Abstract
Background
Comprehensive conjoint characterization of long-term trajectories representing several biological systems is lacking.
Methods
We measured serially indicators representing 14 distinct biological systems in up to 3,453 participants attending four Framingham Study examinations: bone mineral density, body mass index (BMI), C-reactive protein, glomerular filtration rate, forced vital capacity (FVC), 1 second forced expiratory volume/FVC ratio (FEV1/FVC), gait speed, grip strength, glycosylated hemoglobin (HbA1c), heart rate, left ventricular mass, Mini-Mental State Examination (MMSE), pulse pressure, and total/high-density lipoprotein cholesterol ratio (TC/HDL).
Results
We observed that correlations among the 14 sex-specific trajectories were modest (r < .30 for 169 of 182 sex-specific correlations). During follow-up (median 8 years), 232 individuals experienced a cardiovascular disease (CVD) event and 393 participants died. In multivariable regression models, CVD incidence was positively related to trajectories of BMI, HbA1c, TC/HDL, gait time, and pulse pressure (p < .06); mortality risk was related directly to trajectories of gait time, C-reactive protein, heart rate, and pulse pressure but inversely to MMSE and FEV1/FVC (p < .006). A unit increase in the trajectory risk score was associated with a 2.80-fold risk of CVD (95% confidence interval [CI], 2.04–3.84; p < .001) and a 2.71-fold risk of death (95% CI, 2.30–3.20; p < .001). Trajectory risk scores were suggestive of a greater increase in model c-statistic compared with single occasion measures (delta-c compared with age- and sex-adjusted models: .032 vs .026 for CVD; .042 vs .030 for mortality).
Conclusions
Biological systems age differentially over the life course. Longitudinal data on a parsimonious set of biomarkers reflecting key biological systems may facilitate identification of high-risk individuals.
Keywords: Epidemiology, Aging, Trajectories, Cardiovascular disease
Biological systems within the human body can be conceptualized as being organized at the organ or tissue level (eg the circulatory and respiratory systems) or at an ultrastructural level characterized by cells, organelles, and various regulatory pathways (1). Indicators at both levels have been suggested as biomarkers of the aging process. Numerous prior studies have shown that a decline in function of individual biological systems (as reflected by select biomarkers) is associated with an increased risk of mortality and other adverse clinical outcomes (2–14). Certain studies have also demonstrated that longitudinal trajectory patterns of distinct biological systems may offer incremental prognostic value for predicting adverse outcomes over single occasion measures and over other time-averaged measures (not summarized as trajectories) (4, 12–14). Nevertheless, prior investigations evaluated trajectory profiles typically over a short time frame and usually focused on a given biological system; they do not provide a comprehensive conjoint characterization of long-term trajectories representing several biological systems.
Our objective was to gain insight into the characteristics of and outcomes associated with multisystem aging to develop a set of variables that could facilitate identification of high-risk individuals. To reach this objective, we used data from up to 3,453 participants of the Framingham Heart Study Offspring cohort to derive long-term trajectory profiles for 14 distinct biological systems. We hypothesized that trajectories of these biological systems would vary by sex and from each other, and that a set of biomarkers reflecting key biological systems may facilitate identification of individuals at high risk of developing adverse outcomes. We tested our hypothesis using longitudinal data obtained over a 25 year observation in a large community-based sample.
Methods
Study Sample
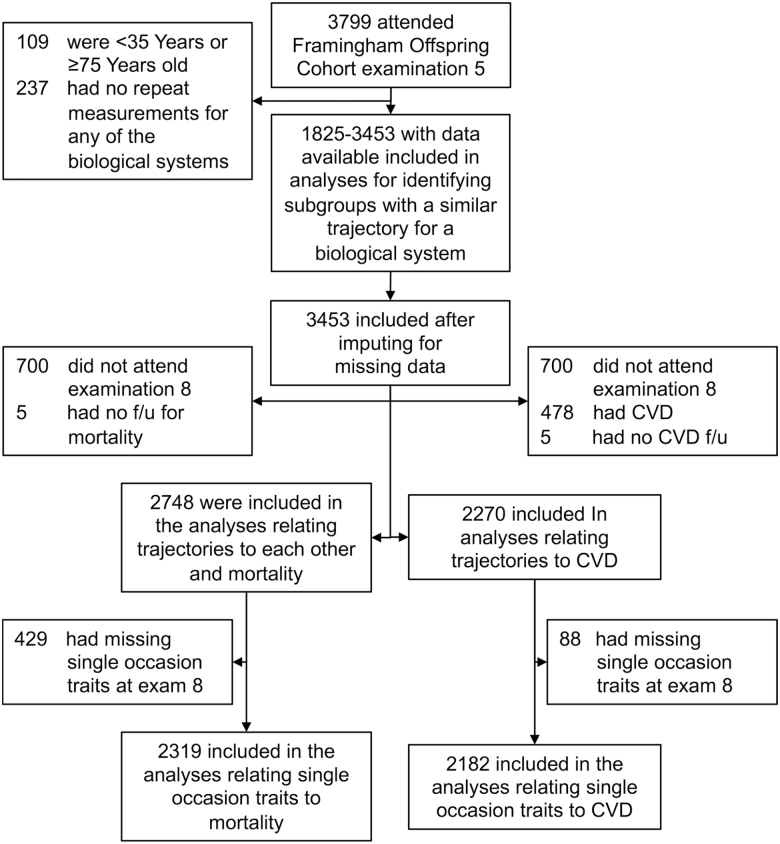
Overall, 3,799 participants attended the fifth examination cycle of the Framingham Offspring cohort, a community-based cohort study (Figure 1 and Supplementary Methods S1) (15, 16). To characterize mid- to late-life biological system trajectories, we focused our investigation on individuals aged 45–74 years who had repeated measurements for at least one of the 14 biological systems between the fifth (1991–1995) and eighth (2005–2008) examination cycles (Figure 1). After deriving sex-specific trajectory patterns for these 14 biological systems, we imputed missing data to achieve a sample of 3,453 persons with no missing data (Figure 1). Of these, 2,748 and 2,270 participants who attended the eighth examination cycle were included in the analyses for either between-trajectory correlations and risk of mortality, or for analyses that related the trajectories to cardiovascular disease (CVD) incidence, respectively (Figure 1). The relations of traits measured on a single occasion (at examination cycle eight) with incident CVD and death were assessed in 2,182 and 2,319 individuals, respectively (Figure 1).
Figure 1.
Flow chart showing sample selection. The range of sample sizes (1,825–3,453) indicates the unimputed sample sizes. CVD = Cardiovascular disease.
Boston University Medical Center’s institutional review board approved all study protocols, and all participants gave written informed consent.
