jamison RN.. Validation and clinical application of the screener and opioid assessment for patients with pain (SOAPP). J Pain Symptom Manage. 2006;32(3):287–293. [DOI] [PubMed] [Google Scholar]
- 12. Moore TM, Jones T, Browder JH, Daffron S, Passik SD.. A comparison of common screening methods for predicting aberrant drug-related behavior among patients receiving opioids for chronic pain management. Pain Med. 2009;10(8):1426–1433. [DOI] [PubMed] [Google Scholar]
- 13. Chou R, Fanciullo GJ, Fine PG, Miaskowski C, Passik SD, Portenoy RK.. Opioids for chronic noncancer pain: prediction and identification of aberrant drug-related behaviors: a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009;10(2):131–146. [DOI] [PubMed] [Google Scholar]
- 14.Veterans Affairs Health Services Research and Development. VA Informatics and Computing Infrastructure (VINCI). https://www.hsrd.research.va.gov/. Accessed August 20, 2018.
- 15.Facility Oncology Registry Data Standardss. American College of Surgeon; 2016. https://www.facs.org/%03/media/files/qualityprograms/cancer/ncdb/fords%25. Accessed May 31, 2019.
- 16. Farges O, Fuks D, Le Treut Y-P, et al. AJCC 7th edition of TNM staging accurately discriminates outcomes of patients with resectable intrahepatic cholangiocarcinoma. Cancer. 2011;117(10):2170–2177. [DOI] [PubMed] [Google Scholar]
- 17.U.S. Census Bureau. 2010 Census Urban and Rural Classification and Urban Area Criteria; 2010. http://www.census.gov/2010census/data/. Accessed May 31, 2019.
- 18.Bureau UC. American Community Survey Summary File Data. https://www.census.gov/programs-surveys/acs/data/summary-file.2015.html. Accessed November 6, 2018.
- 19. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. [DOI] [PubMed] [Google Scholar]
- 20. Bryant AK, Sojourner EJ, Vitzthum LK, et al. Prognostic role of p16 in nonoropharyngeal head and neck cancer. JNCI J Natl Cancer Inst. 2018;110(12):1393–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Charlson ME, Pompei P, Ales KL, MacKenzie CR.. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 22. Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264(19):2511–2518. [PubMed] [Google Scholar]
- 23. Flegal KM, Kit BK, Orpana H, Graubard BI.. Association of all-cause mortality with overweight and obesity using standard body mass index categories. JAMA. 2013;309(1):71.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee J-J, Hu HM, Edelman AL, et al. New persistent opioid use among patients with cancer after curative-intent surgery. J Clin Oncol. 2017;35(36):4042–4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. 2017;152(6):e170504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brescia AA, Harrington CA, Mazurek A, et al. Factors associated with new persistent opioid usage after lung resection. Ann Thorac Surg. 2019;107(2):363–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bohnert ASB, Ilgen MA, Trafton JA, et al. Trends and regional variation in opioid overdose mortality among veterans health administration patients, fiscal year 2001 to 2009. Clin J Pain. 2014;30(7):605–612. [DOI] [PubMed] [Google Scholar]
- 28. Sun EC, Darnall BD, Baker LC, Mackey S.. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med. 2016;176(9):1286.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Association AP. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). American Psychiatric Publishing; 2013. [Google Scholar]
- 30. Moore B, Barrett ML. Case Study: Exploring How Opioid-Related Diagnosis Codes Translate From ICD-9-CM to ICD-10-CM; 2017. https://www.hcupus.ahrq.gov/datainnovations/icd10_resources.jsp. Accessed May 31, 2019.
