Abstract
Background/Objective: There is increasing use of computed tomography (CT) in sarcopenia research using a wide variety of techniques. We performed a systematic review of the CT literature to identify the differences between approaches used. Methods: A comprehensive search of PubMed from 1983 to 2017 was performed to identify studies that used CT muscle measurements to assess muscle mass and myosteatosis. The CT protocols were evaluated based on anatomic landmark(s), thresholding, muscle(s) segmented, key measurement (ie, muscle attenuation, cross-sectional area, volume), derived variables, and analysis software. From the described search, 657 articles were identified and 388 studies met inclusion criteria for this systematic review. Results: Muscle mass was more commonly assessed than myosteatosis (330 vs. 125). The most commonly assessed muscle or muscle groups were total abdominal wall musculature (142/330 and 49/125 for muscle mass and myosteatosis, respectively) and total thigh musculature (90/330 and 48/125). The most commonly used landmark in the abdomen was the L3 vertebra (123/142 and 45/49 for muscle mass and myosteatosis, respectively). Skeletal muscle index and intermuscular adipose tissue were the most commonly used measures of abdominal wall muscle mass (114/142) and myosteatosis (27/49), respectively. Cut points varied across studies. A significant majority of studies failed to report important CT technical parameters, such as use of intravenous contrast and slice thickness (94% and 63%, respectively). Conclusions: There is considerable variation in the CT approaches used for the assessment of muscle mass and myosteatosis. There is a need to develop consensus for CT-based evaluation of sarcopenia and myosteatosis.
Keywords: Body composition, Imaging, Muscle, Sarcopenia
Sarcopenia, broadly defined as a significant loss of muscle mass and function, has been associated with a variety of adverse outcomes, including physical disability, falls, and prolonged hospitalization (1–7). Recent studies on sarcopenia have emphasized its high prevalence in various clinical settings (8–11) as well as the significant impact of common medications on muscle mass (12,13). There has also been considerable debate on the clinically relevant cut points for the diagnosis of sarcopenia (14–17). Diagnostic cut points are particularly important in the context of sarcopenia treatment and prevention. Although nutritional supplementation and physical activity have already been shown to positively influence muscle mass and strength in older adults (18–25), many pharmacologic treatments of sarcopenia are under investigation (26–29).
A variety of tests of physical function and imaging techniques are available for assessing muscle mass. Dual X-ray absorptiometry is the most widely used imaging technique for assessment of muscle mass, with appendicular lean mass the most commonly used phenotype in sarcopenia research. Bioelectrical impedance analysis offers a low-cost alternative, but can be confounded by alterations in hydration, soft-tissue edema, exercise status, and food intake (5). More recently, magnetic resonance imaging has been used to quantify muscle volume and composition, allowing for the differentiation of muscle tissue from adipose tissue, edema, and fibrous connective tissue (5).
Current usage of the term sarcopenia in the medical literature is inconsistent. In general, the aging literature reserves the term sarcopenia for low muscle mass (usually measured with dual X-ray absorptiometry or bioelectrical impedance analysis) and low physical function (usually measured with grip strength or gait speed) and considers myosteatosis a related, but distinct, entity (2–4,6–8). In contrast, many cancer journals (and other nonaging journals) use the term sarcopenia for low muscle mass (usually measured with computed tomography [CT]) without any measurement of physical function and occasionally consider myosteatosis as a component of sarcopenia, rather than a distinct entity (1,30–34).
Despite the inconsistent terminology, the use of CT for research on muscle in older adults is becoming more common, due to its ability to measure muscle quality (eg, myosteatosis) and muscle mass (32). CT assessment of muscle mass usually involves measuring muscle cross-sectional area on a single CT image (5). Cross-sectional area is often then indexed for patient’s height, resulting in skeletal muscle index (SMI) (5). CT assessment of myosteatosis usually involves measuring intermuscular adipose tissue (IMAT) area or muscle attenuation (MA) (5). Increased fat infiltration in muscle (ie, myosteatosis) results in lower radiodensity of muscle on CT images (5).
Unfortunately, variation in CT acquisition parameters and image analysis techniques are obstacles to wider adoption of CT and limit opportunities for comparison of data across studies (35–38). We performed a systematic review of the CT-based muscle literature to define the degree of variation in muscle mass and myosteatosis measurements, as the first step in addressing this lack of standardization.
Methods
Two PubMed searches were performed in November 2017, without a limiting date range for the results. The first search was as follows: ((cachexia OR cachexic OR sarcopenia OR sarcopenic OR dynapenia OR dinapenia OR myosteatosis OR myopenia) AND (attenuation OR Hounsfield OR “CT number” OR “CT-values” OR “CT-value” OR HU OR “gray scale” OR “grayscale” OR “CT density” OR “skeletal muscle index” OR “skeletal muscle radiodensity” OR “skeletal muscle density” OR “psoas area” OR sliceomatic)) OR (“Tomography, X-Ray Computed” [MH] AND “Muscle Strength” [MH]), and yielded 429 results. The second search was as follows: (English [Language] AND ((“Tomography, X-Ray Computed” [MH] or CT) AND (“Muscle Strength” [MH] OR “Body Composition” [MH]) NOT (animals [MH] NOT humans [MH])) NOT Review[Publication Type] NOT “Case Reports” [Publication Type]) AND (muscle OR psoas OR intermuscular OR sarcopenia OR myopenia OR dynapenia OR myosteatosis), and yielded 616 results. The two searches were combined, removing duplicates, resulting in 657 studies.
