Understanding the composition and assembly mechanisms of root-associated microbial communities of plants is crucial for understanding the interactions between plants and soil. Most previous studies of the plant root-associated microbiome focused on model and economic plants, with fewer temporal or seasonal investigations. The assembly mechanisms of root-associated bacterial communities in different seasons remain poorly known, especially for the aquatic macrophytes. In this study, we compared the diversity, composition, and relative importance of two different assembly processes (stochastic and deterministic processes) of bacterial communities associated with bulk sediment and the rhizosphere and endosphere of Phragmites australis in summer and winter. While we found apparent differences in composition, diversity, and assembly processes of bacterial communities among different compartments, season played important roles in determining BCCs and their diversity patterns and assemblages. We also found that endosphere bacteria mainly originated from the rhizosphere. The results add new knowledge regarding the plant-microbe interactions in aquatic ecosystems.
KEYWORDS: assembly processes, bacterial community, common reed, endosphere, rhizosphere
ABSTRACT
The common reed (Phragmites australis), a cosmopolitan aquatic macrophyte, plays an important role in the structure and function of aquatic ecosystems. We compared bacterial community compositions (BCCs) and their assembly processes in the root-associated compartments (i.e., rhizosphere and endosphere) of reed and bulk sediment between summer and winter. The BCCs were analyzed using high-throughput sequencing of the bacterial 16S rRNA gene; meanwhile, null-model analysis was employed to characterize their assembly mechanisms. The sources of the endosphere BCCs were quantitatively examined using SourceTracker from bulk sediment, rhizosphere, and seed. We observed the highest α-diversity and the lowest β-diversity of BCCs in the rhizosphere in both seasons. We also found a significant increase in α- and β-diversity in summer compared to that in winter among the three compartments. It was demonstrated that rhizosphere sediments were the main source (∼70%) of root endosphere bacteria during both seasons. Null-model tests indicated that stochastic processes primarily affected endosphere BCCs, whereas both deterministic and stochastic processes dictated bacterial assemblages of the rhizosphere, with the relative importance of stochastic versus deterministic processes depending on the season. This study suggests that multiple mechanisms of bacterial selection and community assembly exist both inside and outside P. australis roots in different seasons.
IMPORTANCE Understanding the composition and assembly mechanisms of root-associated microbial communities of plants is crucial for understanding the interactions between plants and soil. Most previous studies of the plant root-associated microbiome focused on model and economic plants, with fewer temporal or seasonal investigations. The assembly mechanisms of root-associated bacterial communities in different seasons remain poorly known, especially for the aquatic macrophytes. In this study, we compared the diversity, composition, and relative importance of two different assembly processes (stochastic and deterministic processes) of bacterial communities associated with bulk sediment and the rhizosphere and endosphere of Phragmites australis in summer and winter. While we found apparent differences in composition, diversity, and assembly processes of bacterial communities among different compartments, season played important roles in determining BCCs and their diversity patterns and assemblages. We also found that endosphere bacteria mainly originated from the rhizosphere. The results add new knowledge regarding the plant-microbe interactions in aquatic ecosystems.
INTRODUCTION
The rhizosphere—the interface between plants and soil—represents one of the richest microbial ecosystems on Earth. The physicochemical properties and bacterial community compositions (BCCs) of the rhizosphere are strongly influenced by root growth, respiration, and nutrient exchange (1, 2). Bacteria within the endosphere—the microbial habitat inside a plant root (2)—help plants resist phytopathogens (3) and improve plant growth through promoting the production of phytohormones (2). Therefore, the rhizosphere and endosphere are very important microbial habitats for plant-microbe as well as plant-soil interactions.
Unlike the BCCs in bulk soil, root-associated BCCs are influenced by plants’ activities, and they may vary between a plant’s different developmental stages (4–7). Current evidence illustrates that the initial BCCs of the rhizosphere are similar to those of the surrounding soil, and the BCCs become more plant specific as plants grow and develop (4–8). For instance, Dombrowski et al. (4) observed marked differences in BCCs from the Arabidopsis rhizosphere throughout a 28-week greenhouse experiment as the plant passed through three growth periods. In a field study, Zhang et al. (7) observed that the rhizosphere BCCs of rice varied considerably during the initial period of plant growth. Nonetheless, most previous studies focused mainly on annual plants under controlled conditions or during a short interval; thus, these plants and their associated bacterial communities experienced had minimal community variation given the negligible environmental change during the experiment. Perennial plants growing over multiple seasons undergo various growth periods usually associated with marked changes in environmental factors, such as temperature and nutrient availability. In addition, perennial plants usually go into dormancy or a quiescent state during the winter, accompanied by aboveground leaf fading or browning. As a result, the photosynthetic capacity of the aboveground part is also reduced, which may affect the secretory activity of the underground part (i.e., the root). The variability of these environmental factors may lead to strong variations of microbial communities associated with roots of plants between different seasons.
Understanding the mechanisms of microbial community assembly is a central topic in microbial community ecology (9–12). Despite the increased recognition of the taxonomic and phylogenetic diversity of plant microbiomes, the mechanisms underlying assembly of microbial communities remain poorly understood (13). It is generally accepted that both neutral (stochastic) and deterministic processes determine the assembly of microbial communities (9–11, 14). Based on the null-model theory, several studies have reported the recruitment mechanisms of the root-associated microbiome of plants (15–18). For example, Fan et al. (15) found that the importance of deterministic processes in determining diazotrophic communities decreased with distance away from wheat roots; therefore, the rhizosphere can selectively filter the specific functional microbial groups. In contrast, stochastic processes drove the random phylogenetic turnover of root-associated microbial communities of speargrasses subjected to the extreme conditions in deserts (16). These studies suggested that the relative importance of ecological processes (i.e., stochastic and deterministic processes) that drive the assembly of microbial communities may vary depending on the edaphic environment and plant species.
Most previous studies about plant root-associated microbiomes focused on terrestrial plants without regard to temporal and developmental parameters. Aquatic macrophytes, as major components of aquatic ecosystems, serve important functions in purifying water and protecting shores (19, 20). However, few studies have investigated the BCCs in their rhizospheres and endospheres (21–23). Phragmites australis (common reed) is a cosmopolitan aquatic macrophyte and plays an important role in the structure and function of aquatic and wetland ecosystems. We investigated the diversity, composition, and assembly processes of bacterial communities associated with different compartments (i.e., bulk sediment, rhizosphere, and endosphere) of reed in summer and winter. Furthermore, we quantitatively examined the sources (bulk sediment, rhizosphere, and seed) of the endosphere BCCs using SourceTracker. We aimed to determine the (i) variations of BCCs between bulk sediment, the rhizosphere, and the endosphere of reed in summer and winter, (ii) mechanisms underlining the assembly of BCCs among the three compartments during different seasons, and (iii) sources of endosphere BCCs in both seasons.
RESULTS
Physicochemical properties of the bulk sediments.
The measured physicochemical properties of the collected bulk sediments are presented in Table S1 in the supplemental material. Higher temperature was observed in summer (32°C) than in winter (8°C). Pairwise t tests indicated that the winter possessed higher contents of total nitrogen (TN), total phosphorus (TP), and loss on ignition (LOI) than those in summer (P < 0.05) (Table S1).
Richness and diversity of the bacterial community between compartments and seasons.
We obtained a total of 1,537,082 high-quality sequences from 60 samples representing three different compartments (bulk sediment, rhizosphere, and endosphere) across two seasons (summer and winter) after trimming, denoising, and removing chimeras, nonbacterial sequences, and rare sequences. The minimum sequence number (12,753 sequences) was found in the endosphere compartment in summer; therefore, to compare the diversity of each group, the sequences obtained from each sample were subsampled randomly into 12,753 sequences. These high-quality sequences were then clustered into 9,165 operational taxonomic units (OTUs) at a 97% cutoff for all rarefied samples. Rarefaction curves indicated a thorough sampling across the entire bacterial communities (Fig. S1).
The bacterial diversity of bacterial communities was estimated by OTU richness, Faith’s phylogenetic diversities (PD) (24), and the Shannon index, respectively (Fig. 1). Two nonparametric tests (Kruskal-Wallis H test and Mann-Whitney U test) were applied to compare differences in diversities of bacterial communities among the six sample groups. We observed no significant differences in bacterial diversities between the bulk sediment and rhizosphere compartments irrespective of the seasons; however, the bacterial diversity (OTU richness and Faith’s PD) of rhizosphere was slightly higher than that of the bulk sediment in summer (Fig. 1; Fig. S1). Moreover, the bacterial diversities in the endosphere compartment were significantly lower than those in the bulk sediment and rhizosphere compartments, regardless of season (Kruskal-Wallis H test, P < 0.05) (Fig. 1; Fig. S1). The bacterial diversities of the bulk sediment and the rhizosphere in summer were significantly higher than those in winter (Mann-Whitney U test, P < 0.05) (Fig. 1; Fig. S1). In summary, the diversity of bacterial communities outside the root tissue of P. australis was much higher than that inside the tissue, and, except for the endosphere, bacterial assemblages were more diverse in summer than in winter (Fig. 1; Fig. S1).