- 31. Buuren S. V, Groothuis-Oudshoorn K.. Multivariate imputation by chained equations in R. J Stat Softw. 2011;45(3):1–67. [Google Scholar]
- 32. Tibshirani R. Regression shrinkage and selection via the Lasso. J R Stat Soc Ser B. 1996;58(1):267–288. http://www.jstor.org/stable/2346178. Accessed May 31, 2019. [Google Scholar]
- 33. James G, Witten D, Hastie T, Tibshirani R.. An Introduction to Statistical Learning. Vol 112 New York: springer; 2013:18. [Google Scholar]
- 34. Friedman J, Hastie T, Tibshirani R.. The Elements of Statistical Learning. Vol 1 New York: Springer Series in Statistics; 2001. [Google Scholar]
- 35. Tuminello S, Schwartz RM, Liu B, et al. Opioid use after open resection or video-assisted thoracoscopic surgery for early-stage lung cancer. JAMA Oncol. 2018;4(11):1611.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Saraswathula A, Chen MM, Mudumbai SC, Whittemore AS, Divi V.. Persistent postoperative opioid use in older head and neck cancer patients. Otolaryngol Head Neck Surg. 2019;160(3):380–387. [DOI] [PubMed] [Google Scholar]
- 37. Salz T, Lavery JA, Lipitz-Snyderman AN, et al. Trends in opioid use among older survivors of colorectal, lung, and breast cancers. JCO. 2019;37(12):1001–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Califf RM, Woodcock J, Ostroff S.. A proactive response to prescription opioid abuse. N Engl J Med. 2016;374(15):1480–1485. [DOI] [PubMed] [Google Scholar]
- 39.Swarm RA, Paice JA, Anghelescu DL, et al. Adult Cancer Pain, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019;17(8):977–1007. [DOI] [PubMed]
- 40. Argoff CE, Alford DP, Fudin J, et al. Rational urine drug monitoring in patients receiving opioids for chronic pain: consensus recommendations. Pain Med. 2018;19(1):97–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kennedy AJ, Arnold RM, Childers JW.. Opioids for chronic pain in patients with history of substance use disorders, part 2: management and monitoring #312. J Palliat Med. 2016;19(8):890–891. [DOI] [PubMed] [Google Scholar]
- 42. Edlund MJ, Martin BC, Russo JE, DeVries A, Braden JB, Sullivan MD.. The role of opioid prescription in incident opioid abuse and dependence among individuals with chronic noncancer pain: The role of opioid prescription. Clin J Pain. 2014;30(7):557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McDermott JD, Eguchi M, Stokes WA, et al. Short-and Long-term Opioid Use in Patients with Oral and Oropharynx Cancer. Otolaryngol Head Neck Surg. 2019;160(3):409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Boscarino JA, Rukstalis M, Hoffman SN, et al. Risk factors for drug dependence among out-patients on opioid therapy in a large US health-care system. Addiction. 2010;105(10):1776–1782. [DOI] [PubMed] [Google Scholar]
- 45. Ives TJ, Chelminski PR, Hammett-Stabler CA, et al. Predictors of opioid misuse in patients with chronic pain: a prospective cohort study. BMC Health Serv Res. 2006;6(1):46.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Turk DC, Swanson KS, Gatchel RJ.. Predicting opioid misuse by chronic pain patients. Clin J Pain. 2008;24(6):497–508. doi: 10.1097/AJP.0b013e31816b1070 [DOI] [PubMed] [Google Scholar]
- 47. Butler SF, Budman SH, Fernandez KC, et al. Development and validation of the current opioid misuse measure. Pain. 2007;130(1):144–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Butler SF, Fernandez K, Benoit C, Budman SH, Jamison RN.. Validation of the revised screener and opioid assessment for patients with pain (SOAPP-R). J Pain. 2008;9(4):360–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jones, PhD T, Lookatch, Ma S, Moore, PhD T.. Validation of a new risk assessment tool: The Brief Risk Questionnaire. J Opioid Manag. 2015;11(2):171.. [DOI] [PubMed] [Google Scholar]
- 50. Profile of Veterans; 2017. https://www.census.gov/programs-surveys/acs/. Accessed May 31, 2019.
- 51. Bremner JD, Southwick SM, Darnell A, Charney DS.. Chronic PTSD in Vietnam combat veterans: course of illness and substance abuse. Am J Psychiatry. 1996;153(3):369. [DOI] [PubMed] [Google Scholar]
- 52. Pinkerton R, Hardy JR.. Opioid addiction and misuse in adult and adolescent patients with cancer. Intern Med J. 2017;47(6):632–636. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.