Abstract
Objective
To assess the role of CSF chitinase 3-like-1 (CHI3L1), chitinase 3-like-2 (CHI3L2), and neurofilament light chain (NfL) in predicting the course of primary progressive MS (PPMS).
Methods
We analyzed CSF CHI3L1, CHI3L2, and NfL levels in 25 patients with PPMS with disease duration ≤10 years and no disease-modifying therapy for ≥6 months from the prospective Understanding Primary Progressive Multiple Sclerosis cohort study. CSF samples taken at disease diagnosis were analyzed using commercial ELISAs and following the manufacturer's instructions. Data on Expanded Disability Status Scale (EDSS) scores, disability progression, and cognitive function according to the Brief Repeatable Neuropsychological Battery were also assessed throughout the 1-year study follow-up.
Results
Increasing CHI3L1 levels correlated with higher EDSS scores at baseline (ρ = 0.490, 95% CI 0.118–0.742, p = 0.013) and month 12 (ρ = 0.455, 95% CI 0.063–0.725, p = 0.026) and tended to be associated with a higher risk of disability progression according to EDSS scores (OR = 1.008, 95% CI 0.999–1.017, p = 0.089). Increasing CHI3L2 levels also tended to correlate with lower baseline EDSS scores (ρ = −0.366, 95% CI -0.676–0.054, p = 0.086). There was no correlation with regard to NfL levels.
Conclusions
This analysis supports the association between CSF CHI3L1 levels and neurologic disability according to EDSS scores in patients with PPMS. Other chitinase-like proteins such as CHI3L2 may also be involved.
Classification of evidence
This study provides Class II evidence that CSF CHI3L1 is associated with neurologic disability in patients with PPMS.
Several studies have explored CSF biomarkers reflecting inflammatory and neurodegenerative processes underlying MS, suggesting the prognostic role of chitinase 3-like-1 (CHI3L1), chitinase 3-like-2 (CHI3L2), and neurofilament light chain (NfL) in conversion to definite MS.1–4 Reported data have also pointed at measuring CHI3L1 and NfL to anticipate conversion to progressive disease,5 as well as supporting the biomarker use of CHI3L1 to monitor disease activity in secondary progressive MS6 and response to interferon-beta in relapsing-remitting patients.7 These proteins may also be involved in the pathogenesis of the different MS forms, with a progressive increase in CHI3L1 levels with disease stages from clinically isolated syndrome (CIS), and an opposite variation of CHI3L2 expression in relapsing-remitting and progressive forms.1 However, they have not been fully characterized in patients with primary progressive MS (PPMS), and their role as predictive biomarkers of disease course remains unknown.
Therefore, we approached the analysis of CSF CHI3L1, CHI3L2, and NfL levels to assess their role in predicting disease course in patients with PPMS.
Methods
Study design and participants
This pilot CSF analysis included a sample of patients with PPMS from the prospective Understanding Primary Progressive Multiple Sclerosis (UPPMS) cohort study and provides Class II evidence.
Patients were consecutively recruited from neurologic departments at 11 Spanish hospitals between January and July 2017. Key eligibility criteria included age ≥18 years, PPMS diagnosis according to the 2010 McDonald criteria,8 disease duration ≤10 years from neurologic symptom onset, and no disease-modifying therapy within the past 6 months.
Assessments
When CSF samples taken at MS diagnosis were available, an aliquot was sent to the Laboratory of Neuroimmunology “Dr. Francisco Coret”—Institut d’Investigació Sanitària La Fe (Valencia, Spain). Commercially available ELISAs for CHI3L1 (Quantikine ELISA kit, R&D Systems Inc., Minneapolis), CHI3L2 (Human CHI3L2 ELISA kit, Cusabio Technology LLC, Houston), and NfL (NF-light neurofilament ELISA kit, UmanDiagnostics AB, Umea, Sweden) were used according to the manufacturer's instructions. Mean intra-assay variations were 6%, 8.9%, and 1.7% for CHI3L1, CHI3L2, and NfL, respectively; all interassay variation coefficients were <15%.