Exposure Variables
We performed repeated, standardized measurements of surrogate markers representing 14 distinct biological systems at four study cycles between 1991 and 2008 (Supplementary Figure S1 and Supplementary Methods S2). For these 14 biological systems, we derived sex-specific longitudinal trajectories that were used as the exposure variables. The surrogate markers (listed alphabetically) and their respective biological systems (in parentheses) were bone mineral density (BMD; skeletal system), body mass index (BMI; adipose tissue), C-reactive protein (CRP; inflammation), estimated glomerular filtration rate (eGFR; kidney function), forced vital capacity (FVC; pulmonary vital capacity), ratio of forced expiratory volume in 1 second and FVC (FEV1/FVC; obstructive lung function), gait time/speed (neuromotor function), grip strength (muscular function), glycosylated hemoglobin (HbA1c; glucose homeostasis), heart rate (chronotropic function), left ventricular mass index (LVMI; heart mass), Mini-Mental State Examination (MMSE; cognitive function), pulse pressure (vascular pulsatile hemodynamic function), and the ratio of serum total cholesterol and serum high-density lipoprotein cholesterol (TC/HDL; lipid metabolism).
Outcomes
We used incident CVD, non-CVD mortality, and all-cause mortality after examination eight as the outcome variables (Supplementary Methods S3).
Statistical Methods
We used latent class models to identify subgroups that shared a similar underlying trajectory for each of the 14 biological systems (17, 18). These models were fitted separately in men and women using SAS Proc Traj, adjusting for age at examination five. In addition, FEV1/FVC is adjusted for height, FVC is adjusted for height, HbA1C is adjusted for diabetes medication use, heart rate is adjusted for beta blocker/aminoglycoside use, and TC/HDL ratio for lipid-lowering medication use (Supplementary Methods S4) (17, 18). We assigned scores of one (best trajectory with lowest absolute values for harmful risk factors and highest values for protective risk factors), two (intermediate trajectory), or three (worst trajectory with highest absolute values for harmful risk factors and highest values for protective risk factors) if we observed three distinct trajectories for a biological system. We assigned scores of one for best and two for worst if we observed two distinct trajectories. Thus, for BMD, eGFR, FEV1/FVC, FVC, gait time, grip strength, and MMSE, a higher score reflected a more optimal score. To address missing data for trajectories, we imputed 10 complete datasets of trajectory scores using the Markov Chain Monte Carlo method (19, 20). Our imputation model included sex, age, and CVD status at examination eight, all 14 trajectory scores, outcome indicators (death, incident CVD) plus natural log of cumulative hazard for death. After imputation, we rounded trajectory scores to nearest integers and we winsorized extreme values to produce trajectory scores of one, two, and three (Supplementary Methods S4).
We assessed the correlations between biological system trajectories using Spearman’s correlation, separately in men and women. We tested for sex differences in the correlations using Fisher’s r-to-z transformation. In these analyses, we chose a Bonferroni-corrected statistical significance threshold of .0055 (.05/91) to account for comparing 91 pairs of correlation coefficients for each sex.
We related the individual biological system trajectories to the incidence of CVD, mortality, and non-CVD mortality after examination eight using Cox regression models, adjusting for sex and age at examination eight (Supplementary Methods S4). We assessed the differences in hazard ratios between men and women using a z test. We examined the multivariable associations of the 14 biological system trajectory patterns with CVD incidence and with death using proportional hazards (Cox) regression models with forward selection. We forced age at examination 8 and sex in these models and used an entry threshold of p < .05.
To assess the risk associated with several adverse trajectories simultaneously, we defined trajectory risk scores for CVD and mortality for each participant. We calculated the sum of trajectory scores for those biological systems that entered the forward selection models, weighted according to their corresponding regression coefficients. We then used the trajectory risk score as a continuous variable in a sex- and age-adjusted Cox proportional hazards regression model. We used Cox models and unadjusted cumulative incidence functions to illustrate the relations of the trajectory risk scores and traits measured on a single occasion (at examination eight) to the risk of CVD and mortality. Supplementary Methods S4 contains details on the derivation of the trajectory risk score. We assessed model discrimination using the c-statistic. A two-sided p < .05 was considered statistically significant. We performed statistical analyses using SAS software version 9.4 (SAS Institute, Inc.).