FIG 1.
Richness and diversity of the bacterial community of the various compartments and seasons. (A) OTU richness; (B) Faith’s PD; (C) Shannon index. Boxes represent the upper and lower quartiles, horizontal lines indicate the median, whiskers show 95% range, and points are outliers. Uppercase and lowercase letters above the bars indicate significant differences (P < 0.05) between the different compartments in winter and summer, respectively (Kruskal-Wallis H test; multiple comparisons were corrected by Bonferroni correction; n = 10 for each group). Asterisks indicate significant differences between two seasons in the same compartment (Mann-Whitney U test; n = 10 for each group). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Variations of BCCs between compartments and seasons.
We used nonmetric multidimensional scaling (NMDS) based on Bray-Curtis and unweighted UniFrac distance (phylogeny-based) matrices to examine the differences between the BCCs of the six sample groups. We found significant differences in BCCs between the three compartments and two seasons (Fig. 2A and B; Fig. S2). Results of both the analysis of similarity (ANOSIM) (Bray-Curtis dissimilarity, R = 0.783 to 1 and P < 0.001; unweighted UniFrac dissimilarity, R = 0.623 to 1 and P < 0.001) and the permutational multivariate analysis of variance (PERMANOVA) (Fig. S2) confirmed that the clusters were statistically significant among the different groups. Within the same season, the bacterial communities in the endosphere possessed the highest beta dissimilarity, or the highest variation in community composition, across the three compartments (Kruskal-Wallis H test, P < 0.05). The rhizosphere BCCs exhibited the lowest beta dissimilarity in both seasons (Fig. 2C and D). As well, the BCCs of three compartments in summer exhibited higher beta diversities than those in winter (Mann-Whitney U test, P < 0.05) (Fig. 2C and D).
FIG 2.
Comparisons of β-diversity of bacterial community among the various compartments during different seasons. Shown are nonmetric multidimensional scaling (NMDS) plots of the bacterial community composition of the different sample groups based on Bray-Curtis distance (taxonomy dissimilarity) (A) and unweighted UniFrac distance (phylogeny dissimilarity) (B). Distances in each group are compared using boxplots (C and D). Boxes represent the upper and lower quartiles, horizontal lines indicate the median, whiskers show 95% range, and points are outliers. Different letters above the bars indicate significant differences (P < 0.05) among the various sample groups (Kruskal-Wallis H test; multiple comparisons were corrected by Bonferroni correction; n = 10 for each group). Asterisks indicate significant differences between the two seasons for the same compartment (Mann-Whitney U test; n = 10 for each group). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
PERMANOVA (Table 1) showed that season (including its associated environmental factors) explained the greatest amount of BCC variation, accounting for more than 30% (Bray-Curtis dissimilarity, R2 = 0.309 and P < 0.001; unweighted UniFrac dissimilarity, R2 = 0.336 and P < 0.001). This was followed by compartment (Bray-Curtis dissimilarity, R2 = 0.222 and P < 0.001; unweighted UniFrac dissimilarity, R2 = 0.124 and P < 0.001) and interaction between the two factors (Bray-Curtis dissimilarity, R2 = 0.120 and P < 0.001; unweighted UniFrac dissimilarity, R2 = 0.073 and P < 0.001) (Table 1).
TABLE 1.
PERMANOVA results based on Bray-Curtis and unweighted UniFrac distance to partition sources of variation (compartment, season, interaction between compartment and season) for bacterial communitiesb
| Distance | Source of variation | SS | MS | F. model | R2 | Pa |
|---|---|---|---|---|---|---|
| Bray-Curtis | Compartment | 3.628 | 1.814 | 17.210 | 0.222 | 0.001 |
| Season | 5.042 | 5.042 | 47.838 | 0.309 | 0.001 | |
| Interaction | 1.958 | 0.979 | 9.289 | 0.120 | 0.001 | |
| Residuals | 5.692 | 0.105 | 0.349 | |||
| Total | 16.320 | 1 | ||||
| Unweighted UniFrac | Compartment | 1.297 | 0.648 | 7.182 | 0.124 | 0.001 |
| Season | 3.513 | 3.513 | 38.917 | 0.336 | 0.001 | |
| Interaction | 0.760 | 0.380 | 4.206 | 0.073 | 0.001 | |
| Residuals | 4.874 | 0.090 | 0.467 | |||
| Total | 10.443 | 1 |
Statistical significance (P) was calculated based on sequential sums of squares from 999 permutations.
SS, sum of squares; MS, mean squares; F. model, F statistic.
Taxonomic differences across compartments and seasons.
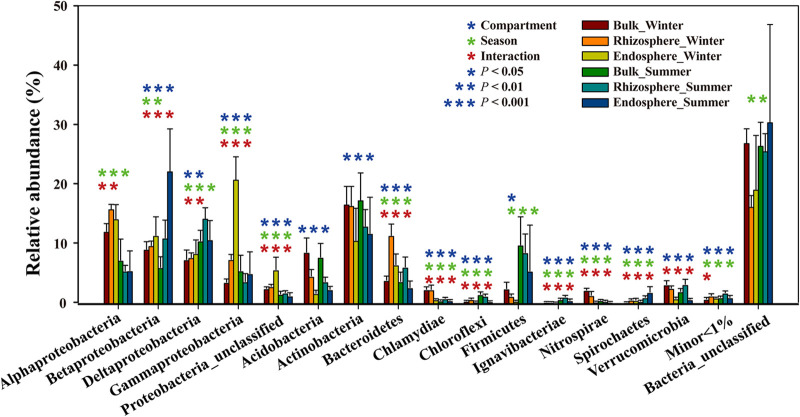
We identified 16 bacterial phyla (and classes for Proteobacteria) (Fig. 3; Fig. S3; Table S2). All phyla having a relative abundance of <1% were defined as minor. The relative abundances of the dominant bacterial phyla varied between compartments and seasons (Fig. 3). Unclassified bacteria—defined as those sequences having a <80% confidence value as assigned to any phylum in SILVA with RDP—accounted for a large proportion of each sample group (16.17% ± 1.72% to 30.38% ± 15.62%). With the exception of the unclassified bacterial group, Actinobacteria (12.85% ± 2.64% to 17.27% ± 4.34%) dominated the bacterial communities of both the bulk sediment and rhizosphere, regardless of the season. Alphaproteobacteria were more abundant in winter (two-way ANOVA, F = 39.399 and P < 0.001), whereas Deltaproteobacteria (two-way ANOVA, F = 16.440 and P < 0.001) and Firmicutes (two-way ANOVA, F = 9.974 and P < 0.001) exhibited a significantly higher abundance in summer than in winter, regardless of the compartment (Fig. 3). The bulk sediment samples were more enriched with Acidobacteria than the other two compartments (two-way ANOVA, F = 36.638 and P < 0.001) (Fig. 3). Within the same season, Bacteroidetes had the greatest relative abundance in the rhizosphere compartment as opposed to the bulk sediment and endosphere compartments (two-way ANOVA, F = 44.027 and P < 0.001) (Fig. 3). Gammaproteobacteria had the greatest relative abundance in the endosphere in winter (two-way ANOVA, F = 71.248 and P < 0.001) (Fig. 3).
FIG 3.
Comparisons of dominant bacterial communities among the sample groups at the level of phyla or subphyla. All phyla having a relative abundance of >1% are shown, and phyla having a relative abundance of < 1% are defined as minor. Each error bar represents the standard deviation of each sample group. Asterisks indicate significant differences between the sample groups (two-way ANOVA; *, P < 0.05; **, P < 0.01; ***, P < 0.001) based on the different factors (blue represents the effects of compartment, green represents the effects of season, and red represents the effects of interaction).
