Abstract
Background
Antipsychotics are prescribed to treat various symptoms in older adults, however, their safety in this context has not been fully evaluated. The objective was to evaluate mortality risks associated with off-label use of antipsychotics among older adults with no preexisting mental illness or dementia relative to those with diagnosis of dementia.
Methods
Data (2007–2015) were derived from Department of Veterans Affairs registries for 730,226 patients (≥65 years) with no baseline serious mental illness, dementia). We estimated the cumulative incidence of antipsychotics prescription and 10-year all-cause mortality. The extended Cox models were used to estimate Hazard Ratios (HRs) associated with antipsychotics prescription, adjusted for time-varying covariates, dementia diagnosis, comorbidity index score, and age at time of first exposure to antipsychotics.
Results
The study included 98% males, 13% African Americans, and 81% Caucasian. Patients with dementia and antipsychotics had the highest risk of mortality (78.0%), followed by (73.0%) for patients with dementia alone and compared with patients without dementia or antipsychotics exposure who had the lowest mortality risk (42.0%). Exposure to typical antipsychotics was associated with (HR: 2.1, confidence interval [CI] 2.0–2.2) compared with atypical antipsychotics (HR: 1.5, CI 1.4–1.5, p = <.0001).
Conclusion
In a large cohort of older adults, antipsychotics were associated with an increased risk of all-cause mortality. While significant increase in mortality was attributable to the diagnosis of dementia, the addition of antipsychotics resulted in added mortality risk among all patients. Antipsychotic medications should be used cautiously in all older adults, not only those with dementia.
Keywords: Cognition, Drug related, Primary care
Typical and atypical antipsychotic medications are approved by the U.S. Food and Drug Administration (FDA) to treat a range of serious mental illnesses, including schizophrenia, bipolar disorder, and depression under specific circumstances (1,2). Unlike the older typical medications, several atypical antipsychotics have a broader spectrum of action and are used to treat bipolar disorder and/or depression that did not respond to antidepressant medication alone (3,4).
Recent studies have found that nearly 58% of Medicaid patients and 60% of veterans have been prescribed antipsychotic medications for an off-label use (ie, not specifically approved by the FDA) (5–7), much of this off-label uptake in atypical antipsychotics prescriptions for older adults were seen in the United States, Canada, and Europe (8–10). Antipsychotics are often prescribed for challenging behavior rather than for diagnosed mental illness (11–15). And they are associated with serious adverse effects, particularly in older adults with dementia (5,16–18). Despite the limited evidence that antipsychotics have behavioral and psychological symptoms of dementia (19), their safety has been challenged by an increase in cerebrovascular adverse side effects and mortality.
Several pooled studies and meta-analyses have demonstrated increased risk of cerebrovascular events and mortality with the use of antipsychotics among persons with dementia (11,17,20,21). This was shown in older adults residing nursing homes, which might be due to advanced dementia; hence, higher baseline mortality risk (22–28).
The American Geriatric Society’s 2015 Beers criteria for safe medication use in older adults recommend avoiding antipsychotics to treat the neuropsychiatric symptoms of dementia because of the increased mortality and stroke risk (29). While the risk associated with the use of antipsychotics in patients with dementia is well established, their safety in the absence of dementia or mental illness is not well studied. In this study, we attempted to evaluate the mortality risks associated with the use of antipsychotics among community-dwelling older adults with or without dementia. We hypothesized that dementia and/or exposure antipsychotic medications, in the absence of preexisting mental illness, is associated with increased risk of mortality
Methods
Study Design
This was a retrospective cohort study of community-dwelling older U.S. veterans. Data were identified and extracted from the Veterans Affairs (VA) Informatics and Computing Infrastructure Corporate Data Warehouse (VINCI), which houses a national repository and is composed of data from several Veterans Health Administration (VHA) sources, including clinical and health utilization data.
Study Population
Veterans ages ≥65 years between January 1, 2007 and January 1, 2015, were identified (730,226 patients). At baseline, patients are classified as having dementia if they were diagnosed as such or if they were prescribed treatment for dementia. The diagnosis is defined by the International Classification of Diseases, Ninth Revision, Clinical Modification codes [ICD9] for dementia (ICD9:290.0-290.4,294.20,331.1,331.8), schizophrenia (ICD9: 292.0–295.9), schizoaffective disorder and bipolar disorder (ICD9: 296.0, 296.1, 296.4–296.8), delusional disorder (ICD9: 297–297.9), or other nonorganic psychosis (ICD9: 298–298.9). Patients residing in a nursing home or hospice prior to or during the study were excluded. To determine whether patients received care through VHA, they were included only if they had at least two outpatient VHA encounters during the study.
