Abstract
FLAG-tags are commonly used for protein abundance measurements and for identification of protein-protein interactions in living cells. We have observed that the cholera pathogen Vibrio cholerae encodes a FLAG-antibody-reactive protein and identified this protein as an outer membrane porin, Porin4, which contains a sequence very similar to the 3×FLAG epitope tag. We have demonstrated the binding affinity of the conserved peptide sequence (called Porin 4 tag) in Porin4 against monoclonal anti-FLAG M2 antibody. In addition, we created a porin4 deletion mutant, which can be used for background-less FLAG antibody detection experiments.
Keywords: Affinity tag, epitope tag, non-specific binding, 3×FLAG tag, monoclonal anti-FLAG M2 antibody and Porin4
Introduction
Affinity tags have been developed to improve production of recombinant proteins and well-characterized epitope-tag systems have become major tools for life science research. Antibodies for the detection of such affinity tags are commercially available without the need for costly and time-intensive generation of custom antibodies. Relatively small epitope tags such as His, c-Myc, Strep, HA (hemagglutinin), Spot, T7, Glu-Glu and particularly the FLAG-tag are widely used for the detection of fusion proteins to facilitate protein abundance measurements, purification and interaction studies, in vitro and in cell culture (1–7). Their short, linear recognition motifs rarely affect the properties of the heterologous protein of interest and are usually very specific for their respective primary antibodies. The FLAG system has been used in a variety of cell types, including bacteria, (8–9), yeast (10–11), and mammalian cells (12–13). The FLAG-tag system utilizes a short, hydrophilic eight amino acid peptide that is fused to the protein of interest at the C- or N- terminus of the target protein (14).
Protein quantification via Western Blot and identification of binding complexes by immunoprecipitation rely on the specificity of the antibody for binding its cognate epitope tag and, for the most common tags, non-specific background binding is generally low. Where non-specific background does occur, it is often ignored or used as an internal loading control, assuming that background detection is stable over many conditions tested (e.g., when comparing protein abundances during a growth time course).
Sometimes, however, non-specific signal confounds analysis of the intended target signals. For example, the Fur (Fe2+ uptake transcriptional regulator) subfamily transcription factors (Fur-Fe2+; PerR-Fe2+; Mur-Mn2+; Nur-Ni2+; Zur-Zn2+ and Irr-heme), which are well-conserved in bacteria, contain at least 3 or 7 direct repeated histidines and thus contaminate 6×His tag fusion protein purification after binding to Ni-NTA resin (15).
Here, we report a FLAG-reactive protein in V. cholerae, the causative agent of cholera disease. We identified this protein as an outer membrane chitoporin that contains an amino acid sequence with striking similarity to the 3×FLAG tag. Our data should be informative to researchers using FLAG tags in Vibrio cholerae.
MATERIALS AND METHODS
Bacterial strains and culture conditions
All V. cholerae strains used in this study (see Table S1) are derivatives of wild type El Tor strain N16961. Vibrio cholerae cells were grown on Lysogeny Broth (LB) medium supplemented with 200μg/ml streptomycin sulfate (TCI, cat# S0585) at 37°C with shaking at 200 rpm. E. coli DH5α λpir was used for routine DNA cloning. Sub-cloning was conducted in E. coli SM10 λpir for conjugal gene transfer into V. cholerae. E. coli BL21 DE3/pLysS (Novagen) and its derivative strain, Rosetta-gami2 (Novagen) were used for overproduction of proteins.
Construction of strains and plasmids
For 3×FLAG tag fusion strains and Δporin4 mutant strains were constructed using the suicide vector pCVD442 (16). 500 to 800 bps of flanking sequences were cloned into this vector digested with SphI and XmaI in E. coli DH5α λpir, then transformed into the donor strain SM10 λpir and mated for 5 hours at 37°C with V. cholerae by mixing equal volumes (1 ml) of exponential cultures, concentrating into 100 μl, and spot-plating. Successful first cross-over recipients were selected on LB agar plates containing 200 μg/ml streptomycin and 50 μg/ml carbenicillin, re-streaked to single colony and then plated on LB agar containing 10% sucrose and streptomycin. Final transformants were tested by plating on carbenicillin for loss of pCVD442 and by diagnostic PCR for successful double crossover. The following primers were used to construct knockout plasmid for porin4 (TD-JHS273/274) or 3×FLAG-tagging plasmids for protein A-vxrA (TD-JHS029/030), protein B-shyA (by amplifying ShyA-flag from ShyAflag_gBlock using primers TDP233/234), and protein C-shyC (by amplifying ShyC-flag from ShyCgblock_up using primers TDP515/516 and downstream homologies using primers TDP 517/18).
