Abstract
Ashortage of NIOSH-approved respirators is predicted during an influenza pandemic and other infectious disease outbreaks. Healthcare workers may use surgical masks instead of respirators due to non-availability and for economical reasons. This study investigated the filtration performance of surgical masks for a wide size range of submicron particles including the sizes of many viruses. Five models of FDA-cleared surgical masks were tested for room air particle penetrations at constant and cyclic flow conditions. Penetrations of polydisperse NaCl aerosols (75±20 nm, count median diameter), monodisperse NaCl aerosols (20–400 nm range) and particles in the 20–1000 nm range were measured at 30 and 85 liters/min. Filtration performance of surgical masks varied widely for room air particles at constant flow and correlated with the penetration levels measured under cyclic flow conditions. Room air particle penetration levels were comparable to polydisperse and monodisperse aerosol penetrations at 30 and 85 liters/minute. Filtration performance of FDA-cleared surgical masks varied widely for room air particles, and monodisperse and polydisperse aerosols. The results suggest that not all FDA-cleared surgical masks will provide similar levels of protection to wearers against infectious aerosols in the size range of many viruses.
Keywords: Surgical mask, Filtration performance, Particle penetration, Virus particles, Nanoparticles
INTRODUCTION
The term “surgical mask” is used to refer to Food and Drug Administration (FDA)-cleared surgical, laser, isolation, dental, medical procedure or face masks with or without a face shield. Healthcare personnel often wear various types of surgical masks to provide protection against body fluid splashes to the nose and mouth. They are also worn by surgeons and other operating room personnel to prevent organisms in their noses and mouths from falling into the sterile field and potentially causing surgical site infections. Infection control guidance recommends placing surgical masks on potentially infectious patients to limit the dissemination of infectious respiratory secretions from patients to others. Surgical masks are often confused with filtering facepiece respirators (FFRs), because surgical masks look similar to respirators and both are worn on the face. The differences between surgical masks and respirators were discussed in a 2008 Institute of Medicine report on personal protective equipment for healthcare workers during an influenza pandemic (Goldfrank et al. 2008).
The FDA does not test and certify surgical masks, but clears them for sale after reviewing the manufacturer’s test data and proposed claims (FDA 2004). Manufacturers test surgical masks for particle filtration efficiency (PFE), bacterial filtration efficiency (BFE), fluid resistance, differential pressure and flammability, and submit the results for FDA clearance. For BFE measurements, surgical masks are tested with non-neutralized Staphylococcus aureus of 3 ± 0.3 μm diameter at a flow rate of 28.3 liters/minute (ASTM 2001; FDA 2004). Some types of surgical masks are also tested with 100 nm diameter non-neutralized polystyrene latex spheres (PSL) at 1 to 25 cm/second face velocity for PFE (ASTM 1989; FDA 2004). FDA-cleared surgical masks can be categorized into three types of medical face mask materials as specified in ASTM F 2100–04 (ASTM 2004). The high and moderate barrier masks are cleared with >98% filtration efficiency levels for both BFE and PFE tests, while the low barrier masks require >95% for the BFE test only (ASTM 2004). On the other hand, NIOSH certified respirators are tested under “near worst case” test conditions using charge-neutralized polydisperse aerosol particles at a high flow rate (85 liters/minute) (Federal Register 1995; NIOSH 2005). N class respirators are tested using NaCl aerosol with a count median diameter (CMD) of 75±20 nm, and P and R class respirators with dioctyl phthalate aerosol with a CMD of 185±20 nm. Class N, R and P respirators are certified at <5, <1 and <0.03% penetration levels. Unlike surgical masks, respirators are designed to fit and seal tightly to the face. Beginning in 2004, a new surgical mask category called “surgical N95 respirator” was also cleared by FDA for sale. The surgical N95 respirator is a NIOSH-approved N95 FFR, which also meets FDA-required fluid resistance and differential pressure tests (FDA 2008).
In response to the need for improved Mycobacterium tuberculosis infection control methods in the 1990s, several studies compared the filtration efficiency of surgical masks to respirators (Brosseau et al. 1997; Chen and Willeke 1992; Lenhart et al. 2004; Weber et al. 1993; Willeke et al. 1996). In one study, penetration of particles in the 150–4000 nm range at different flow rates using a manikin fitted with a mask or respirator were measured (Chen and Willeke 1992). Surgical masks showed penetration levels of approximately 55–85% and 70–90% at flow rates of 30 and 100 liters/minute, respectively, for 300 nm particles. The most penetrating particle size (MPPS) was in the 200–500 nm range. Surgical masks were found to be less efficient compared to dust-mist (DM) and dust-mist-fume (DMF) respirators. DM and DMF respirators were classifications of particulate respirators approved under 30 CFR 11 which was superseded by the current 42 CFR 84 regulations. Subsequent studies with eight different surgical masks showed penetration levels of 15–100% and 6–100% for 200 nm and 1000 nm size particles, respectively (Weber et al. 1993). Another study compared the efficiency of surgical masks to DM and DMF respirators against 550 nm polystyrene latex particles at 45 liters/minute and Mycobacterium abscessus (1.0–2.5 μm length × 0.5 μm width) particles at 45 and 85 liters/minute flow rates (Brosseau et al. 1997). Their results also confirmed that the efficiency level of surgical masks was less than that of DM and DMF respirators.
More recently, the filtration efficiency of surgical masks and N95 FFRs against MS2 virus particles in the 10–80 nm range was reported (Balazy et al. 2006). Penetration levels of one of the two surgical mask models tested increased with increasing particle size from 10 to 50 nm, and then plateaued at 20% up to 80 nm diameter at 85 liters/minute flow rates. A similar penetration pattern was obtained at 30 liters/minute flow rate, with a maximum penetration level of 13%. Another surgical mask showed a steady increase in penetration levels up to approximately 80% with increase in particle size from 10 nm to 80 nm.
Another recent study investigated particle filtration and face fit performance of surgical masks (Oberg and Brosseau 2008). This study reported high penetration levels for 9 surgical masks (5 were FDA-cleared surgical masks) commonly used in hospital and dental settings. Filtration efficiencies of various surgical masks were measured using monodisperse polystyrene latex spheres (PSL) of 895, 2000, and 3100 nm diameters at 6 liters/minute. A wide range of particle penetration levels (0–84%) was obtained for the three different size PSL particles. Surgical masks were also tested with polydisperse NaCl aerosol with a count median diameter of 75 nm using a TSI 8130 similar to the NIOSH particulate respirator certification test protocol. A wide range of penetration levels (4–90%) was obtained similar to PSL particles. The authors also reported that fit factor measurements using a Bitrex qualitative fit test failed all test subjects when masks were donned without assistance. After receiving assistance, the test failed all but two male subjects. Subjects were also tested for quantitative fit using a PortaCount® Plus (TSI). Maximum fit factors of 6.9 for unassisted donning and 9 for assisted donning were reported.
A detailed study on the filtration performance of surgical masks for particles in the submicron size range is lacking and is needed to confirm the earlier studies discussed above. This knowledge gap needs to be addressed, because, there is increased concern of human exposure to harmful airborne virus particles during pandemic events. A shortage of FFRs is predicted during an influenza pandemic (Bailar et al. 2006; CDC 2006). Workers and the general public may be tempted to use surgical masks instead of NIOSH-approved filtering facepieces (FFRs) for protection against airborne influenza virus when there is a shortage of FFRs during an influenza pandemic. For these reasons, filtration performance of FDA-cleared surgical masks was investigated for room air particles in the 20–1000 nm range and compared with the NIOSH particulate respirator test method using polydisperse NaCl, as well as ten different size monodisperse NaCl particles in the 20–400 nm range. It was hypothesized that the FDA-cleared surgical masks studied here would exhibit a wide range of filtration efficiencies against submicron particles across the various test methods employed, confirming the earlier studies.
MATERIALS AND METHODS
Surgical Masks
FDA-cleared surgical masks from five manufacturers were selected randomly and only one model from each manufacturer was used in the study. The manufacturer and model of the evaluated surgical masks were 3M (1800), Busse (370), CrossTex (GCS), Precept (1510), and Primed (PG4–1073). Of these five surgical masks, one model (A) was classified as high, two models as moderate (B and C) and the other two models as low barrier types (D and E) (ASTM 2004). Table I shows manufacturer provided penetration levels for the FDA-cleared surgical masks. These surgical masks are not certified by NIOSH for respiratory protection.
Table I.