Exposure
Outpatient prescription records were obtained from the VHA Pharmacy Benefits Management Strategic Healthcare Group and were reviewed from January 2007 to January 2015. These prescription files are extracted monthly from electronic prescriptions at each VA medical center and clinic. Patients who had been prescribed any antipsychotic prior to study entry were excluded. To control for length of exposure, we defined exposure as the period between date of first antipsychotics medication prescription and last date of antipsychotics prescription. The atypical antipsychotic medications included were aripiprazole, clozapine, olanzapine, quetiapine, risperidone, and ziprasidone. The typical antipsychotic medications included were chlorpromazine, fluphenazine, haloperidol, perphenazine, thioridazine, and trifluoperazine. In this cohort, 37,110 patients were identified as first taking antipsychotic medication during the study based on two or more prescriptions for antipsychotics.
Thirteen thousand three hundred and eighty-five patients developed dementia and/or were prescribed donepezil, galantamine, rivastigmine, or memantine during the study. 683,072 had no dementia and no antipsychotics exposure during the study.
Outcome Variables
The primary endpoint for this study was mortality after age 65 years. Date of death was extracted from the national VHA Vital Status File (VSF), which includes records of death for veterans who have received care from the VHA since 1992 and were enrolled in VHA or received compensation or pension benefits from the Veterans Benefit Administration since 2002. Multiple VHA and non-VHA data sources contribute to the VHA VSF, including the Beneficiary Identification Records Locator Subsystem Death File, VHA Medicare VSF, Social Security Administration Death Master File, and inpatient discharge records (30).
Baseline Covariates
Baseline demographic information such as gender, race, ethnicity, marital status, and rurality was extracted. Rurality was defined according to a custom map based on the Goldsmith Modification of the Office of Management and Budget definition urban, rural, and highly rural land areas (31).
Urban is any land area that the Census Bureau formally defines as an urbanized area (UA). All areas that are not classified as urban are classified as rural. Those areas in counties with a population density of less than seven persons per square mile. These areas are designated as highly rural.
Comorbidities
To control for the presence of comorbidities, we calculated a time-varying comorbidity index score at each age interval based on the Elixhauser comorbidity index, which includes 18 medical comorbidities (excluding dementia) (30).
Statistical Analysis
Bivariate analyses were conducted using chi-square tests, Fisher’s Exact Test, and t tests to examine the association of baseline characteristics with survival.
Kaplan–Meier method was used to estimate the probability of each end point according to the age of the patient, and differences between the antipsychotics medication and the control group were tested using two-sided log-rank tests and stratified according to dementia.
The extended Cox model was used to generate hazard ratios and confidence intervals for antipsychotic use versus no antipsychotic use (time-varying covariate), and dementia versus no dementia (time-varying covariate), age at first exposure to antipsychotics, and Elixhauser comorbidity index (time-varying covariate).
Left Truncation
For incidence of antipsychotic use, data for each patient was left truncated at age 65.
For incidence of all-cause mortality, data for patients was left censored at time of first antipsychotic prescription or at age 65 for patients not prescribed antipsychotics.
Right Censoring
For incidence of antipsychotic use, each patient was right censored at time of first exposure to an antipsychotic. Patients who were not prescribed antipsychotics were censored at time of last encounter.
For incidence of all-cause mortality, patients were right censored at time of death or administratively censored at end of follow-up period.
Results
Of the 730,226 veterans who met inclusion criteria, 98% were male, 81% were Caucasian, and 13% were African American (Table 1). During the study period, of the 716,841 veterans identified for this study, 13,385 (1.8%) were diagnosed with dementia, of which 48.8% were prescribed antipsychotic medications (10-year cumulative incidence of 51.5%, N = 6,538). Antipsychotics use among patients who were not diagnosed with dementia was approximately 4.7%.
Table 1.
