Dozens of bacterial endosymbiont species have been described and estimated to infect about half of all insect species. However, only a few them are tractable in vitro, which hampers our understanding of the bacterial determinants of the host-symbiont interaction. Developing a transformation method for S. poulsonii is a major step toward genomic engineering of this symbiont, which will foster basic research on endosymbiosis. This could also open the way to practical uses of endosymbiont engineering through paratransgenesis of vector or pest insects.
KEYWORDS: endosymbiosis, insect, male killing, oriC, spiroplasma
ABSTRACT
Insects are frequently infected by bacterial symbionts that greatly affect their physiology and ecology. Most of these endosymbionts are, however, barely tractable outside their native host, rendering functional genetics studies difficult or impossible. Spiroplasma poulsonii is a facultative bacterial endosymbiont of Drosophila melanogaster that manipulates the reproduction of its host by killing its male progeny at the embryonic stage. S. poulsonii, although a very fastidious bacterium, is closely related to pathogenic Spiroplasma species that are cultivable and genetically modifiable. In this work, we present the transformation of S. poulsonii with a plasmid bearing a fluorescence cassette, leveraging techniques adapted from those used to modify the pathogenic species Spiroplasma citri. We demonstrate the feasibility of S. poulsonii transformation and discuss approaches for mutant selection and fly colonization, which are persisting hurdles that must be overcome to allow functional bacterial genetics studies of this endosymbiont in vivo.
IMPORTANCE Dozens of bacterial endosymbiont species have been described and estimated to infect about half of all insect species. However, only a few them are tractable in vitro, which hampers our understanding of the bacterial determinants of the host-symbiont interaction. Developing a transformation method for S. poulsonii is a major step toward genomic engineering of this symbiont, which will foster basic research on endosymbiosis. This could also open the way to practical uses of endosymbiont engineering through paratransgenesis of vector or pest insects.
INTRODUCTION
Insects are frequently infected by vertically transmitted symbiotic bacteria. These endosymbionts have a major impact on their insect hosts’ physiology and ecology, affecting their reproduction or their nutrition or conferring upon them selective advantages such as protection against natural enemies (1). Despite the high prevalence of endosymbionts in insects, functional studies are hampered by the lack of techniques to manipulate them, particularly in the field of molecular biology. Host-symbiont coevolution leads to an erosion of the bacterial genome and thus to a lack of adaptability to changing environments (2). As a consequence, most endosymbionts are uncultivable and untransformable (3). Nevertheless, the field of endosymbiont manipulation has been rapidly expanding over the past few years with the development of new culture media (4–6), and an example of successful genetic modification of the facultative tsetse fly endosymbiont Sodalis glossinidius followed by host recolonization proved that endosymbiont manipulation is possible (7, 8). Yet, there is still a technical gap preventing the tractability of most models and impeding the discovery and understanding of bacterial genes involved in endosymbiosis.
Spiroplasma is a group of host-associated bacteria belonging to the Mollicutes, a Gram-positive-derived class that lost its cell wall by regressive evolution (9). Spiroplasmas are long, helical, and highly motile bacteria that include species that are commensal, mutualistic, and pathogenic for plants and arthropods (10). Some species such as Spiroplasma citri and Spiroplasma kunkelii are considered major agricultural problems, as they are vectored by leafhoppers toward the phloem sap of citrus trees and maize, where they cause citrus stubborn disease and corn stunt disease, respectively (11). Because of their economic importance, these species received significant research attention for almost 40 years and are the most well-known representatives of the genus. S. citri in particular benefited from constant development of new techniques that made it cultivable and genetically tractable (12), allowing for the discovery of bacterial factors and metabolic pathways involved in both plant and insect pathogenicity (13–15). Other species include strict pathogens, also cultivable, such as Spiroplasma melliferum, which infects honeybees (16), and Spiroplasma eriocheiris, an emerging pathogen of marine arthropods (17).