Filtration efficiency of FDA-cleared surgical masks
| Manufacturer | FDA Clearance | Barrier Type | Filtration efficiency (%) | ||
|---|---|---|---|---|---|
| Non-neutralized | |||||
| Bacterial aerosol | 100 nm polystyrene latex spheres | ~100 nm room air particles | |||
| A | Yes | High | >98 | >98 | 92.0 |
| B | Yes | Medium | >98 | >98 | 85.5 |
| C | Yes | Medium | >98 | >98 | 83.4 |
| D | Yes | Low | >95 | NR | NR |
| E | Yes | Low | >95 | NR | NR |
BFE and PFE values cited in ASTM F2100–04.
NR - not required by ASTM F2100–04.
Room Air Particle Penetration at Constant Flow Condition
Figure 1 shows the schematic of the room air particle penetration test system. Briefly, laboratory room air particles were passed into a Plexiglas test box (20 cm × 20 cm × 10 cm) mounted with a surgical mask placed between the upstream and downstream filter chucks as described previously (Rengasamy et al. 2007). Upstream and downstream aerosol particles were counted by an ultra-fine condensation particle counter (UCPC, TSI 3025A) by sampling through ports, off each filter chuck, alternately. Particle counting was continued for 100 seconds irrespective of the number of particles downstream of the surgical masks. Percentage particle penetration was calculated by multiplying the ratio of the number of particles downstream to the number of particles upstream by 100.
Figure 1.
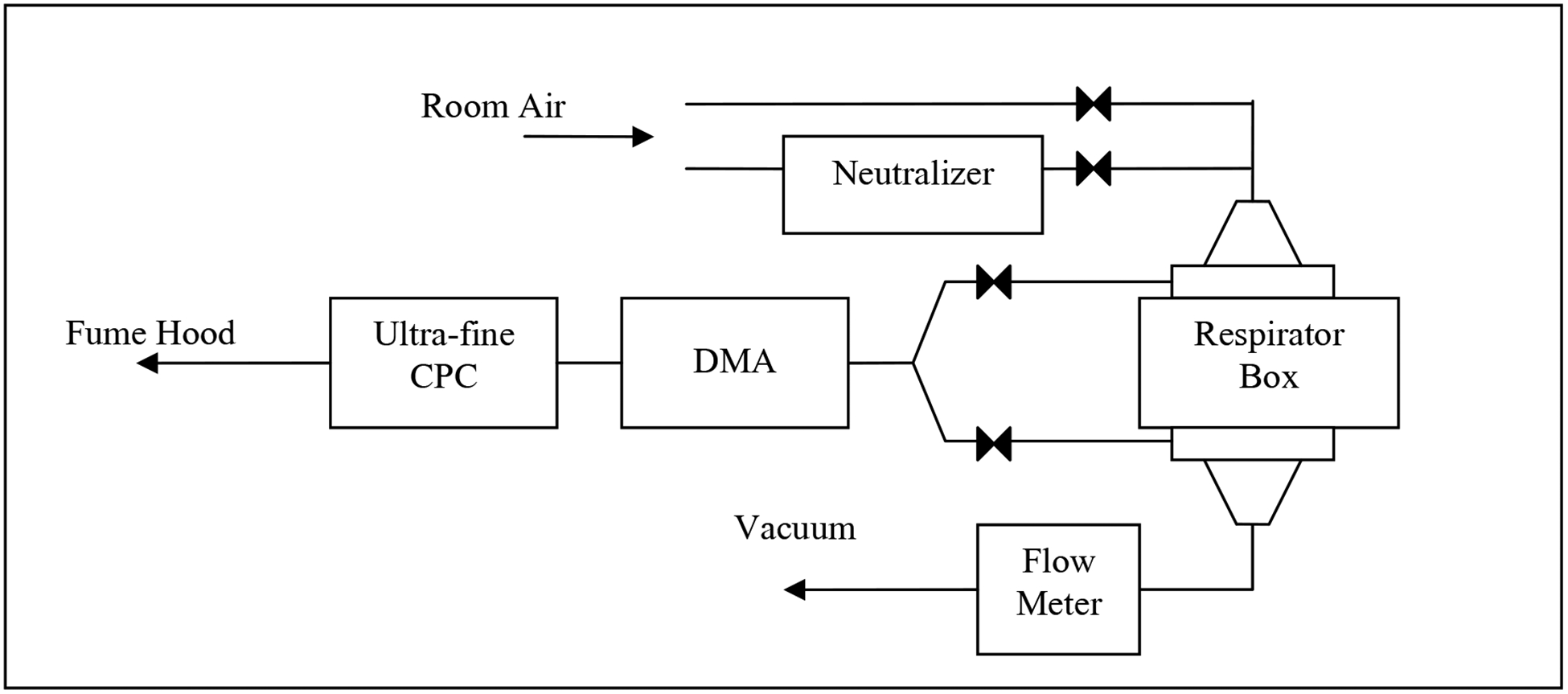
Schematic diagram of constant flow room air particle penetration test system.
Four samples of each surgical mask model were tested for the penetration of room air particles (control) at a constant flow rate. The same surgical masks were again tested for charge-neutralized particles by passing room air through a 85Kr source (TSI 3012), and then into the Plexiglas test box. When changing from control to neutralized particles, a 5 minute time was allowed for equilibration. Penetration levels at three different flow rates (6, 30, and 85 liters/minute) were measured and a separate set of four masks was tested for each flow rate.
Room Air Particle Penetration as a Function of Particle Size
Percentage penetration for each surgical mask was also measured as a function of particle size from 20–1000 nm using a Scanning Mobility Particle Sizer (SMPS, TSI, Inc.) in the scan mode. Particle concentration upstream and downstream of the surgical mask was measured using the Plexiglas box set up at 85 liters/minute. Room air particle concentrations for the 20–1000 nm size range particles were measured for 135 seconds for upstream and downstream samples, alternately. Percentage particle penetration was calculated by multiplying the ratio of the particle concentration downstream to upstream of the mask by 100.
Particle Penetration Measurement at Cyclic Flow Condition
Particle penetration levels for each surgical mask were measured under cyclic flow conditions. The set up used in this study allowed room air to go in and out of a rubber bladder, similar to a human lung, through a mask sealed on to a manikin (Figure 2). The volume of air going through the mask per minute can be compared to minute volumes of human breathing, but not the cyclic pattern. Unlike human breathing, the tidal volume remains the same at different flow rates. Briefly, a surgical mask was fitted with a sampling port similar to that used for fit factor measurement for respirators with a PortaCount® Plus (TSI, Inc.). The surgical mask was sealed to a manikin using a silicone adhesive to eliminate any leakage around the seal, and was connected to the breathing pump (Figure 2). Particle concentrations inside and outside of the mask were analyzed by sampling through the sampling port and outside of the manikin head, respectively, using two condensation particle counters. A set of four masks was tested for particle concentration against control room air particles at 6 and 30 liter minute volumes under cyclic flow conditions. Percentage penetration was calculated by multiplying the ratio of concentration of particles inside to outside of the mask by 100.
Figure 2.
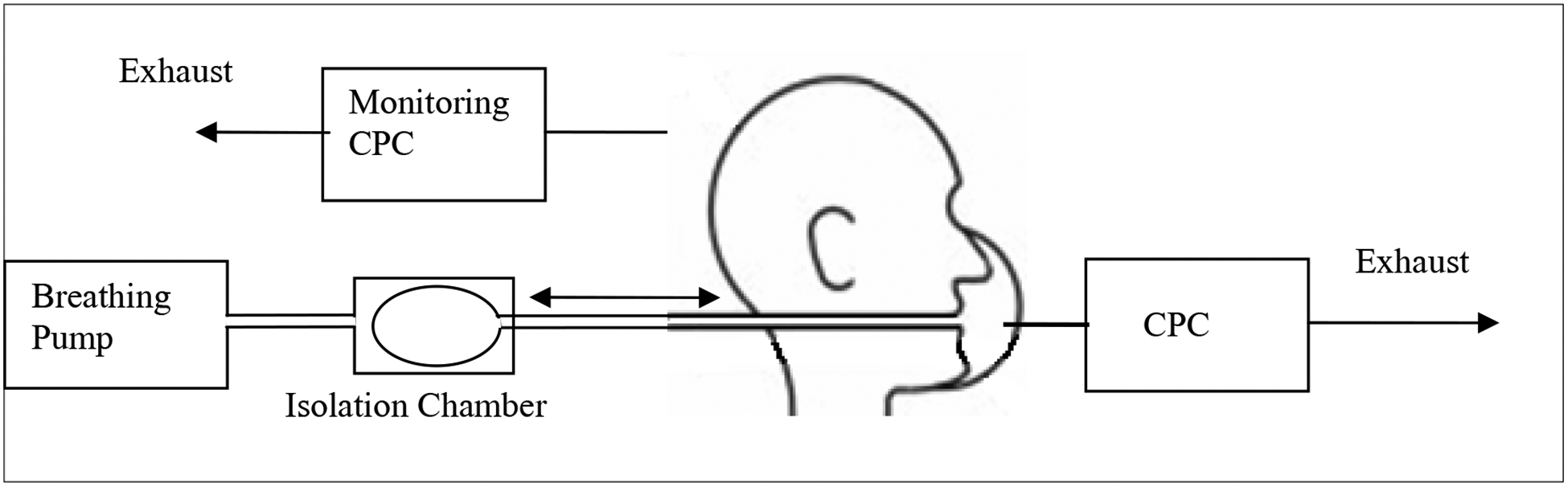
Schematic diagram of cyclic flow room air particle test system.