Baseline Characteristics of Community-Dwelling Older Adult U.S. Veterans
| No Dementia (N = 716,841) N (%) |
Dementia (N = 13,385)N (%) | |||||
|---|---|---|---|---|---|---|
| No Meds (N = 683,072) | Meds (N = 33,769) | p Value* | No Meds (N = 6,847) | Meds (N = 6,538) | p Value* | |
| Gender | ||||||
| Male | 671,437 (98.3%) | 33,007 (97.7%) | .535 | 6,745 (98.5%) | 6,415 (98.1%) | .533 |
| Race | ||||||
| African American/Black | 87,681 (12.8%) | 5,260 (15.6%) | <.001 | 1,229 (17.9%) | 1,080 (16.5%) | .076 |
| Caucasian/White | 552,001 (80.8%) | 26,403 (78.2%) | <.001 | 5,217 (76.2%) | 5,095 (77.9%) | .043 |
| American Indian/Native Alaskan | 5,491 (0.8%) | 281 (0.8%) | .574 | 40 (0.6%) | 47 (0.7%) | .193 |
| Asian | 4,463 (0.7%) | 139 (0.4%) | <.001 | 25 (0.4%) | 24 (0.4%) | 1 |
| Ethnicity | ||||||
| Hispanic | 31,483 (4.6%) | 1,530 (4.5%) | .515 | 280 (4.1%) | 350 (5.4%) | <.001 |
| Marital Status | ||||||
| Married | 430,713 (63.1%) | 18,817 (55.7%) | <.001 | 4,049 (59.1%) | 4,052 (62%) | .003 |
| Divorced | 152,750 (22.4%) | 9,141 (27.1%) | <.001 | 1,627 (23.8%) | 1,502 (23%) | .398 |
| Separated | 15,496 (2.3%) | 1,050 (3.1%) | <.001 | 177 (2.6%) | 176 (2.7%) | .895 |
| Single | 665 (0.1%) | 27 (0.1%) | .369 | 12 (0.2%) | 10 (0.2%) | .412 |
| Widow | 32,318 (4.7%) | 1,771 (5.2%) | <.001 | 402 (5.9%) | 286 (4.4%) | .073 |
| Rurality | 35,631 (5.2%) | 1,557 (4.6%) | <.001 | 296 (4.3%) | 236 (3.6%) | .111 |
Note: *Fisher’s test, Significant difference (p < .05).
Patients with dementia generally had significantly higher prevalence of comorbid conditions as compared with the patients without dementia. Diabetes was the most prevalent condition amongst patients with dementia at 43 versus 60% in patients without dementia. Cerebrovascular disease was the second most prevalent (37 vs 14%), and chronic kidney disease at (19 vs 13%) (Table 2).
Table 2.
Distributing of Comorbidities Among Community-Dwelling Older Adult U.S. Veterans
| No Dementia (N = 716,841) N (%) |
Dementia (N = 13,385) N (%) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Controls (N = 683,072) | Meds (N = 33,769) | All (N = 716,841) | p Value* | Controls (N = 6,847) | Meds (N = 6,538) | All (N = 13,385) | p Value* | |
| Cerebrovascular Disease | 98,075 (14.4%) | 2594 (7.7%) | 100,669 (14%) | <.001 | 2,776 (40.5%) | 2140 (32.7%) | 4,916 (36.7%) | <.001 |
| Heart Failure | 45,770 (6.7%) | 1138 (3.4%) | 46,908 (6.5%) | <.001 | 850 (12.4%) | 552 (8.4%) | 1,402 (10.5%) | <.001 |
| Chronic Kidney Disease | 88,075 (12.9%) | 2256 (6.7%) | 90,331 (12.6%) | <.001 | 1,391 (20.3%) | 1087 (16.6%) | 2,478 (18.5%) | <.001 |
| Connective Tissue Disease | 378 (0.1%) | 16 (0%) | 394 (0.1%) | .634 | 5 (0.1%) | 3 (0%) | 8 (0.1%) | .671 |
| Diabetes | 418,985 (61.3%) | 7,892 (23.4%) | 426,877 (59.5%) | <.001 | 3,105 (45.3%) | 2,600 (39.8%) | 5,705 (42.6%) | <.001 |
| Cancer | 91,199 (13.4%) | 1,962 (5.8%) | 93,161 (13%) | <.001 | 700 (10.2%) | 646 (9.9%) | 1,346 (10.1%) | .483 |
| Peptic Ulcer Disease | 30,995 (4.5%) | 830 (2.5%) | 31,825 (4.4%) | <.001 | 362 (5.3%) | 300 (4.6%) | 662 (4.9%) | .178 |
| Liver Disease | 24,380 (3.6%) | 728 (3.2%) | 25,108 (3.5%) | <.001 | 408 (6.0%) | 307 (4.7%) | 715 (5.3%) | .011 |
*Fisher’s test, Significant difference (p < .05).