Finally, some Spiroplasma species evolved as facultative inherited endosymbionts of insects. Spiroplasma poulsonii MSRO (melanogaster sex ratio organism) is one of the two natural endosymbionts infecting Drosophila melanogaster, along with Wolbachia (18). It lives in the hemolymph of the flies and is maternally inherited through transovarial transfer (19). It manipulates Drosophila reproduction by causing early male killing (that is, the death of male offspring at embryonic stages) through the secreted toxin SpAID (20). Male killing fosters the spread of the bacterium by reducing the inbreeding depression of infected females (21). S. poulsonii also protects Drosophila against parasitoid wasps and nematodes (22–24) by metabolic competition against wasps (25) and by producing toxins of the ribosome-inactivating protein family against wasps and nematodes (23, 26). The recent development of a culture medium for S. poulsonii allowed us to identify at least six other putative virulence factors suspected to participate in the interaction with Drosophila (27). None of these genes has been functionally characterized yet, partly because of the complete genetic intractability of the model. In this work, we addressed this hurdle: by taking advantage of previously developed methods for S. citri transformation (28, 29) and from the recently successful in vitro culture of S. poulsonii (27), we were able to transform the latter with a plasmid carrying a fluorescent marker. This represents a first step toward the development of genomic engineering methods for S. poulsonii that will greatly foster research on the bacterial side of endosymbiosis.
RESULTS
Marker choice and plasmid design.
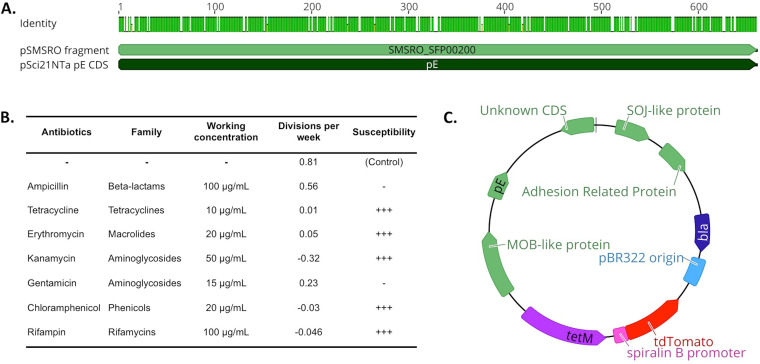
The genome sequencing of S. poulsonii revealed the presence of extrachromosomal DNA, including pSMSRO, a 36-kb plasmid (27). The replication sequences, however, are not yet characterized, and the low copy number and large size of this natural plasmid render it hardly tractable for molecular biology. We thus took advantage of the extensive characterization of natural plasmids of S. citri (29, 30) and used as a starting point the pSci21NT plasmid built by Breton et al. in 2008 (29). This plasmid derives from the natural plasmid pSci2, which was combined with a tetracycline resistance cassette (tetM), a pBlueScript replication origin, and an ampicillin resistance marker (bla) for cloning and replication in Escherichia coli. This plasmid is transformable and replicative in S. citri and has a moderate size (13.5 kb). In S. citri, plasmid replication relies on a plasmid-encoded sequence, pE, that needs to be borne on the plasmid to ensure its replication (29). S. citri pE on pSci2 has a high level of similarity with an uncharacterized coding sequence on pSMSRO (Fig. 1A), suggesting that the pE-based replication system in S. citri would be functional in S. poulsonii. An antibiogram of representative antibiotics of six major families revealed that S. poulsonii MSRO Ug-1 was susceptible to tetracycline, kanamycin, rifampin, and chloramphenicol during in vitro growth (Fig. 1B). This result indicates that the S. citri tetM resistance gene present on pSci21NT would be a suitable choice for selecting S. poulsonii transformants.
FIG 1.