LEfSe (linear discriminant analysis effect size) analysis indicated strong variation in BCCs at the genus level. We listed the top 20 genera with the highest relative abundances on a heat map (Fig. S4). The relative abundances of two genera, Bacillus and Paenibacillus (both affiliated with Firmicutes), significantly decreased from bulk to rhizosphere and to endosphere (Fig. S4, Wilcoxon rank sum test, P < 0.05, and linear discriminant analysis [LDA] scores > 2). Only a few genera, including Dechloromonas, Denitratisoma, Methylibium, and Leptothrix (all affiliated with Betaproteobacteria), were found to be significantly abundant in both the rhizosphere and endosphere compartments (Fig. S4, Wilcoxon rank sum test, P < 0.05, and LDA scores > 2). In contrast, relatively more genera (Agromyces, Arthrobacter, Mycobacterium, Streptomyces, Devosia, Kaistobacter, Phenylobacterium, and Steroidobacter) were abundant exclusively in the rhizosphere or endosphere compartment in different seasons (Fig. S4, Wilcoxon rank sum test, P < 0.05, and LDA scores > 2). A Venn diagram illustrates the distribution of different OTUs for the three compartments in both seasons (Fig. S5). The number of shared OTUs between two compartments decreased from the bulk sediment to the rhizosphere compartment and then to the endosphere compartment in both seasons, as did the number of unique OTUs within each compartment (Fig. S5).
Assembly processes of the bacterial communities between different seasons.
We calculated the beta nearest taxon index (βNTI) values to compare the relative importance of two kinds of assembly processes (deterministic and stochastic) of the BCCs in the bulk sediment, rhizosphere, and endosphere compartments of each season (Fig. 4). Both deterministic and stochastic processes drove the assembly of BCCs (Fig. 4). In winter, deterministic processes were the main force that determined the bacterial community assembly of the bulk and rhizosphere compartments (62.2% for the bulk sediment and 84.4% for the rhizosphere) (Fig. 4). Stochastic processes, however, played more important roles in driving the assembly of bacterial communities in both the bulk sediment (66.7%) and the rhizosphere compartment (73.3%) in summer (Fig. 4). Surprisingly, the majority of the βNTI values for the endosphere BCCs during both seasons fell between −2 and 2 (62.2% in winter and 86.7% in summer) (Fig. 4), suggesting that stochastic processes dominated the assembly of the endosphere BCCs in both seasons.
FIG 4.

Distribution of beta nearest taxon index (βNTI) based on the different compartments and seasons. Positive (or negative) βNTI values indicate greater (or less) than expected turnover in phylogenetic composition. The horizontal dotted blue line (above +2 or below −2 are statistically significant) shows the 95% confidence intervals around the expectation under neutral community assembly.
Sources of the bacterial community in the endosphere.
We quantitatively determined the sources of the bacterial community found within the endosphere compartment in both seasons. Rhizosphere sediments were more likely to be the primary source of the bacterial community for the root invasion, contributing, on average, between 71.06% and 67.65% of the BCCs in winter and summer (Fig. 5). In addition, a small proportion of the endosphere bacteria originated from seed endophytic bacteria (14.66% in winter and 7.86% in summer) (Fig. 5). Only 2% to 4% of the endosphere bacteria originated from bulk sediment (3.35% in the winter and 2.03% in summer) (Fig. 5). However, these three sources did not differ significantly between the two seasons (Mann-Whitney U test, P > 0.05) (Fig. 5).
FIG 5.

Sources of bacterial community in the root endosphere compartment in summer and winter as predicted by SourceTracker. Each error bar represents the standard deviation of each source. Uppercase and lowercase letters above the bars indicate significant differences (P < 0.05) among different compartments in winter and summer, respectively (Kruskal-Wallis H test; multiple comparisons were corrected by Bonferroni correction; n = 10 for each group).
DISCUSSION
Phragmites australis (common reed) is a cosmopolitan perennial emergent plant in aquatic ecosystems (20, 25) and has been widely used in constructed wetlands for pollution control (19, 20, 25–27). It was found that the rate of removal of nitrogen, potassium, and chemical oxygen demand (COD) by reed differed significantly between different seasons (28, 29). These differences may be partly attributable to the different BCCs associated with its root and plant-bacterial interactions. In this study, while we observed apparent differences in composition, diversity, and assembly processes of bacterial communities among the bulk sediment, rhizosphere, and endosphere compartments, season accompanied by changes in environmental factors explained the largest amount of variation (accounting for more than 30%) of the BCCs.
Higher α- and lower β-diversity of BCCs in the rhizosphere microhabitat.
Although the rhizosphere occupies the same medium as the bulk sediment, bacterial diversity in the rhizosphere was slightly higher than that in the bulk sediment in summer (Fig. 1; Fig. S1). In a hypersaline pond, Borruso et al. (30) also found that bacterial diversity in the rhizosphere of P. australis was higher than that in bulk sediment. Similarly, several macrophyte species, including Acorus calamus (31), Spartina alterniflora (32), and Avicennia marina (33), also exhibited a greater bacterial diversity in the rhizosphere than in bulk sediment. Therefore, the results showing higher diversity in the reed rhizosphere than in the bulk sediment are consistent with previous studies of different aquatic macrophytes (30–33). Previous studies have focused on several terrestrial plants that illustrate how root exudates of maize (34) and barley (35) enrich and filter for microbes maintaining specific functions (36); therefore, they observed a reduced diversity of the bacterial community in the rhizosphere compartment (36). Unlike the terrestrial soil, lake sediments are usually anaerobic a few millimeters below the surface. An emergent plant not only provides nutrients through root exudates (37) but also releases oxygen into the surrounding sediments to enhance microbial colonization (37, 38). Armstrong (39) found that emergent plant roots release oxygen through the aerenchyma to the rhizosphere, a process known as root radial oxygen loss (ROL). Other research suggested that wetland plants transfer a portion of the oxygen—produced through photosynthesis within the aerenchyma to the roots—to the wetland substrate (40, 41). Many previous studies have found direct and indirect evidence of aeration in the rhizosphere of Phragmites australis, including increased oxygen partial pressure and oxidation reduction potential (ORP) (42–45). Therefore, we speculated that increased oxygen content might be a major reason for the increased bacterial diversity in the rhizosphere in summer. In addition, the lower bacterial β-diversity (i.e., variation in community composition) in the rhizosphere (Fig. 2C and D) may suggest filtration effects in the root-sediment interface, which have been observed for many terrestrial plants (8, 46).
Enrichment of specific bacterial taxa in the rhizosphere and endosphere.
For both seasons, Proteobacteria, Actinobacteria, Acidobacteria, Bacteroidetes, and Firmicutes were the most abundant phyla (Fig. 3; Fig. S3; Table S2), consistent with previous studies (22, 30, 47–49). We selected the significantly different abundant genera influenced by seasons and compartments and found that some of these groups were related to specific functions. In our study, the relative abundances of Arthrobacter (affiliated with Actinobacteria) in the rhizosphere and Streptomyces (affiliated with Actinobacteria) and Devosia (affiliated with Alphaproteobacteria) in the endosphere were significantly higher than those in the bulk sediment (Fig. S4, Wilcoxon rank sum test, P < 0.05, and LDA scores > 2). These genera have been reported as important plant growth-promoting rhizobacteria (PGPR) (50–52). PGPR could greatly expand host adaptations and performance by various promoting activities, including indole acetic acid (IAA) production, siderophore production, phosphate solubilization, and induced systemic resistance (53, 54). In addition, Agromyces (affiliated with Actinobacteria) and Mycobacterium (affiliated with Actinobacteria) were enriched in the rhizosphere, while Methylibium (affiliated with Betaproteobacteria) was enriched in both the rhizosphere and endosphere; these taxa all play important roles in the degradation of a wide spectrum of substrates, such as sugars, acids, cellulose, and xylan, as well as many aromatic compounds that can be excreted by the root (55, 56). Dechloromonas (affiliated with Betaproteobacteria) organisms are autotrophic, denitrifying, and phosphate-accumulating bacteria (57, 58) which have been found in the seeds of P. australis and Typha angustifolia L. (48). This genus was more abundant in the rhizosphere and endosphere compartments in summer than in winter (Fig. S4, Wilcoxon rank sum test, P < 0.05, and LDA scores > 2). Therefore, the growth of P. australis may promote the enrichment of Dechloromonas around the root and enhance denitrification processes and phosphorus absorption by the root. The respective abundances of these genera suggest that the rhizosphere and endosphere of P. australis tend to resist adverse external factors by enriching bacteria that are beneficial for the plant’s growth and health (59, 60).
Season accompanied by the changes in environmental factors influences the composition and assembly of root-associated bacterial communities.
