Abstract
Novel coronavirus infection [coronavirus disease 2019 (COVID-19)] has spread to more than 203 countries of various regions including Africa, America, Europe, South East Asia and Western Pacific. The WHO had declared COVID-19 as the global public health emergency and subsequently as pandemic because of its worldwide spread. It is now one of the top-priority pathogens to be dealt with, because of high transmissibility, severe illness and associated mortality, wide geographical spread, lack of control measures with knowledge gaps in veterinary and human epidemiology, immunity and pathogenesis. The quick detection of cases and isolating them has become critical to contain it. To meet the increasing demand of the diagnostic services, it is necessary to enhance and expand laboratory capabilities since existing laboratories cannot meet the emerging demand. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a BSL-2 (Biosafety Level 2) agent and needs to be handled in biosafety cabinet using standard precautions. This review highlights minimum requirements for the diagnostic laboratories opting testing of material for the diagnosis of COVID-19 and associated biorisk to the individuals and to the community.
Keywords: Biorisk, biosafety, diagnosis, infrastructure, laboratories, novel coronavirus
Introduction
Coronaviruses are enveloped viruses with non-segmented positive-sense RNA, widely distributed in humans and animals1,2. Initially, infections caused by several human coronaviruses (HCoVs) were only mild and hence were considered as neglected pathogens. After the emergence of highly pathogenic severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1) (2002 and 2003) and Middle East respiratory syndrome coronavirus (MERS-CoV, 2012)3, it has become obvious that coronaviruses can cross the species barrier and cause life-challenging infections in human, thus needing greater attention than the initial HCoVs4.
Recently, another pathogenic HCoV was identified in Wuhan, People's Republic of China, which was initially named as 2019 novel coronavirus (2019-nCoV)5. But later, it has been named as SARS-CoV-2 by the International Committee on Taxonomy of Viruses (ICTV)6. This virus causes coronavirus disease 2019 (COVID-19). The clinical presentation of infection ranges from asymptomatic to very severe pneumonia with acute respiratory distress syndrome, septic shock and multi-organ failure resulting in death7. As of March 2020, SARS-CoV-2 has spread to more than 203 countries of various regions including Africa, America, Europe, South East Asia and Western Pacific alarming public health authorities around the world8.
Since, the first case reported on December 31, 2019, the WHO has been notified with more than 100,000 confirmed cases including 3,380 deaths globally as on March 6, 2020, of whom 90 per cent (3,045) were from China itself, while the remaining 10 per cent (335) were from other countries9. The SARS-CoV-2 is now one of the top-priority pathogens to be dealt with, because of high fatality rate in severe cases, spread in a wide geographical area, lack of control measures and knowledge gaps in its epidemiology, immunity and pathogenesis. Currently, there are no licensed vaccines or therapies specific to COVID-19. Hence, the WHO has initially declared COVID-19 as the global public health emergency10 and subsequently as pandemic11.
Because of the rapid spread of this virus, it has become necessary to enhance laboratory capabilities to provide immediate diagnostic assistance as the load of samples from suspected patients is increasing on a daily basis and existing laboratories cannot meet the demands. The objective of this review is to highlight various requirements for the diagnostic laboratories involved in the testing of SARS-CoV-2 and also describe measures to mitigate the risk factors involved in laboratories that are providing molecular diagnosis, so that more laboratories become available for providing quick diagnosis under all safety precautions.
Sample of choice
Coronaviruses are mainly responsible for respiratory tract infections resulting in symptoms such as common flu. Choice of sample for detection will be respiratory samples including clinical material from the upper and lower respiratory tracts depending on the symptoms and condition of the patient12,13. For SARS-CoV-2, shedding patterns are not well understood and further investigations are required to understand the timing, compartmentalization and magnitude of virus shedding. However, the virus may be detectable in other specimens including blood and urine as in cases of SARS-CoV-1 and MERS-CoV14,15,16. The mean incubation period for SARS-CoV-2 is 5.2 days; however, it may vary widely depending on severity of illness17.
Specimen collection
Only trained staff should be allowed for appropriate specimen collection, storage, packaging and transport, ensuring that adequate standard operating procedures in consonance with the national or the WHO guidelines18 are in use, and all specimens should be treated as potentially infectious.