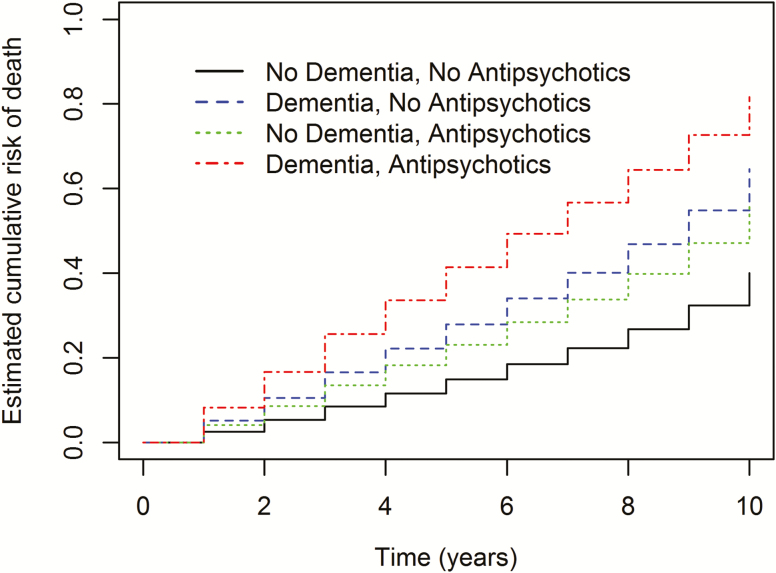
Kaplan–Meier curves demonstrated that patients without dementia or antipsychotics exposure had the lowest mortality risk (51.0%), followed by patients without dementia who were prescribed antipsychotics (55.0%), then patients with dementia and antipsychotics prescription had the highest risk of mortality (78.0%), while patients with dementia alone (73.0%) (log rank p-value = <.0001) (Figure 1;Table 3).
Figure 1.
Kaplan–Meier estimates of the cumulative risk of death by 10 years. Shown is the probability of death among patients who no exposure to antipsychotics or diagnosis of dementia (black curve), among patients who were exposed to antipsychotics with no dementia diagnosis (green curve), among patients who were not exposed to antipsychotics with dementia diagnosis (blue curve), and among patients who were exposed to antipsychotics with dementia diagnosis (red curve). Rates were compared with the use of the log-rank test.
Table 3.
Incidence of Mortality Among Community-Dwelling Older Adult U.S. Veterans by Dementia Diagnosis and Antipsychotics Use
| Age | Controls | Meds | Dementia | Dementia and Meds | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number at Risk | Number of Events | Risk | Number at Risk | Number of Events | Risk | Number at Risk | Number of Events | Risk | Number at Risk | Number of Events | Risk | |
| 66 | 683,072 | 16,286 | 0.02 | 40,307 | 325 | 0.01 | 6,847 | 140 | 0.02 | 6,538 | 34 | 0.01 |
| 67 | 532,764 | 17,280 | 0.06 | 21,266 | 422 | 0.03 | 4,912 | 205 | 0.07 | 1,704 | 53 | 0.04 |
| 68 | 456,823 | 16,553 | 0.09 | 19,968 | 640 | 0.06 | 4,582 | 286 | 0.13 | 1,625 | 84 | 0.09 |
| 69 | 369,935 | 14,789 | 0.13 | 17,734 | 728 | 0.10 | 4,055 | 353 | 0.20 | 1,477 | 110 | 0.15 |
| 70 | 280,598 | 12,616 | 0.17 | 14,897 | 747 | 0.14 | 3,384 | 342 | 0.28 | 1,257 | 102 | 0.22 |
| 71 | 194,872 | 9,921 | 0.21 | 11,614 | 687 | 0.19 | 2,518 | 286 | 0.36 | 994 | 124 | 0.32 |
| 72 | 124,492 | 7,009 | 0.26 | 8,589 | 572 | 0.25 | 1,769 | 234 | 0.45 | 694 | 104 | 0.42 |
| 73 | 71,157 | 4,784 | 0.31 | 6,057 | 508 | 0.31 | 1,065 | 173 | 0.54 | 430 | 87 | 0.54 |
| 74 | 28,276 | 2,609 | 0.37 | 3,806 | 408 | 0.38 | 450 | 108 | 0.65 | 204 | 61 | 0.68 |
| 75 | 2,917 | 229 | 0.42 | 1,629 | 257 | 0.48 | 34 | 8 | 0.73 | 19 | 6 | 0.78 |
Overall, exposure to typical antipsychotic medications was associated with an adjusted (hazard ratio [HR]: 2.12; confidence interval [CI] 2.00–2.24) compared with no exposure, and exposure to atypical antipsychotic medications was associated with an adjusted HR of 1.46 (CI 1.41–1.51) compared with no exposure (Table 4). A diagnosis of dementia was associated with an (HR: 2.26; CI 2.19–2.34) compared with no dementia (Table 4).