To test the anti-FLAG antibody binding activity of proteins identified by Immunoprecipitation and LC-MS/MS analysis (referred in Table 1), entire (ompA: vc2213) or truncated ORFs (porin4:vc0972) of top 5 candidates were amplified and cloned into pET15b vector (Novagen) after NdeI and BamHI digestion for 6xHis tag and IPTG inducible promoter fusion. Each plasmid was transformed into Rosetta-gami2 for the pulse induction. For confirmation of the binding activity of anti-FLAG antibody against Porin4 tag and for the binding affinity comparison between 3×FLAG tag and Porin4 tag, Porin4 or 3×FLAG tag was fused at C-terminus region of OmpA, VxrB and, BsuMntR protein. The resulting recombinant plasmids were transformed into E. coli BL21 (DE3/pLysS) strain for protein overexpression. The following primers were used to construct overexpression vectors for pET15b VxrB (TD-JHS001/002), OmpA (TD-JHS193/194), Porin4 (TD-JHS 191/192), tShyA (TD-JHS 062/063), BsuMntR (TD-JHS 389/390), OmpA + Porin4 tag (TD-JHS 193/246), VxrB + Porin4 tag (TD-JHS 001/247), VxrB + 3×FLAG (TD-JHS 001/312), BsuMntR + Porin4 tag (TD-JHS 389/313), and BsuMntR + 3×FLAG tag (TD-JHS 389/314). All oligos are summarized in Table S2.
Table 1.
Top 5 candidates among the common target proteins (37) both WT and 3xFLAG+ProteinA.
| Rank | Gene no/Protein ID | Function | Mass (kDa) | Hits (# of peptide spectral matches) | Subcellular location | |
|---|---|---|---|---|---|---|
| WT | 3xFLAG+ProteinA | |||||
| 1 | VC0972(Porin4)/Q9KTD0 | Porin | 37.9 | 65 | 204 | Outer membrane |
| 2 | VC0633(OmpU)/P0C6Q6 | Porin | 36.6 | 14 | 38 | Outer membrane |
| 3 | VC2000/Q9KQJ8 | Glyceraldehyde-3-phosphate dehydrogenase | 35.2 | 11 | 21 | Cytosol |
| 4 | VC2213(OmpA)/H9L4R9 | Porin | 34.2 | 5 | 13 | Outer membrane |
| 5 | VC1179(RluB)/Q9KSS7 | Ribosomal large subunit pseudouridine synthase B | 36.6 | 8 | 9 | Cytosol |
Western blotting
Harvested cell pellets were resuspended in Immunoblotting cell lysis buffer pH7.8 [50 mM Tris-HCl, 150 mM NaCl, 1% (wt/vol) SDS, 0.4% (vol/vol) beta-mercaptoethanol (BME)] and resuspensions were boiled for 2 min at 95°C and then sonicated 10× (Qsonica sonicators) at 30% amplitude for 5 s each. Lysates were quantified by using Bradford assay kit (Protein assay dye reagent, cat# 5000006, Bio-Rad). Whole-cell extracts of V. cholerae (30 μg) or E. coli (5 or 7 μg) were resolved by 10% SDS-PAGE gel and transferred to a nitrocellulose membrane by using a semi-drying transfer system (I Blot 2, NC Regular Stacks, cat# IB23001-Invitrogen). The membrane was then blocked with 4% milk in the buffer [20 mM Tris-HCl (pH7.8), 150 mM NaCl, 0.1% Triton X-100] for more than 2 hours. The blocked membrane was incubated with mouse monoclonal anti-FLAG M2 antibody (1:12000 dilutions, cat# F1804, Sigma-Aldrich), anti-His antibody (1:12000 dilutions, cat# 66005–1-lg, Proteintech), or anti-RNA polymerase alpha subunit antibody (1:15000 dilutions, cat# 663104, BioLegend) for 2 hours in 0.5% skim milk buffer and then further incubated with IRDye 800CW Goat anti-Mouse IgG (1:15000 dilutions, cat# 926–32210, LI-COR) for 1 hour. Finally, all blots were washed three times with TBST buffer [20 mM Tris-HCl (pH7.8), 150 μM NaCl, 0.1% Triton X-100] and scanned on an Odyssey CLx imaging device (LI-COR Biosciences) and signal was visualized by using image studio lite Ver 5.2 (LI-COR) software.