Season explained the majority of the variation in the BCCs of root-associated compartments in P. australis (Table 1). In this study, the season encompasses multiple factors, including the growth condition of P. australis and changes in environmental variables (e.g., temperature) (Table S1). In winter, reeds may go into the dormant stage due to relative lower temperatures and photosynthetic capacity. While in summer, reeds may recover into the vegetative stage with new grown leaves. Significant differences in the diversity and community composition of the bulk sediments between the two seasons (Fig. 1B; Fig. 2C and D) were found in the present study; therefore, we cannot ignore the effects of the external environment on the composition of the bacterial community. However, differences in diversity (α-diversity and β-diversity) (Fig. 1; Fig. 2C and D) of the bacterial community between the rhizosphere and bulk compartments were higher at the vegetative period (summer) than those of the dormant period (winter); this pattern relates to the plant itself, as the rhizosphere and bulk compartments shared the same environment during the same season.
For the plant, the amount and composition of root exudate varied between the seasons, which could affect the respective responses of root-associated bacterial communities (61). In a laboratory experiment, the root exudates of reed increased with plant growth during the experimental period (50 days) (ranging from 0.03 to 1.53 μmol g−1 day−1) (62). Therefore, increased amounts of root exudate (e.g., organic acids) may be produced during the vegetative stage (summer) in our study. Nutrient enrichment accelerates the growth of some rare bacterial species; therefore, the diversity of the bacterial community is increased, and the relative importance of stochastic processes is also enhanced in the assembly of the bacterial community (63).
Except for the specific effects of the plant itself, temperature is likely the most important factor contributing to differences in the bacterial communities given that it changed markedly between the two seasons (8°C in winter and 32°C in summer) (Table S1). Temperature is one of the major determinants of bacterial assemblage composition, as the temperature optima of bacterial taxa differ greatly (64, 65). In this study, the wide temperature difference between the two sampling times (24°C) could have resulted in the differences of bacterial community between the seasons. Moreover, almost all biological processes are affected by temperature increases, as the metabolic rates increase exponentially as temperatures rise (26, 65, 66). Increased temperature promotes the kinetic metabolic rate of microorganisms, thereby increasing the diversity and the stochasticity of the colonization and extinction of members of the bacterial community (65). Therefore, we can explain why the bacterial communities within the same compartments were more affected by stochastic processes in summer marked by higher temperatures.
Composition and source of the bacterial community in the endosphere.
Previous studies have indicated that endosphere bacteria originated mainly from the soil environment because root exudates and rhizodeposits attract microorganisms (3, 59, 67). Our results were in line with this idea, as 69.68% to 74.41% of the endosphere bacteria originated from the rhizosphere and bulk compartments (Fig. 5). Most of the observed endosphere bacterial community originated from the rhizosphere sediment (71.06% in winter and 67.65% in summer) (Fig. 5), consistent with the idea that the invasion of root endophytic microbiome follows a two-step selection model in which microorganisms first colonize the rhizosphere and then invade the roots (2, 8, 46). In addition, the endophytic bacterial community of the seed also contributed to the composition of endosphere bacteria (Fig. 5). This is consistent with recent discoveries (68–70) that endophytes could be vertically transmitted through seeds (59). Despite the fact that significant differences in the bacterial communities were observed in the bulk sediment and rhizosphere compartments between the two seasons (Fig. 1 and 2), the contributions of these sources (bulk sediment, rhizosphere, and seed) to the endosphere bacterial community were consistent between the two seasons (Mann-Whitney U test, P > 0.05) (Fig. 5); thus, the assembly of the endosphere bacterial community remained stable.
It is generally assumed that roots are effective habitat filters, restricting membership to progressively more narrowly defined lineages as environments deviate from soil to roots (1, 71). Therefore, the lowest diversity of the bacterial community was observed within the root tissue (Fig. 1), in line with previous studies (8, 71–73). More surprising, however, the community composition of the endosphere bacteria showed a high heterogeneity, including a higher β-diversity (Fig. 2C and D), regardless of the season. Gottel et al. (73) also observed more variable microbial communities (including bacterial and fungal communities) in the endosphere than those of the rhizosphere of Populus deltoides. They attributed these differences to the sporadic and nonuniform colonization of roots by the bacterial community of the rhizosphere. In our study, stochastic processes dominated the assembly of the endosphere bacterial community (Fig. 4), seemingly supporting this hypothesis. A passive mode of transmission has bacteria survive after they enter the plant—often via natural breaks or wounds in roots (59, 67, 74); these breaks or wounds may be caused by stochastic processes during the growth of the plant. These random processes provide opportunities for the colonization of specific bacterial species within individual plants.
In conclusion, this study revealed the differences in diversity, composition, and assembly processes of root-associated bacterial communities of P. australis in two specific seasons. We showed that season played more important roles in the diversity, composition, and assembly processes of the root-associated bacterial communities. In addition, our results suggested that the surrounding sediments provide the primary sources of bacteria that invade the roots. While we could explain the possible assembly mechanisms of bacterial communities in the rhizosphere and endosphere, there remain uncertainties regarding the considerable effect of environment variables within in situ field conditions. Therefore, further larger-scale investigations or experiments under controlled conditions are required.
MATERIALS AND METHODS
Study sites and sample collection.
The sampling site (118.870°E, 32.052°N) is located near a lake on the Zijin Mountain in Nanjing, Jiangsu Province, China, where Phragmites australis is the dominant aquatic macrophyte and relatively few anthropogenic activities affect the lake. A more detailed description of the sampling sites can be found in our previous study (75) and Fig. S6. Samples in winter representing the dormant stage were collected in December of 2017 at 8°C (air temperature), and samples in summer representing the vegetative stage were collected in July of 2018 at 32°C (air temperature). To reduce bias related to random environmental variation and ensure a sufficient number of samples for subsequent analyses, we selected two 10-m by 10-m plots for sampling. Within each plot, samples were collected using a five-point sampling method (center point and four vertex positions) (Fig. S7). We collected 10 sediment cores that were each 30 cm in diameter and 5 to 15 cm deep for each P. australis station. And at least one taproot of the same length as the core was present in the center of each sediment core. The collected sediment core containing root was transported to the laboratory intact for further processing. Bulk sediments were collected at the same sediment depth using a core sampler (model no. DM60; China Mingyu Holdings Group, Shanghai, China) at about 30 cm away from each sampled P. australis. Moreover, 10 seed samples were also collected from the ears of 10 individual P. australis plants. All samples were kept at 4°C and transported to the laboratory within 4 h of collection. Samples were stored at –80°C until further processing.
All samples, except the seed samples, were freeze-dried using a vacuum freeze dryer (FD-1A-50; Beijing Boyikang Lab Instrument Co. Ltd., Beijing, China). Then the roots and rhizosphere samples were separated as described by Bulgarelli et al. (1). Briefly, for each sediment core, the loosely attached sediments were shaken off. After that, the fibrous roots scattering over the taproot, which was covered by ca. 1-mm freeze-dried sediments, were collected of all the depths by sterilized scissors and put into a 50-ml sterile Falcon tube with 35 ml of sterile phosphate-buffered saline (PBS) (130 mM NaCl, 7 mM Na2HPO4, 3 mM NaH2PO4 [pH 7.0], and 0.02% Silwet L-77). Then the whole Falcon tube containing roots and PBS was put onto a shaking platform for 20 min at 180 rpm. The washing buffer was then centrifuged (1,500 × g, 20 min); the collected particles were homogenized and defined as the rhizosphere sample (i.e., all the freeze-dried sediment adhered tightly to the whole root, a layer about 1 mm thick) and stored at –80°C. We repeated the washing steps (20 min at 180 rpm) twice more. If the supernatant was still cloudy or colored, we continued washing the roots until the supernatant was clear. The roots were then transferred to another 50-ml Falcon tube (containing 35 ml of PBS) and sonicated for 10 cycles consisting of 30-s pulses at 160 W and breaks of 30 s using an ultrasonic cleaner (model no. KH-500DB; Hechuang Ultrasonic, China). After all washing and sonicating steps, the remaining roots were frozen and defined as the endosphere compartment. We confirmed that the surfaces of all root samples were sterile by rubbing a subsampled root from each collection onto LB plates and incubating the plates overnight at 30°C (73). Note that each rhizosphere and endosphere sample that we obtained was specific to the same plant. Seed samples were surface sterilized following the protocol of Gao and Shi (48).
Sediment properties.
Sediment properties of the bulk samples, including pH, total nitrogen (TN), total phosphorus (TP), nitrate nitrogen (NO3−-N), nitrite nitrogen (NO2−-N), and loss on ignition (LOI), were measured following the methods of Zeng et al. (76).
DNA extraction, PCR amplification, and high-throughput sequencing.