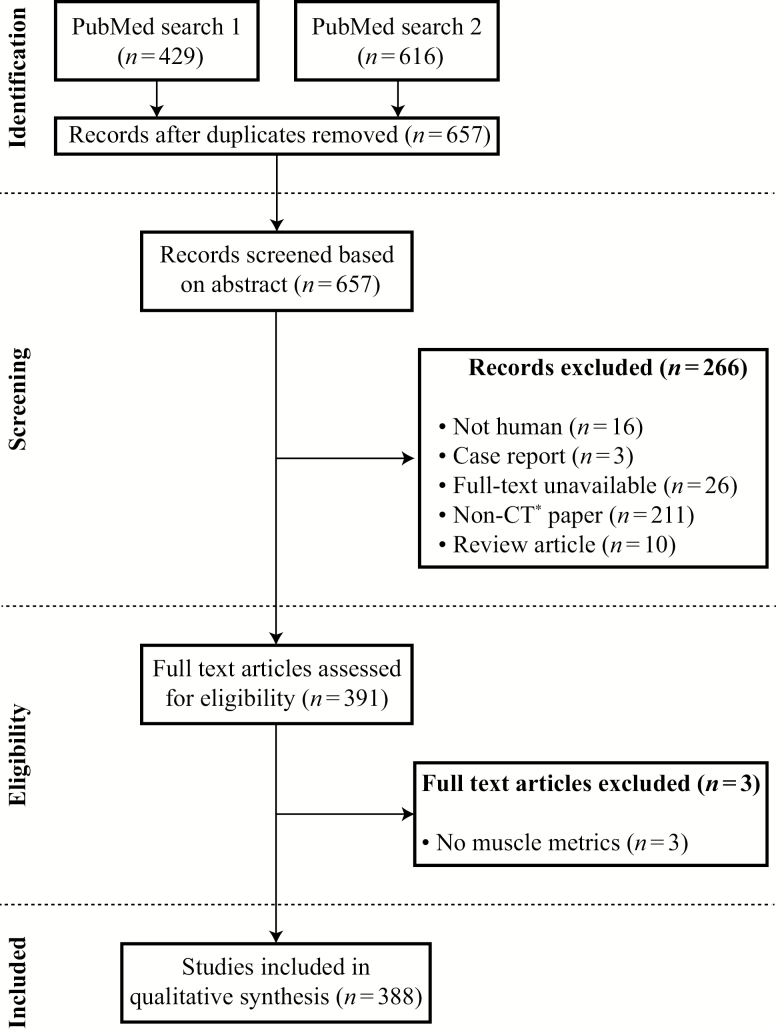
The abstracts were reviewed by two authors to select studies that reported on muscle metrics assessed using CT. Three hundred and eighty-eight studies were selected for the full-text analysis. Figure 1 shows the flow diagram for the identification, screening, and inclusion of articles in the systematic review.
Figure 1.
Flow diagram on identification, screening, eligibility, and inclusion of full-text articles. *Non-computed tomography (CT) article refers to articles that did not use CT to assess body composition metrics as a primary modality (eg, magnetic resonance imaging).
The data collected included publication year, segmentation software, segmentation method, number of readers, number of slices used, approach to thresholding, threshold values, anatomic landmark (eg, L3, L4, femoral neck, etc.), muscle(s) segmented, measured variable (eg, attenuation, area, or volume), derived variable (eg, SMI), CT examination (eg, abdomen, pelvis, etc.), and image analysis software.
Risk of bias was low, as this was primarily a review of image analysis methodology. We were not assessing diagnostic test accuracy, outcomes, adverse events, or other variables that would be subjected to selective reporting of positive results.
Results
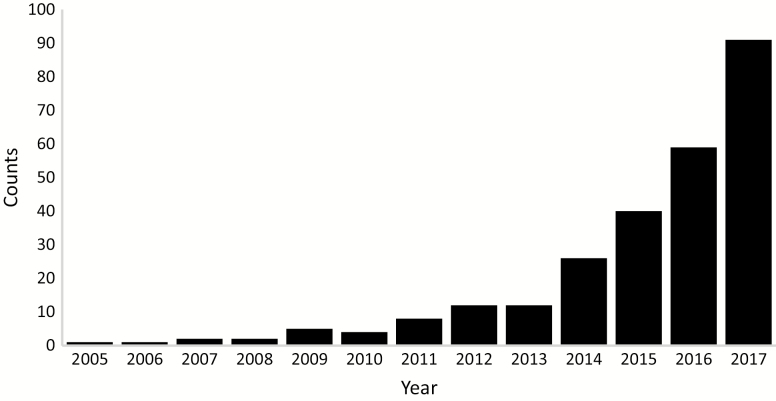
Three hundred and eighty-eight studies met inclusion criteria for this systematic review. Figure 2 shows the number of publications by year, indicating the most rapid increase since 2013. The earliest study was from Maughan and colleagues in 1984, measuring the cross-sectional area of various muscle compartments of the forearm using CT (39). The number of subjects in the studies varied, with the majority (54%) having fewer than 100 (median = 92).
Figure 2.
Number of publications per year.
Tables 1 and 2 summarize the studies that used CT to measure muscle mass and myosteatosis, respectively. More studies assessed muscle mass (n = 330) than myosteatosis (n = 125). Because some studies performed both assessments, the results in the two tables do not add to 388.
Table 1.