Design of a fluorescence plasmid for S. poulsonii transformation. (A) Alignment of the sequence coding for the cis-acting plasmid replication protein pE in S. citri-derived plasmid pSci21NTa to the uncharacterized coding sequence SMSRO_SFP00200 located on S. poulsonii natural plasmid pSMSRO. A green mark on the “Identity” track indicates an identical amino acid for the considered position. The two sequences share 87% identity, suggesting that S. citri pE is likely to be functional in S. poulsonii. (B) Antibiogram of S. poulsonii in liquid culture. −, resistant; +++, susceptible. (C) Scheme of the pSpo-tdTomato plasmid. Blue indicates E. coli-optimized sequences for cloning, and green indicates S. citri coding sequences present on the original plasmid pSci2. Purple and red are Spiroplasma-optimized sequences. bla, beta-lactamase coding gene (ampicillin resistance marker for E. coli); tetM, gene encoding tetracycline resistance protein M (tetracycline resistance marker for Spiroplasma).
We decided to perform the proof-of-concept transformation using a fluorescent protein-coding gene. Since Spiroplasma species use an alternative genetic code whereby UGA encodes tryptophan rather than a stop (31), we synthesized a Spiroplasma codon-optimized tdTomato coding sequence under the control of the S. poulsonii spiralin B gene promoter. This promoter was chosen based on RNA sequencing data showing that the spiralin B gene is strongly expressed by S. poulsonii in vivo (in the fly hemolymph) as well as in in vitro culture (27). The spiBpromoter-tdTomato cassette was then cloned into pSci21NT to produce the pSpo-tdTomato vector usable for S. poulsonii transformation (Fig. 1C).
Transformation of Spiroplasma poulsonii and selection.
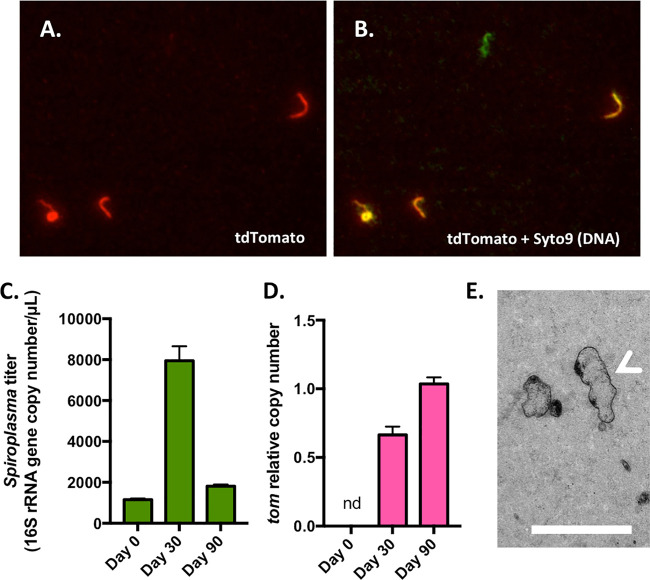
pSpo-tdTomato was transformed in S. poulsonii cells in mid-growth phase using an electroporation protocol previously designed for S. citri transformations (28). Microscopy screening of tdTomato fluorescence revealed the presence of transformants after 2 to 3 weeks (Fig. 2A; see Movie S1 in the supplemental material). The presence of pSpo-tdTomato and its replication were confirmed by quantitative PCR (qPCR) targeting the tdTomato coding gene, which showed that the gene copy number relative to the 16S rRNA gene copy number increased between 30 and 90 days after transformation while it remained under the detection limit in untransformed cultures (Fig. 2D). This increase could represent either an increase in the prevalence of transformants in the culture, an increase in plasmid copy number per cell, or both. Microscopy revealed, however, that liquid cultures remained highly contaminated with nonfluorescent bacteria, which are likely to be spontaneous tetracycline-resistant mutants (Fig. 2B). We then tried to select transformants using a colony-based selection approach on solid BSK medium. The acidification of the medium over 3 weeks, evidenced by the pH indicator phenol red, revealed that the bacteria were alive on this medium, but no colony was visible. Randomly cutting a piece of medium and incubating it in liquid medium allowed the development of a new culture within 2 weeks, further indicating that S. poulsonii survives on solid medium. Further investigations by electron microscopy showed that S. poulsonii cells were dispersed inside the agar gel (Fig. 2E). The use of higher concentrations of gelling agent led to no pH change in the medium over a 3-week time course, suggesting that S. poulsonii does not survive on stiff surfaces. The absence of a suitable agar concentration range, where S. poulsonii survives without being able to dig into the substrate and swim inside the gel, renders solid BSK medium unusable for the selection of individual clones.