Table 4.
The Extended Cox Model Hazard Modelling Prescription for Incidence of Death Among Community-Dwelling Older Adult U.S. Veteran
| Unadjusted HR | 95% | 95% | p Value | Adjusted HRa | 95% | 95% | p Value | |
|---|---|---|---|---|---|---|---|---|
| LCL | UCL | LCL | UCL | |||||
| Elixhauser comorbidity index Score 3 vs 0 | 2.75 | 2.69 | 2.84 | <.001 | 2.98 | 2.92 | 3.03 | <.001 |
| Elixhauser comorbidity index Score 2 vs 0 | 1.71 | 1.7 | 1.73 | <.001 | 1.55 | 1.53 | 1.58 | <.001 |
| Dementia | 2.91 | 2.814 | 2.998 | <.001 | 2.26 | 2.19 | 2.34 | <.001 |
| Conventional Antipsychotics | 2.54 | 2.41 | 2.68 | <.001 | 2.12 | 2 | 2.24 | <.001 |
| Atypical Antipsychotics | 1.48 | 1.44 | 1.51 | <.001 | 1.46 | 1.41 | 1.51 | <.001 |
| Age (at time of antipsychotics exposure) | 1.13 | 1.12 | 1.14 | <.001 | 1.06 | 1.05 | 1.07 | <.001 |
Note: LCL = Lower confidence limit; UCL = Upper confidence limit.
aAdjusted for Elixhauser comorbidity index (time-varying), Dementia (time-varying), Conventional Antipsychotics (time-varying), Atypical Antipsychotics (time-varying), Age (at time of antipsychotics exposure).
*Significant difference (p < .05).
Discussion
This study demonstrated that in a large cohort of community-dwelling older veterans, antipsychotic use was associated with an increased risk of all-cause mortality in patients with and without dementia and without preexisting serious mental illness. A significant increase in mortality risk was attributable to dementia, and the addition of antipsychotics increased that risk. To the best of our knowledge, this is the largest sample to date of this cohort and the first study to explore the association between antipsychotics and all-cause mortality in older veterans without dementia or serious mental illness.
In the last several decades, antipsychotics have been frequently prescribed as a psychopharmacologic treatment for a variety of symptoms in older adults (32). These medications are frequently prescribed to treat delusions, hallucinations, aggression, and agitation in older adult patients with dementia. Antipsychotics have a small but statistically significant effect in treating agitation, psychosis, and behavioral symptoms associated with dementia (11,33).
Furthermore, atypical antipsychotics have been found to be effective as an adjuvant medication in major depressive disorder in patients with inadequate responses to selective serotonin reuptake inhibitors/serotonin and norepinephrine reuptake inhibitors, and quetiapine has been found to be effective as a monotherapy for major depressive disorder (34). Limited data suggest possible efficacy in other conditions, such as obsessive-compulsive disorder and posttraumatic stress disorder (35), but in general these medications tend to be used frequently for off-label conditions and nonspecific symptoms in a variety of contexts, including insomnia (6,36). Widespread use of atypical antipsychotics is of concern given the observed risk for adverse outcomes and mortality, particularly in older adults (5,28,37).
Given that research has mostly focused on the use of antipsychotic medications in the context of dementia, it has been difficult to separate the baseline mortality risk that may be attributed to dementia itself (7,8). However, one study that examined the behavioral symptoms of dementia separately found that they independently increased mortality risk (9). When samples of patients with dementia are further categorized as those receiving and not receiving antipsychotics, it is likely that underlying differences in the severity of the dementia may partly account for the mortality risk between the groups.
Vulnerable groups, including older adults, are often prescribed off-label use of antipsychotics, and this use has occurred in the absence of clear efficacy data (10). However, older adults, including those without dementia, may be at increased risk for serious adverse events when exposed to antipsychotics. Older adults are more sensitive to many of the known side effects of these medications, such as their anticholinergic effects. Older adults also are more likely to have preexisting comorbidities that interact negatively with complications associated with these medications, such as weight gain, metabolic syndrome, and hyperlipidemia. Older persons with cardiovascular disease, hypercholesterolemia, or diabetes mellitus are predisposed to worsening of these conditions as a result of antipsychotic use. Furthermore, the prescription of these medications in an older population may be a proxy for more serious underlying illness, and thus a harbinger of increased risk.