Muscle Mass Assessment
| N = 330a | Sarcopenia Cut Points (cm2/m2, Unless Indicated Otherwise)b | ||||||
|---|---|---|---|---|---|---|---|
| Anatomic Landmarks | Main Muscle Mass Measurement | Sarcopenia Cut Points Used | Malec | Female | Muscle Thresholding Used | Threshold Range (HU) | |
| Total abdominal wall musculature (n = 142)A1 | L3 (n = 123) | SMI (n = 114) | Yes (n = 84) | SMI < 43 or 53 (n = 27)d | SMI < 39 (n = 44) | Yes (n = 125) | −29 to 150 (n = 95) |
| L3–L4 (n = 7) | CSA (n = 31) | SMI < 52 (n = 23) | SMI < 41 (n = 32) | −30 to 150 (n = 5) | |||
| L4 (n = 7) | SMI < 55 (n = 20) | −30 to 110 (n = 4) | |||||
| L4–L5 (n = 7) | 0 to 100 (n = 4) | ||||||
| Total thigh musculature (n = 90)A2 | Midthigh (n = 69) | CSA (n = 89) | Yes (n = 1) | CSA < 84 cm2 (n = 1) | CSA < 84 cm2 (n = 1) | Yes (n = 59) | 0 to 100 (n = 23) |
| Proximal thigh (n = 5) | SMI (n = 1) | −29 to 150 (n = 4) | |||||
| Distal thigh (n = 2) | −49 to 100 (n = 3) | ||||||
| Psoas only (n = 48)A3 | L3 (n = 24) | SMI (n = 27) | Yes (n = 4) | SMI < 6 (n = 2) | SMI < 4 (n = 2) | Yes (n = 23) | −30 to 110 (n = 8) |
| L4 (n = 21) | CSA (n = 22) | SMI < 7 (n = 1) | SMI < 6 (n = 2) | −29 to 150 (n = 6) | |||
| L3–L4 (n = 2) | SMI < 8 (n = 1) | −30 to 150 (n = 2) | |||||
| L4–L5 (n = 2) | |||||||
| Other (n = 91)e,A4 | Variablee | CSA (n = 78) | Yes (n = 1) | Pectoralis muscle SMI < 44 (n = 1) | Pectoralis muscle SMI < 31 (n = 1) | Yes (n = 60) | −29 to 150 (n = 10) |
| SMI (n = 8) | 0 to 100 (n = 6) | ||||||
| 0 to 200 (n = 3) | |||||||
| −29 to 151 (n = 3) |
Notes: CSA = cross-sectional area; HU = Hounsfield units; SMI = skeletal muscle index; WBFFM = whole-body fat free mass.
A1–A4Please refer to Supplementary Appendix for references.
aThree hundred and thirty of the 388 publications assessed muscle mass. Numbers in table sum to more than 330, as many publications assessed more than one muscle group. Many publications assessed both muscle mass and quality, and the numbers in Tables 1 and 2 total to more than 388. bNumbers for cut points rounded to nearest integer. cThree studies only assessed women (31,33,34). dDepending on body mass index. eThese included head and neck, lower leg, individual thigh muscles, upper extremity muscles, unspecified abdominal muscle, anterior abdominal wall musculature, chest musculature, dorsal group muscles (not including psoas), and psoas and at least one dorsal group muscle.
Table 2.
Myosteatosis Assessment
| N = 125a | Myosteatosis Cut Pointsb | ||||||
|---|---|---|---|---|---|---|---|
| Anatomic Landmarks | Main Myosteatosis Measurement | MyosteatosIs Cut Points Used | Male | Female | Used HU Threshold | Threshold Range (HU) | |
| Total abdominal wall musculature (n = 49)A5 | L3 (n = 45) | IMAT (n = 27) | Yes (n = 11) | MA < 41 or 33 HUc (n = 8) | MA < 41 or 33 HUc (n = 8) | Yes (n = 48) | −190 to −30 (n = 36) |
| L4–L5 (n = 3) | MA (n = 12) | ||||||
| Total thigh musculature (n = 48)A6 | Midthigh (n = 32) | IMAT (n = 38) | None | N/A | N/A | Yes (n = 34) | −190 to −30 (n = 20) |
| Proximal thigh (n = 5) | LDLT (n = 19) | 0 to 34 (n = 9) | |||||
| All thigh (n = 3) | 0 to 30 (n = 6) | ||||||
| Psoas (n = 12)A7 | L3 (n = 8) | MA (n = 17) | Yes (n = 2) | MA < 33.3 HU (n = 1) | SMD < 31.1 HU (n = 1) | Yes (n = 7) | −190 to −30 (n = 4) |
| L4–L5 (n = 2) | LDLT (n = 3) | Quartile based (n = 1) | Quartile based (n = 1) | 0 to 29 (n = 3) | |||
| L4 (n = 1) | HDLT (n = 3) | 0 to 29 (n = 1) | |||||
| IMAT (n = 2) | |||||||
| Others (n = 21)d,A8 | Variabled | IMAT (n = 15) | None | N/A | N/A | Yes (n = 20) | −190 to −30 (n = 5) |
| HDLT (n = 8) | −200 to −1 (n = 3) | ||||||
| LDLT (n = 7) | 0 to 29 (n = 6) |
Notes: IMAT = intermuscular fat (cm2); LDLT = low-density lean tissue (cm2); HU = Hounsfield units; MA = muscle attenuation; N/A = not applicable.