Data on Expanded Disability Status Scale (EDSS) scores, disability progression, and cognitive function were collected throughout the 1-year study follow-up. Disability progression was assessed and confirmed ≥3 months later according to the EDSS score (≥1-point increase when the baseline score was ≤5.0 or ≥0.5-point increase when the baseline score was ≥5.5), Timed 25-Foot Walk (T25-fw; ≥20% increase from baseline), 9-Hole Peg Test (9-HPT; ≥20% increase from baseline), and a composite of previously mentioned variables. The Rao Brief Repeatable Neuropsychological Battery was used to assess cognitive function.9
Statistical analyses
CHI3L1, CHI3L2, and NfL levels were correlated with EDSS scores at enrollment (baseline) and month 12 using Spearman rank correlations. Associations of these levels with 12-month disability progression and cognitive assessments were analyzed using Spearman rank correlations and logistic regressions. Indeterminate/missing data were not considered in the analyses, which were performed by Dynamic Science S.L. (Madrid, Spain) with the Statistical Package for the Social Sciences (SPSS) version 22.0 (SPSS Inc, Chicago) and a significance level of 0.05.
Standard protocol approvals, registrations, and patient consents
The study was conducted according to Good Pharmacoepidemiology Practices, the Declaration of Helsinki, and national regulations. The ethics committee of Hospital Universitario 12 de Octubre approved it, and all patients gave their written informed consent.
Data availability
All data are reported within the article and available anonymized by request from qualified investigators.
Results
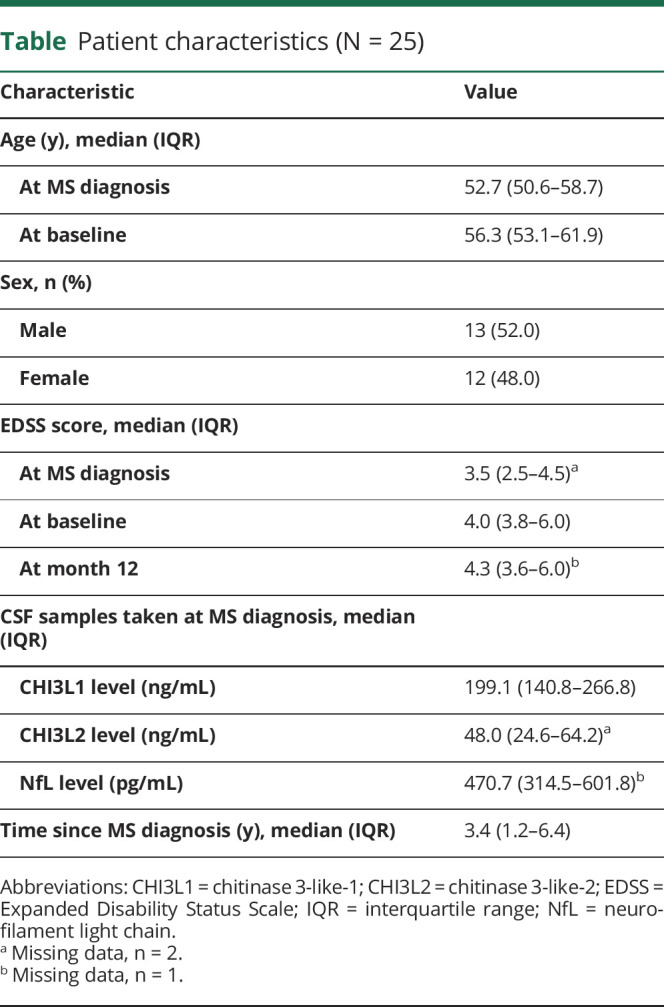
Twenty-five of the 55 patients enrolled in the UPPMS study had CSF samples taken at disease diagnosis (table).
Table.