Immunoprecipitation for binding protein against anti-FLAG antibody
Wild type V. cholerae and 3×FLAG tag+ ProteinA fusion strains were grown in LB medium to mid-logarithmic phase (at an OD600 ~0.5) and then 20 ml cell cultures were spun down at 4830 rcf for 10 min. The cell pellets were resuspended with 1 ml IP lysis buffer pH7.5 (50 mM Tris-HCl, 0.2% Tergitol, 150 mM NaCl, 5 mM EDTA, and 1× EDTA free protease inhibitor) and cells were lysed by sonication with 30% amplitude for a total 2 minutes’ pulse time (3 s pulse/5 s on ice). Supernatant was transferred in a new 2 ml tube after centrifugation at 16000 rcf for 20 minutes and quantified by Bradford assay. 50 μl anti-FLAG M2 agarose beads (EZview Red ANTI-FLAG M2 Affinity Gel, cat# F2426, Sigma-Aldrich) were washed three times with 2-bed volume of lysis buffer. Total 4 mg of cell extract was incubated with the washed anti-FLAG M2 agarose beads for three hours at 4°C under constant agitation. The resin was recovered at 600 rcf for 30 seconds and washed three times with a chilled IP lysis buffer. Finally, all proteins on the resin were eluted with 150 μl of Elution buffer pH8.0 (100 mM Tris-HCl and 1% SDS) at 95°C for 5 min and then each 0.05% of input and flow-through (F.T) or 10% of eluted samples were resolved on 10% SDS-PAGE gel and visualized by silver staining (Pierce Silver Stain Kit, cat#24612, Thermo-Scientific).
In-gel trypsin digestion
Each half volume of eluted sample from IP was loaded in two wells of 10% SDS-PAGE gel; one for positive control of western blotting and the other for in-gel tryptic digestion. The ~37–31kDa area was excised after zinc staining and the zinc stain was then removed using the Pierce Zinc Reversible Stain Kit (cat #24582, ThermoScientific). All proteins were digested using Pierce In-Gel Tryptic Digestion Kit (cat #89871, ThermoScientific) following the manufacturer’s instructions. In brief, cysteine residues of proteins were reduced by TCEP [Tris (2-carboxyethyl) phosphine] and then alkylated by IAA (lodoacetamide). The excised gel was further dehydrated by acetonitrile (ACN) and incubated with activated trypsin overnight at 30°C. To confirm the correct gel slice, all remaining protein bands on the gel were transferred onto a nitrocellulose membrane for immunoblotting.
LC-MS/MS analysis
Peptides extracted from the gel were desalted with 50mg Sep-Pak C18 column. The C18 column was conditioned with 5 column volumes of 80% acetonitrile and 0.1% acetic acid and washed with 5 column volumes of 0.1% trifluoroacetic acid before loading samples. After samples were loaded, the column was washed with 5 column volumes of 0.1% acetic acid followed by elution with 4 column volumes of 80% acetonitrile and 0.1% acetic acid. The elution was dried in a SpeedVac evaporator. Dried samples were reconstituted in 0.1% trifluoroacetic acid and subjected to LC-MS/MS analysis using a 20-cm-long 125-μm inner diameter column packed in-house with 3μm C18 reversed-phase particles (Magic C18 AQ beads, Bruker). Separated peptides were electrosprayed into a QExactive Orbitrap mass spectrometer (Thermo Fisher Scientific). Xcalibur software (Thermo Fischer Scientific) was used for the data acquisition and the Q Exactive was operated in data-dependent mode. Survey scans were acquired in the Orbitrap mass analyzer over the range of 380 to 1800 m/z with a mass resolution of 70,000 (at m/z 200). MS/MS spectra was performed selecting up to the 10 most abundant ions with a charge state using of 2, 3 or 4 within an isolation window of 2.0 m/z. Selected ions were fragmented by Higher-energy Collisional Dissociation (HCD) with normalized collision energies of 27 and the tandem mass spectra was acquired in the Orbitrap mass analyzer with a mass resolution of 17,500 (at m/z 200). Repeated sequencing of peptides was kept to a minimum by the dynamic exclusion of the sequenced peptides for 30 seconds. For MS/MS, AGC target was set to 1e5 and max injection time was set to 120ms.