Diagnosis
Suspected cases should be tested for the virus with nucleic acid amplification tests, such as real-time reverse transcription - polymerase chain reaction (RT-PCR) with confirmation by nucleic acid sequencing when needed. Viral RNA extraction should be done in a biosafety cabinet in a BSL-2 or equivalent facility, which will be used further for amplification of genes targeted including nucleocapsid (N), spike (S), envelope (E), and RNA-dependent RNA polymerase (RdRp)18. Serological tests are still under development, and once these become available, field surveys will aid in better understanding of the outbreak, implementation of control measures and also understanding cross-reactivity with other viruses.
Infrastructure needed
Suspected samples should be handled at initial phase in a biosafety cabinet by well-trained staff with respect to standard BSL-2 facility. National guidelines on laboratory biosafety should be followed in all circumstances19. At present, very limited information is available on the risk posed by COVID-19, therefore, all procedures should be undertaken based on risk assessment.
Specimen handling for molecular testing of COVID-19 would require BSL-2 or equivalent facilities. These facilities include separate hand and eye wash sinks, and these also need to have automatic door locking systems. The BSL-2 laboratories should have access to facility of decontamination, including an autoclave19.
It is recommended that good microbiological laboratory practices and universal precautions must be followed in all laboratories where primary specimens (such as sputum, throat swab, nasopharyngeal swab, oropharyngeal swab, and stool) that may contain SARS-CoV-2 virus, are handled. While working with suspected patient's samples, laboratory personnel should be supervised by staff competent in handling infectious agents and related standard procedures19. The list of basic laboratory equipment and reagents required for providing laboratory diagnosis for COVID-19 is provided in Table I.
Table I.
List of laboratory equipments and reagents required for laboratory diagnosis of coronavirus disease 2019 (COVID-19) and personal protective equipment (PPE) for carrying out COVID-19 molecular test
| Sr. No. | Details of PPE and equipment |
|---|---|
| 1. | Disposable, back closure laboratory gowns |
| 2. | Face mask and head cap |
| 3. | Disposable gloves |
| 4. | Closed-toe footwear |
| 5. | Protective eyewear |
| 6. | Protective laboratory coats |
| 7. | Disposable shoe covers |
| 8. | Centrifuge tube 15 ml sterile (250 tubes/pack) |
| 9. | Centrifuge tube 50 ml sterile (150 tubes/pack) |
| 10. | Microcentrifuge tube (1.5 ml) |
| 11. | Micropipettes of variable volumes |
| 12. | Sterilized filter tips |
| 13. | Vortex |
| 14. | Mini spin |
| 15. | Small high-speed centrifuge for RNA extraction process |
| 16. | Cold centrifuge for sample processing |
| 17. | Plate spinner |
| 18. | Real-time PCR machine |
| 19. | Biosafety cabinet class 2 type II |
Biosafety measures
In most cases, SARS-CoV-2 is transmitted from human to human through inhalation or deposition on mucosal surfaces of large respiratory droplets. Other routes identified are contact with contaminated fomites and inhalation of aerosol, generated during handling of large volumes, etc20. For the laboratories involved in the diagnosis of COVID-19, it is necessary that staff should be well trained in the implementation of appropriate biosafety measures. The rational, correct and consistent use of available personal protective equipment (PPE) and appropriate hand hygiene help to reduce the spread of the pathogens. Though PPE is considered as a primary prevention strategy, it should not be completely relied upon for complete prevention for virus transmission. The effectiveness of PPE depends upon proper handling of PPEs by trained staff, hand hygiene practices and human factor21,22,23,24. Immunization policy for influenza would also help in giving protection to laboratory workers and reduce the suspicion of the staff to be getting infection in such emergency situation. Basic biosafety requirements for the laboratories that include important features of procedures and processes to be followed during processing the samples for COVID-19 laboratory diagnosis are provided in Table II.
Table II.