A5–A8Please refer to Supplementary Appendix for references.
aOne hundred and twenty-five of the 388 publications assessed muscle quality. Numbers in table sum to more than 125, as many publications assessed more than one muscle group. Many publications assessed both muscle mass and quality, and the numbers in Tables 1 and 2 total to more than 388. bNumbers for cut points rounded to nearest integer. cDepending on body mass index. dThese included individual muscles, as well as muscle groups. Individual muscles: adductor longus, adductor magnus, biceps femoris, gluteus maximus, gracilis, rectus femoris, sartorius, vastus lateralis, and vastus medialis. Muscle groups: hip abductors (gluteus medius, gluteus minimus and piriformis), hamstrings (semitendinosus, semimembranosus, and biceps femoris), hip adductors (adductor magnus, adductor brevis, adductor longus, quadratus femoris, quadratus externus, pectineus), hip flexors (rectus femoris and sartorius), knee flexor compartment (hamstrings, gracilis, sartorius, and adductors), gluteus medius/minimus combination, semitendinosis/semimembranosis combination, quadratus femoris muscles (rectus femoris, vastus lateralis, vastus intermedius, vastus medialis).
Table 1 summarizes studies of muscle mass (n = 330). The most commonly assessed muscle or muscle group was total abdominal wall musculature (142 of 330). The most commonly used landmark for locating the CT image for muscle measurement was L3 vertebra (123/142). SMI was the most commonly used measure of abdominal wall muscle mass (114/142). Most of these studies (84/142) applied cut points for sarcopenia diagnosis based on SMI of abdominal wall musculature. However, these cut points varied across studies: in men, SMI cut points of 43, 53, and 55 cm2/m2 were used in 27, 23, and 20 studies, respectively; in women, SMI cut points of 39 and 41 cm2/m2 were used in 44 and 32 studies, respectively.
During muscle segmentation, muscle thresholding was used in the majority of studies (261/325), with the thresholds range of −29 to 150 Hounsfield units (HU) most commonly used (117/261).
The approaches used to measure muscle mass in other muscle groups including the thigh, psoas, and other muscle groups are also given in Table 1. These approaches are less standardized than what was described previously for total abdominal muscles.
Table 2 summarizes CT studies that included measurements of myosteatosis (n = 125). The most commonly assessed muscle or muscle groups were total abdominal and total thigh musculature (49 and 48/125, respectively). In the abdomen, common CT measures of myosteatosis included IMAT and MA in 27 and 12 of 49, respectively. In the thigh, common CT measures of myosteatosis included IMAT and low-density lean tissue in 38 and 19 of 48, respectively. The most common thresholds used for assessment of IMAT were −190 to −30 HU (36/48 in the abdomen and 20/34 in the thigh). The most common thresholds for low-density lean tissue were 0–34 and 0–30 HU. In the abdomen, diagnostic cut points were used for MA in 11 of 12 studies that assessed this measure. In these studies, values of less than 41 HU or less than 33 HU used as an indicator for myosteatosis in both men and women depending on body mass index.
Table 3 shows details of CT image analysis methods. A single reader was used in 38% of studies, whereas a similar number failed to specify the number of readers. The majority of studies (59%) used semiautomated methods for segmentation, and of these studies, most (58%) used commercial software.
Table 3.
Image Analysis Details
| Assessed Feature | Result | Number (%) |
|---|---|---|
| Number of readers | 1 | 97 (38) |
| 2 | 53 (21) | |
| 3 | 7 (3) | |
| Not specified | 98 (38) | |
| Segmentation methods | ||
| Manual | 107 (27) | |
| Semiautomated | 235 (59) | |
| Automated | 6 (1.5) | |
| Not specified | 50 (12.5) | |
| Segmentation software | ||
| Commercial | 231 (58) | |
| Slice-O-matic | 85 (37) | |
| Other | 77 (33) | |
| Osirix | 22 (10) | |
| ImageJ | 18 (8) | |
| AW (GE) | 16 (7) | |
| MIPAV | 13 (6) | |
| Custom | 31 (8) | |
| PACS | 18 (5) | |
| More than 1 | 1 (<1) | |
| None | 1 (<1) | |
| Not specified | 114 (29) |
Table 4 shows the acquisition parameters for CT scans. The majority (89%) of studies failed to indicate the type of CT examination (eg, stating “CT used for pancreatic cancer follow-up” instead of “CT abdomen” or “CT abdomen and pelvis”). Most studies (94%) did not mention if intravenous contrast was administered and what slice thickness was used (63%).
Table 4.
CT Acquisition Parameters
| Parameters | Number (%) |
|---|---|
| CT exam | |
| Abdomen alone or with another part | 32 (8) |
| Lower extremity | 4 (1) |
| Chest | 3 (1) |
| Other | 3 (1) |
| Not specified | 346 (89) |
| IV contrast given | |
| Yes | 11 (3) |
| No | 10 (3) |
| Variable | 3 (1) |
| Not specified | 364 (94) |
| Slice thickness | |
| >10 mm | 5 (1) |
| 10 mm | 53 (14) |
| Between 5 and 10 mm | 7 (2) |
| 5 mm | 31 (8) |
| <5 mm | 33 (9) |
| Variable | 15 (4) |
| Not specified | 244 (63) |
| Number of slices used | |
| 1 | 243 (60) |
| 2 | 49 (12) |
| 3 | 15 (4) |
| >3 | 35 (9) |
| Variable | 24 (6) |
| Not specified | 40 (10) |
Notes: CT = computed tomography; IV = intravenous.