Polydisperse NaCl Aerosol Penetration Measurement
A different set of three surgical masks was tested for polydisperse NaCl aerosol (75±20 nm, count median diameter) penetrations with a TSI 8130 Automated Filter Tester (TSI 8130) used for NIOSH particulate respirator tests (Federal Register 1995; NIOSH 2005). Initial penetration levels of NaCl particles were measured for 1 min, instead of conducting the entire NIOSH 42 CFR 84 test protocol. Percentage penetration was measured using the Plexiglas box set up as described previously (Rengasamy et al. 2007). Penetrations were measured at 30 and 85 liters/minute flow rates using separate sets of surgical masks to avoid any loading effects.
Monodisperse Aerosol Test Method
Another set of four surgical masks from the same models were tested against monodisperse NaCl particles using a TSI 3160 Fractional Efficiency Tester (TSI 3160) as described previously (Rengasamy et al. 2007). Initial percentage penetration levels of ten different monodisperse aerosols (20, 30, 40, 50, 60, 80, 100, 200, 300 and 400 nm) were measured for each mask at 30 liters/minute and then at 85 liters/minute.
Effect of Isopropanol Treatment on Monodisperse Aerosol Penetrations
To better understand particle filtration by electrostatic mechanism, the surgical mask models tested for monodisperse particle penetrations were carefully removed from the Plexiglas box and dipped into isopropanol for 1 min, removed and allowed to dry in a fume hood overnight. Monodisperse aerosol penetrations were again measured for each of these surgical masks as described previously. Previous studies showed that liquid isopropanol treatment of electret filters reduced or removed electrical charges associated with fibrous filters and increased particle penetration in laboratory experiments (Chen and Huang 1998; Chen et al. 1993; Martin and Moyer 2000).
Data Analysis
The data were analyzed using the SigmaStat® (Jandel Corporation) computer program. Average and 95% confidence interval penetration levels were calculated for each model. Correlation coefficients between variable parameters were calculated using the Pearson Product Moment Correlation method.
RESULTS
Room Air Particle Penetration at Constant Flow Condition
Percentage penetration levels of control and charge-neutralized room air particles were measured for four samples from each of the surgical mask models at 6, 30 and 85 liters/minute constant flow rates. Figure 3 shows that the percentage penetrations of control room air particles were less than 10% for four models (A, B, C, and D) at 6 liters/minute and for two models (A, and B) at both 30 and 85 liters/minute. Model E showed >46% at 6 liters/minute, which increased to 76% at 85 liters/minute flow rate. In general, penetration levels of control room air particles did not differ from the penetrations obtained for charge-neutralized room air particles at 6, 30 and 85 liters/minute flow rates.
Figure 3.
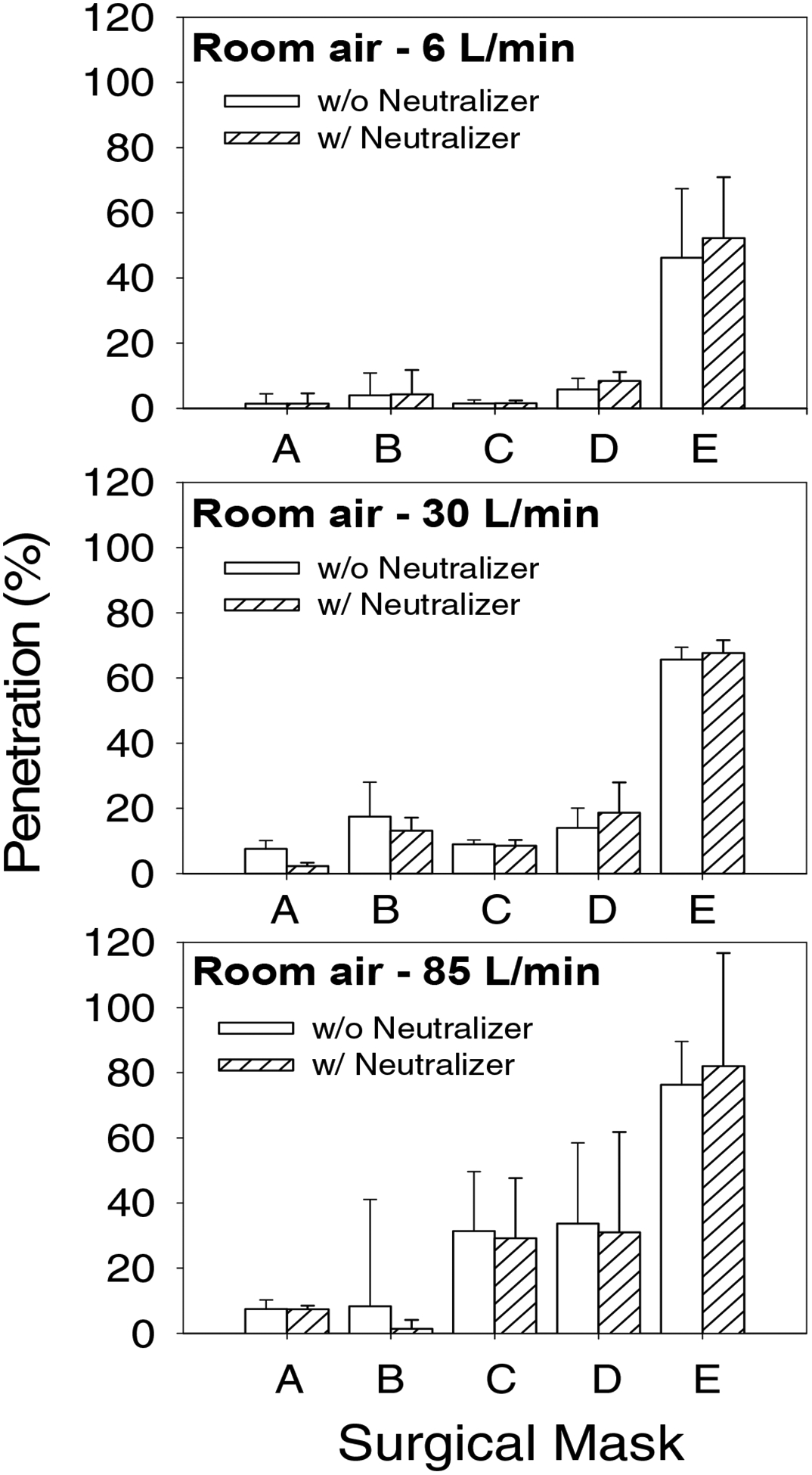
Percentage penetration levels of control (empty bars) and charge neutralized (hatched bars) room air particles for surgical masks at 6, 30 and 85 liters/minute constant flow rates.
Room Air Particle Penetration as a Function of Particle Size
Room air particles were size classified using an SMPS and the penetration levels of particles in the 20–1000 nm was measured at 6, 30 and 85 liters/minute flow rates. In general, percentage penetration levels increased from 20 nm, reached a maximum and then decreased up to 1000 nm. Figure 4 shows that the penetration levels at 85 liters/minute flow rate peaked at 50 nm for one model (A) and at ~130 nm for two models (B and C) and ~200–400 nm for the other two models. Control and charge-neutralized room air particles showed more or less similar penetration levels for the different size particles in the 20–1000 nm range. The mean penetration values for non-neutralized room air particles in the range of 95–105 nm were integrated to represent the penetration levels for 100 nm size particles to allow comparisons with test methods specified in ASTM 2100–04. Percentage penetration levels were 8.0, 24.5, 27.6, 45.1 and 89.8 for surgical mask models A, B, C, D and E, respectively. In other words, the efficiency levels were 92% for the high barrier mask (A), 83.4–85.5% for the moderate barrier masks (B and C) and 11.2–54.9% for the low barrier masks (D and E) (Table I). Similarly, the filtration efficiency levels integrated for 500–1000 nm particles were 97.7% for high barrier mask (A), 77.6–86.2% for moderate barrier masks (B and C) and 57.9–88.6% for low barrier masks (D and E).
Figure 4.