The management of late-life behavioral disorders is clinically complex and must include assessment of the risks versus benefits of the use of antipsychotic medications. In its recent practice guidelines, the American Psychiatric Association recommended prescribing antipsychtics to patients with dementia (11,38) only when behavioral problems are severe, potentially harmful to self or others, or resulting in significant distress. Clinicians should be made aware of that potential for adverse outcomes in such patients are increased with the use of psychotropic medications. Given the absence of prospective studies, guideline-based clinical judgment in prescribing must be patient-centered and focused on quality of life. It is essential to understand that dementia itself is a life-limiting illness. Medications should be used in the lowest effective dose, for the shortest clinically indicated duration. Medications are only one part of a comprehensive care plan that emphasizes quality and compassionate care. In older adults without dementia, the off-label use of antipsychotic medications should receive even more caution. Clinicians should recognize the increased risk for mortality that is associated with the use of these agents in this population.
This study was limited by several factors; the cohort was predominantly male and while representative of the VA population may not be generalizable to nonveteran groups. Additionally, the retrospective design relied on data that had already been collected, and not all pertinent risk factors may have been identified. For example, dementia was based on clinician diagnosis, which is likely to be under-recognized in the clinical setting. Consequently, some of the patients receiving antipsychotic medication may have been receiving treatment for early symptoms of undiagnosed dementia or subclinical delirium; these conditions would be independently associated with mortality regardless of antipsychotic exposure (39,40).
In conclusion, in this large cohort of community-dwelling U.S. veterans, off-label antipsychotics exposure after age 65, in the absence of baseline serious mental illness or dementia, was associated with an increased risk of all-cause mortality. Although the association between antipsychotic medication and all-cause mortality was significant in patients with dementia, the increased risk was independent of the dementia. Future prospective studies should be conducted to validate the current results and test generalizability outside the VA population.
Funding
R.E.K. received funds from the Veterans Affairs Capitol Health Care Network (VISN 5) to establish the Center for Health and Aging at the Washington DC Veterans Affairs Medical Center. Research reported in this publication was also supported by the National Center for Advancing Translational Science of the National Institutes of Health under award number UL1‐TR001409. The opinions are the authors’ and do not reflect those of the Veterans Health Administration.
Conflict of Interest
None reported.
References
- 1. Alvarez-Jiménez M, Parker AG, Hetrick SE, McGorry PD, Gleeson JF. Preventing the second episode: a systematic review and meta-analysis of psychosocial and pharmacological trials in first-episode psychosis. Schizophr Bull. 2011;37:619–630. doi: 10.1093/schbul/sbp129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Glick ID, Murray SR, Vasudevan P, Marder SR, Hu RJ. Treatment with atypical antipsychotics: new indications and new populations. J Psychiatr Res. 2001;35:187–191. doi: 10.1016/S0022-3956(01)00020-6 [DOI] [PubMed] [Google Scholar]
- 3. Fusar-Poli P, Kempton MJ, Rosenheck RA. Efficacy and safety of second-generation long-acting injections in schizophrenia: a meta-analysis of randomized-controlled trials. Int Clin Psychopharmacol. 2013;28:57–66. doi: 10.1097/YIC.0b013e32835b091f [DOI] [PubMed] [Google Scholar]
- 4. Spore D, Mor V, Larrat EP, Hiris J, Hawes C. Regulatory environment and psychotropic use in board-and-care facilities: results of a 10-state study. J Gerontol A Biol Sci Med Sci. 1996;51:M131–M141. [DOI] [PubMed] [Google Scholar]