Discussion
CT-derived measures of muscle mass and myosteatosis have been associated with adverse outcomes in many populations, including older adults (1,30–33,40–45). Increasing use of CT for deriving these muscle metrics was highlighted at two recent conferences of the National Institutes of Health (NIH): The National Cancer Institute Workshop, “Understanding the Role of Muscle and Body Composition in Studies of Cancer Risk and Prognosis in Cancer Survivors,” on September 25–26, 2017 (46) and the National Institute of Aging Workshop, “Myosteatosis in the Context of Skeletal Muscle Function Deficit,” on September 14, 2018. Both conferences called for increased standardization for measurements of muscle mass and myosteatosis using CT.
This systematic review reveals important trends in the literature on CT assessment of muscle mass and myosteatosis, including the muscles analyzed, analysis techniques, and use of diagnostic cut points. At the same time, it highlights the need for further standardization in the field.
This review shows that muscle mass and myosteatosis are commonly assessed on CT but that different muscle groups are preferentially measured for each purpose. For muscle mass measurement, the total abdominal wall muscles are favored (n = 142) over the thigh muscles (n = 90). For myosteatosis, the total thigh muscles are measured almost as often (n = 48) as total abdominal wall muscles (n = 49).
There is emerging consensus on the preferred anatomic levels (ie, CT image location) used for muscle measurements. Our review shows that L3 vertebra level is most commonly used for the measurement of abdominal muscles while the midthigh level is most commonly used for the thigh muscles. However, there is variability in the exact definitions of these anatomic sites. For example, an L3 landmark may refer to the upper, mid, or lower vertebral body, whereas the midthigh CT landmark may be defined as the midpoint between the medial edge of the greater trochanter and the intercondyloid fossa of the patella (47), the midpoint of the femur (48), 20 cm distal to the greater trochanter (49), or the midpoint between the femur and the lateral condyle (50). Further studies are needed to determine the impact of such variations in measurement location on measurements of muscle mass (ie, SMI) and myosteatosis (ie, MA, IMAT, low-density lean tissue).
We also find an emerging consensus on the diagnostic cut points for muscle mass (ie, SMI). Our findings on diagnostic cut points for abdominal wall muscles are in agreement with a recent review on the use of CT in cancer cohorts by Daly and colleagues (30). That review summarized 12 different diagnostic thresholds for sarcopenia (30). Based on SMI, the cut points of 52.4 cm2/m2 for men and 38.5 cm2/m2 for women were the most common (33% of studies) (30). Our study supports their findings: most common SMI cut points were 52–55 cm2/m2 for men and 39–41 cm2/m2 for women (rounded to the nearest integer). However, for muscle groups outside the abdomen, we find that the use of diagnostic cut points is much less standardized.
Unlike cut points for SMI, cut points for myosteatosis were only used in a few studies (13/125). Daly and colleagues (30) concluded that the prevalence of sarcopenia in cancer studies is highly dependent on diagnostic thresholds and should be standardized. Our review points to additional need for standardization of CT-derived diagnostic thresholds for muscle mass and myosteatosis, and not just in cancer studies, but also in other cohorts, including older adults.
Our study shows important trends in the techniques used for analysis of muscles on CT images (ie, segmentation). Majority of studies employed semiautomated software for muscle segmentation, a trend that will probably continue as these tools become less expensive and more accurate. However, 27% of studies still used manual segmentation of muscles. It is worth emphasizing that both manual and semiautomated approaches to muscle segmentation require human input. For this reason, it is surprising that 38% of studies failed to report the number of readers who performed muscle measurements. In the future, fully automated techniques for muscle segmentation will be available, limiting human intervention to quality control. However, in existing studies, the lack of detail on readers is concerning, making the findings in these studies less generalizable.
The most unexpected finding of the present review is that many publications provide insufficient details concerning CT acquisition protocols to allow for future studies to reproduce their work. At the most basic level, the majority of studies fail to report the examination type (eg, CT abdomen), although the type of examination could be deduced from the anatomy studied. More importantly, many studies fail to specify the use of intravenous contrast or the slice thickness, both of which have been shown to affect muscle measurements (35,37).
When interpreting the CT literature, CT acquisition parameters should be viewed as confounding factors when determining the associations between muscle metrics and clinical outcomes. For example, lower MA values will be obtained on unenhanced CTs, compared with contrast-enhanced CTs. Because unenhanced CTs are more commonly performed in patients with renal insufficiency, this difference in attenuation could bias the observed association between MA and patient prognosis.
Strengths and Limitations
Our study has several strengths and limitations. We only used a single database (PubMed), which led to the exclusion of publications in nonindexed journals and may have affected the results. However, we believe the overall trends described here would generalize to the larger data set. The major strengths of our review were the large number of studies included and the detail with which the CT measurements of muscle mass and myosteatosis were evaluated.
Future Directions
As the diagnosis and treatment of sarcopenia in older adults transitions from research settings to become a routine part of patient care, better tools for noninvasive measurement of muscle mass and myosteatosis will be needed. These CT measurements have already been validated in many studies of older adults. Existing obstacles to wider CT usage include cost, access to equipment, and radiation exposure. However, muscle mass and myosteatosis could be measured opportunistically on clinical CT scans obtained as part of routine patient care, without additional cost or radiation exposure to patients. For that to happen, further standardization on diagnostic thresholds using CT is needed. The NIH has played a major role in standardizing approaches to diagnosing sarcopenia using gait speed, grip strength, and dual X-ray absorptiometry–derived lean mass, most recently convening a Sarcopenia Definitions and Outcomes (SDOC) Position Development Conference on November 13, 2018 (17,51) A similar process for standardizing the CT measurements of muscle mass and myosteatosis is needed.