Results
The characteristics of participants who were included to derive the biological system trajectories or in the main analyses are shown in Table 1.
Table 1.
Characteristics of The Framingham Heart Study Offspring Cohort Participants Included in Analyses for Deriving the Trajectory Patterns (maximum n = 3,453) or in the Cross-sectional and Outcome Analyses (maximum n = 2,748)
| Characteristics of the Trajectory Derivation Sample at in 1991–1995 | Characteristics of the Cross-sectional and Outcome Analysis Sample at in 2005–2008 | |||||||
|---|---|---|---|---|---|---|---|---|
| Women | Men | Women | Men | |||||
| Characteristic | n | Measure | n | Measure | n | Measure | n | Measure |
| Age, y | 1,852 | 54.6 (9.3) | 1,601 | 54.9 (9.2) | 1,516 | 67.2 (8.9) | 1,232 | 67.1 (8.6) |
| BMD, g/cm2 | 1,578* | 0.91 (0.15) | 1,243 | 1.04 (0.15) | 925 | 0.90 (0.13) | 792 | 1.0 (0.2) |
| BMI, kg/m2 | 1,837 | 26.8 (5.5) | 1,596 | 28.2 (4.1) | 1,457 | 27.7 (5.8) | 1,202 | 29.0 (4.7) |
| CRP, mg/dL, median (IQR) | 1,786 | 1.7 (0.3, 5.6) | 1,568 | 1.5 (0.4, 4.3) | 1,443 | 1.6 (0.8, 3.5) | 1,199 | 1.5 (0.8, 2.9) |
| eGFR, mL/min/1.73 m2 | 1,542 | 89.1 (19.4) | 1,353 | 88.9 (18.7) | 1,442 | 77.3 (16.4) | 1,199 | 77.7 (16.5) |
| FEV1/FVC, % | 1,593 | 0.74 (0.07) | 1,383 | 0.73 (0.07) | 1,308 | 0.73 (0.07) | 1,052 | 0.71 (0.08) |
| FVC, mL | 1,593 | 3313 (595) | 1,383 | 4611 (833) | 1,308 | 3037 (590) | 1,052 | 4341 (904) |
| Gait time, s | 1,222† | 3.4 (1.1) | 1,059 | 3.3 (0.7) | 1,452 | 3.6 (1.0) | 1,192 | 3.5 (0.9) |
| Grip strength, kg | 1,116† | 24.3 (7.6) | 966 | 42.9 (10.1) | 1,474 | 23.3 (6.0) | 1,212 | 40.5 (9.7) |
| Hba1c, % | 1,328 | 5.4 (0.9) | 1,126 | 5.5 (1.1) | 1,449 | 5.7 (0.6) | 1,203 | 5.7 (0.7) |
| Heart rate, 1/min | 1,852 | 67.3 (10.7) | 1,601 | 63.2 (11.3) | 1,516 | 63.9 (10.2) | 1,231 | 60.8 (10.9) |
| LVMI, g/m2 | 1,492 | 86.8 (18.6) | 1,132 | 103.9 (20.7) | 1,227 | 86.6 (18.4) | 900 | 103.6 (19.9) |
| MMSE, points | 1,845 | 29.1 (1.3) | 1,593 | 28.6 (1.6) | 1,505 | 28.6 (2.5) | 1,221 | 28.2 (2.1) |
| Pulse pressure, mmHg | 1,852 | 51.7 (16.1) | 1,601 | 52.6 (14.0) | 1,514 | 58.7 (17.6) | 1,231 | 57.3 (16.1) |
| TC/HDL ratio | 1,829 | 4.0 (1.4) | 1,590 | 5.0 (1.5) | 1,449 | 3.3 (0.9) | 1,202 | 3.7 (1.1) |
| BP medication, No. (%) | 1,844 | 315 (17.1) | 1,595 | 312 (19.6) | 1,515 | 706 (46.6) | 1,230 | 674 (54.8) |
| Lipid medication, No. (%) | 1,852 | 105 (5.7) | 1,600 | 143 (8.9) | 1,515 | 578 (38.2) | 1,230 | 615 (50.0) |
| Diabetes medication, No. (%) | 1,851 | 51 (2.8) | 1,601 | 62 (3.9) | 1,466 | 117 (8.0) | 1,159 | 142 (12.3) |
Note: BMD = Bone mineral density; BMI = Body mass index: CRP = C-reactive protein; eGFR = Estimated glomerular filtration rate; FEV1 = 1 Second forced expiratory volume; FVC = Forced vital capacity; HbA1c = Glycated hemoglobin; HDL = High-density lipoprotein cholesterol; LVMI = Left ventricular mass index; MMSE = Mini-Mental State Examination; TC = Total cholesterol. *Presenting examination was six instead of five. †Presenting examination was seven instead of five.
Modeling the Biological System Trajectories
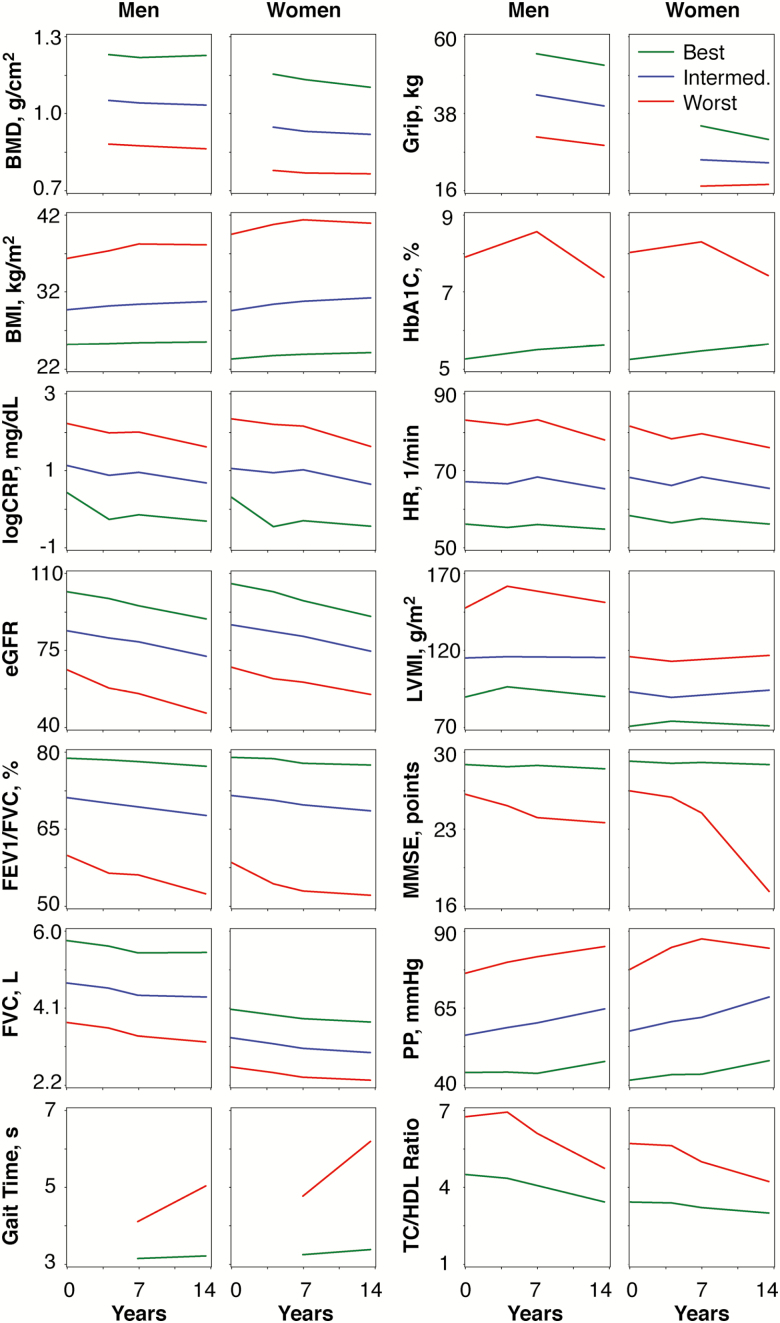
We observed two or three distinct underlying longitudinal sex-specific trajectories for the 14 biological systems (Supplementary Table S1, Figure 2). Most trajectories were parallel; however, a few trajectories showed nonlinearity, notably gait time, and MMSE (Figure 2). In general, most participants were on the best and intermediate trajectories (Supplementary Table S2).
Figure 2.
Biological system trajectories between examinations five and eight by sex. All trajectory models were adjusted for age at examination five. In addition, FEV1/FVC is adjusted for height, FVC is adjusted for height, HbA1C is adjusted for diabetes medication use, heart rate is adjusted for beta blocker/aminoglycoside use, and TC/HDL ratio for lipid-lowering medication use. Only observed, and not predicted, values are shown. BMD = Bone mineral density; BMI = Body mass index: CRP = C-reactive protein; eGFR = Estimated glomerulal filtration rate; FEV1 = 1 Second forced expiratory volume; FVC = Forced vital capacity; Grip = Grip strength; HbA1c = Glycated hemoglobin; HR = Heart rate, LVMI = Left ventricular mass index; MMSE = Mini-Mental State Examination; PP = Pulse pressure; TC = Total cholesterol; HDL = High-density lipoprotein cholesterol.