- 5. Steinberg M, Lyketsos CG. Atypical antipsychotic use in patients with dementia: managing safety concerns. Am J Psychiatry. 2012;169:900–906. doi: 10.1176/appi.ajp.2012.12030342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Victor I. Reus, Laura J. Fochtmann, A. Evan Eyler, et al. The American Psychiatric Association Practice Guideline on the Use of Antipsychotics to Treat Agitation or Psychosis in Patients With Dementia: American Psychiatric Association, 2016. doi:10.1176/appi.books.9780890426807 [DOI] [PubMed] [Google Scholar]
- 7. Onder G, Bonassi S, Abbatecola AM, et al. ; Geriatrics Working Group of the Italian Medicines Agency High prevalence of poor quality drug prescribing in older individuals: a nationwide report from the Italian Medicines Agency (AIFA). J Gerontol A Biol Sci Med Sci. 2014;69:430–437. doi: 10.1093/gerona/glt118 [DOI] [PubMed] [Google Scholar]
- 8. Leslie DL, Mohamed S, Rosenheck RA. Off-label use of antipsychotic medications in the department of Veterans Affairs health care system. Psychiatr Serv. 2009;60:1175–1181. doi: 10.1176/ps.2009.60.9.1175 [DOI] [PubMed] [Google Scholar]
- 9. Leslie DL, Rosenheck R. Off-label use of antipsychotic medications in Medicaid. Am J Manag Care. 2012;18:e109–e117. [PubMed] [Google Scholar]
- 10. Maglione M, Maher AR, Hu J, et al. Off-Label Use of Atypical Antipsychotics: An Update. Rockville, MD, 2011. /books/n/hscompeffcollect/AHRQ Comparative Effectiveness Reviews. [PubMed] [Google Scholar]
- 11. Schneider LS, Tariot PN, Dagerman KS, et al. ; CATIE-AD Study Group Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med. 2006;355:1525–1538. doi: 10.1056/NEJMoa061240 [DOI] [PubMed] [Google Scholar]
- 12. Doan TN, Lennox NG, Taylor-Gomez M, Ware RS. Medication use among Australian adults with intellectual disability in primary healthcare settings: a cross-sectional study. J Intellect Dev Disabil. 2013;38:177–181. doi: 10.3109/13668250.2013.778968 [DOI] [PubMed] [Google Scholar]
- 13. Matson JL, Bamburg JW, Mayville EA, et al. Psychopharmacology and mental retardation: a 10 year review (1990–1999). Res Dev Disabil. 2000;21:263–296 doi: 10.1016/S0891-4222(00)00042-1 [DOI] [PubMed] [Google Scholar]
- 14. Tsiouris JA. Pharmacotherapy for aggressive behaviours in persons with intellectual disabilities: treatment or mistreatment? J Intellect Disabil Res. 2010;54:1–16. doi: 10.1111/j.1365-2788.2009.01232.x [DOI] [PubMed] [Google Scholar]
- 15. Brylewski J, Duggan L. Antipsychotic medication for challenging behaviour in people with learning disability. Cochrane Database Syst Rev. 2001; 3:CD000377 doi: 10.1002/14651858.CD000377. [DOI] [PubMed] [Google Scholar]
- 16. Kales HC, Valenstein M, Kim HM, et al. Mortality risk in patients with dementia treated with antipsychotics versus other psychiatric medications. Am J Psychiatry. 2007;164:1568–76; quiz 1623. doi: 10.1176/appi.ajp.2007.06101710 [DOI] [PubMed] [Google Scholar]
- 17. Rossom RC, Rector TS, Lederle FA, Dysken MW. Are all commonly prescribed antipsychotics associated with greater mortality in elderly male veterans with dementia? J Am Geriatr Soc. 2010;58:1027–1034. doi: 10.1111/j.1532-5415.2010.02873.x [DOI] [PubMed] [Google Scholar]
- 18. Ballard C, Howard R. Neuroleptic drugs in dementia: benefits and harm. Nat Rev Neurosci. 2006;7:492–500. doi: 10.1038/nrn1926 [DOI] [PubMed] [Google Scholar]
- 19. Liperoti R, Pedone C, Corsonello A. Antipsychotics for the treatment of behavioral and psychological symptoms of dementia (BPSD). Curr Neuropharmacol. 2008;6:117–124. doi: 10.2174/157015908784533860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maust DT, Kim HM, Seyfried LS, et al. Antipsychotics, other psychotropics, and the risk of death in patients with dementia: number needed to harm. JAMA Psychiatry. 2015;72:438–445. doi: 10.1001/jamapsychiatry.2014.3018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wooltorton E. Olanzapine (Zyprexa): increased incidence of cerebrovascular events in dementia trials. CMAJ 2004;170:1395 doi: 10.1503/cmaj.1040539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wooltorton E. Risperidone (Risperdal): increased rate of cerebrovascular events in dementia trials. CMAJ. 2002;167:1269–1270. [PMC free article] [PubMed] [Google Scholar]