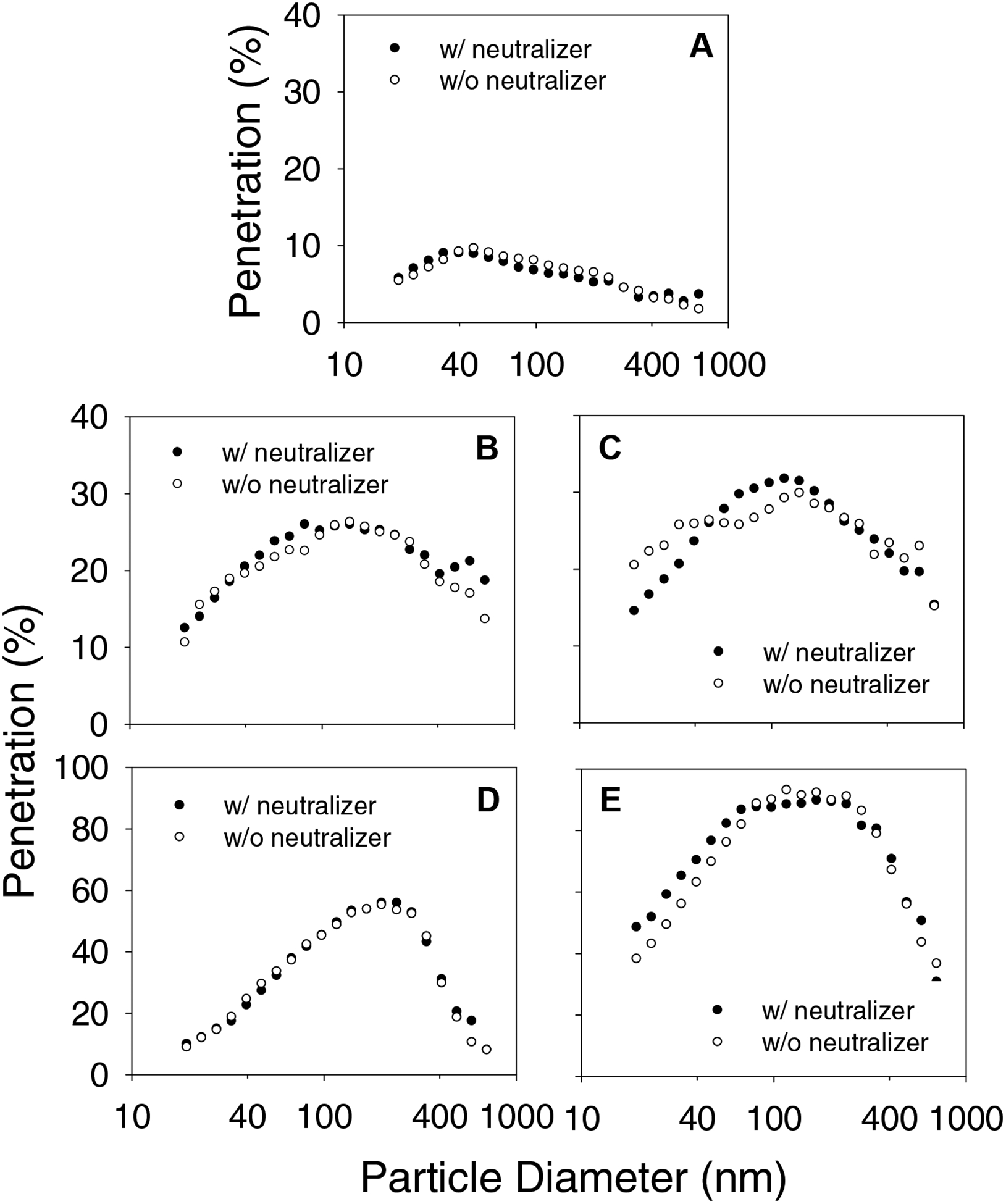
Size dependent penetration data from SMPS measurements of room aerosol at 85 liters/minute flow rate. A, B, C, D and E represent different surgical mask models.
Room Air Particle Penetrations at Constant and Cyclic Flow Conditions
Room air particle penetrations of surgical masks under constant flow using the Plexiglas box set up and cyclic flow using the manikin set up are compared in Figure 5. Percentage penetration levels for different models varied between 0.7–51 and 2.2–67.7 at 6 and 30 liters/minute constant flow rates, respectively. These values were compared with the range of filter penetration levels 1.3–50.6% and 5.1–60.9% measured at 6 and 30 liters/minute cyclic flow conditions, respectively. In general, penetration levels measured at constant flow rates showed good correlations (r > 0.96 and r>0.97) with the penetration levels measured under cyclic flow rates.
Figure 5.
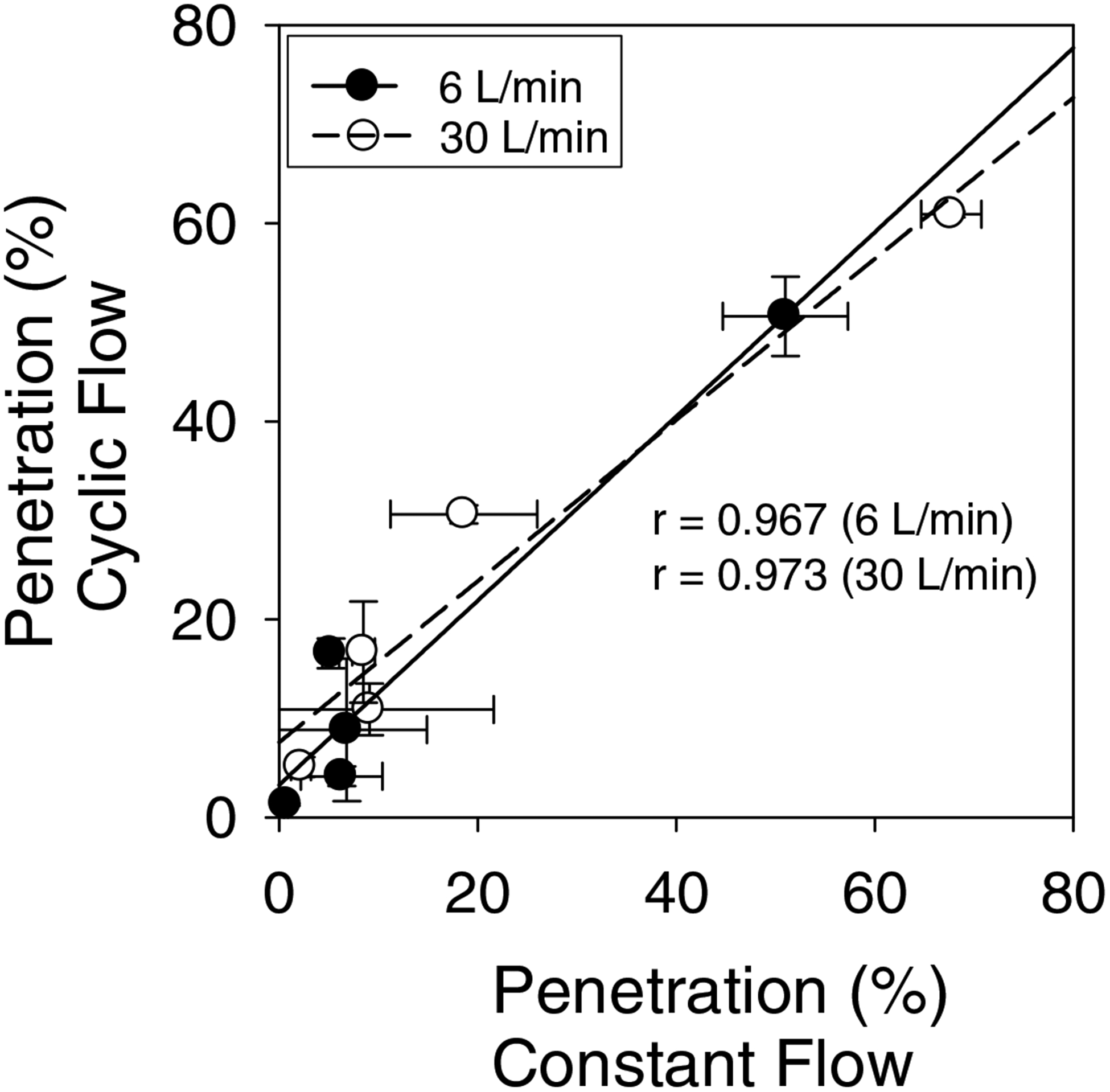
Correlation of surgical mask penetration levels at constant flow rates with penetration levels measured at cyclic flow conditions. Straight lines are linear best fit lines of the two data sets.