Among-Trajectory Correlations
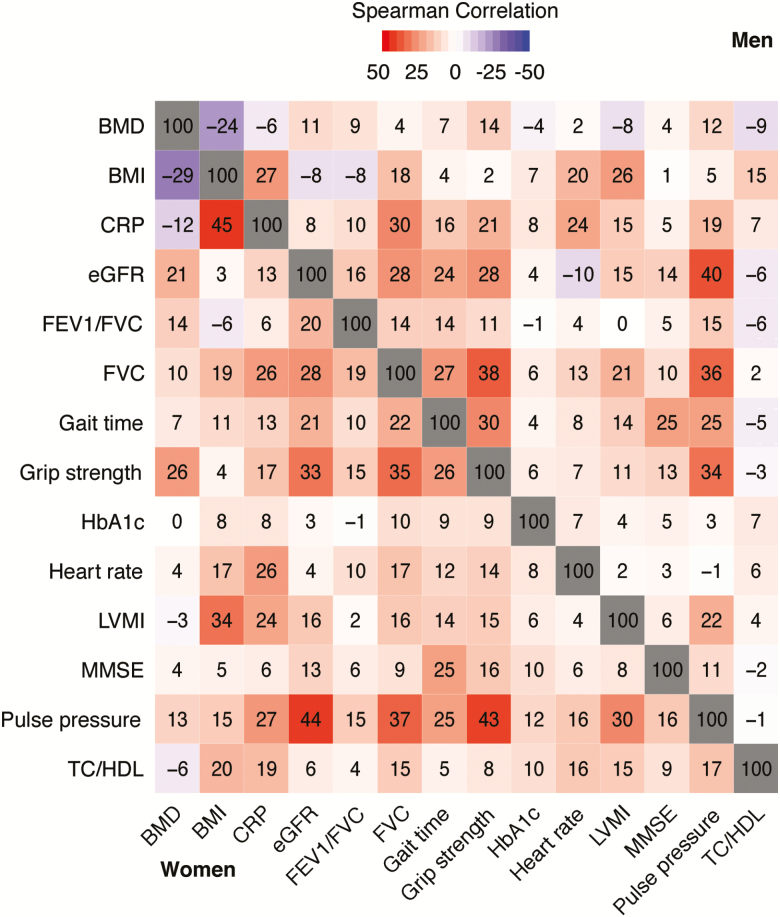
Correlations among biological system trajectory patterns, within sex, are shown in Figure 3 and corresponding p values are shown in Supplementary Table S3. Most correlations were weak in both sexes (r < .30 for 169 of 182 sex-specific Spearman correlations), except among pulse pressure, eGFR, FVC, and grip strength (ie Spearman r ≥ 0.30). In addition, BMI was moderately related to LVMI and CRP. We observed higher correlations in women than in men: for TC/HDL ratio with CRP, eGFR, FEV1/FVC, FVC, grip strength, LVMI, and pulse pressure; and for BMI-CRP, BMD-grip strength, eGFR-heart rate, and heart-rate pulse pressure associations (p < .005 for all).
Figure 3.
Correlations between biological system trajectory patterns by sex (n = 2,748). Numbers represent Spearman correlation coefficients × 100. BMD = Bone mineral density; BMI = Body mass index; CRP = C-reactive protein; eGFR = Estimated glomerular filtration rate; FEV1 = 1 Second forced expiratory volume; FVC = Forced vital capacity; HbA1c = Glycated hemoglobin; LVMI = Left ventricular mass index; MMSE = Mini-Mental State Examination; TC = total cholesterol; HDL = High-density lipoprotein cholesterol.
Relation of Biological System Trajectories With CVD Incidence
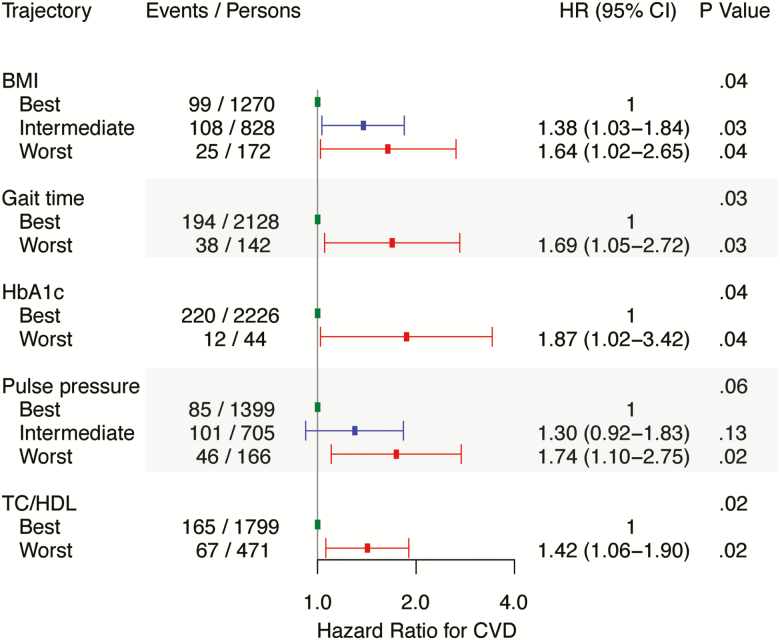
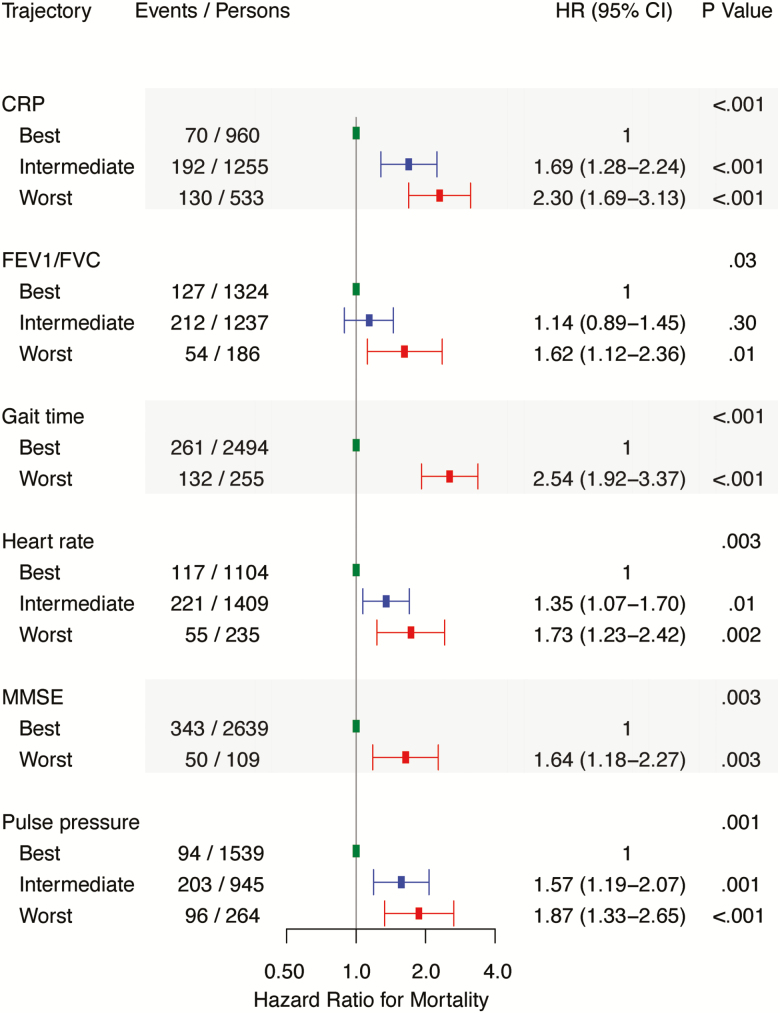
During a median follow-up of 8 years, 232 individuals experienced a CVD event. Relations of individual biological system trajectories and CVD events after examination 8 are shown in Supplementary Figure S2. BMI, CRP, gait time, HbA1c, heart rate, pulse pressure, and TC/HDL trajectories were positively associated with incident CVD. In a multivariable model with forward selection of variables, TC/HDL, BMI, gait time, HbA1c, and pulse pressure (in order of statistical significance) were associated with CVD incidence (Figure 4).
