Abstract
We present the case of a 55-year-old man who was admitted with new abdominal ascites and change in stool caliber. The colonoscopy examination revealed a severe stricture with inflamed friable mucosa measuring 8 cm in length in the rectosigmoid colon. Histologically, poorly differentiated adenocarcinoma with signet ring cells was seen. The patient underwent esophagogastroduodenoscopy (EGD), which was suggestive of linitis plastica of the stomach. On microscopic examination, biopsy reported poorly differentiated adenocarcinoma with occasional signet ring cells as the primary source.
Keywords: signet ring cell type carcinoma, gastric cancer, colonic stricture
Introduction
Gastric cancers are the third most common cause of cancer-related deaths worldwide [1]. Among the various gastric malignancies, signet ring cell carcinoma (SRC) is well-known to have an aggressive course and, therefore, poor prognosis. Linitis plastica is the macroscopic presentation of a diffuse infiltrating carcinoma of a hollow organ, causing it to retain its shape but remain stiff and contracted. A study by Piessen et al. had a total of 27 cases of linitis plastica, of which 21 had a histological diagnosis of SRC [2]. It is important not to consider linitis plastica and SRC as synonyms.
There have been very few cases of gastric SRC with metastasis to the large intestine presenting in the form of polyps, ulcerations, and depressed lesions. We present a case of gastric SRC with metastasis to the rectosigmoid junction presenting as a stricture.
Case presentation
A 55-year-old male presented to our emergency room with left-sided, intermittent, dull, lower abdominal pain, and progressive abdominal distension of five months duration. The patient reported intermittent constipation with a change in stool caliber. He otherwise denied weight loss, loss of appetite, dark stools, and blood in stools. His medical conditions included hypertension, fatty liver, and gastroesophageal reflux disease. Surgical history was significant for umbilical and left inguinal hernia repair. He never used tobacco or recreational drugs. He had been consuming up to eight beers every day for many years and quit three months prior to his presentation. He denied the use of any over-the-counter medications or herbal medications. There was no history of gastrointestinal (GI) malignancies in the family. His medications included pantoprazole, atenolol, and stool softeners as needed.
He presented to the emergency room with stable vitals. Physical examination was significant for mild abdominal distention, fluid trill, and shift. Laboratory findings revealed normal complete blood count and liver function tests. Computed tomography (CT) of the abdomen (Figure 1) showed mild wall thickening of the sigmoid colon and rectum. He was also noted to have a moderate-to-large amount of simple density ascites, a mildly enlarged liver, and a normal spleen.
Figure 1. Computed axial tomography of the abdomen and pelvis showed rectosigmoid thickening.
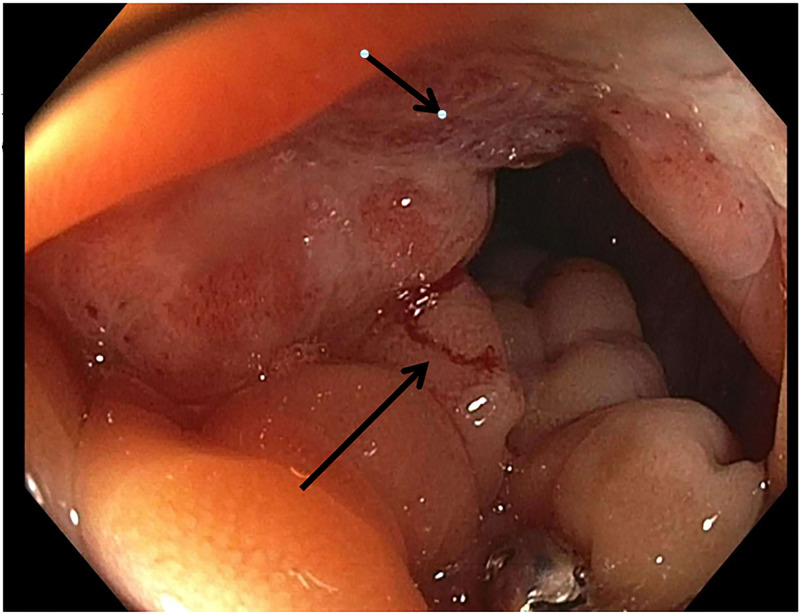
A diagnostic paracentesis showed cloudy fluid with a serum to ascites albumin gradient (SAAG) ratio of 1.4, lymphocytic predominance, and total protein of 4.4. Cytology revealed highly atypical epithelial cells, suspicious for carcinoma. The patient underwent colonoscopy, which revealed a severe stricture with inflamed friable mucosa measuring 8 cm in length in the rectosigmoid colon located 12-20 cm from the anal verge (Figure 2). The standard adult colonoscope was not able to be traverse due to severe stenosis; it was switched to an ultrathin scope, which was advanced with ease to the ileocecal valve. There were diffuse erythema and granularity in the descending, transverse, and ascending colon. Multiple biopsies were performed throughout the entire colon, including the stenotic lesion.