Polydisperse Aerosol Penetrations
Figure 6 shows penetration levels for polydisperse aerosols measured using the TSI 8130. One surgical mask model (A) showed less than 5% penetration at both 30 and 85 liters/minute flow rates. Penetration levels were in the 5–20% range for three models (B, C and D) at 30 liters/minute and two models (B and C) at 85 liters/minute. Model E had 63.3% penetration at 30 liters/minute, which increased to 88.0% at 85 liters/minute.
Figure 6.
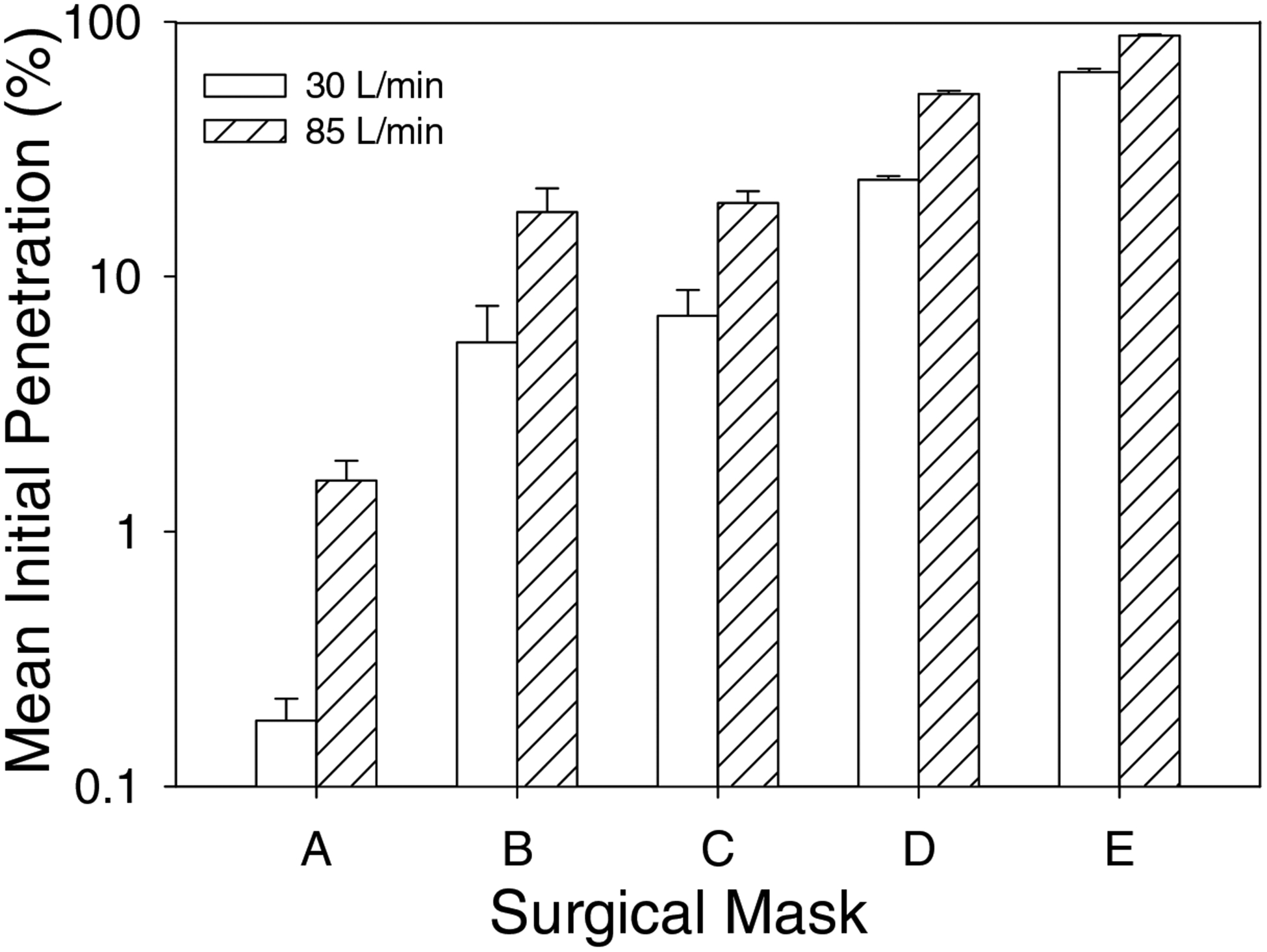
Polydisperse aerosol penetration levels of five surgical mask models as measured by a TSI 8130 at 30 and 85 liters/minute.
Monodisperse Aerosol Penetrations
Another set of five surgical masks from each manufacturer were tested against ten different size monodisperse aerosol particles in the 20–400 nm range. Their initial penetrations levels were measured at 30 and 85 liters/minute flow rates. In general, penetration levels increased from 20 nm, reached a maximum at 40–400 nm (Figure 7). Penetration levels for the different size monodisperse particles obtained at 85 liters/minute were higher than that at 30 liters/minute for all surgical masks. The MPPS was in the 40–60 nm range for three surgical mask models (A, B, and C) and 200–400 nm for two other models (D and E) at both 30 and 85 liters/minute flow rates.
Figure 7.
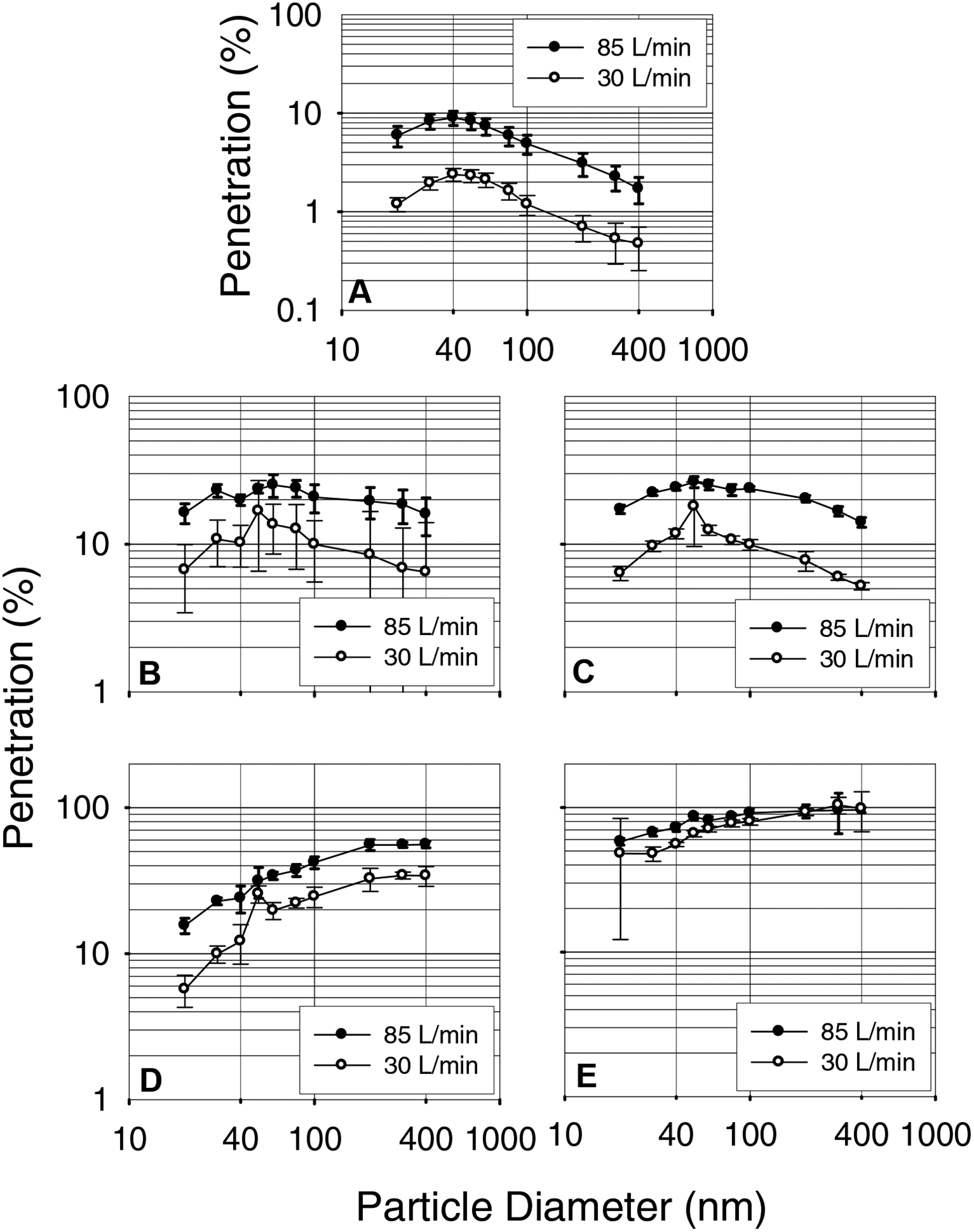
Monodisperse aerosol penetration levels of surgical masks as recorded by a TSI 3160. A, B, C, D and E represent surgical mask models.