Figure 4.
Multivariable associations between biological system trajectories and cardiovascular disease events after examination eight (n = 2,270). All hazard ratios are adjusted for age at examination eight and sex. Number of events and persons may not add up as they are based on the means of 10 imputed datasets. BMD, CRP, eGFR, FEV1/FVC, FVC, grip strength, heart rate, LVMI, and MMSE did not reach the entry threshold of p < .05 and were not included in the multivariable model. The biological systems in the multivariable model are reported in the order of significance. BMI = Body mass index; CI = Confidence interval; HR = Hazard ratio; HbA1c = Glycated hemoglobin; TC = Total cholesterol; HDL = High-density lipoprotein cholesterol.
Relation of Biological System Trajectories With Mortality
During a median follow-up of 8 years, 393 participants died. Distributions of CVD and non-CVD deaths after examination 8 by biological system trajectory are shown in Supplementary Table S4. Relations of biological system trajectories with all-cause mortality are shown in Supplementary Figure S3. Generally, being on a worse trajectory was associated with increased risk of mortality. Trends in risk were similar for men and women (Supplementary Table S5). We observed sex-specific differences only for MMSE, for which the worst trajectory was more strongly associated with mortality in women compared with men (p = .02 for difference in hazard ratios). In a multivariable model with forward selection of variables, mortality risk was directly associated with gait time, CRP, heart rate, pulse pressure, MMSE, and FEV1/FVC (in order of statistical significance, Figure 5). We also performed a similar analysis for non-CVD mortality, in which we observed that non-CVD mortality was related to gait speed, CRP, MMSE, FEV1/FVC, HbA1C, and pulse pressure (in order of statistical significance) in multivariable models (Supplementary Table S6).
Figure 5.
Multivariable associations between biological system trajectories and risk of death after examination eight (n = 2,748). All hazard ratios are adjusted for age at examination eight and sex. Number of events and persons may not add up as they are based on the means of 10 imputed datasets. BMD, BMI, eGFR, FEV1/FVC, FVC, grip strength, LVMI, and MMSE did not reach the entry threshold of p < .05 and were not included in the multivariable model. The biological systems in the multivariable model are reported in the order of significance. CI = Confidence interval; CRP = C-reactive protein; FEV1 = 1 Second forced expiratory volume; FVC = Forced vital capacity; HR = Hazard ratio; MMSE = Mini-Mental State Examination.
Relation of Biological System Trajectory Risk Scores With CVD and All-Cause Mortality
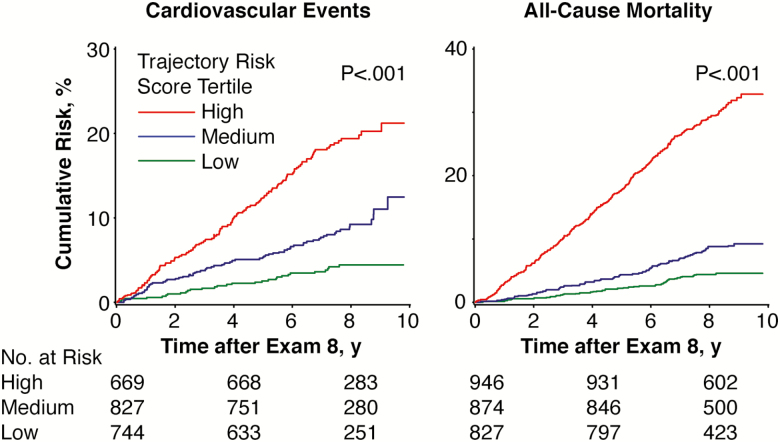
We estimated two biological system trajectory risk scores for CVD incidence and mortality based on the four and seven biological system trajectories that were significantly related to their respective outcomes in the multivariable models (Figures 4 and 5). In the trajectory risk score for CVD (mean: 0.39 ± 0.37; limits 0.00–2.54), we observed that a one-unit increase was related to a 2.80-fold risk of incident CVD (95% confidence interval [CI], 2.04–3.84; p < .001). Likewise, for the mortality risk score (mean: 1.02 ± 0.69; limits 0.00–3.92), a one-unit increase was associated with a 2.71-fold risk of death (95% CI, 2.30–3.20; p < .001). The cumulative incidence of CVD and death according to tertiles of the corresponding trajectory risk score is shown in Figure 6.
Figure 6.
Cumulative incidence of All-cause mortality and cardiovascular disease events after examination eight by tertiles of the corresponding biological system trajectory risk scores (n = 2,270 for cardiovascular events and n = 2,748 for mortality). The lines represent unadjusted cumulative incidence function curves. Log-rank p < .001 for all 10 imputed datasets.