Basic biosafety requirements for the laboratories (some important features of procedures and processes to be followed during processing samples for coronavirus disease 2019 laboratory diagnosis)
| Sr. No. | Requirements |
|---|---|
| 1. | Personnel wear dedicated laboratory clothing (e.g., scrubs) which should not be worn outside the laboratory, anteroom or change room |
| 2. | Primary containment devices should always be used in this procedure, these should be validated/certified and well-maintained and there are procedures in place for proper use |
| 3. | Type of material to be used in this procedure for diagnostic samples should be up to 250 ml volumes. Absorbent materials should be used on the bench or BSC to contain spills and reduce splashing |
| 4. | Proper practices for reducing/eliminating aerosols should be identified in the laboratory procedures; should be taught and verified on a regular schedule |
| 5. | The measures should be in place to reduce infectious aerosols exiting the laboratory, all the aerosolization procedures and processes should be conducted in the biosafety cabinets and, during open bench, proper PPEs should be worn; depending on the risk assessment, respirators (e.g., N95, N100 and PAPR), goggles and face shield should be used |
| 6. | Since all such procedures will be performed in biosafety cabinets, and being small volumes of samples handled, there will be very low potential and extent of a splash or spill in this procedure, however, personnel must be trained on biosafety and should have laboratory procedures in place during spill or splash |
| 7. | Biosafety cabinets should always be used, these should be routinely validated/certified and well-maintained and there are procedures in place for proper use |
| 8. | Contaminated waste should be safely and efficiently treated within laboratory and should be stored in the laboratory, till disposed properly |
| 9. | No sharps should be used in these laboratory procedures |
| 10. | All surfaces in the laboratory should be easy to clean and decontaminated. No equipment should be maintained or repaired without decontamination, and the process should be documented and validated |
| 11. | The laboratory should have a complete and well-maintained inventory system. It should also have an active shipping and receiving programme and well-defined procedures and plans in place |
| 12. | There should be medical surveillance programme in place |
| 13. | Laboratory should implement standard laboratory practices for safety |
| 14. | There should be defined procedures in place for entry into the laboratory |
| 15. | Institution/laboratory should have defined roles and responsibilities for biosafety and should also be commitment to safety as well as comprehensive biosafety documentation and should conduct biosafety drills or exercises |
BSC, biological safety cabinet; PAPR, powered air-purifying respirator
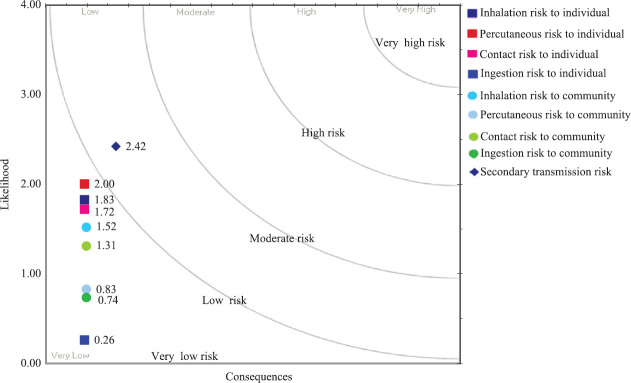
Biorisk assessment carried out for individual laboratory personnel and to the community with regard to providing molecular diagnosis for COVID-19 is provided in the Figure This biorisk assessment will also provide guidance for the future laboratories that are opting to provide laboratory diagnosis for this infection.
Figure.
Biorisk assessment for individual laboratory personnel and community with regard to providing molecular diagnosis for coronavirus disease 2019. Likelihood of secondary transmission to human is on moderate risk, in case laboratory personnel get infected while handling infected material.
Footnotes
Financial support & sponsorship: None
Conflicts of Interest: None.
References
- 1.Richman DD, Whitley RJ, Hayden FG. Clinical virology. New York: John Wiley & Sons; 2016. [Google Scholar]
- 2.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fauci AS, Lane HC, Redfield RR. Covid-19 - Navigating the Uncharted. N Engl J Med. 2020;382:1268–9. doi: 10.1056/NEJMe2002387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song Z, Xu Y, Bao L, Zhang L, Yu P, Qu Y, et al. From SARS to MERS, thrusting coronaviruses into the spotlight. Viruses. 2019;11:59. doi: 10.3390/v11010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paules CI, Marston HD, Fauci AS. Coronavirus Infections- more than just the common cold. JAMA. 2020;323:707–8. doi: 10.1001/jama.2020.0757. [DOI] [PubMed] [Google Scholar]
- 6.Gorbalenya AE, Baker SC, Baric RS, de Groot RJ, Drosten C, Gulyaeva AA, et al. The species severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–44. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.European Centre for Disease Prevention and Control. Infection prevention and control for COVID-19 in healthcare settings - March 2020. Stockholm: ECDC; 2020. [Google Scholar]
- 8.Centers for Disease Control and Prevention. COVID-19 Situation Summary CDC. 2020. [accessed on March 12, 2020]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/locationsconfirmed-cases.html .