Seeds and roots were pulverized with a mortar and pestle in liquid nitrogen prior to DNA extraction. Freeze-dried sediment samples (including rhizosphere and bulk compartments) were ground and homogenized before DNA extraction. DNA was extracted from all samples using the FastDNA SPIN for soil kit (MP Biomedicals, Solon, OH) following the manufacturer’s instructions. After checking that DNA was extracted successfully from each sample via electrophoresis on a 0.8% (wt/vol) agarose gel, the concentration and quality (purity) of the extracted DNA were evaluated by measuring the absorbance at 260 nm and 280 nm using a BioPhotometer (Eppendorf, Hamburg, Germany). Each sample was extracted twice, and duplicate DNAs from each sample were pooled and kept at –80°C until further analysis.
We selected the primers 799F (5′-AACMGGATTAGATACCCKG-3′) and 1115R (5′-AGGGTTGCGCTCGTTG-3′), which target the V5-V6 hypervariable regions of the bacterial 16S rRNA gene, for PCR amplification to avoid contamination of chloroplast DNA (77, 78). PCR amplification was performed in a 20-μl mixture that including 10 ng of template DNA, 4 μl of 5× FastPfu buffer, 2 μl of 2.5 mM deoxynucleoside triphosphates (dNTPs), 0.8 μl of 5 μM (each) concentrations of the forward and reverse primers, and 0.4 μl of FastPfu polymerase. Thermal cycling conditions were set at a 2-min initial denaturation at 95°C, 25 cycles of denaturation at 95°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 30 s, and a final extension at 72°C for 5 min. Following amplification, the PCR products were verified by 2% (wt/vol) agarose gel electrophoresis. After amplifying each sample in triplicate, we purified the combined PCR products with the AxyPrepDNA gel purification kit (Axygen Biotechnology Hangzhou Ltd., Hangzhou, China). High-throughput sequencing was performed using an Illumina MiSeq platform at Shanghai Biozeron Co., Ltd.
Sequence analysis.
The following standard operating procedure of QIIME (v.1.9.1) (79) was applied to filter the sequencing quality for each forward and reverse fastq file. First, libraries (paired-end data) were demultiplexed into each sample based on the index sequences downloaded from the Illumina MiSeq platform. Next, paired-end sequences of each sample were trimmed for their quality and length using Trimmomatic (v.0.33) (80). The 250-bp reads were truncated at any site having an average quality score of <20 over a 10-bp moving window, discarding the truncated reads that were <50 bp. Reads with any barcode mismatch or any nucleotide mismatch in primer matching or that contained ambiguous characters were removed. Trimmed sequences overlapping only by >10 bp and having a mismatch of <0.25 were assembled based on the overlap sequences with FLASH (v.1.2.11) (81). We discarded pair-end reads that could not be assembled.
We then detected and removed chimera reads from downstream analyses using USEARCH software v.4.2.52 based on the UCHIME algorithm with a de novo strategy (82). Finally, operational taxonomic units (OTUs) were clustered using the script pick_otus.py of QIIME via the uclust method at a similarity cutoff of 97%. Rare OTUs (sequence number < 17) were removed from downstream analyses (83). In addition, representative reads of each OTU were aligned against the SILVA v.132 databases (16S rRNA genes) and alignments were applied to calculate the phylogenetic tree in FastTree v.2.1 (84). Taxonomic information of each OTU was retrieved using the online RDP (Ribosomal Database Project) classifier with a bootstrap cutoff of 80% (85). Chloroplast, archaeal, mitochondrial, and unclassified reads were discarded. All samples were randomly rarefied to the lowest number of sequences (12,753 sequences) for further analysis.
Statistical analysis.
We calculated α-diversity (including OTU richness, Faith’s phylogenetic diversities [PD] [24], and Shannon index) using the command alpha_diversity.py in QIIME (79). β-Diversity of the bacterial community was calculated based on Bray-Curtis (86) and unweighted UniFrac (87) distances. We assessed significant differences in the diversity indices between the two seasons and three compartments via two nonparametric tests (Kruskal-Wallis H test and Mann-Whitney U test) (88). Nonmetric multidimensional scaling (NMDS) provided a visualization of the bacterial community composition of the sample groups. The bacterial community differences across the compartments and seasons were identified using analysis of similarities (ANOSIM) and permutational multivariate analysis of variance (PERMANOVA). Moreover, PERMANOVA tested whether compartment or season had a greater effect on the composition of the bacterial community (89). The relative abundances of dominant phyla and subphyla were compared between the compartments using SPSS v22.0 software (IBM-SPSS, Chicago, IL). We applied linear discriminant analysis effect size (LEfSe) (90), and only significant genera (Wilcoxon rank sum test, P < 0.05, and LDA scores > 2) were accepted if enriched in the three compartments. The relative abundances of significantly different genera were compared using a heat map. Similarity and clustering relationships of distinctive OTUs for each compartment were calculated and presented in a Venn diagram. All these analyses were performed using the functions in the vegan package (91) of R v.3.3.1 (92). SourceTracker (v.0.9.5), based on a combination of Bayes’ theorem and Gibbs sampling (93), was used to predict the sources of bacterial communities in the endosphere compartments. Bulk sediments, the rhizosphere compartments, and seed compartments were set as sources; the endosphere samples were set as sinks. Source predictions were run with 100 burn-ins, 10 random restarts, and a rarefaction depth of 20,000 (94).
Analysis of bacterial community assembly.
We applied a null-model theory to evaluate the assembly processes of bacterial communities associated with the three compartments (bulk sediment, rhizosphere, and endosphere). The mean nearest taxon distance metric (βMNTD) for each compartment of both seasons was calculated based on the phylogenetic turnover between communities in pairs (14) using the package picante (95) in R. In addition, we calculated the beta nearest taxon index (βNTI)—the difference between observed βMNTD and mean of the null distribution of βMNTD normalized by its standard deviation—to evaluate the dominant assembly processes (deterministic or stochastic) of the bacterial communities (96). βNTI values between −2 and +2 indicate the dominance of stochastic processes, while βNTI values above +2 or below −2 indicate the dominance of deterministic processes (14, 97).
Accession number(s).
The raw sequences obtained in this study have been submitted to the National Center for Biotechnology Information (NCBI) Sequence Read Archive database under BioProject accession number PRJNA528336. Please note that 10 sequences of rhizosphere community of July 2018 were involved in our previous study (75) to compare the differences of bacterial communities in the rhizosphere and phyllosphere compartments of Phragmites australis. These two batches of the data share the same BioProject number.
Supplementary Material
ACKNOWLEDGMENTS
We thank Shuren Wang, Xiaowei He, Qi Zhou, Xiaomin Zhang, Huimin Xu, and Xinyi Cao for the sampling work and data analysis.
This work was supported by the National Natural Science Foundation of China (31730013, 41621002, 41671078, 41871096, and 31971478), the Science & Technology Basic Resources Investigation Program of China (2017FY100300), the Second Tibetan Plateau Scientific Expedition and Research Program (STEP) (2019QZKK0503), the Key Research Program of Frontier Science, CAS (QYZDJ-SSW-DQC030), the Natural Science Foundation of Jiangsu Province, China (BK20181311), the Fundamental Research Funds for the Central Universities (2018B43414 and 2019B17814), and the Belt and Road Special Foundation of the State Key Laboratory of Hydrology-Water Resources and Hydraulic Engineering (2018490211).