We assessed the changes in c-statistics when trajectory risk scores, instead of the same traits measured on a single occasion (last available measurement, ie at the eighth examination cycle), were included as predictors of incident CVD and death after examination eight resulted in greater increments in the model c-statistic (relative to models incorporating age and sex alone). For incident CVD, the model with age and sex produced a c-statistic, c = .704, but when we added the trajectory risk score for CVD it gave c = .736 (change in c-statistic, .032). The c-statistic for death was .756 in a model with age and sex, which increased to .798 (change in c-statistic, .042) when we added the trajectory risk score for death. Similarly, we added single occasion traits (measured at the eighth examination cycle [last available measurement in our analyses] and corresponding to biological system trajectories that were significant in multivariable models, Figures 4 and 5) to models with age and sex, observing c-statistic increases of 0.026 and 0.030 for incident CVD and death, respectively (Supplementary Table S7).
Discussion
The World Health Organization defines health as a multidimensional concept and “not merely the absence of disease and infirmity” (21). Therefore, optimizing health requires a holistic approach characterized by the optimal conjoint functioning of all biological systems. Our principal findings on the characteristics of and outcomes associated with multisystem aging over the adult life course are four-fold. First, although the individual trajectories of distinct biological systems are mainly parallel (within system comparison), their differing slopes (across systems comparison) suggest that these biological systems may age at a different rate. Second, sex-specific differences in biological system trajectories are modest. Third, membership in a worse trajectory group in virtually all biological systems is related to increased risk of mortality. Fourth, a few select biological system trajectories can be used as indicators to capture the essence of risk related to death and CVD.
Although numerous studies have assessed the association between biomarkers of single biological systems and adverse outcomes (2–14), few have simultaneously characterized longitudinal trajectories for multiple biological systems (22, 23). However, the results of prior studies are not easily comparable to ours as these investigations often used absolute change in biomarker values, instead of trajectory profiles, as the exposure variables. Furthermore, these studies did not always relate the trajectory patterns to clinical outcomes (22, 23).
Our results demonstrate that function of different biological systems during mid- to late-life follows two or three main trajectories in men and women (Figure 1). We also observed that most individuals were on the best and intermediate trajectories for each organ system, for which there are several plausible reasons, such as measurement error, lack of drastic change over the time period measured, or a healthy state being more predominant than a diseased state for each individual organ system. Only cognitive (MMSE) and neuromotor (gait speed) function demonstrated clearly nonparallel trajectory patterns with a faster decline in function for individuals who were on the worst trajectory. Rapid decline in these traits, and particularly in gait speed, was also strongly related to increased risk of mortality, a finding also supported by some previous studies (13, 24). For most traits, however, we observed that individual trajectories of a biological system during mid- to late-life are often parallel. Biological systems have a functional reserve to serve beyond the regular needs, but after age 30 years, loss of this reserve begins (25). Our findings highlight the importance of the acme of biological system function that may be achieved around age 20 to 30 years, a decade that likely reflects a critical transition point of development and aging (26). After this point, the decline in biological system function seems to be mainly linear. Overall, the parallel pattern of trajectories underscores the importance of prevention efforts early in young adulthood to promote and maintain optimal risk factor profiles, ie before the establishment of adverse trajectory profiles.
Although the trajectories for distinct biological systems were mainly parallel (Figure 1), the correlations between the trajectory patterns of various biological systems were mostly weak (Figure 2). Only vascular (pulse pressure) and muscular (grip strength) function demonstrated moderate (r ≥ 0.30) correlations with other biological systems. Interestingly, the correlations in biological system trajectory patterns were generally similar in both magnitude and direction in men versus women, with very few sex-specific differences (Figure 2). This phenomenon may be partly explained by both the randomness in error accumulated over time and the possibility that different biological systems may not age synchronously (using select biomarkers as indicators for the systems evaluated). Thus, an individual’s select biological system may be on a poor trajectory, whereas others may simultaneously be on an optimal trajectory. The mechanisms underlying the nonsynchronous aging of biological systems remain unclear, although a recent animal study by Ori and colleagues may offer some insight (27). In this study, the investigators characterized gene expression, bulk translation, and cell biology in the brains and livers of young and old rats. Although some protein-level differences appeared to be a generic property of the rats’ chronological age, the majority were organ-specific. The authors speculated that the observed differences could be a consequence of the organ’s physiology or the chronological age of the cells within the tissue (27). Additional studies of these phenomena are clearly warranted.
We observed in univariable analyses that being on a worse trajectory for virtually any organ system was associated with increased risk of mortality. However, this finding did not apply to CVD risk as only trajectories for neuromotor function (gait speed) and six other biological systems that used established CVD risk factors as surrogates were related to CVD outcomes. In multivariable models, five and six biological system trajectories were related to CVD incidence and mortality risk, respectively. Our results, therefore, indicate that a relatively parsimonious set of biological system trajectories can be effectively used to capture the risk of adverse outcomes.
Of all the variables related to outcomes, gait speed in particular stands out as it was the strongest predictor of mortality while being simultaneously associated with CVD outcomes. Instead of being a marker of driven predominantly by central and peripheral neuromotor function, gait speed is influenced also by vision, cardio-respiratory fitness, and individual motivation (to accomplish task). Gait speed could, therefore, also be used as an outcome, instead of as a predictor, when studying the biological reasons for aging. Our finding extends from prior work demonstrating that predicted 10 year survival across the range of cross-sectionally measured gait speeds ranged from 19 to 91 per cent, and that predicted survival based on age, sex, and gait speed was as accurate as predicted survival based on age, sex, and eight other clinical variables (28). Furthermore, gait speed has been shown to be a summary index measuring impairments across multiple physiological systems (29). Gait speed and gait speed trajectories can, therefore, be considered as one of the simplest and most accessible indicators of the health, especially in older age (30). The association of gait speed trajectories with CVD risk is particularly intriguing. It is likely that gait speed is both a marker of frailty of vascular aging and also may reflect lower skeletal muscle oxygen extraction capacity. In select forms of CVD such as overt heart failure, symptomatology localized to skeletal muscles may be a marker of mortality risk (31, 32).
Our results analyzing tertiles of trajectory risk scores highlight that conjoint membership in several suboptimal organ system trajectories is related to considerably elevated risk of CVD incidence and mortality. For instance, in our study, being in the highest tertile of a biological system trajectory risk score for mortality was associated with over fivefold increase in cumulative incidence of 10 year mortality compared with the lowest tertile (Figure 5). Furthermore, including trajectory risk scores, instead of the same traits measured on a single occasion, as predictors of incident CVD and death resulted in numerically greater increments in the model c-statistic (relative to models incorporating age and sex alone). This result is in line with those from previous studies that have demonstrated that longitudinal trajectory patterns of distinct biological systems may offer incremental prognostic value for predicting adverse outcomes over single occasion measures (4, 12–14). However, other studies have also concluded that repeated measures may offer only little additional value (33). Our results suggest that long-term trajectories of select biological systems may facilitate the discrimination of individuals at varying risk.