Overexpression of tag fused proteins in E. coli strains
An overnight culture from a single colony was used to inoculate 5 ml of LB medium. Cells were grown with vigorous shaking at 37°C to an optical density at 600 nm (OD600) of 0.5. For the protein induction, 200 μM isopropyl-β-D-thiogalactopyranoside [IPTG (Gold Bio, cat# I2481C25)] was added to E. coli Rosetta-gami2 for 30 min at 30°C and 1 mM IPTG was used for BL21 DE3/pLysS for full induction of proteins at 30°C for 2 hours. Cell cultures were harvested at 4830 rcf for 10 min and kept at −80°C.
Spotting Assay
Cells were grown to logarithmic phase (OD600~ 0.4) in LB medium at 37°C. 5 μl of serially diluted cell culture were spotted on each stress plate containing 0.7 mM EDTA (Ethylene diamine Tetra Acetic Acid, cat# S311–500, Fisher Chemical), 0.3 mM H2O2(Hydrogen Peroxide Solution, cat#95321–100ML, Sigma-Aldrich), 250 mM NaCl(Sodium Chloride, cat#BP358–10, Fisher BioReagents), 200 μg/ml Vancomycin (Vancomycin Hydrochloride, cat#V-200–25, GoldBio), 0.02% SDS(Sodium Dodecyl Sulfate, cat#BP166–500, Fisher BioReagents), 50 μg/ml Polymyxin B(Polymyxin B sulfate salt, cat#P4932, Sigma-Aldrich), or low salt LB (Yeast Extract, cat#BP1422–500 and Tryptone, cat#BP1421–500, Fisher BioReagents). All plates were incubated at 30°C for 24 hours and images were taken by Cannon ds126–151.
Results and Discussion
Vibrio cholerae encodes a FLAG-antibody-reactive protein, which is differentially regulated by growth phase
During Western Blotting experiments with anti-Flag antibody aimed at mapping the growth-phase-dependent expression pattern of a 3×FLAG-tagged protein (Fig. 1A) in V. cholerae, we noticed a background band at around ~36 kDa (Fig. 1B). We at first hypothesized that this band represented a product of proteolytic degradation of our target protein. However, the same band appeared as background in several other strains carrying 2 different FLAG-tagged proteins, as well as in an untagged wild-type background. Non-specific background bands can in principle be used as a loading control; however, the FLAG-reactive protein appeared at varying abundance during different growth phases, i.e., strongly diminished in stationary and lag phases of growth and strong expression during exponential phase (Fig. 1C). Thus, V. cholerae encodes a growth-phase-regulated protein that reacts with FLAG-antibody.
Figure 1 |. Vibrio cholerae encodes a FLAG-antibody-reactive protein that is upregulated in exponential growth phase.
(A) Construction of 3×FLAG tag fusion proteins. 3×FLAG peptide was fused after N-terminus signal sequences of Protein A and B, or at C-terminus region of Protein C for quantification of protein levels. (B) Detection of non-specific protein by anti-FLAG antibody in both wild type and 3×FLAG fusion strains (C) Growth phase dependent induction of anti-FLAG antibody binding protein. Lag, lag phase; Log, exponential phase; Sta, stationary phase.
An outer membrane porin co-precipitates with FLAG-tagged proteins and contains 3×FLAG-peptide-like sequences
Since the presence of a strongly FLAG-reactive protein could potentially confound analyses of protein abundances (a Uniprot proteome search revealed ~180 proteins in V. cholerae in the size range 36 – 39 kDa) when using the common FLAG-tag, we sought to identify and remove this background peptide. To this end, we subjected lysates from an untagged wild-type strain and a derivative strain expressing a 3× FLAG-tagged protein to anti-FLAG immunoprecipitation. After precipitation with a Flag-antibody resin, we excised a gel slice in the size range expected for the background band (Fig. 2AB) and confirmed its absence after excision using Western Blot (Fig. 2C). We then identified proteins contained in the gel slice using mass spectrometry. A total of 37 proteins occurred in both the WT and the FLAG-tagged mutant (Fig. 2D); among these proteins, VC0972 was most abundant (Table 1). This protein is annotated as an outer membrane porin (henceforth: Porin4) and we hypothesized that this was the FLAG-reactive background protein. VC0972 is a chitoporin that allows uptake of chitin breakdown products in its natural environment (17).