Conclusion
This is the first systematic review of CT-derived measures of muscle mass and myosteatosis that includes noncancer cohorts. Although there is considerable variation in CT assessment of muscle metrics, there is emerging consensus on various aspects of muscle measurement. Our results support the need for further standardization of CT measurements to allow for future clinical trials and ultimately, clinical practice.
Funding
This work was supported by the National Institutes of Health Pepper Center (P30 AG021332).
Author Contributions
B.A. contributed to conception and design, data analysis, and manuscript writing and editing. S.P.B. contributed to data collection and manuscript editing. R.D.B. and L.L. contributed to conception and design, and manuscript writing and editing.
Conflict of Interest
None reported.
Supplementary Material
Acknowledgments
The authors acknowledge the expert advice of medical physicists J. Anthony Seibert, PhD and John M. Boone, PhD in determining the most relevant CT technical factors to analyze in this systematic review. This study was presented at ICFSR meeting on March 3, 2018.
References
- 1. Boutin RD, Bamrungchart S, Bateni CP, et al. CT of patients with hip fracture: muscle size and attenuation help predict mortality. Am J Roentgenol. 2017;208:W208–W215. doi: 10.2214/AJR.16.17226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Clynes MA, Edwards MH, Buehring B, Dennison EM, Binkley N, Cooper C. Definitions of sarcopenia: associations with previous falls and fracture in a population sample. Calcif Tissue Int. 2015;97:445–452. doi: 10.1007/s00223-015-0044-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sousa AS, Guerra RS, Fonseca I, Pichel F, Amaral TF. Sarcopenia and length of hospital stay. Eur J Clin Nutr. 2016;70:595–601. doi: 10.1038/ejcn.2015.207 [DOI] [PubMed] [Google Scholar]
- 4. Tyrovolas S, Koyanagi A, Olaya B, et al. The role of muscle mass and body fat on disability among older adults: a cross-national analysis. Exp Gerontol. 2015;69:27–35. doi: 10.1016/j.exger.2015.06.002 [DOI] [PubMed] [Google Scholar]
- 5. Boutin RD, Yao L, Canter RJ, Lenchik L. Sarcopenia: current concepts and imaging implications. Am J Roentgenol. 2015;205:W255–W266. doi: 10.2214/AJR.15.14635 [DOI] [PubMed] [Google Scholar]
- 6. Schaap LA, van Schoor NM, Lips P, Visser M. Associations of sarcopenia definitions, and their components, with the incidence of recurrent falling and fractures: the longitudinal aging study Amsterdam. J Gerontol A Biol Sci Med Sci. 2018;73:1199–1204. doi: 10.1093/gerona/glx245 [DOI] [PubMed] [Google Scholar]
- 7. Tang TC, Hwang AC, Liu LK, et al. FNIH-defined Sarcopenia predicts adverse outcomes among community-dwelling older people in Taiwan: results from I-Lan Longitudinal Aging Study. J Gerontol A Biol Sci Med Sci. 2018;73:828–834. doi: 10.1093/gerona/glx148 [DOI] [PubMed] [Google Scholar]
- 8. Bianchi L, Abete P, Bellelli G, et al. ; GLISTEN Group Investigators Prevalence and clinical correlates of sarcopenia, identified according to the EWGSOP definition and diagnostic algorithm, in hospitalized older people: the GLISTEN study. J Gerontol A Biol Sci Med Sci. 2017;72:1575–1581. doi: 10.1093/gerona/glw343 [DOI] [PubMed] [Google Scholar]
- 9. Bianchi L, Ferrucci L, Cherubini A, et al. The predictive value of the EWGSOP definition of sarcopenia: results from the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2016;71:259–264. doi: 10.1093/gerona/glv129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Han P, Kang L, Guo Q, et al. Prevalence and factors associated with sarcopenia in suburb-dwelling older Chinese using the Asian working group for sarcopenia definition. J Gerontol A Biol Sci Med Sci. 2016;71:529–535. doi: 10.1093/gerona/glv108 [DOI] [PubMed] [Google Scholar]
- 11. Cooper AB, Slack R, Fogelman D, et al. Characterization of anthropometric changes that occur during neoadjuvant therapy for potentially resectable pancreatic cancer. Ann Surg Oncol. 2015;22:2416–2423. doi: 10.1245/s10434-014-4285-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Henderson RM, Lovato L, Miller ME, et al. ; LIFE Study Investigators Effect of statin use on mobility disability and its prevention in at-risk older adults: the LIFE study. J Gerontol A Biol Sci Med Sci. 2016;71:1519–1524. doi: 10.1093/gerona/glw057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. König M, Spira D, Demuth I, Steinhagen-Thiessen E, Norman K. Polypharmacy as a risk factor for clinically relevant sarcopenia: results from the berlin aging study II. J Gerontol A Biol Sci Med Sci. 2017;73:117–122. doi: 10.1093/gerona/glx074 [DOI] [PubMed] [Google Scholar]
- 14. Choe YR, Joh JY, Kim YP. Clinically relevant cut-off points for the diagnosis of sarcopenia in older Korean people. J Gerontol A Biol Sci Med Sci. 2017;72:1724–1731. doi: 10.1093/gerona/glx052 [DOI] [PubMed] [Google Scholar]
- 15. Cheung CL, Lam KS, Cheung BM.. Evaluation of cutpoints for low lean mass and slow gait speed in predicting death in the national health and nutrition examination survey 1999–2004. J Gerontol A Biol Sci Med Sci. 2016;71:90–95. doi: 10.1093/gerona/glv112 [DOI] [PubMed] [Google Scholar]