Patient characteristics (N = 25)
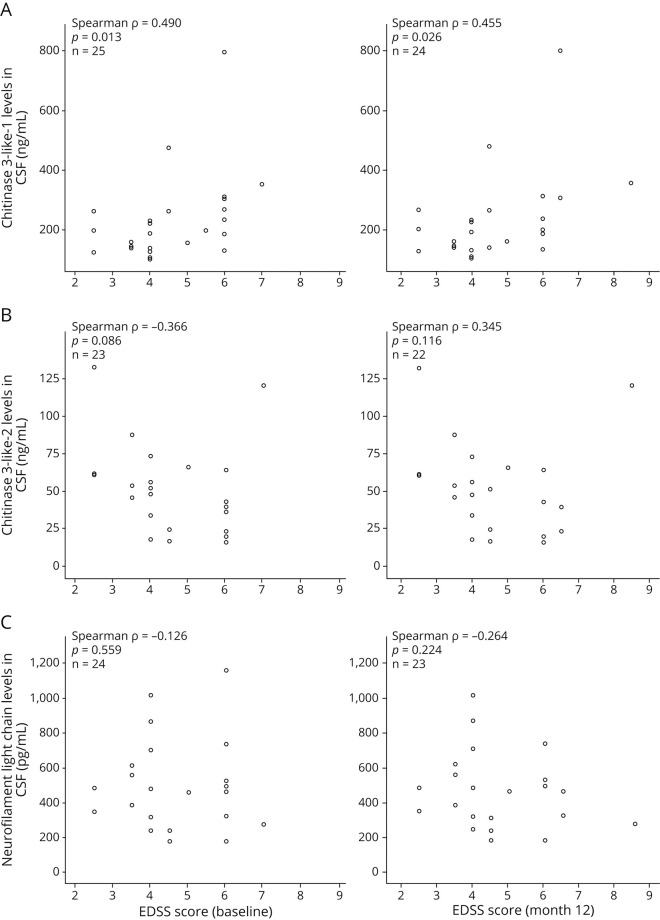
Increasing CHI3L1 levels correlated with higher EDSS scores at baseline (Spearman ρ = 0.490, 95% CI 0.118–0.742, p = 0.013) and month 12 (Spearman ρ = 0.455, 95% CI 0.063–0.725, p = 0.026) (figure). Conversely, increasing CHI3L2 levels tended to correlate with lower baseline EDSS scores (Spearman ρ = −0.366, 95% CI −0.676 to 0.054, p = 0.086) (figure). There was no significant Spearman rank correlation between NfL levels and EDSS scores at baseline or month 12 (figure).
Figure. Correlations between biomarker levels and Expanded Disability Status Scale scores.
This figure shows the results of the Spearman rank correlations of chitinase 3-like-1 (A), chitinase 3-like-2 (B), and neurofilament light chain (C) levels with Expanded Disability Status Scale scores.
Three (12.0%) patients experienced disability progression according to EDSS scores at month 12, 1 (4.0%) according to T25-fw, none according to 9-HPT, and 4 (16.0%) according to a composite of EDSS scores, T25-fw, or 9-HPT. Higher CHI3L1 levels tended to be associated with a higher risk of disability progression according to this composite (OR = 1.008, 95% CI 0.999–1.018, p = 0.092) and EDSS scores (OR = 1.008, 95% CI 0.999–1.017, p = 0.089), although there was no association regarding CHI3L2 (OR = 1.014, 95% CI 0.978–1.051, p = 0.450) and NfL levels (OR = 0.996, 95% CI 0.988–1.004, p = 0.327). There was no association with other disability progression measurements or cognitive assessments (data not shown).
Discussion
This pilot CSF analysis showed that CHI3L1 levels at PPMS diagnosis were related to EDSS scores at study follow-up. Higher CHI3L1 levels also tended to be associated with a higher risk of disability progression according to EDSS scores, although the relatively short study follow-up and the reduced sample size limited its power to detect significant differences. These results are in line with a previous report showing that CSF CHI3L1 correlated with EDSS scores through years 1–4 after the CIS episode.4 In addition, increasing CSF CHI3L1 expression was associated with a higher and earlier risk of developing a sustained EDSS score ≥3 within 5 years after CIS.3 The CHI3L1 is a member of the glycoside hydrolase 18 chitinase family, whose increase throughout MS stages seems to derive from the extension of diffuse brain inflammation associated with neurologic impairment rather than acute inflammation during relapses1 and the detrimental role of astrocyte activation in disease pathogenesis.3
In addition, there was a trend toward finding lower CHI3L2 levels at disease diagnosis to be related with higher EDSS scores at study baseline and vice versa, suggesting a potential predictive role in PPMS that deserves further confirmation. CHI3L2 is another member of the glycoside hydrolase 18 chitinase family that was reported to predict MS development in patients with CIS.10 Contrasting to CH3L1, CHI3L2 levels are lower in progressive vs relapsing MS, which might result from their distinct temporal pattern of expression.1 However, we found no evidence on its role in predicting EDSS scores either in this patient population or in patients with PPMS.
Furthermore, our analyses provide no evidence to suggest the predictive value of NfL in patients with PPMS. As NfL is a cytoskeletal polypeptide of the axon whose release denotes axonal impairment, the lacking association may result from the underlying progressive neurodegenerative process rather than axonal damage outbreaks.5
We acknowledge that the analysis has limitations that may affect its statistical power, including the limited study follow-up, sample size, and patients with disability progression. However, we identified significant differences and tendencies deserving confirmation. Despite the absence of significant differences or tendencies according to NfL levels, additional data on larger sample sizes, longer follow-ups, and using SIMOA technology, preferably in serum, are needed to confirm its role in patients with PPMS. In addition, the observational, prospective, and multicenter nature of the study supports the generalizability of analysis findings to clinical practice.