Effect of Liquid Isopropanol Treatment on Monodisperse Aerosol Penetrations
Figure 8 shows that liquid isopropanol treatment increased the penetration levels of monodisperse particles in the 60–400 nm range for three surgical mask models (A, B, and C). The MPPS for these surgical mask models was shifted from 40–60 nm to the 200–400 nm range. The penetration levels of model D and E remained mostly at levels obtained for control surgical masks with no change in the MPPS.
Figure 8.
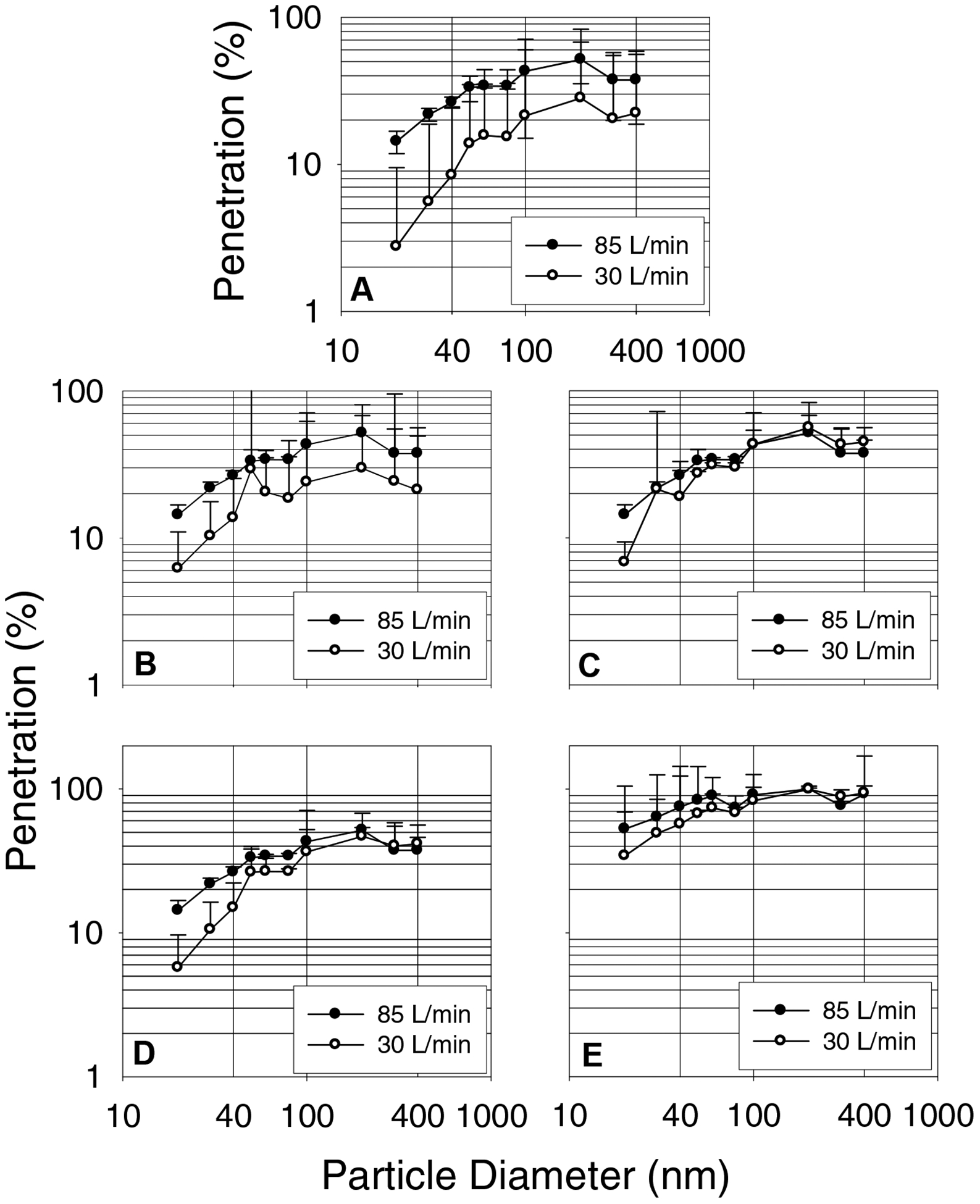
Monodisperse aerosol penetration data for isopropanol (IP) treated surgical masks measured with a TSI 3160. A, B, C, D and E represent surgical mask models.
Surface Area of Surgical Masks and Face Velocity
The surface area of the five surgical mask models tested in the study ranged from 135 –294 cm2 with an average of 230 cm2. This value was used for calculating the face velocities of 2.2 and 6.2 cm/second corresponding to 30 and 85 liters/minute flow rates.
DISCUSSION
Results from this study using five models of FDA-cleared surgical masks showed a wide variation in filtration performance from the four filtration test methods employed. Initial percentage penetrations for room air particles were in the range of 1.4 – 46.2, 6.7 – 65.7, and 7.5 – 76.3 at 6, 30 and 85 liters/minute constant flow rates, respectively. Similarly, polydisperse NaCl particle penetrations measured similar to the NIOSH certification test protocol were in the range of 0.2–63.3% and 1.6 – 88.1% at 30 and 85 liters/minute flow rates. Similar variation in filtration performance for surgical masks was reported in the literature (Chen and Willeke 1992; McCullough et al. 1997; Oberg and Brosseau 2008; Weber et al. 1993; Willeke et al. 1996). The wide variation in penetration levels can be partly explained by the particle penetration tests employed for testing the three different categories of surgical masks. For particulate filtration efficiency measurements, the low barrier surgical masks are tested for only BFE. The moderate and high level barrier surgical masks are not only tested for BFE, but also for PFE using 100 nm non-neutralized latex sphere particles at 1 to 25 cm/second face velocity (ASTM 1989; ASTM 2004). The high and moderate barrier masks are cleared for >98% filtration efficiency levels for PFE and BFE tests, while the low barrier type for >95% level for BFE tests (ASTM 2004). In our study, moderate and high barrier surgical masks showed filtration efficiency levels between 83.4–85.5% and 92% for 100 nm size NaCl particles at a face velocity of 6.2 cm/second (85 liters/minute). These filtration efficiency levels for moderate and high barrier surgical masks are less than those levels expected given that these surgical masks had been tested previously by manufacturers for performance using the test methods specified in ASTM F 2100–04 (ASTM 2004). The discrepancy in the penetration levels obtained for surgical masks in this study and the FDA specified penetration levels may be explained partly by the difference in the face velocity employed for testing surgical masks. For example, FDA requires surgical mask testing to be conducted according to ASTM F 2100–04 specifications. ASTM F 2100–04 describes a test method for surgical mask filter media at face velocities in the 1 to 25 cm/second range. This indicates that manufacturers can submit surgical mask penetration results obtained from tests conducted at any face velocity in the 1 to 25 cm/second range which can be a potential source for the wide variation in penetration values. Indeed, surgical mask model A was tested at a face velocity higher than that employed for the other models as informed by the manufacturers. A wide variation in penetration levels is expected because of the lack of FDA requirement for testing surgical masks at a specified face velocity.
Monodisperse aerosol particle penetrations were measured to determine the most penetrating particle size range for the surgical masks studied. The results for the different surgical masks showed markedly different penetration levels for ten different size monodisperse particles in the 20–400 nm range. The MPPS was in the 40–50 nm range with penetration levels <10% for one model and 20–30% for two other models at 85 liters/minute flow rate. The other two models showed that the MPPS was in the 200–400 nm range with penetration levels of 50–80%. The MPPS obtained with the TSI 3160 measurement was compared to the penetration values obtained for polydisperse aerosols with the TSI 8130. A good correlation (r=0.99) was obtained between the two penetration values (data not shown) similar to previous reports for N95 FFRs (Rengasamy et al. 2007) and non-certified dust masks (Rengasamy et al. 2008). The penetration levels at the MPPS obtained with the TSI 3160 measurement were compared to the MPPS values obtained with the room air particle penetration measurements using the SMPS in the scanning mode. The MPPS obtained for three surgical models (A, D and E) were similar by the two methods at 85 liters/minute flow rate. Models B and C showed that the MPPS was in the 40–60 nm range using the TSI 3160 and approximately 130 nm by the SMPS scanning data. The discrepancy can be explained partly by the filtration characteristics of surgical masks. For example, the slopes for penetration levels for monodisperse 40 to 100 nm is less for surgical mask models B and C compared to other models (Figure 7).