We declare no conflict of interest.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Bulgarelli D, Rott M, Schlaeppi K, van Themaat EVL, Ahmadinejad N, Assenza F, Rauf P, Huettel B, Reinhardt R, Schmelzer E, Peplies J, Gloeckner FO, Amann R, Eickhorst T, Schulze-Lefert P. 2012. Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota. Nature 488:91–95. doi: 10.1038/nature11336. [DOI] [PubMed] [Google Scholar]
- 2.Bulgarelli D, Schlaeppi K, Spaepen S, van Themaat EVL, Schulze-Lefert P. 2013. Structure and functions of the bacterial microbiota of plants. Annu Rev Plant Biol 64:807–838. doi: 10.1146/annurev-arplant-050312-120106. [DOI] [PubMed] [Google Scholar]
- 3.Philippot L, Raaijmakers JM, Lemanceau P, van der Putten WH. 2013. Going back to the roots: the microbial ecology of the rhizosphere. Nat Rev Microbiol 11:789–799. doi: 10.1038/nrmicro3109. [DOI] [PubMed] [Google Scholar]
- 4.Dombrowski N, Schlaeppi K, Agler MT, Hacquard S, Kemen E, Garrido-Oter R, Wunder J, Coupland G, Schulze-Lefert P. 2017. Root microbiota dynamics of perennial Arabis alpina are dependent on soil residence time but independent of flowering time. ISME J 11:43–55. doi: 10.1038/ismej.2016.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi SJ, Nuccio E, Herman DJ, Rijkers R, Estera K, Li JB, da Rocha UN, He ZL, Pett-Ridge J, Brodie EL, Zhou JZ, Firestone M. 2015. Successional trajectories of rhizosphere bacterial communities over consecutive seasons. mBio 6:e00746-15. doi: 10.1128/mBio.00746-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sugiyama A, Ueda Y, Zushi T, Takase H, Yazaki K. 2014. Changes in the bacterial community of soybean rhizospheres during growth in the field. PLoS One 9:e100709. doi: 10.1371/journal.pone.0100709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J, Zhang N, Liu YX, Zhang X, Hu B, Qin Y, Xu H, Wang H, Guo X, Qian J, Wang W, Zhang P, Jin T, Chu C, Bai Y. 2018. Root microbiota shift in rice correlates with resident time in the field and developmental stage. Sci China Life Sci 61:613–621. doi: 10.1007/s11427-018-9284-4. [DOI] [PubMed] [Google Scholar]
- 8.Edwards J, Johnson C, Santos-Medellin C, Lurie E, Podishetty NK, Bhatnagar S, Eisen JA, Sundaresan V. 2015. Structure, variation, and assembly of the root-associated microbiomes of rice. Proc Natl Acad Sci U S A 112:E911–E920. doi: 10.1073/pnas.1414592112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nemergut DR, Schmidt SK, Fukami T, O’Neill SP, Bilinski TM, Stanish LF, Knelman JE, Darcy JL, Lynch RC, Wickey P, Ferrenberg S. 2013. Patterns and processes of microbial community assembly. Microbiol Mol Biol Rev 77:342–356. doi: 10.1128/MMBR.00051-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou J, Ning D. 2017. Stochastic community assembly: does it matter in microbial ecology? Microbiol Mol Biol Rev 81:e00002-17. doi: 10.1128/MMBR.00002-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ning D, Deng Y, Tiedje JM, Zhou J. 2019. A general framework for quantitatively assessing ecological stochasticity. Proc Natl Acad Sci U S A 116:16892–16898. doi: 10.1073/pnas.1904623116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chase JM, Myers JA. 2011. Disentangling the importance of ecological niches from stochastic processes across scales. Philos Trans R Soc Lond B Biol Sci 366:2351–2363. doi: 10.1098/rstb.2011.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cordovez V, Dini-Andreote F, Carrión VJ, Raaijmakers JM. 2019. Ecology and evolution of plant microbiomes. Annu Rev Microbiol 73:69–88. doi: 10.1146/annurev-micro-090817-062524. [DOI] [PubMed] [Google Scholar]
- 14.Stegen JC, Lin X, Konopka AE, Fredrickson JK. 2012. Stochastic and deterministic assembly processes in subsurface microbial communities. ISME J 6:1653–1664. doi: 10.1038/ismej.2012.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan KK, Weisenhorn P, Gilbert JA, Shi Y, Bai Y, Chu HY. 2018. Soil pH correlates with the co-occurrence and assemblage process of diazotrophic communities in rhizosphere and bulk soils of wheat fields. Soil Biol Biochem 121:185–192. doi: 10.1016/j.soilbio.2018.03.017. [DOI] [Google Scholar]
- 16.Marasco R, Mosqueira MJ, Fusi M, Ramond JB, Merlino G, Booth JM, Maggs-Kolling G, Cowan DA, Daffonchio D. 2018. Rhizosheath microbial community assembly of sympatric desert speargrasses is independent of the plant host. Microbiome 6:215. doi: 10.1186/s40168-018-0597-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mendes LW, Kuramae EE, Navarrete AA, van Veen JA, Tsai SM. 2014. Taxonomical and functional microbial community selection in soybean rhizosphere. ISME J 8:1577–1587. doi: 10.1038/ismej.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiao S, Yang Y, Xu Y, Zhang J, Lu Y. 2020. Balance between community assembly processes mediates species coexistence in agricultural soil microbiomes across eastern China. ISME J 14:202–216. doi: 10.1038/s41396-019-0522-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiviat E. 2013. Ecosystem services of Phragmites in North America with emphasis on habitat functions. AoB Plants 5:plt008. doi: 10.1093/aobpla/plt008. [DOI] [Google Scholar]
- 20.Bacci G, Cerri M, Lastrucci L, Ferranti F, Ferri V, Foggi B, Gigante D, Venanzoni R, Viciani D, Mengoni A, Reale L, Coppi A. 2018. Applying predictive models to decipher rhizobacterial modifications in common reed die-back affected populations. Sci Total Environ 642:708–722. doi: 10.1016/j.scitotenv.2018.06.066. [DOI] [PubMed] [Google Scholar]
- 21.Huang R, Zeng J, Zhao D, Cook KV, Hambright KD, Yu Z. 2020. Sediment microbiomes associated with the rhizosphere of emergent macrophytes in a shallow, subtropical lake. Limnol Oceanogr 65:S38–S48. doi: 10.1002/lno.11325. [DOI] [Google Scholar]
- 22.Pietrangelo L, Bucci A, Maiuro L, Bulgarelli D, Naclerio G. 2018. Unraveling the composition of the root-associated bacterial microbiota of Phragmites australis and Typha latifolia. Front Microbiol 9:1650. doi: 10.3389/fmicb.2018.01650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yarwood SA, Baldwin AH, Mateu MG, Buyer JS. 2016. Archaeal rhizosphere communities differ between the native and invasive lineages of the wetland plant Phragmites australis (common reed) in a Chesapeake Bay subestuary. Biol Invasions 18:2717–2728. doi: 10.1007/s10530-016-1144-z. [DOI] [Google Scholar]
- 24.Faith D. 1992. Conservation evaluation and phylogenetic diversity. Biol Conserv 61:1–10. doi: 10.1016/0006-3207(92)91201-3. [DOI] [Google Scholar]
- 25.Meyerson LA, Cronin JT, Pysek P. 2016. Phragmites australis as a model organism for studying plant invasions. Biol Invasions 18:2421–2431. doi: 10.1007/s10530-016-1132-3. [DOI] [Google Scholar]
- 26.Bowen JL, Kearns PJ, Byrnes JEK, Wigginton S, Allen WJ, Greenwood M, Tran K, Yu J, Cronin JT, Meyerson LA. 2017. Lineage overwhelms environmental conditions in determining rhizosphere bacterial community structure in a cosmopolitan invasive plant. Nat Commun 8:433. doi: 10.1038/s41467-017-00626-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li YH, Zhu JN, Liu QF, Liu Y, Liu M, Liu L, Zhang Q. 2013. Comparison of the diversity of root-associated bacteria in Phragmites australis and Typha angustifolia L. in artificial wetlands. World J Microbiol Biotechnol 29:1499–1508. doi: 10.1007/s11274-013-1316-2. [DOI] [PubMed] [Google Scholar]
- 28.Baldantoni D, Ligrone R, Alfani A. 2009. Macro- and trace-element concentrations in leaves and roots of Phragmites australis in a volcanic lake in Southern Italy. J Geochem Explor 101:166–174. doi: 10.1016/j.gexplo.2008.06.007. [DOI] [Google Scholar]
- 29.Liu XL, Zhang Y, Li XH, Fu CY, Shi TH, Yan PP. 2018. Effects of influent nitrogen loads on nitrogen and COD removal in horizontal subsurface flow constructed wetlands during different growth periods of Phragmites australis. Sci Total Environ 635:1360–1366. doi: 10.1016/j.scitotenv.2018.03.260. [DOI] [PubMed] [Google Scholar]
- 30.Borruso L, Bacci G, Mengoni A, De Philippis R, Brusetti L. 2014. Rhizosphere effect and salinity competing to shape microbial communities in Phragmites australis (Cav.) Trin. ex-Steud. FEMS Microbiol Lett 359:193–200. doi: 10.1111/1574-6968.12565. [DOI] [PubMed] [Google Scholar]