Although our study has several strengths, several limitations of our study also should be acknowledged. First, data for BMD, grip strength, and gait speed were missing for several individuals. However, we used a multiple imputation approach to incorporate information for participants with such missing data. Second, only individuals who attended examination cycle eight were included in the analyses relating trajectories to risk of clinical outcomes, undoubtedly leading to survivorship bias. However, the trajectories themselves were derived from datasets of individuals who had at least two measurements available between examination cycles five and eight, thereby possibly minimizing the effects of attrition. Third, our sample included predominantly white adults of European ancestry, and thus the generalizability of our results to other ethnic groups remains unknown. Fourth, we did not study immune/hematopoetic system function or hepatic function, which may be predictors of CVD and mortality risk (34–37); biomarkers of these systems were not ascertained longitudinally on multiple occasions to render the construction of trajectories feasible. Fifth, trajectories that did not reach statistical significance in our multivariable models, such as kidney function (eGFR), may still be related to outcomes in case of more extreme trajectory values of a magnitude not seen in our ambulatory free-living community-based sample. Furthermore, it is also conceivable that in individuals with poor organ system function, the lack of a measure of organ system function may be actually more informative than the individual’s trajectory by itself. Sixth, we cannot exclude the possibility of residual confounding, despite including trajectories of all 14 biological systems in the multivariable forward selection models. Seventh, we used MMSE, a relatively crude marker to test cognitive function as it was the only cognitive test available for several sequential Framingham Offspring cohort examinations. Eighth, because the Framingham Offspring cohort has been broadly phenotyped over a long period of time, only partial replication of our results is currently possible in other cohorts. Ninth, age, sex, and the 14 biological system trajectories were the only covariates considered in the multivariable models because we observed either significant associations (eg education or calory intake) or differential missingness (eg physical activity) for others.
Although the correlates and predictors of incident CVD and mortality are well known, our findings shed new light on the inherent features of multisystem aging over the adult life course. In brief, our study demonstrates that the different biological systems do not age in parallel, with modest sex-related differences—a finding supported by a prior study which demonstrated that changes in circulating aging-related biomarkers were poorly correlated (38). Furthermore, longitudinal data on only a limited number of biological systems trajectories are adequate to capture the risk of adverse outcomes better than by using single occasion measurements. Our findings describe the characteristics of and outcomes associated with multisystem aging. However, with the increasing use of “big data” derived from electronic health records, our findings could also be potentially used in the future as a framework to enable automatic, longitudinal health surveillance using trajectories of measures of common biological systems that are routinely ascertained. Such a scenario could facilitate personalized care and decision-making and provide targeted interventions to individuals at the highest risk of morbid outcomes, a premise that warrants further evaluation (39, 40).
Funding
The Framingham Heart Study acknowledges the support of contracts NO1-HC-25195 and HHSN268201500001I from the National Heart, Lung and Blood Institute for this research.
Conflict of interest statement
None declared.
Supplementary Material
Acknowledgments
We also acknowledge the dedication of the FHS study participants without whom this research would not be possible.
References
- 1. Westerhoff HV, Palsson BO. The evolution of molecular biology into systems biology. Nat Biotechnol. 2004;22:1249–1252. doi: 10.1038/nbt1020 [DOI] [PubMed] [Google Scholar]
- 2. Goldman N, Glei DA. Quantifying the value of biomarkers for predicting mortality. Ann Epidemiol. 2015;25:901–6.e1. doi: 10.1016/j.annepidem.2015.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Verdecchia P, Schillaci G, Borgioni C, et al. Prognostic significance of serial changes in left ventricular mass in essential hypertension. Circulation. 1998;97:48–54.doi: 10.1161/01.CIR.97.1.48 [DOI] [PubMed] [Google Scholar]
- 4. Allen NB, Siddique J, Wilkins JT, et al. Blood pressure trajectories in early adulthood and subclinical atherosclerosis in middle age. JAMA. 2014;311:490–497. doi: 10.1001/jama.2013.285122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nguyen ND, Center JR, Eisman JA, Nguyen TV. Bone loss, weight loss, and weight fluctuation predict mortality risk in elderly men and women. J Bone Miner Res. 2007;22:1147–1154. doi: 10.1359/jbmr.070412. [DOI] [PubMed] [Google Scholar]
- 6. Coresh J, Turin TC, Matsushita K, et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA. 2014;311:2518–2531. doi: 10.1001/jama.2014.6634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mannino DM, Reichert MM, Davis KJ. Lung function decline and outcomes in an adult population. Am J Respir Crit Care Med. 2006;173:985–990. doi: 10.1164/rccm.200508-1344OC [DOI] [PubMed] [Google Scholar]
- 8. Cooper R, Kuh D, Hardy R; Mortality Review Group; FALCon and HALCyon Study Teams Objectively measured physical capability levels and mortality: systematic review and meta-analysis. BMJ. 2010;341:c4467.doi:10.1136/bmj.c4467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pekkanen J, Nissinen A, Vartiainen E, Salonen JT, Punsar S, Karvonen MJ. Changes in serum cholesterol level and mortality: a 30-year follow-up. The Finnish cohorts of the seven countries study. Am J Epidemiol. 1994;139:155–165.doi: 10.1093/oxfordjournals.aje.a116977 [DOI] [PubMed] [Google Scholar]
- 10. Nauman J, Janszky I, Vatten LJ, Wisløff U. Temporal changes in resting heart rate and deaths from ischemic heart disease. JAMA. 2011;306:2579–2587. doi: 10.1001/jama.2011.1826 [DOI] [PubMed] [Google Scholar]
- 11. Schupf N, Tang MX, Albert SM, et al. Decline in cognitive and functional skills increases mortality risk in nondemented elderly. Neurology. 2005;65:1218–1226. doi: 10.1212/01.wnl.0000180970.07386.cb [DOI] [PubMed] [Google Scholar]