Figure 2 |. Identification of anti-FLAG antibody binding protein by Immunoprecipitation and LC-MS/MS.
(A-B) Silver-stained (A) or zinc-stained (B) protein gel demonstrating effective IP with FLAG antibody resin. (C) Western Blot showing excision of non-specific protein-containing region from the gel in (B). Each half volume of eluted sample from IP was loaded in two wells of 10% SDS-PAGE gel; one for positive control of western blotting and the other for in-gel tryptic digestion. (D) Venn diagram showing number of detected total proteins from IP/LC-MS/MS of the size-selected gel band in wild type and 3×FLAG fusion strain.
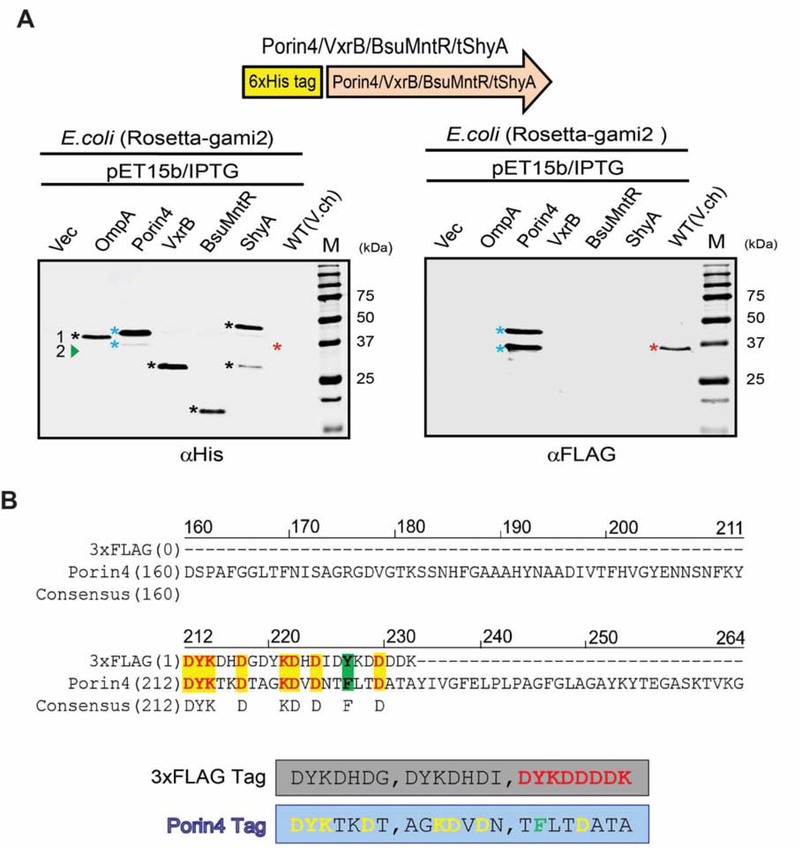
To confirm FLAG-antibody-reactivity of Porin4, we overexpressed it heterologously in E. coli and performed Western Blotting analyses using anti-Flag antibody. We also included an N-terminal His-tag (to control for the presence of the protein) as well as several His-tagged control proteins (OmpA, VxrB, MntRBsub, ShyA and crude lysate from wild-type V. cholerae). Coomassie staining revealed that all proteins were expressed at the expected sizes (Fig. S1A). All His-tagged proteins could be detected using His-antibody (Fig. 3A). In contrast, an anti-Flag Western Blot only detected Porin4 and the expected band in WT V. cholerae lysate (Fig. 3A). Thus, full-length Porin4 can be detected with anti-Flag antibody. Interestingly, Porin4 presented as two bands with different molecular weights in E. coli, likely the result of proteolytic processing. A primary amino acid sequence alignment of Porin4 with the 3× FLAG sequence revealed a striking similarity between a region of Porin4 and the epitope tag (Fig. 3B). We designated this sequence (DYKTKDT, AGKDVDN, TFLTDATA, overlap with 3×FLAG sequence in bold) the “Porin 4 tag”.