- 23. Huybrechts KF, Gerhard T, Crystal S, et al. Differential risk of death in older residents in nursing homes prescribed specific antipsychotic drugs: population based cohort study. BMJ. 2012;344:e977. doi: 10.1136/bmj.e977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294:1934–1943. doi: 10.1001/jama.294.15.1934 [DOI] [PubMed] [Google Scholar]
- 25. Kales HC, Kim HM, Zivin K, et al. Risk of mortality among individual antipsychotics in patients with dementia. Am J Psychiatry. 2012;169:71–79. doi: 10.1176/appi.ajp.2011.11030347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goff DC, Cather C, Evins AE, et al. Medical morbidity and mortality in schizophrenia: guidelines for psychiatrists. J Clin Psychiatry. 2005;66:183–94; quiz 147, 273. [DOI] [PubMed] [Google Scholar]
- 27. Nerius M, Johnell K, Garcia-Ptacek S, Eriksdotter M, Haenisch B, Doblhammer G. The impact of antipsychotic drugs on long-term care, nursing home admission, and death in dementia patients. J Gerontol A Biol Sci Med Sci. 2018;73:1396–1402. doi: 10.1093/gerona/glx239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wei YJ, Simoni-Wastila L, Lucas JA, Brandt N. Fall and fracture risk in nursing home residents with moderate-to-severe behavioral symptoms of Alzheimer’s disease and related dementias initiating antidepressants or antipsychotics. J Gerontol A Biol Sci Med Sci. 2017;72:695–702. doi: 10.1093/gerona/glw095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American geriatrics society 2015 updated beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227–46. doi: 10.1111/jgs.13702 [DOI] [PubMed] [Google Scholar]
- 30. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83 [DOI] [PubMed] [Google Scholar]
- 31. Stewart MK, Beachler M, Slayton D. Improving access to capital for health care infrastructure: the experience of the Southern Rural Access Program’s revolving loan fund. J Rural Health. 2003;19 Suppl:391–396. [DOI] [PubMed] [Google Scholar]
- 32. Jeste DV, Blazer D, Casey D, et al. ACNP White Paper: update on use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology. 2008;33:957–970. doi: 10.1038/sj.npp.1301492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Devanand DP, Mintzer J, Schultz SK, et al. Relapse risk after discontinuation of risperidone in Alzheimer’s disease. N Engl J Med. 2012;367:1497–1507. doi: 10.1056/NEJMoa1114058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cuijpers P, Sijbrandij M, Koole SL, Andersson G, Beekman AT, Reynolds CF 3rd. Adding psychotherapy to antidepressant medication in depression and anxiety disorders: a meta-analysis. World Psychiatry. 2014;13:56–67. doi: 10.1002/wps.20089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ahearn EP, Krohn A, Connor KM, Davidson JR. Pharmacologic treatment of posttraumatic stress disorder: a focus on antipsychotic use. Ann Clin Psychiatry. 2003;15:193–201. [DOI] [PubMed] [Google Scholar]
- 36. Bakouni H, Berbiche D, Vasiliadis HM. Off-label use of antipsychotics and associated factors in community living older adults. Aging Ment Health. 2017:1–8 doi: 10.1080/13607863.2017.1401583 [DOI] [PubMed] [Google Scholar]
- 37. Sahlberg M, Holm E, Gislason GH, Køber L, Torp-Pedersen C, Andersson C. Association of selected antipsychotic agents with major adverse cardiovascular events and noncardiovascular mortality in elderly persons. J Am Heart Assoc. 2015;4:e001666. doi: 10.1161/JAHA.114.001666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Michel JP, Pautex S, Zekry D, Zulian G, Gold G. End-of-life care of persons with dementia. J Gerontol A Biol Sci Med Sci. 2002;57:M640–M644. [DOI] [PubMed] [Google Scholar]
- 39. Michaud L, Burnand B, Stiefel F. Taking care of the terminally ill cancer patient: delirium as a symptom of terminal disease. Ann Oncol. 2004;15(Suppl 4):iv199–iv203. doi: 10.1093/annonc/mdh927 [DOI] [PubMed] [Google Scholar]
- 40. Gnjidic D, Agogo GO, Ramsey CM, Moga DC, Allore H. The impact of dementia diagnosis on patterns of potentially inappropriate medication use among older adults. J Gerontol A Biol Sci Med Sci. 2018;73:1410–1417. doi: 10.1093/gerona/gly078 [DOI] [PMC free article] [PubMed] [Google Scholar]