Surgical mask models B and C showed an increase in polydisperse aerosol penetration levels by about 3-fold compared to 12-fold for model A after isopropanol treatment, suggesting that models B and C are weaker electrostatic filters with mild mechanical characteristics (data not shown). The mild mechanical nature of surgical masks B and C with a slope of close to zero for 40–100 nm monodisperse particle penetrations might have shifted the MPPS to 130 nm.
The dependence of surgical mask filtration efficiency on electrical charges of room air particles and filter media was investigated. Two approaches were attempted to gain insight into the role of electrical charges in capturing room air particles. The first one was aimed to understand whether room air particles carry net electrical charges and influence penetration levels. For this reason, penetrations were measured against room air particles with and without charge neutralization. The results from this set of experiments showed no significant difference in the penetration levels for control and charge-neutralized room air particles at three different flow rates (6, 30 and 85 liters/minute). This suggests that room air particles do not carry significant net charges to alter particle penetration levels. A previous study which investigated the electric charge for workplace aerosols in several factories, quarries and a coal mine showed approximately equal number of positive and negative charges (Johnston et al. 1985). This is consistent with the notion that comparatively aged ambient aerosol particles are neutralized to Boltzmann equilibrium (John 1980).
Secondly, the presence of electrical charges on surgical mask fibers used to enhance particle capturing was investigated. Liquid isopropanol is known to remove electrical charges from filter media fibers as revealed by a shift in the MPPS to a larger size and an increase in particle penetration level (Chen and Huang 1998; Chen et al. 1993; Martin and Moyer 2000). Results from this study showed that three of the five surgical masks showed a shift in the MPPS from 40–60 nm to 200–400 nm range suggesting the presence of electrical charges. The incorporation of electrical charges on filter fiber media is known to increase filtration efficiency without increasing the resistance (Barrett and Rousseau 1998). At the same time, the other two surgical masks showed neither a shift in the MPPS nor an increase in the penetration levels for different size monodisperse particles after isopropanol treatment suggesting the lack of electrical charges on the fiber media. The results suggest that the electrostatic surgical masks are more efficient in capturing submicron size particles compared to the mechanical type. Mechanical type filters can be made more efficient, but this increases the pressure drop making them harder to breathe through. Similar observations have been made for other types of filter media including those used for respiratory protection (Barrett and Rousseau 1998).
Interestingly, the penetration results obtained for FDA-cleared surgical masks in this study are similar to non-approved dust masks from local home improvement/hardware stores (Rengasamy et al. 2008). Surgical mask models tested in this study and dust mask models used in a previous study (Rengasamy et al. 2008) were randomly selected for investigation. The manufacturers of surgical masks were mostly different from the manufacturers of dust masks. Results showed that three models of surgical and dust masks were electrostatic and the rest were mechanical type. To our surprise, one electrostatic surgical model in this study and one dust mask model tested in the previous study (Rengasamy et al. 2008) obtained from different manufacturers, showed <5% penetration level when tested similar to NIOSH respirator certification test conditions at 85 liters/minute flow rate. The other two electrostatic surgical masks and two electrostatic dust mask models showed average penetration levels of 17.9–19.4% and 10–12%, respectively. On the other hand, the mechanical type surgical and dust masks showed penetration levels in the range of 51–89%.
The protection provided by a surgical mask is also dependent on face seal leakage of particles in addition to penetration through filter media. Leakage at the face/mask interface reduces the protection levels against particles. In this study, the penetration levels of surgical masks sealed to the manikin with a silicone sealant to prevent leakage varied widely. This suggests that a surgical mask user would be expected to get protection levels far less than that observed in this study, because a complete sealing of a surgical mask to a human face cannot be achieved during use. Indeed, none of the six surgical models tested in a previous study had good fitting characteristics (Lawrence et al. 2006). Another study showed that measurement of the protection factor using an Electrical Low Pressure Impactor (ELPI) did not exceed 10 for 9 different surgical mask models, which was 8–12 times lower than that obtained for N95 FFRs (Lee et al. 2008). Similarly, the quantitative fit factors measured using a PortaCount® Plus showed average fit factors ranging from 2.5 to 6.9 for unassisted donning and 2.8 to 9.6 for assisted donning (Oberg and Brosseau 2008).
A shortage of NIOSH-approved FFRs is predicted during an influenza pandemic (Bailar et al. 2006; CDC 2006). For respiratory protection, users may select surgical masks instead of respirators due to non-availability and for economical reasons. Use of surgical masks will not provide respiratory protection against airborne virus particles expelled by humans during talking, coughing, breathing or sneezing. For example, a recent study on the exhaled breath of influenza infected patients contained about 70% of influenza virus particles in the 300–500 nm range (Fabian et al. 2008). In addition, exhaled breath of normal subjects contained aerosol particles predominantly in the 150–199 nm range as measured by a six channel optical counter (Edwards et al. 2004). Similarly another study on normal subjects reported a majority of exhaled aerosol particles were <300 nm when measured using a laser spectrometer (Fairchild and Stampfer 1987). This suggests that droplet nuclei containing an influenza virion can potentially be <300 nm. Thus, a more aggressive standard filtration performance requirement (e.g. using neutralized submicron particles in the MPPS range) for surgical masks may be useful to discriminate between products that currently perform equivalently using the existing test methods cited by ASTM 2100–04.
FDA describes the purpose of using surgical masks as follows: “If worn properly, a facemask is meant to help block large-particle droplets, splashes, sprays or splatter that may contain germs (viruses and bacteria) from reaching your mouth and nose. Facemasks may also help reduce exposure of your saliva and respiratory secretions to others” (FDA 2008). The size of droplets and droplet nuclei generated by breathing, talking, and coughing (whatever the studies have looked at) vary among individuals (Fairchild and Stampfer 1987; Papineni and Rosenthal 1997; Yang et al. 2007). In addition, healthy human subjects and patients generate not only droplets, but also submicron size particles in the exhaled breath (Edwards et al. 2004; Fabian et al. 2008; Fairchild and Stampfer 1987; Papineni and Rosenthal 1997). In our study, moderate and high barrier level surgical masks showed filtration efficiency values of 77.6–97.7% for room air particles in the 500–1000 nm range. Wide variations (54.9–92%) in filtration efficiency levels were obtained for moderate and high barrier level surgical masks when challenged with 100 nm size room air particles at a face velocity of 6.2 cm/second. These categories of surgical masks were expected to have both BFE and PFE filtration efficiencies of >98 as is prescribed under the ASTM test protocols. The results from this study are consistent with the wide range of penetration values reported for different size particles in other studies (Brosseau et al. 1997; Chen and Willeke 1992; Oberg and Brosseau 2008). This suggests that not all FDA-cleared surgical masks will provide similar protection levels to the wearer of the mask to submicron particles even within the same barrier level category. The lack of an aggressive standard submicron particle penetration test method and performance requirement allows wide variations in penetration levels for FDA-cleared surgical masks. Setting standard penetration levels using aggressive test conditions for bacterial and virus size particles can improve the level of protection of surgical masks.
There are some limitations to the results obtained in the study. For example, only one high barrier, and two each of moderate and low barrier surgical masks were used to measure particle penetration levels. Other surgical mask models in the market may perform better or worse. Several surgical mask models should be tested to strengthen the conclusions. Similarly, the test data obtained in this study with the non-neutralized 100 nm size room air particles is not the same as those obtained with the monodisperse polystyrene latex particles cited in ASTM F2100–04 (ASTM 2004). Penetration of room air particles was measured as a function of a range of different size particles (20–1000 nm) in a shorter time (130 seconds), which is as accurate as the penetration levels measured with monodisperse aerosols. Thus, the results obtained in this study cannot be directly compared with the penetration values for FDA-cleared surgical masks.
CONCLUSIONS
Five FDA-cleared surgical mask models tested in the study showed wide variation in particle penetrations by the different test methods. Room air particle penetration for the different surgical models varied between 1.4–46.2%, 7.6–65.7% and 7.5–76.3% at 6, 30 and 85 liters/minute constant flow rates. Filtration efficiency of moderate and high barrier level surgical masks when challenged with room air particles in the 100 nm as well in the 500–1000 nm sizes were less than the expected >98% filtration efficiency. Room air particle penetrations under constant flow conditions correlated with penetration levels obtained at similar flow rates under cyclic flow conditions. Similar wide variations in penetrations for polydisperse as well as for different size monodisperse aerosols were obtained for the different surgical mask models. The MPPS size was in the 40–60 nm range for the three surgical mask models which shifted to 200–400 nm, after isopropanol treatment, suggesting that the masks contained electrically charged filter media. The electrostatic surgical masks showed better filtration performance compared to the mechanical types. The wide variation in penetration levels for room air particles, which included particles in the same size range of viruses confirms that surgical masks should not be used for respiratory protection. The wide variation in filtration performance for submicron size particles can be reduced by setting standard penetration levels for surgical masks using a more aggressive test procedure for submicron aerosols (e.g. charge-neutralized particles at the MPPS, higher flow rates, etc.).