- 9.World Health Organization. Coronavirus disease 2019 (COVID-2019) Situation Report - 46. WHO; 2020. [accessed on March 6, 2020]. Available from: https, https://wwwwhoint/emergencies/diseases/novel-coronavirus-2019/situation-reports . [Google Scholar]
- 10.World Health Organization. Novel Coronavirus (2019-nCoV) Situation Report - 11. WHO: 2020. [accessed on March 12, 2020]. Available from: http://wwwwhoint/docs/default-source/coronaviruse/situation-reports . [Google Scholar]
- 11.World Health Organization. Coronavirus disease 2019 (COVID-2019) Situation Report - 51. WHO; 2020. [accessed on March 12, 2020]. Available from: http://wwwwhoint/docs/default-source/coronaviruse/situation-reports . [Google Scholar]
- 12.Xu K, Cai H, Shen Y, Ni Q, Chen Y, Hu S, et al. Management of corona virus disease-19 (COVID-19): The Zhejiang experience. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020:49. doi: 10.3785/j.issn.1008-9292.2020.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Chen C, Zhu S, Shu C, Wang D, Song J, et al. Isolation of 2019-nCoV from a stool specimen of a laboratory-confirmed case of the coronavirus disease 2019 (COVID-19) CCDC Weekly. 2020;2:123–4. [PMC free article] [PubMed] [Google Scholar]
- 14.Shi X, Gong E, Gao D, Zhang B, Zheng J, Gao Z, et al. Severe acute respiratory syndrome associated coronavirus is detected in intestinal tissues of fatal cases. Am J Gastroenterol. 2005;100:169–76. doi: 10.1111/j.1572-0241.2005.40377.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhou J, Li C, Zhao G, Chu H, Wang D, Yan HHN, et al. Human intestinal tract serves as an alternative infection route for Middle East respiratory syndrome coronavirus. Sci Adv Am Assoc Adv Sci. 2017;3:eaao4966. doi: 10.1126/sciadv.aao4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding Y, He L, Zhang Q, Huang Z, Che X, Hou J, et al. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol. 2004;203:622–30. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in Wuhan, China of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. Laboratory testing for coronavirus disease 2019 (COVID-19) in suspected human cases: Interim guidance. WHO; 2020. [accessed on March 12, 2020]. Available from: https://appswhoint/iris/handle/10665/331329 . [Google Scholar]
- 19.National Centre for Disease Control. National guidelines for infection prevention and control in healthcare facilities Ministry of Health & Family Welfare, Government of India. 2020. [accessed on March 12, 2020]. Available from: https://wwwmohfwgovin/pdf/National%20Guidelines%20for%20IPC%20in%20HCF%20-%20final%281%29pdf .
- 20.Mourya DT, Yadav PD, Majumdar TD, Chauhan DS, Katoch VM. Establishment of Biosafety Level-3 (BSL-3) laboratory: Important criteria to consider while designing, constructing, commissioning & operating the facility in Indian setting. Indian J Med Res. 2014;140:171–83. [PMC free article] [PubMed] [Google Scholar]
- 21.Yin Y, Wunderink RG. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology. 2018;23:130–7. doi: 10.1111/resp.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization. How to put on and take off personal protective equipment (PPE) WHO; 2008. [accessed on March 12, 2020]. Available from: https://appswhoint/iris/handle/10665/70066 . [Google Scholar]
- 23.World Health Organization. WHO guidelines on hand hygiene in health care: A summary. WHO; 2009. [accessed on March 12, 2020]. Available from: https://wwwwhoint/gpsc/5may/tools/9789241597906/en/ [Google Scholar]
- 24.World Health Organization. Infection prevention and control of epidemic- and pandemic prone acute respiratory infections in health care. WHO Guidelines WHO; 2014. [accessed on March 12, 2020]. Available from: https://wwwwhoint/csr/bioriskreduction/infection_control/publication/en/ [PubMed] [Google Scholar]