Figure 3 |. Validation of VC0972 (Porin4) as the putative anti-FLAG antibody binding protein.
(A) Construction of overexpression vectors for 6xHis tag fusion proteins. A 6xHis tag was fused to the N-terminus region of all proteins (OmpA, Porin4, VxrB, ShyA, and Bacillus subtilis MntR) and overproduced in E. coli followed by detection via anti-His and anti-Flag Western Blot. Vec, empty vector. Black stars or red stars indicate bands recognized by anti-His antibody or anti-FLAG antibody, respectively. Blue star presents the band detected by both antibodies. (B) Amino acid sequence alignment between 3×FLAG tag and the relevant parts of Porin4 protein reveals the putatively FLAG-antibody-reactive Porin4 tag. Identical residues are highlighted yellow.
To demonstrate that the Porin4 tag was sufficient to allow detection via Flag-antibody Western Blot, we created two different fusion proteins (VxrB and OmpA of V. cholerae) containing an N-terminal 6xHis tag with or without an additional C-terminal Porin 4 tag (Fig. 4A) and expressed them in Escherichia coli (which does not contain a FLAG-antibody-reactive background band). Coomassie staining revealed that all proteins were robustly overproduced at the expected sizes (Fig. S1B). As expected, all fusion proteins were detectable via Western Blots with His-antibody; in contrast, only the Porin4-tagged proteins were detectable via FLAG antibody Western (Fig. 4A). Thus, the Porin4 tag sequence is sufficient for detection with anti-FLAG antibody. Lastly, we confirmed that Porin4 was indeed the background band observed in wild-type V. cholerae. We created a Porin4 deletion mutant in both, a wild-type background and in a strain carrying a chromosomal 3×FLAG-VxrA fusion and conducted Western Blotting experiments with anti-FLAG antibody. As expected, deletion of Porin4 completely removed the FLAG-reactive background in both the WT and a strain carrying FLAG-tagged VxrA (Fig. 4B).
Figure 4 |. Detection of Porin4-tagged proteins by anti-FLAG antibody.
(A) Construction of 6xHis and Porin4 dual tag fusion proteins. To determine anti-FLAG antibody binding, the Porin4 tag (DYKTKDT AGKDVDN TFLTDATA) was fused to the C-termini of OmpA or VxrB. A 6xHis tag was added to the N-termini. Bands were detected by immunoblotting with anti-His antibody (with RNA polymerase as loading control). Blue stars indicate protein bands detected by both anti-His and anti-FLAG antibodies. Black stars or red arrowheads present bands recognized by only anti-His or anti-FLAG antibody, respectively. (B) Absence of non-specific FLAG-antibody reactive bands in Δporin4 backgrounds.
A Porin4 mutant has wild-type stress resistance
We next sought to describe phenotypes associated with a Porin4 deletion (in addition to the known phenotype of chitin degradation product uptake deficiency) and subjected the mutant to detailed phenotypic analysis of cell envelope stresses. The Aporin4 strain exhibited wild-type level resistance against all tested stress conditions, including EDTA, H2O2, high salt, low salt, vancomycin (a readout for OM integrity), SDS, and Polymyxin B (Fig. S2). Thus, Porin4 is not essential for wild-type stress resistance (at least in the standard laboratory growth medium LB). Porin4 is widely conserved within Vibrionaceae (Fig. S3) and we recommend deleting the Portin4 ORF in Vibrio species when conducting experiments using anti-Flag antibody.
Supplementary Material
Highlights.
The cholera pathogen Vibrio cholerae encodes a protein, Porin 4, that reacts with Flag-antibodies.
Porin 4 causes a background band in Western Blots aimed at 3×FLAG tag detection.
Porin 4 contains a sequence of striking similarity to 3×FLAG tag.
Deletion of Porin 4 removes the 3×FLAG-reactive background band.