- 12. Norby FL, Soliman EZ, Chen LY, et al. Trajectories of cardiovascular risk factors and incidence of atrial fibrillation over a 25-year follow-up: the ARIC study (Atherosclerosis Risk in Communities). Circulation. 2016;134:599–610. doi: 10.1161/CIRCULATIONAHA.115.020090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. White DK, Neogi T, Nevitt MC, et al. Trajectories of gait speed predict mortality in well-functioning older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2013;68:456–464. doi: 10.1093/gerona/gls197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kagura J, Adair LS, Munthali RJ, Pettifor JM, Norris SA. Association between early life growth and blood pressure trajectories in Black South African children. Hypertension. 2016;68:1123–1131. doi: 10.1161/HYPERTENSIONAHA.116.08046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Splansky GL, Corey D, Yang Q, et al. The third generation cohort of the national heart, lung, and blood institute’s Framingham heart study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–1335. doi: 10.1093/aje/kwm021 [DOI] [PubMed] [Google Scholar]
- 16. Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–290.doi: 10.1093/aje/kwx110 [DOI] [PubMed] [Google Scholar]
- 17. Jones BL, Nagin DS. Advances in group-based trajectory modeling and an SAS procedure for estimating them. Sociol Methods Res. 2007;35:542–571. doi: 10.1177/0049124106292364 [DOI] [Google Scholar]
- 18. Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol. 2010;6:109–138. doi: 10.1146/annurev.clinpsy.121208.131413 [DOI] [PubMed] [Google Scholar]
- 19. Schunk D. A Markov chain Monte Carlo algorithm for multiple imputation in large surveys. AStA Adv Stat Anal. 2008;92:101–114. doi: 10.1007/s10182-008-0053-6 [DOI] [Google Scholar]
- 20. Wolf DA, Freedman VA, Ondrich JI, Seplaki CL, Spillman BC. Disability trajectories at the end of life: a “Countdown” model. J Gerontol B Psychol Sci Soc Sci. 2015;70:745–752. doi: 10.1093/geronb/gbu182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. International Health Conference. Constitution of the World Health Organization. Bull World Health Organ. 2002;80:983–984. [PMC free article] [PubMed] [Google Scholar]
- 22. Belsky DW, Caspi A, Houts R, et al. Quantification of biological aging in young adults. Proc Natl Acad Sci USA. 2015;112:E4104–E4110. doi: 10.1073/pnas.1506264112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moayyeri A, Hart DJ, Snieder H, Hammond CJ, Spector TD, Steves CJ. Aging trajectories in different body systems share common environmental etiology: the healthy aging twin study (HATS). Twin Res Hum Genet. 2016;19:27–34. doi: 10.1017/thg.2015.100 [DOI] [PubMed] [Google Scholar]
- 24. Hui JS, Wilson RS, Bennett DA, Bienias JL, Gilley DW, Evans DA. Rate of cognitive decline and mortality in Alzheimer’s disease. Neurology. 2003;61:1356–1361.doi: 10.1212/01.WNL.0000094327.68399.59 [DOI] [PubMed] [Google Scholar]
- 25. Sehl ME, Yates FE. Kinetics of human aging: I. Rates of senescence between ages 30 and 70 years in healthy people. J Gerontol A Biol Sci Med Sci. 2001;56:B198–B208.doi: 10.1093/gerona/56.5.B198 [DOI] [PubMed] [Google Scholar]
- 26. Kuh D, Karunananthan S, Bergman H, Cooper R. A life-course approach to healthy ageing: maintaining physical capability. Proc Nutr Soc. 2014;73:237–248. doi: 10.1017/S0029665113003923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ori A, Toyama BH, Harris MS, et al. Integrated transcriptome and proteome analyses reveal organ-specific proteome deterioration in old rats. Cell Syst. 2015;1:224–237. doi: 10.1016/j.cels.2015.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rosso AL, Sanders JL, Arnold AM, et al. Multisystem physiologic impairments and changes in gait speed of older adults. J Gerontol A Biol Sci Med Sci. 2015;70:319–324. doi: 10.1093/gerona/glu176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Peel NM, Kuys SS, Klein K. Gait speed as a measure in geriatric assessment in clinical settings: a systematic review. J Gerontol A Biol Sci Med Sci. 2013;68:39–46. doi: 10.1093/gerona/gls174 [DOI] [PubMed] [Google Scholar]
- 31. Narumi T, Watanabe T, Kadowaki S, et al. Sarcopenia evaluated by fat-free mass index is an important prognostic factor in patients with chronic heart failure. Eur J Intern Med. 2015;26:118–122. doi: 10.1016/j.ejim.2015.01.008 [DOI] [PubMed] [Google Scholar]
- 32. von Haehling S, Ebner N, Dos Santos MR, Springer J, Anker SD. Muscle wasting and cachexia in heart failure: mechanisms and therapies. Nat Rev Cardiol. 2017;14:323–341. doi: 10.1038/nrcardio.2017.51 [DOI] [PubMed] [Google Scholar]
- 33. Hillier TA, Stone KL, Bauer DC, et al. Evaluating the value of repeat bone mineral density measurement and prediction of fractures in older women: the study of osteoporotic fractures. Arch Intern Med. 2007;167:155–160. doi: 10.1001/archinte.167.2.155 [DOI] [PubMed] [Google Scholar]
- 34. Kovanen PT, Mänttäri M, Palosuo T, Manninen V, Aho K. Prediction of myocardial infarction in dyslipidemic men by elevated levels of immunoglobulin classes A, E, and G, but not M. Arch Intern Med. 1998;158:1434–1439.doi: 10.1001/archinte.158.13.1434 [DOI] [PubMed] [Google Scholar]
- 35. Tsimikas S, Willeit P, Willeit J, et al. Oxidation-specific biomarkers, prospective 15-year cardiovascular and stroke outcomes, and net reclassification of cardiovascular events. J Am Coll Cardiol. 2012;60:2218–2229. doi: 10.1016/j.jacc.2012.08.979 [DOI] [PubMed] [Google Scholar]
- 36. Danesh J, Collins R, Peto R, Lowe GD. Haematocrit, viscosity, erythrocyte sedimentation rate: meta-analyses of prospective studies of coronary heart disease. Eur Heart J. 2000;21:515–520. doi: 10.1053/euhj.1999.1699 [DOI] [PubMed] [Google Scholar]
- 37. Jaiswal S, Fontanillas P, Flannick J, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371:2488–2498. doi: 10.1056/NEJMoa1408617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sanders JL, Ding V, Arnold AM, et al. Do changes in circulating biomarkers track with each other and with functional changes in older adults?J Gerontol A Biol Sci Med Sci. 2014;69:174–181. doi: 10.1093/gerona/glt088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ohno-Machado L. Mining electronic health record data: finding the gold nuggets. J Am Med Inform Assoc. 2015;22:937. doi: 10.1093/jamia/ocv119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rumsfeld JS, Joynt KE, Maddox TM. Big data analytics to improve cardiovascular care: promise and challenges. Nat Rev Cardiol. 2016;13:350–359. doi: 10.1038/nrcardio.2016.42 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.