SARS-CoV-2 is an emergent virus responsible for the coronavirus disease 2019 (COVID-19) outbreak. Reported neurologic manifestations associated with SARS-CoV-2 include hyposmia, headache, and consciousness disturbances.1 We describe 2 patients with COVID-19 presenting with ophthalmoparesis and characteristic MRI findings.
Case description
Case 1
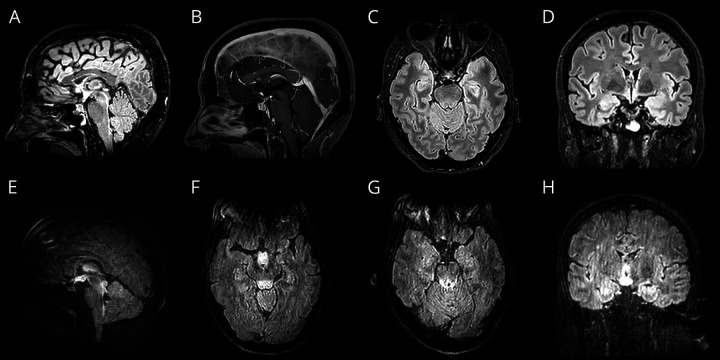
A 60-year-old woman presented with diplopia and right hemicranial headache after 10 days of fever, hyposmia, nausea, and cough. Neurologic examination revealed right abducens nerve palsy. The patient was hypoxemic (PaO2 67 mm Hg) in the absence of dyspnea. Blood investigations showed lymphopenia (430/µL) and increased C-reactive protein (C-RP; 85 mg/L), with thiamine, pyridoxine, and D-dimer levels within the normal range. Chest x-ray revealed bilateral pneumonia. CSF showed 1 cell/mm3, normal protein levels (0.32 g/L), and elevated lactic acid dehydrogenase (LDH; 54 U/L). SARS-CoV-2 RNA was detected by RT-PCR in a nasopharyngeal swab specimen but not in CSF. Antiganglioside antibodies were negative, and oligoclonal bands were not detected in the CSF. Brain MRI (figure 1, A–D and figure e-1 links.lww.com/NXI/A276) showed diffuse fluid-attenuated inversion recovery (FLAIR)/T2-hyperintensity (HI) in the pontine tegmentum and focal HI in the right VI cranial nerve nuclei. The mammillary bodies and hypothalamus were HI, the pituitary gland was enlarged, and the upper pituitary stalk seemed globular. Treatment with hydroxychloroquine and azithromycin was started. Diplopia persisted 1 month after admission.
Figure 1. MRI findings in case 1 (A–D) and case 2 (E–H).
Case 1: (A) Sagittal fluid-attenuated inversion recovery (FLAIR) demonstrates an enlarged, hyperintense pituitary gland, thickened pituitary stalk, and hyperintense hypothalamus. T2/FLAIR-hyperintensity (HI) is also seen in the dorsal midbrain and pons. (B) Sagittal postcontrast 3D magnetization-prepared rapid gradient echo imaging T1 shows a heterogeneously enhancing pituitary and upper stalk. (C) Axial FLAIR shows HI in the tegmentum, involving the right abducens nucleus. (D) Coronal FLAIR shows a hyperintense pituitary gland, prominent HI in the periventricular region of the III ventricle, and subtle HI in the medial temporal lobes. Case 2: (E) Sagittal FLAIR demonstrates extensive HI in the hypothalamus and dorsal brainstem. (F) Axial FLAIR image shows HI and swelling of the hypothalamus, mammillary bodies, and dorsal midbrain. (G) Axial FLAIR demonstrates HI in the tectum of the midbrain and subtle HI in the medial temporal lobes. (H) Coronal FLAIR shows a prominent HI in the periventricular region of the III ventricle and in the mammillary bodies, and subtle HI in the medial temporal lobes.
Case 2
A 35-year-old woman with a history of bulimia and a 3-week history of vomiting was admitted. During her hospital stay, she developed diplopia and paresthesia. Vomiting disappeared few days after admission, and she was discharged. Three days later, the patient presented with progressive encephalopathy and was again admitted. She had neither fever nor respiratory symptoms. Neurologic examination showed decreased arousal, disorientation, episodic memory deficits, bilateral abducens nerve palsy, and mild paraparesis with normal reflexes. Hypoxemia (PaO2 61 mm Hg), decreased lymphocytes (740/µL), increased C-RP (42 mg/L), and creatinine kinase (346 U/L) were detected; LDH, D-dimer, thiamine, pyridoxine, and ion levels were within the normal range. CSF revealed 0.53 g/L proteins, 2 cell/mm3, and normal LDH. Chest x-ray was normal. SARS-CoV-2 RNA was detected by RT-PCR in a nasopharyngeal swab specimen but not in CSF. Anti-AQP4 and antiganglioside antibodies were negative, and oligoclonal bands were not detected in the CSF. MRI (figure 1, E–H and figure e-1) demonstrated T2/FLAIR HI in the brainstem, including the VI cranial nerve nuclei, thalami, medial temporal lobes, mammillary bodies, and hypothalamus. The upper pituitary stalk seemed swollen and HI. The patient was diagnosed with Wernicke encephalopathy (WE) and was supplemented with thiamine and pyridoxine despite normal vitamin levels. The patient's mental status partially improved during the following days. A control MRI (1 week after) also demonstrated partial improvement of the MRI findings. After 1 month, ophthalmoparesis and paraparesis as well as her mental status improved, but she had persistent episodic memory loss and depression.