Acknowledgements
The authors acknowledge NIOSH colleagues including Roland BerryAnn, Raymond Roberge and Lisa Delaney for their critical review of the manuscript and suggestions. This research work was supported by NIOSH funding-CAN #927 Z1NT.
Footnotes
Publisher's Disclaimer: Disclaimer
Mention of commercial product or trade name does not constitute endorsement by the National Institute for Occupational Safety and Health. The findings and conclusions of this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
REFERENCES
- ASTM. (1989). Standard test method for determining the initial efficiency of a flatsheet filter medium in an airflow using latex spheres. ASTM Standards (F 1215 – 89). [Google Scholar]
- ASTM. (2001). Standard test method for evaluating the bacterial filtration efficiency (BFE) of medical face mask materials, using a biological aerosol of Staphylococcus aereus. Annual Book of ASTM Standards (F2101–01), 1553–1557. [Google Scholar]
- ASTM. (2004). Standard specification for performance of materials used in medical face masks. Annual Book of ASTM Standards (F2100–04). [Google Scholar]
- Bailar JC, Brosseau LM, Cohen HJ, Gallagher EJ, Gensheimer KF, Hack AL, Jayaraman S, Karasz FE, Liu Y, McGeer A, and Osterholm MT (2006). Reusability of facemasks during an influenza pandemic. Institute of Medicine, National Academies Press, Washington, D.C. [Google Scholar]
- Balazy A, Toivola M, Adhikari A, Sivasubramani SK, Reponen T, and Grinshpun SA (2006). Do N95 respirators provide 95% protection level against airborne viruses, and how adequate are surgical masks? Am J Infect Control, 34, 51–57. [DOI] [PubMed] [Google Scholar]
- Barrett LW, and Rousseau AD (1998). Aerosol loading performance of electret filter media. Am Ind Hyg Assoc J, 59, 532–539. [Google Scholar]
- Brosseau LM, McCullough NV, and Vesley D (1997). Mycobacterial aerosol collection efficiency of respirator and surgical mask filters under varying conditions of flow and humidity. Appl Occup Environ Hyg, 12, 435–445. [DOI] [PubMed] [Google Scholar]
- CDC. (2006). Estimated number of masks needed, by type and setting, for a severe influenza pandemic. Atlanta, Centers for Disease Control and Prevention. [Google Scholar]
- Chen CC, and Huang SH (1998). The effects of particle charge on the performance of a filtering facepiece. Am Ind Hyg Assoc J, 59, 227–233. [DOI] [PubMed] [Google Scholar]
- Chen CC, Lehtimaki M, and Willeke K (1993). Loading and filtration characteristics of filtering facepieces. Am Indust Hyg Assoc J, 54, 51–60. [DOI] [PubMed] [Google Scholar]
- Chen CC, and Willeke K (1992). Aerosol penetration through surgical masks. Am J Infect Cont, 20, 177–84. [DOI] [PubMed] [Google Scholar]
- Edwards DA, Man JC, Brand P, Kastra JP, Sommerer K, Stone HA, Nardell EA, and Scheuch G (2004). Inhaling to mitigate exhaled bioaerosols. Proc Natl Acad Sci U S A, 101, 17383–17388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian P, McDevitt JJ, DeHaan WH, Fung ROP, Cowling BJ, Chan KH, Leung GM, and Milton DK (2008). Influenza Virus in Human Exhaled Breath: An Observational Study. PloS ONE, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairchild CI, and Stampfer JF (1987). Particle concentration in exhaled breath. Am Indus Hyg Assoc J, 48, 948–958. [DOI] [PubMed] [Google Scholar]
- FDA. (2004). Guidance for industry and FDA staff. Surgical masks - premarket notification [510(k)] submissions; Guidance for industry and FDA. Washington, D.C. US Department of Health and Human Services, Food and Drug Administration, Center for Devices and Radiological Health. http://www.fda.gov/cdrh/ode/guidance/094.html. [Google Scholar]
- FDA. (2008). Personal protective equipment (PPE) and patient care. Masks and N95 Respirators. Washington, D.C. US Department of Health and Human Services, Food and Drug Administration, Center for Devices and Radiological Health. http://www.fda.gov/cdrh/ppe/masksrespirators.html. [Google Scholar]
- Federal Register. (1995). Respiratory Protective Devices. Final Rules and Notice. U.S. Government Printing Office, Office of Federal Register, Washington, D.C. 60, 30335–30398. [Google Scholar]
- Goldfrank LR, Liverman CT, and eds. (2008). Preparing for an Influenza Pandemic: Personal Protective Equipment for Healthcare Workers. Institute of Medicine Report. National Academies Press, Washington, D.C. [Google Scholar]
- John W (1980). Particle charge effects. In Generation of aerosols, Willeke K, (Ed.), Ann Arbor Science, Ann Arbor, Michigan, 141–151. [Google Scholar]
- Johnston AM, Vincent JH, and Jones AD (1985). Measurements of electric charge for workplace aerosols. Ann Occup Hyg, 29, 271–84. [DOI] [PubMed] [Google Scholar]
- Lawrence RB, Duling MG, Calvert CA, and Coffey CC (2006). Comparison of performance of three different types of respiratory protection devices. J Occup Environ Hyg, 3, 465–74. [DOI] [PubMed] [Google Scholar]
- Lee S-A, Grinshpun SA, and Reponen T (2008). Respiraory performance offered by N95 respirators and surgical masks: Human subject evaluation with NaCl aerosol representing bacterial and viral particle size range. Ann Occup Hyg, 3, 177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenhart SW, Seitz T, Trout D, and Bollinger N (2004). Issues affecting respirator selection for workers exposed to infectious aerosols: Emphasis on healthcare settings. Appl Biosafety, 9, 20–36. [Google Scholar]
- Martin SB Jr., and Moyer ES (2000). Electrostatic respirator filter media: filter efficiency and most penetrating particle size effects. Appl Occup Environ Hyg, 15, 609–617. [DOI] [PubMed] [Google Scholar]
- McCullough NV, Brosseau LM, and Vesley D (1997). Collection of three bacterial aerosols by respirator and surgical mask filters under varying conditions of flow and relative humidity. Ann Occup Hyg, 41, 677–690. [DOI] [PubMed] [Google Scholar]
- NIOSH. (2005). Procedure No. RCT-APR-STP-0057, 0058, 0059, Revision 1.1. Determination of particulate filter penetration to test against solid particulates for negative pressure, airpurifying respirators Standard testing procedure (STP). DHHS, Centers; for Disease Control and Prevention, National Institute for Occupational Safety and Health, National Personal Protective Technology Laboratory, Pittsburgh. http://www.cdc.gov/niosh/npptl/stps/pdfs/RCT-APR-0057%2058%2059.pdf. [Google Scholar]
- Oberg T, and Brosseau LM (2008). Surgical mask filter and fit performance. Am J Infect Cont, 36, 276–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papineni RS, and Rosenthal FS (1997). The size distribution of droplets in the exhaled breath of health human subjects. J Aero Med, 10, 105–116. [DOI] [PubMed] [Google Scholar]
- Rengasamy A, Verbofsky R, King WP, and Shaffer RE (2007). Nanoparticle penetration through NIOSH-approved N95 filtering-facepiece respirators. J Int Soc Res Prot, 24, 49–59. [Google Scholar]
- Rengasamy S, Eimer B, and Shaffer RE (2008). Nanoparticle filtration performance of commercially available dust masks. J Int Soc Res Prot. , 25, 27–41. [PMC free article] [PubMed] [Google Scholar]
- Weber A, Willeke K, Marchioni R, Myojo T, McKay R, Donnelly J, and Liebhaber F (1993). Aerosol penetration and leakage characteristics of masks used in the health care industry. Am J Infect Control, 21, 167–173. [DOI] [PubMed] [Google Scholar]
- Willeke K, Qian Y, Donnelly J, Grinshpun S, and Ulevicius V (1996). Penetration of airborne microorganisms through a surgical mask and a dust/mist respirator. Am Indust Hyg Assoc J, 57, 348–355. [DOI] [PubMed] [Google Scholar]
- Yang S, Lee GWM, Chen C-M, Wu C-C, and Yu K-P (2007). The size and concentration of droplets generated by coughing in human subjects. J Aero Med, 20, 484–494. [DOI] [PubMed] [Google Scholar]