- 16. Cesari M, Rolland Y, Abellan Van Kan G, Bandinelli S, Vellas B, Ferrucci L. Sarcopenia-related parameters and incident disability in older persons: results from the “invecchiare in Chianti” study. J Gerontol A Biol Sci Med Sci. 2015;70:457–463. doi: 10.1093/gerona/glu181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69:547–558. doi: 10.1093/gerona/glu010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Englund DA, Kirn DR, Koochek A, et al. Nutritional supplementation with physical activity improves muscle composition in mobility-limited older adults, the VIVE2 study: a randomized, double-blind, placebo-controlled trial. J Gerontol A Biol Sci Med Sci. 2017;73:95–101. doi: 10.1093/gerona/glx141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bradlee ML, Mustafa J, Singer MR, Moore LL. High-protein foods and physical activity protect against age-related muscle loss and functional decline. J Gerontol A Biol Sci Med Sci. 2017;73:88–94. doi: 10.1093/gerona/glx070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reid KF, Martin KI, Doros G, et al. Comparative effects of light or heavy resistance power training for improving lower extremity power and physical performance in mobility-limited older adults. J Gerontol A Biol Sci Med Sci. 2015;70:374–380. doi: 10.1093/gerona/glu156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fragala MS, Dam TT, Barber V, et al. Strength and function response to clinical interventions of older women categorized by weakness and low lean mass using classifications from the Foundation for the National Institute of Health sarcopenia project. J Gerontol A Biol Sci Med Sci. 2015;70:202–209. doi: 10.1093/gerona/glu110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee SY, Jung SH, Lee SU, Ha YC, Lim JY. Can bisphosphonates prevent recurrent fragility fractures? A systematic review and meta-analysis of randomized controlled trials. J Am Med Dir Assoc. 2018;19:384–390.e1. doi: 10.1016/j.jamda.2018.02.005 [DOI] [PubMed] [Google Scholar]
- 23. Dimori S, Leoni G, Fior L, Gasparotto F. Clinical nutrition and physical rehabilitation in a long-term care setting: preliminary observations in sarcopenic older patients. Aging Clin Exp Res. 2018;30:951–958. doi: 10.1007/s40520-017-0859-8 [DOI] [PubMed] [Google Scholar]
- 24. Fielding RA, Travison TG, Kirn DR, et al. Effect of structured physical activity and nutritional supplementation on physical function in mobility-limited older adults: results from the VIVE2 randomized trial. J Nutr Health Aging. 2017;21:936–942. doi: 10.1007/s12603-017-0936-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yoshiko A, Kaji T, Sugiyama H, Koike T, Oshida Y, Akima H. Effect of 12-month resistance and endurance training on quality, quantity, and function of skeletal muscle in older adults requiring long-term care. Exp Gerontol. 2017;98:230–237. doi: 10.1016/j.exger.2017.08.036 [DOI] [PubMed] [Google Scholar]
- 26. Vinel C, Lukjanenko L, Batut A, et al. The exerkine apelin reverses age-associated sarcopenia. Nat Med. 2018;24:1360–1371. doi: 10.1038/s41591-018-0131-6 [DOI] [PubMed] [Google Scholar]
- 27. Rooks D, Praestgaard J, Hariry S, et al. Treatment of sarcopenia with bimagrumab: results from a phase II, randomized, controlled, proof-of-concept study. J Am Geriatr Soc. 2017;65:1988–1995. doi: 10.1111/jgs.14927 [DOI] [PubMed] [Google Scholar]
- 28. Alway SE, McCrory JL, Kearcher K, et al. Resveratrol enhances exercise-induced cellular and functional adaptations of skeletal muscle in older men and women. J Gerontol A Biol Sci Med Sci. 2017;72:1595–1606. doi: 10.1093/gerona/glx089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Long DE, Peck BD, Martz JL, et al. Metformin to Augment Strength Training Effective Response in Seniors (MASTERS): study protocol for a randomized controlled trial. Trials. 2017;18:192. doi: 10.1186/s13063-017-1932-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Daly LE, Prado CM, Ryan AM. A window beneath the skin: how computed tomography assessment of body composition can assist in the identification of hidden wasting conditions in oncology that profoundly impact outcomes. Proc Nutr Soc. 2018;77:135–151. doi: 10.1017/S0029665118000046 [DOI] [PubMed] [Google Scholar]
- 31. Kumar A, Moynagh MR, Multinu F, et al. Muscle composition measured by CT scan is a measurable predictor of overall survival in advanced ovarian cancer. Gynecol Oncol. 2016;142:311–316. doi: 10.1016/j.ygyno.2016.05.027 [DOI] [PubMed] [Google Scholar]
- 32. Lenchik L, Lenoir KM, Tan J, et al. Opportunistic measurement of skeletal muscle size and muscle attenuation on computed tomography predicts one-year mortality in Medicare patients. J Gerontol A Biol Sci Med Sci. 2018. doi: 10.1093/gerona/gly183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rier HN, Jager A, Sleijfer S, van Rosmalen J, Kock MCJM, Levin MD. Low muscle attenuation is a prognostic factor for survival in metastatic breast cancer patients treated with first line palliative chemotherapy. Breast. 2017;31:9–15. doi: 10.1016/j.breast.2016.10.014 [DOI] [PubMed] [Google Scholar]