Acknowledgments
Research in the Dörr laboratory is supported by National Institutes of Health (NIH) grants R01AI143704 and R01GM130971. Research in the Smolka lab is funded by NIH grants R01HD095296 and R01GM123018.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Biochemical and Biophysical Research Communications
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Jarvik JW, Telmer CA, Epitope tagging, Annu Rev Genet 32 (1998) 601–618. 10.1146/annurev.genet.32.1.601. [DOI] [PubMed] [Google Scholar]
- [2].Kimple ME, Brill AL, Pasker RL, Overview of affinity tags for protein purification, Curr Protoc Protein Sci 73 (2013) 9 9 1–9 9 23. 10.1002/0471140864.ps0909s73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Terpe K, Overview of tag protein fusions: from molecular and biochemical fundamentals to commercial systems, Appl Microbiol Biotechnol 60 (2003) 523–533. 10.1007/s00253-002-1158-6. [DOI] [PubMed] [Google Scholar]
- [4].Maue RA, Understanding ion channel biology using epitope tags: progress, pitfalls, and promise, J Cell Physiol 213 (2007) 618–625. 10.1002/jcp.21259. [DOI] [PubMed] [Google Scholar]
- [5].Zhao X, Li G, Liang S, Several affinity tags commonly used in chromatographic purification, J Anal Methods Chem 2013 (2013) 581093 10.1155/2013/581093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Schembri L, Dalibart R, Tomasello F, Legembre P, Ichas F, De Giorgi F, The HA tag is cleaved and loses immunoreactivity during apoptosis, Nat Methods 4 (2007) 107–108. 10.1038/nmeth0207-107. [DOI] [PubMed] [Google Scholar]
- [7].Zakeri B, Fierer JO, Celik E, Chittock EC, Schwarz-Linek U, Moy VT, Howarth M, Peptide tag forming a rapid covalent bond to a protein, through engineering a bacterial adhesin, Proc Natl Acad Sci U S A 109 (2012) E690–697. 10.1073/pnas.1115485109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Blanar MA, Rutter WJ, Interaction cloning: identification of a helix-loop-helix zipper protein that interacts with c-Fos, Science 256 (1992) 1014–1018. 10.1126/science.1589769. [DOI] [PubMed] [Google Scholar]
- [9].Su X, Prestwood AK, McGraw RA, Production of recombinant porcine tumor necrosis factor alpha in a novel E. coli expression system, Biotechniques 13 (1992) 756–762. [PubMed] [Google Scholar]
- [10].Einhauer A, Schuster M, Wasserbauer E, Jungbauer A, Expression and purification of homogenous proteins in Saccharomyces cerevisiae based on ubiquitin-FLAG fusion, Protein Expr Purif 24 (2002) 497–504. 10.1006/prep.2001.1595. [DOI] [PubMed] [Google Scholar]
- [11].Schuster M, Wasserbauer E, Einhauer A, Ortner C, Jungbauer A, Hammerschmid F, Werner G, Protein expression strategies for identification of novel target proteins, J Biomol Screen 5 (2000) 89–97. 10.1177/108705710000500205. [DOI] [PubMed] [Google Scholar]
- [12].Kunz D, Gerard NP, Gerard C, The human leukocyte platelet-activating factor receptor. cDNA cloning, cell surface expression, and construction of a novel epitope-bearing analog, J Biol Chem 267 (1992) 9101–9106. [PubMed] [Google Scholar]
- [13].Zhang XK, Wills KN, Husmann M, Hermann T, Pfahl M, Novel pathway for thyroid hormone receptor action through interaction with jun and fos oncogene activities, Mol Cell Biol 11 (1991) 6016–6025. 10.1128/mcb.11.12.6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hopp TP, Prickett KS, Price VL, Libby RT, March CJ, Cerretti DP, Urdal DL, Conlon PJ, A Short Polypeptide Marker Sequence Useful for Recombinant Protein Identification and Purification, Bio/Technology 6 (1988) 1204–1210. 10.1038/nbt1088-1204. [DOI] [Google Scholar]
- [15].Shin JH, Jung HJ, An YJ, Cho YB, Cha SS, Roe JH, Graded expression of zinc-responsive genes through two regulatory zinc-binding sites in Zur, Proc Natl Acad Sci U S A 108 (2011) 5045–5050. 10.1073/pnas.1017744108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Donnenberg MS, Kaper JB, Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector, Infect Immun 59 (1991) 4310–4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Meibom KL, Li XB, Nielsen AT, Wu CY, Roseman S, Schoolnik GK, The Vibrio cholerae chitin utilization program, Proc Natl Acad Sci U S A 101 (2004) 2524–2529. 10.1073/pnas.0308707101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.