Figure 2. Colonoscopic view of the rectosigmoid colon stricture with inflamed friable mucosa.
The pathology of the stenotic lesion was reported as a poorly differentiated adenocarcinoma with signet ring cells. The rest of the biopsies were reported to be colonic mucosa with preserved crypt architecture.
The patient underwent esophagogastroduodenoscopy (EGD; Figure 3), which showed linitis plastica of the stomach with a non-bleeding gastric ulcer in the body. Multiple areas of the stomach were biopsied given the appearance of linitis plastic, which was reported to be poorly differentiated adenocarcinoma with occasional signet ring cells. CT of the chest did not reveal any metastasis. The patient was referred to oncology for further management and was lost to follow-up.
Figure 3. Upper endoscopic view showing the gastric fundus revealing thickened gastric folds with diffuse granular mucosa.
Discussion
Gastric SRC is uncommon and tends to have poor survival. Histologically, SRC is primarily composed of more than 50% tumor cells with predominantly mucinous cytoplasm and an eccentrically located crescent-shaped nucleus [3]. There has been an overall decrease in the incidence of gastric cancers over the years; however, the incidence of SRC has been on the rise in the United States [4]. A review article by Antonioli DA showed a rise in SRC cases from 9% to 39% in their newer series on gastric cancers done from 1975-1978 as compared to their previous similar series from 1938-1942 [5]. Theuer et al. reported a histological pattern of SRC in 3% to 39% of gastric cancer cases [6].
SRC is known to have a female predominance, is more common in the younger population, and is usually located in the middle and distal stomach [5,7]. In addition, it is predominant among blacks, Asians, American Indians, and Hispanics [8]. Patients are usually diagnosed at late stages (American Joint Committee on Cancer (AJCC) Stages 3 and 4) or with nodal or distant metastasis, one of the reasons being the infiltrative pattern leading to the late onset of clinical symptoms [8].
SRC occurs primarily in the stomach with common sites of metastasis being the lymph nodes, peritoneum, and intestines. Most of the time, when there is a large intestinal metastasis, the primary malignancy is usually the stomach [9]. Our case has been one among such where the patient was found to have ascites and a rectosigmoid stricture, which led to further investigation and tracing of the primary malignant lesion to the stomach. The literature review revealed various macroscopic presentations of the metastatic intestinal lesions. They included mostly polyps [10-13], ulcerations [14-15], and depressed lesions [9,16-17]. However, literature on metastatic lesions of a gastric SRC presenting as a colonic stricture is rare.
Another unique feature of our case is the location of SRC metastasis. A literature review done by Sonoda et al. showed 11 cases of primary gastric SRC with metastasis to the large intestine, out of which six cases had metastasis to the sigmoid colon and only one case of metastasis to the rectum [9]. Our patient had an uncommon metastasis to the rectosigmoid junction [18-19].
Conclusions
Gastric SRC metastasis to the rectosigmoid junction in the form of stricture formation is a rare presentation. The literature review has revealed mostly gastric SRC metastasis to the colon as ulcerations or depressed or flat, elevated lesions. Given the rarity of gastric SRC metastasis to the colon with stricture formation at the rectosigmoid junction, this case helps build awareness and increase knowledge among physicians to look for possible gastric SRC in patients with colonic strictures.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained by all participants in this study
References
- 1.Signet-ring cell carcinoma of the stomach: Impact on prognosis and specific therapeutic challenge. Pernot S, Voron T, Perkins G, Lagorce-Pages C, Berger A, Taieb J. World J. Gastroenterol. 2018;21:11428–11438. doi: 10.3748/wjg.v21.i40.11428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Signet ring cell histology is an independent predictor of poor prognosis in gastric adenocarcinoma regardless of tumoral clinical presentation. Piessen G, Messager M, Leteurtre E, Jean-Pierre T, Mariette C. Ann Surg. 2009;250:878–887. doi: 10.1097/SLA.0b013e3181b21c7b. [DOI] [PubMed] [Google Scholar]
- 3.Clinicopathological characteristics and prognosis of signet ring cell carcinoma of the stomach. Kwon K-J, Song E-M, Choi J-C, Choi J-Y, Kim S-E, Jung H-K, Jung S-A. https://link.springer.com/article/10.1007/s10120-013-0234-1. Gastric Cancer. 2013;17:43–53. doi: 10.1007/s10120-013-0234-1. [DOI] [PubMed] [Google Scholar]