- 34. Shachar SS, Deal AM, Weinberg M, et al. Skeletal muscle measures as predictors of toxicity, hospitalization, and survival in patients with metastatic breast cancer receiving taxane-based chemotherapy. Clin Cancer Res. 2017;23:658–665. doi: 10.1158/1078-0432.CCR-16-0940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Boutin RD, Kaptuch JM, Bateni CP, Chalfant JS, Yao L. Influence of IV contrast administration on CT measures of muscle and bone attenuation: implications for sarcopenia and osteoporosis evaluation. AJR Am J Roentgenol. 2016;207:1046–1054. doi: 10.2214/AJR.16.16387 [DOI] [PubMed] [Google Scholar]
- 36. Derstine BA, Holcombe SA, Goulson RL, et al. Quantifying sarcopenia reference values using lumbar and thoracic muscle areas in a healthy population. J Nutr Health Aging. 2017;21:180–185. doi: 10.1007/s12603-017-0983-3 [DOI] [PubMed] [Google Scholar]
- 37. Fuchs G, Chretien YR, Mario J, et al. Quantifying the effect of slice thickness, intravenous contrast and tube current on muscle segmentation: implications for body composition analysis. Eur Radiol. 2018;28:2455–2463. doi: 10.1007/s00330-017-5191-3 [DOI] [PubMed] [Google Scholar]
- 38. Morsbach F, Zhang YH, Nowik P, et al. Influence of tube potential on CT body composition analysis. Nutrition. 2018;53:9–13. doi: 10.1016/j.nut.2017.12.016 [DOI] [PubMed] [Google Scholar]
- 39. Maughan RJ, Watson JS, Weir J. The relative proportions of fat, muscle and bone in the normal human forearm as determined by computed tomography. Clin Sci. 1984;66:683–689. doi: 10.1042/cs0660683 [DOI] [PubMed] [Google Scholar]
- 40. Moisey LL, Mourtzakis M, Cotton BA, et al. ; Nutrition and Rehabilitation Investigators Consortium (NUTRIC) Skeletal muscle predicts ventilator-free days, ICU-free days, and mortality in elderly ICU patients. Crit Care. 2013;17:R206. doi: 10.1186/cc12901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Paknikar R, Friedman J, Cron D, et al. Psoas muscle size as a frailty measure for open and transcatheter aortic valve replacement. J Thorac Cardiovasc Surg. 2016;151:745–751. doi: 10.1016/j.jtcvs.2015.11.022 [DOI] [PubMed] [Google Scholar]
- 42. Cruz RJ Jr, Dew MA, Myaskovsky L, et al. Objective radiologic assessment of body composition in patients with end-stage liver disease: going beyond the BMI. Transplantation. 2013;95:617–622. doi: 10.1097/TP.0b013e31827a0f27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Locke JE, Carr JJ, Nair S, Terry JG, Reed RD, Smith GD, et al. Abdominal lean muscle is associated with lower mortality among kidney waitlist candidates. Clin Transpl. 2017;31:1–8. doi: 10.1111/ctr.12911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Leeper CM, Lin E, Hoffman M, Fombona A, Zhou T, Kutcher M, et al. Computed tomography abbreviated assessment of sarcopenia following trauma: the CAAST measurement predicts 6-month mortality in older adult trauma patients. J. Trauma Acute Care Surg. 2016;80:805–811. doi: 10.1097/TA.0000000000000989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Oakland K, Nadler R, Cresswell L, Jackson D, Coughlin PA. Systematic review and meta-analysis of the association between frailty and outcome in surgical patients. Ann R Coll Surg Engl. 2016;98:80–85. doi: 10.1308/rcsann.2016.0048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. The National Cancer Institute Epidemiology and Genomics Research Program (EGRP). Understanding the Role of Muscle and Body Composition in Studies of Cancer Risk and Prognosis in Cancer Survivors 2017. https://epi.grants.cancer.gov/events/sarcopenia. Accessed April 4, 2018.
- 47. Goodpaster BH, Carlson CL, Visser M, et al. Attenuation of skeletal muscle and strength in the elderly: the Health ABC Study. J Appl Physiol (1985). 2001;90:2157–2165. doi: 10.1152/jappl.2001.90.6.2157 [DOI] [PubMed] [Google Scholar]
- 48. Brooks N, Cloutier GJ, Cadena SM, et al. Resistance training and timed essential amino acids protect against the loss of muscle mass and strength during 28 days of bed rest and energy deficit. J Appl Physiol. 2008;105:241–248. doi: 10.1152/japplphysiol.01346.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cauza E, Strehblow C, Metz-Schimmerl S, et al. Effects of progressive strength training on muscle mass in type 2 diabetes mellitus patients determined by computed tomography. Wien Med Wochenschr. 2009;159:141–147. doi: 10.1007/s10354-009-0641-4 [DOI] [PubMed] [Google Scholar]
- 50. Breda AP, Pereira de Albuquerque AL, Jardim C, et al. Skeletal muscle abnormalities in pulmonary arterial hypertension. PLoS One. 2014;9:e114101. doi: 10.1371/journal.pone.0114101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. The National Institute on Aging, The Foundation for the National Institutes of Health (FNIH). SDOC Position Development Conference 2018. https://sdocpdc.squarespace.com. Accessed April 4, 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.