- 31.Zhao LY, Tao JX, Liu M. 2015. Effects of aquatic macrophyte planting on functional diversity of microbial community in sediment. J Yangtze River Sci Res Inst 32:81–86. [Google Scholar]
- 32.Hong YW, Liao D, Hu AY, Wang H, Chen JS, Khan S, Su JQ, Li H. 2015. Diversity of endophytic and rhizoplane bacterial communities associated with exotic Spartina alterniflora and native mangrove using Illumina amplicon sequencing. Can J Microbiol 61:723–733. doi: 10.1139/cjm-2015-0079. [DOI] [PubMed] [Google Scholar]
- 33.Alzubaidy H, Essack M, Malas TB, Bokhari A, Motwalli O, Kamanu FK, Jamhor SA, Mokhtar NA, Antunes A, Simoes MF, Alam I, Bougouffa S, Lafi FF, Bajic VB, Archer J. 2016. Rhizosphere microbiome metagenomics of gray mangroves (Avicennia marina) in the Red Sea. Gene 576:626–636. doi: 10.1016/j.gene.2015.10.032. [DOI] [PubMed] [Google Scholar]
- 34.Fan KK, Cardona C, Li YT, Shi Y, Xiang XJ, Shen CC, Wang HF, Gilbert JA, Chu HY. 2017. Rhizosphere-associated bacterial network structure and spatial distribution differ significantly from bulk soil in wheat crop fields. Soil Biol Biochem 113:275–284. doi: 10.1016/j.soilbio.2017.06.020. [DOI] [Google Scholar]
- 35.Bulgarelli D, Garrido-Oter R, Munch PC, Weiman A, Droge J, Pan Y, McHardy AC, Schulze-Lefert P. 2015. Structure and function of the bacterial root microbiota in wild and domesticated barley. Cell Host Microbe 17:392–403. doi: 10.1016/j.chom.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garcia J, Kao-Kniffin J. 2018. Microbial group dynamics in plant rhizospheres and their implications on nutrient cycling. Front Microbiol 9:1516. doi: 10.3389/fmicb.2018.01516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toyama T, Furukawa T, Maeda N, Inoue D, Sei K, Mori K, Kikuchi S, Ike M. 2011. Accelerated biodegradation of pyrene and benzo[a]pyrene in the Phragmites australis rhizosphere by bacteria-root exudate interactions. Water Res 45:1629–1638. doi: 10.1016/j.watres.2010.11.044. [DOI] [PubMed] [Google Scholar]
- 38.Herrmann M, Saunders AM, Schramm A. 2009. Effect of lake trophic status and rooted macrophytes on community composition and abundance of ammonia-oxidizing prokaryotes in freshwater sediments. Appl Environ Microbiol 75:3127–3136. doi: 10.1128/AEM.02806-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Armstrong W. 1964. Oxygen diffusion from the roots of some British bog plants. Nature 204:801–802. doi: 10.1038/204801b0.14235692 [DOI] [Google Scholar]
- 40.Brix H, Schierup HH. 1990. Soil oxygenation in constructed reed beds—the role of macrophyte and soil-atmosphere interface oxygen-transport, p 53–66. In Cooper PF, Findlater BC (ed), Constructed wetlands in water pollution control. Proceedings of the International Conference on the Use of Constructed Wetlands in Water Pollution Control, 24 to 28 September 1990. Elsevier, Cambridge, UK. doi: 10.1016/B978-0-08-040784-5.50010-3. [DOI] [Google Scholar]
- 41.Tanaka N, Yutani K, Aye T, Jinadasa K. 2007. Effect of broken dead culms of Phragmites australis on radial oxygen loss in relation to radiation and temperature. Hydrobiologia 583:165–172. doi: 10.1007/s10750-006-0483-7. [DOI] [Google Scholar]
- 42.Armstrong W, Cousins D, Armstrong J, Turner DW, Beckett PM. 2000. Oxygen distribution in wetland plant roots and permeability barriers to gas-exchange with the rhizosphere: a microelectrode and modelling study with Phragmites australis. Ann Bot 86:687–703. doi: 10.1006/anbo.2000.1236. [DOI] [Google Scholar]
- 43.Rehman F, Pervez A, Khattak BN, Ahmad R. 2018. Plant growth promoting rhizobacteria impact on Typha latifolia and Phragmites australis growth and dissolved oxygen. Clean (Weinh) 46:1700353. doi: 10.1002/clen.201700353. [DOI] [Google Scholar]
- 44.Toyama T, Nishimura Y, Ogata Y, Sei K, Mori K, Ike M. 2016. Effects of planting Phragmites australis on nitrogen removal, microbial nitrogen cycling, and abundance of ammonia-oxidizing and denitrifying microorganisms in sediments. Environ Technol 37:478–485. doi: 10.1080/09593330.2015.1074156. [DOI] [PubMed] [Google Scholar]
- 45.Wang Q, Cao ZF, Hu YB, Kong Q, Xu F, Du YD, Zhao CC. 2019. Season effects on subsurface constructed wetlands performance: role of radial oxygen loss of Phragmites australis. Clean (Weinh) 47:1800428. doi: 10.1002/clen.201800428. [DOI] [Google Scholar]
- 46.Zarraonaindia I, Owens SM, Weisenhorn P, West K, Hampton-Marcell J, Lax S, Bokulich NA, Mills DA, Martin G, Taghavi S, van der Lelie D, Gilbert JA. 2015. The soil microbiome influences grapevine-associated microbiota. mBio 6:e02527-14. doi: 10.1128/mBio.02527-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bickford WA, Goldberg DE, Kowalski KP, Zak DR. 2018. Root endophytes and invasiveness: no difference between native and non-native Phragmites in the Great Lakes region. Ecosphere 9:e02526. doi: 10.1002/ecs2.2526. [DOI] [Google Scholar]
- 48.Gao T, Shi XY. 2018. Taxonomic structure and function of seed-inhabiting bacterial microbiota from common reed (Phragmites australis) and narrowleaf cattail (Typha angustifolia L.). Arch Microbiol 200:869–876. doi: 10.1007/s00203-018-1493-3. [DOI] [PubMed] [Google Scholar]
- 49.Soares MA, Li HY, Kowalski KP, Bergen M, Torres MS, White JF. 2016. Functional role of bacteria from invasive Phragmites australis in promotion of host growth. Microb Ecol 72:407–417. doi: 10.1007/s00248-016-0793-x. [DOI] [PubMed] [Google Scholar]
- 50.Pontes AP, de Souza R, Granada CE, Passaglia L. 2015. Screening of plant growth promoting bacteria associated with barley plants (Hordeum vulgare L.) cultivated in South Brazil. Biota Neotrop 15:e20140105. [Google Scholar]
- 51.Chen SM, Waghmode TR, Sun RB, Kuramae EE, Hu CS, Liu BB. 2019. Root-associated microbiomes of wheat under the combined effect of plant development and nitrogen fertilization. Microbiome 7:136. doi: 10.1186/s40168-019-0750-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Upadhyay SK, Singh JS, Saxena AK, Singh DP. 2012. Impact of PGPR inoculation on growth and antioxidant status of wheat under saline conditions. Plant Biol (Stuttg) 14:605–611. doi: 10.1111/j.1438-8677.2011.00533.x. [DOI] [PubMed] [Google Scholar]
- 53.Liu F, Hewezi T, Lebeis SL, Pantalone V, Grewal PS, Staton ME. 2019. Soil indigenous microbiome and plant genotypes cooperatively modify soybean rhizosphere microbiome assembly. BMC Microbiol 19:201. doi: 10.1186/s12866-019-1572-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shameer S, Prasad T. 2018. Plant growth promoting rhizobacteria for sustainable agricultural practices with special reference to biotic and abiotic stresses. Plant Growth Regul 84:603–615. doi: 10.1007/s10725-017-0365-1. [DOI] [Google Scholar]
- 55.Liu X, Li YJ, Ren XJ, Chen BH, Zhang Y, Shen CW, Wang F, Wu DF. 29 October 2019. Long-term greenhouse cucumber production alters soil bacterial community structure. J Soil Sci Plant Nutr doi: 10.1007/s42729-019-00109-9. [DOI] [Google Scholar]
- 56.Wang R, Zhang HC, Sun LG, Qi GF, Chen S, Zhao XY. 2017. Microbial community composition is related to soil biological and chemical properties and bacterial wilt outbreak. Sci Rep 7:343. doi: 10.1038/s41598-017-00472-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ginige MP, Hugenholtz P, Daims H, Wagner M, Keller J, Blackall LL. 2004. Use of stable-isotope probing, full-cycle rRNA analysis, and fluorescence in situ hybridization-microautoradiography to study a methanol-fed denitrifying microbial community. Appl Environ Microbiol 70:588–596. doi: 10.1128/aem.70.1.588-596.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu Y, Zhang T, Fang H. 2005. Microbial community analysis and performance of a phosphate-removing activated sludge. Bioresour Technol 96:1205–1214. doi: 10.1016/j.biortech.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 59.Hardoim PR, van Overbeek LS, Berg G, Pirttila AM, Compant S, Campisano A, Doring M, Sessitsch A. 2015. The hidden world within plants: ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol Mol Biol Rev 79:293–320. doi: 10.1128/MMBR.00050-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lareen A, Burton F, Schafer P. 2016. Plant root-microbe communication in shaping root microbiomes. Plant Mol Biol 90:575–587. doi: 10.1007/s11103-015-0417-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vančura V, Hovadik A. 1965. Root exudates of plants: II. Composition of root exudates of some vegetables. Plant Soil 22:21–32. doi: 10.1007/BF01377686. [DOI] [Google Scholar]