Discussion
To our knowledge, these are the 2 first patients with COVID-19 presenting with ophthalmoparesis and involvement of the hypothalamus and mesencephalic tegmentum with some radiologic features resembling those of WE.2 However, thiamine levels in both our patients were normal, and there was no condition leading to thiamine deficiency in patient 1 and some radiologic features in case 2 (limbic involvement) were not typical of WE. Concurrency of both patients, both diagnosed with COVID-19, and the important hypothalamic involvement suggested a relationship with the underlying infection. Other cases of ophthalmoparesis associated with COVID-19 have recently been reported,3,4 one of them with enlargement and contrast enhancement of the oculomotor nerve that we did not observe in our patients. Acute hemorrhagic necrotizing encephalopathy has also been reported in association with confirmed COVID-19 on MRI.5 Similarly, encephalomyelitis associated with SARS-CoV-2 has been recently reported in a patient with unconsciousness and epileptic seizures in which SARS-CoV-2 was detected in the CSF, and MRI demonstrated T2-HI in the right medial temporal lobe.6 Our current cases show prominent hypothalamus and pituitary stalk involvement on MRI. Indeed, SARS-CoV (a related coronavirus causing the 2002–2004 SARS outbreak) has also been detected in hypothalamic neurons of SARS autopsies,7 and hypothalamic-pituitary-adrenal axis dysfunction has been observed in almost 40% of SARS survivors, suggesting that SARS-CoV could cause hypophysitis and/or hypothalamic damage.8 Furthermore, animal models have shown that after intranasal inoculations, SARS-CoV enters the brain through the olfactory bulb and via the olfactory nerve with subsequent trans-synaptic spread, causing selective neuronal infection and death in the absence of inflammation in the amygdala, raphe nuclei, and paramedial hypothalamus, a topographic distribution of lesions similar to that from our cases.9 The olfactory bulb involvement is supported by the high rates of hyposmia in patients with COVID-19 and would enable the trans-synaptic spread hypothesis. However, other mechanisms, arising from the inflammatory response or the metabolic demands to the susceptible regions, could also be playing a role in the development of our patients' lesions.
In conclusion, we report 2 cases of COVID-19-associated neurologic manifestations with abducens palsy, encephalopathy, and characteristic MRI findings that suggest selective vulnerability of the involved regions. Whether this vulnerability is related to direct viral tropism, the inflammatory cascade, or to an increase of local metabolic/vitamin demands remains to be elucidated.
Appendix. Authors


Study funding
No targeted funding reported.
Disclosure
E.P.-G. received speaking fees from Roche and Biogen. J.F., A. M.-D., N.R., M.T., and C.G.-O. declare no conflicts. L.Q. has received speaker honoraria from Merck, Sanofi-Genzyme, Roche, Biogen, Grifols, and CSL Behring; provided expert testimony for Grifols, Johnson and Johnson, Annexin Pharmaceuticals, Alexion, Sanofi-Genzyme, Novartis, and CSL Behring; and received research funds from Roche and Grifols. B.G.-A. declares no conflicts. Go to Neurology.org/NN for full disclosures.
References
- 1.Mao L, Wang M, Chen S, et al. Neurological manifestations of hospitalized patients with COVID-19 in Wuhan, China: a retrospective case series study. SSRN Electron J Epub 2020 Feb 25.
- 2.Zuccoli G, Cruz DS, Bertolini M, et al. MR imaging findings in 56 patients with wernicke encephalopathy: nonalcoholics may differ from alcoholics. Am J Neuroradiol 2009;30:171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gutiérrez-Ortiz C, Méndez A, Rodrigo-Rey S, et al. Miller Fisher Syndrome and polyneuritis cranialis in COVID-19. Neurology Epub 2020 Apr 27. 10.1212/WNL.0000000000009619. [DOI] [PubMed] [Google Scholar]
- 4.Dinkin M, Gao V, Mbbs JK, et al. COVID-19 presenting with ophthalmoparesis from cranial nerve palsy. Neurology Epub 2020 May 1. 10.1212/WNL.0000000000009700. [DOI] [PubMed] [Google Scholar]
- 5.Poyiadji N, Shahin G, Noujaim D, Stone M, Pate P, Griffith B. COVID-19–associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology 2019;2:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis 2020;94:55–58. 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gu J, Gong E, Zhang B, et al. Multiple organ infection and the pathogenesis of SARS. J Exp Med 2005;202:415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leow MKS, Kwek DSK, Ng AWK, Ong KC, Kaw GJL, Lee LSU. Hypocortisolism in survivors of severe acute respiratory syndrome (SARS). Clin Endocrinol (Oxf) 2005;63:197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol 2008;82:7264–7275. [DOI] [PMC free article] [PubMed] [Google Scholar]