- 4.Differential trends in the intestinal diffuse types of gastric carcinoma in the United States, 1973-2000: increase in the signet ring cell type. Henson DE, Dittus C, Younes M, Nguyen H, Albores-Saavedra J. https://www.archivesofpathology.org/doi/full/10.1043/1543-2165%282004%29128%3C765%3ADTITIA%3E2.0.CO%3B2. Arch Pathol Lab Med. 2004;128:765–770. doi: 10.5858/2004-128-765-DTITIA. [DOI] [PubMed] [Google Scholar]
- 5.Changes in the location and type of gastric adenocarcinoma. Antonioli DA, Goldman H. Cancer. 1982;50:775–781. doi: 10.1002/1097-0142(19820815)50:4<775::aid-cncr2820500425>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 6.Signet ring cell histology is associated with unique clinical features but does not affect gastric cancer survival. Theuer CP, Nastanski F, Brewster WR, Butler JA. https://search.proquest.com/openview/76bd5e99187357c3c3c817da2a3f40f4/1?pq-origsite=gscholar&cbl=49079. Am Surg. 1999;65:915–921. [PubMed] [Google Scholar]
- 7.Signet ring cell carcinoma of the stomach. Maehara Y, Sakaguchi Y, Moriguchi S, Orita H, Korenaga D, Kohnoe S, Sugimachi K. https://acsjournals.onlinelibrary.wiley.com/doi/abs/10.1002/1097-0142(19920401)69:7%3C1645::AID-CNCR2820690702%3E3.0.CO;2-X. Cancer. 1992;69:1645–1650. doi: 10.1002/1097-0142(19920401)69:7<1645::aid-cncr2820690702>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 8.Prognostic significance of signet ring gastric cancer. Taghavi S, Jayarajan SN, Davey A, Willis AI. J Clin Oncol. 2012;30:3493–3498. doi: 10.1200/JCO.2012.42.6635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lymphogenous metastasis to the transverse colon that originated from signet-ring cell gastric cancer: a case report and review of the literature. Sonoda H, Kawaia K, Yamaguchi H, et al. Clin Res Hepatol Gastroenterol. 2017;41:0. doi: 10.1016/j.clinre.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Metastases of a gastric adenocarcinoma presenting as colonic polyposis report of a case. Metayer P, Antonietti M, Oumrani M, Hemet J, Lemoine F, Basuyau J. https://link.springer.com/article/10.1007/BF02049905. Dis Colon Rectum. 1991;34:622–623. doi: 10.1007/BF02049905. [DOI] [PubMed] [Google Scholar]
- 11.Metastases from gastric adenocarcinoma presenting as multiple colonic polyps: report of a case. Ogiwara H, Konno H, Kitayama Y, Kino I, Baba S. https://link.springer.com/article/10.1007/BF01427044. Surg Today. 1994;24:473–475. doi: 10.1007/BF01427044. [DOI] [PubMed] [Google Scholar]
- 12.Colonic anastomosis and colonic polyp mucosal metastasis of signet ring cell gastric adenocarcinoma. Rodríguez Salas N, González Paz CG, Rivera T, Lopez Alfonso A, Martin Marino M, Lara Álvarez MA. https://link.springer.com/article/10.1007/s12094-010-0496-6. Clin Transl Oncol. 2010;12:238–239. doi: 10.1007/s12094-010-0496-6. [DOI] [PubMed] [Google Scholar]
- 13.Polypoid colonic metastases from gastric stump carcinoma: a case report. Gao B, Xue X, Tai W, et al. Oncol Lett. 2014;8:1119–1122. doi: 10.3892/ol.2014.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colonic metastasis of diffuse signet ring cells gastric carcinoma [Article in Spanish] Reyes-Díaz ML, Torres-Arcos C, Oliva-Mompeán F, Curado-Soriano A, Lizarralde-Gómez CJ, Cuaresma-Soriano F. Rev Esp Enferm Dig. 2012;104:442–443. doi: 10.4321/s1130-01082012000800012. [DOI] [PubMed] [Google Scholar]
- 15.Colonic metastasis from gastric cancer. Oh SY, Cunningham J, Saif MW. https://einstein.pure.elsevier.com/en/publications/colonic-metastasis-from-gastric-cancer#:~:text=The%20most%20common%20sites%20of,from%20gastric%20cancer%20are%20rare. Clin Colorectal Cancer. 2014;13:442–443. doi: 10.1016/j.clcc.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 16.A solitary colonic metastasis from gastric cancer detected at an early stage. Nakamura H, Fu K, Fukui H, Hurlstone DP, Kaji Y, Ishikawa T, Fujimori T. Gastrointest Endosc. 2008;67:1000–1004. doi: 10.1016/j.gie.2007.10.052. [DOI] [PubMed] [Google Scholar]
- 17.Metastases from gastric carcinoma to colon in the form of multiple flat elevated lesions: a case report. Lee H-C, Yang M-T, Lin K-Y, Tu H-S, Zhang T-A, Chen P-H. Kaohsiung J Med Sci. 2004;20:552–557. doi: 10.1016/S1607-551X(09)70257-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laparoscopic low anterior resection for hematogenous rectal metastasis from gastric. Lim SW, Huh JW, Kim YJ, Kim HR. World J Surg Oncol. 2011;9:148. doi: 10.1186/1477-7819-9-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Solitary rectal metastasis from primary gastric cancer. Hamada Y, Tanaka K, Katsurahara M, Baba Y. J-Stage. 2019;58:1037–1038. doi: 10.2169/internalmedicine.1902-18. [DOI] [PMC free article] [PubMed] [Google Scholar]