- 62.Duan XN, Wang XK, Ouyang ZY. 2009. Influence of common reed (Phragmites australis) on CH4 production and transport in wetlands: results from single-plant laboratory experiments. Water Air Soil Pollut 197:185–191. doi: 10.1007/s11270-008-9802-0. [DOI] [Google Scholar]
- 63.Zhou JZ, Deng Y, Zhang P, Xue K, Liang YT, Van Nostrand JD, Yang YF, He ZL, Wu LY, Stahl DA, Hazen TC, Tiedje JM, Arkin AP. 2014. Stochasticity, succession, and environmental perturbations in a fluidic ecosystem. Proc Natl Acad Sci U S A 111:E836–E845. doi: 10.1073/pnas.1324044111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu LM, Yang J, Yu XQ, Chen GJ, Yu Z. 2013. Patterns in the composition of microbial communities from a subtropical river: effects of environmental, spatial and temporal factors. PLoS One 8:e81232. doi: 10.1371/journal.pone.0081232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ren LJ, He D, Chen Z, Jeppesen E, Lauridsen TL, Sondergaard M, Liu ZW, Wu QL. 2017. Warming and nutrient enrichment in combination increase stochasticity and beta diversity of bacterioplankton assemblages across freshwater mesocosms. ISME J 11:613–625. doi: 10.1038/ismej.2016.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schulte PM. 2015. The effects of temperature on aerobic metabolism: towards a mechanistic understanding of the responses of ectotherms to a changing environment. J Exp Biol 218:1856–1866. doi: 10.1242/jeb.118851. [DOI] [PubMed] [Google Scholar]
- 67.Compant S, Clement C, Sessitsch A. 2010. Plant growth-promoting bacteria in the rhizo- and endosphere of plants: their role, colonization, mechanisms involved and prospects for utilization. Soil Biol Biochem 42:669–678. doi: 10.1016/j.soilbio.2009.11.024. [DOI] [Google Scholar]
- 68.Johnston-Monje D, Lundberg DS, Lazarovits G, Reis VM, Raizada MN. 2016. Bacterial populations in juvenile maize rhizospheres originate from both seed and soil. Plant Soil 405:337–355. doi: 10.1007/s11104-016-2826-0. [DOI] [Google Scholar]
- 69.Johnston-Monje D, Mousa WK, Lazarovits G, Raizada MN. 2014. Impact of swapping soils on the endophytic bacterial communities of pre-domesticated, ancient and modern maize. BMC Plant Biol 14:233. doi: 10.1186/s12870-014-0233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Johnston-Monje D, Raizada MN. 2011. Conservation and diversity of seed associated endophytes in Zea across boundaries of evolution, ethnography and ecology. PLoS One 6:e20396. doi: 10.1371/journal.pone.0020396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu H, Carvalhais LC, Crawford M, Singh E, Dennis PG, Pieterse CMJ, Schenk PM. 2017. Inner plant values: diversity, colonization and benefits from endophytic bacteria. Front Microbiol 8:2552. doi: 10.3389/fmicb.2017.02552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Beckers B, De Beeck MO, Weyens N, Boerjan W, Vangronsveld J. 2017. Structural variability and niche differentiation in the rhizosphere and endosphere bacterial microbiome of field-grown poplar trees. Microbiome 5:25. doi: 10.1186/s40168-017-0241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gottel NR, Castro HF, Kerley M, Yang Z, Pelletier DA, Podar M, Karpinets T, Uberbacher E, Tuskan GA, Vilgalys R, Doktycz MJ, Schadt CW. 2011. Distinct microbial communities within the endosphere and rhizosphere of Populus deltoides roots across contrasting soil types. Appl Environ Microbiol 77:5934–5944. doi: 10.1128/AEM.05255-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Turner TR, James EK, Poole PS. 2013. The plant microbiome. Genome Biol 14:209. doi: 10.1186/gb-2013-14-6-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou Q, Zhang XM, He RJ, Wang SR, Jiao CC, Huang R, He XW, Zeng J, Zhao DY. 2019. The composition and assembly of bacterial communities across the rhizosphere and phyllosphere compartments of Phragmites australis. Diversity (Basel) 11:98. doi: 10.3390/d11060098. [DOI] [Google Scholar]
- 76.Zeng J, Jiao C, Zhao D, Xu H, Huang R, Cao X, Yu Z, Wu QL. 2019. Patterns and assembly processes of planktonic and sedimentary bacterial community differ along a trophic gradient in freshwater lakes. Ecol Indic 106:105491. doi: 10.1016/j.ecolind.2019.105491. [DOI] [Google Scholar]
- 77.Redford AJ, Bowers RM, Knight R, Linhart Y, Fierer N. 2010. The ecology of the phyllosphere: geographic and phylogenetic variability in the distribution of bacteria on tree leaves. Environ Microbiol 12:2885–2893. doi: 10.1111/j.1462-2920.2010.02258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Laforest-Lapointe I, Messier C, Kembel SW. 2017. Tree leaf bacterial community structure and diversity differ along a gradient of urban intensity. mSystems 2:e00087-17. doi: 10.1128/mSystems.00087-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Magoc T, Salzberg SL. 2011. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27:2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. 2011. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27:2194–2200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bokulich NA, Subramanian S, Faith JJ, Gevers D, Gordon JI, Knight R, Mills DA, Caporaso JG. 2013. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat Methods 10:57–59. doi: 10.1038/nmeth.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Price MN, Dehal PS, Arkin AP. 2009. FastTree: computing large minimum evolution trees with profiles instead of a distance matrix. Mol Biol Evol 26:1641–1650. doi: 10.1093/molbev/msp077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang Q, Garrity GM, Tiedje JM, Cole JR. 2007. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bray JR, Curtis JT. 1957. An ordination of the upland forest communities of southern Wisconsin. Ecol Monogr 27:325–349. doi: 10.2307/1942268. [DOI] [Google Scholar]
- 87.Lozupone C, Knight R. 2005. UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol 71:8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Siegel S, Castellan NJ Jr.. 1988. Nonparametric statistics for the behavioural sciences, 2nd ed, p 240–261. McGraw-Hill, New York, NY. [Google Scholar]
- 89.Cregger MA, Veach AM, Yang ZK, Crouch MJ, Vilgalys R, Tuskan GA, Schadt CW. 2018. The Populus holobiont: dissecting the effects of plant niches and genotype on the microbiome. Microbiome 6:31. doi: 10.1186/s40168-018-0413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C. 2011. Metagenomic biomarker discovery and explanation. Genome Biol 12:R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dixon P. 2003. VEGAN, a package of R functions for community ecology. J Veg Sci 14:927–930. doi: 10.1111/j.1654-1103.2003.tb02228.x. [DOI] [Google Scholar]
- 92.R Core Team. 2017. R: a language and environment for statistical computing. http://www.r-project.org/.
- 93.Knights D, Kuczynski J, Charlson ES, Zaneveld J, Mozer MC, Collman RG, Bushman FD, Knight R, Kelley ST. 2011. Bayesian community-wide culture-independent microbial source tracking. Nat Methods 8:761–763. doi: 10.1038/nmeth.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sun RB, Dsouza M, Gilbert JA, Guo XS, Wang DZ, Guo ZB, Ni YY, Chu HY. 2016. Fungal community composition in soils subjected to long-term chemical fertilization is most influenced by the type of organic matter. Environ Microbiol 18:5137–5150. doi: 10.1111/1462-2920.13512. [DOI] [PubMed] [Google Scholar]
- 95.Kembel SW, Cowan PD, Helmus MR, Cornwell WK, Morlon H, Ackerly DD, Blomberg SP, Webb CO. 2010. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26:1463–1464. doi: 10.1093/bioinformatics/btq166. [DOI] [PubMed] [Google Scholar]
- 96.Tripathi BM, Stegen JC, Kim M, Dong K, Adams JM, Lee YK. 2018. Soil pH mediates the balance between stochastic and deterministic assembly of bacteria. ISME J 12:1072–1083. doi: 10.1038/s41396-018-0082-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dini-Andreote F, Stegen JC, van Elsas JD, Salles JF. 2015. Disentangling mechanisms that mediate the balance between stochastic and deterministic processes in microbial succession. Proc Natl Acad Sci U S A 112:E1326–E1332. doi: 10.1073/pnas.1414261112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.