This analysis supports the association between CSF CHI3L1 levels and neurologic disability according to EDSS scores in patients with PPMS. Other chitinase-like proteins such as CHI3L2 may also be involved. These findings warrant further confirmatory assessment in larger samples of patients with PPMS.
Acknowledgment
The authors are extremely grateful to all the patients and their families for making the UPPMS study possible. Medical writing support was provided by Esther Álvarez-García (Dynamic Science S.L.) during the preparation of this article (funded by Roche Farma S.A), who contributed to drafting, editing, and word processing the manuscript as well as responding to the editor and reviewers' comments.
Glossary
- 9-HPT
9-Hole Peg Test
- CHI3L1
chitinase 3-like-1
- CHI3L2
chitinase 3-like-2
- CIS
clinically isolated syndrome
- EDSS
Expanded Disability Status Scale
- NfL
neurofilament light chain
- PPMS
primary progressive MS
- T25-fw
Timed 25-Foot Walk
- UPPMS
Understanding Primary Progressive Multiple Sclerosis
Appendix. Authors
Study funding
The authors disclosed receipt of the following financial support for the research, authorship, and publication of this article: This work was supported by Roche Farma S.A. (ML39253).
Disclosure
F. Pérez-Miralles was part of the steering committee of the UPPMS study and has received compensation for serving on scientific advisory boards or speaking honoraria from Almirall, Biogen Idec, Genzyme, Merck Serono, Mylan, Novartis, Roche, Sanofi-Aventis, and Teva, outside the submitted work. D. Prefasi is an employee of Roche Farma S.A. A. García-Merino has received consultant and/or lecture fees from Merck, Teva, Biogen, Novartis, Roche, and Sanofi. F. Gascón-Giménez reports no disclosures. N. Medrano is an employee of Roche Farma S.A. J. Castillo-Villalba, L. Cubas, C. Alcalá, and S. Gil-Perotín report no disclosures. R. Gómez-Ballesteros and J. Maurino are employees of Roche Farma S.A. E. Álvarez-García is an employee of Dynamic Science S.L. B. Casanova reports no disclosures. Go to Neurology.org/NN for full disclosures.
References
- 1.Hinsinger G, Galeotti N, Nabholz N, et al. Chitinase 3-like proteins as diagnostic and prognostic biomarkers of multiple sclerosis. Mult Scler 2015;21:1251–1261. [DOI] [PubMed] [Google Scholar]
- 2.Arrambide G, Espejo C, Eixarch H, et al. Neurofilament light chain level is a weak risk factor for the development of MS. Neurology 2016;87:1076–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Canto E, Tintore M, Villar LM, et al. Chitinase 3-like 1: prognostic biomarker in clinically isolated syndromes. Brain 2015;138:918–931. [DOI] [PubMed] [Google Scholar]
- 4.Comabella M, Fernandez M, Martin R, et al. Cerebrospinal fluid chitinase 3-like 1 levels are associated with conversion to multiple sclerosis. Brain 2010;133:1082–1093. [DOI] [PubMed] [Google Scholar]
- 5.Gil-Perotin S, Castillo-Villalba J, Cubas-Nunez L, et al. Combined cerebrospinal fluid neurofilament light chain protein and chitinase-3 like-1 levels in defining disease course and prognosis in multiple sclerosis. Front Neurol 2019;10:1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burman J, Raininko R, Blennow K, Zetterberg H, Axelsson M, Malmestrom C. YKL-40 is a CSF biomarker of intrathecal inflammation in secondary progressive multiple sclerosis. J Neuroimmunol 2016;292:52–57. [DOI] [PubMed] [Google Scholar]
- 7.Matute-Blanch C, Rio J, Villar LM, et al. Chitinase 3-like 1 is associated with the response to interferon-beta treatment in multiple sclerosis. J Neuroimmunol 2017;303:62–65. [DOI] [PubMed] [Google Scholar]
- 8.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boringa JB, Lazeron RH, Reuling IE, et al. The brief repeatable battery of neuropsychological tests: normative values allow application in multiple sclerosis clinical practice. Mult Scler 2001;7:263–267. [DOI] [PubMed] [Google Scholar]
- 10.Mollgaard M, Degn M, Sellebjerg F, Frederiksen JL, Modvig S. Cerebrospinal fluid chitinase-3-like 2 and chitotriosidase are potential prognostic biomarkers in early multiple sclerosis. Eur J Neurol 2016;23:898–905. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are reported within the article and available anonymized by request from qualified investigators.