FIG 2.
Detection of S. poulsonii transformant by microscopy and molecular methods. (A) Representative image of S. poulsonii transformants producing the red fluorescent protein tdTomato. (B) Overlay of panel A with a Syto9 signal (DNA staining) showing all bacteria. A fluorescent-green-only, probable spontaneously tetracycline-resistant bacterium is visible in the center. (C) Absolute quantification of S. poulsonii 16S rRNA gene copy number in the transformant culture. (D) Quantification of the tdTomato gene copy number relative to the 16S rRNA gene copy number. nd, not detected. (E) Transmission electron microscopy image of BSK-H-spiro solid medium showing the transformant cells scattered inside the agar gel. The white arrow indicates a cell that has been cut longitudinally through the agar matrix, revealing the characteristic helical morphology of Spiroplasma. Scale bar, 500 nm.
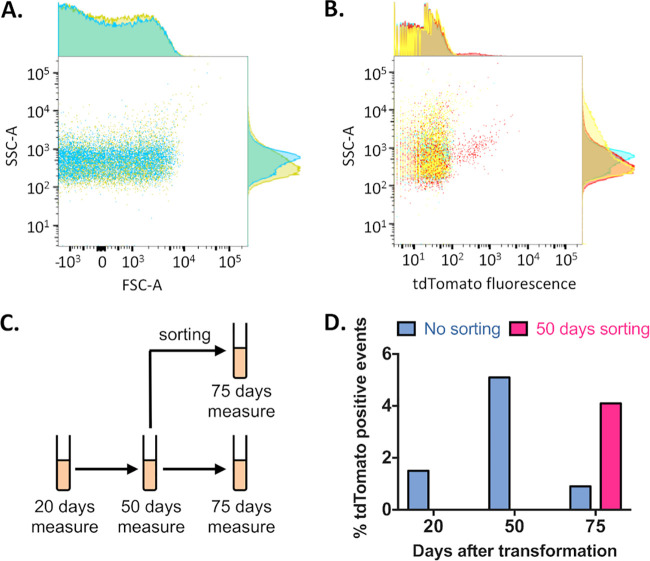
We thus tried to visualize transformants by flow cytometry in order to estimate the transformant prevalence in tetracycline-treated cultures (Fig. 3) and eventually to select transformants by cell sorting based on fluorescence (fluorescence-activated cell sorting [FACS]). Comparison of the forward scatter (FSC) parameter between the culture and axenic BSK medium indicated that this proxy for particle size was not able to discriminate S. poulsonii cells from debris or protein aggregates present in the medium. However, the side scatter (SSC) parameter, a proxy for particle complexity, discriminates some S. poulsonii cells from medium debris (Fig. 3A). Based on this observation, we measured the transformation culture for SSC and tdTomato fluorescence (Fig. 3B). We did measurements at 25, 50, and 75 days after transformation, with the culture being kept under tetracycline selection. On day 50, we sorted a tdTomato-positive subpopulation that we kept in a separate culture. We assessed the enrichment ability of this day 50 sorting with a new round of FACS at day 75 (Fig. 3C). The prevalence of tdTomato-positive cells was estimated to be 1.5% at 25 days, 4.5% at 50 days, and 0.9% at 75 days without sorting at day 50 (Fig. 3D), indicating that transformants were positively selected for at least 50 days before the emergence of spontaneous tetracycline-resistant (nonfluorescent) individuals that take over the population. Sorting at day 50 yielded 4.1% tdTomato-positive cells at day 75, demonstrating that FACS allowed for enrichment of the tdTomato-positive subpopulation but did not provide a fully reliable way to purify it of spontaneous mutants.
FIG 3.
Fluorescence-activated cell sorting enrichment of transformant S. poulsonii culture. (A) Dot plot of forward scatter (FSC-A) versus side scatter (SSC-A) of a wild-type S. poulsonii culture (yellow) versus axenic BSH-H-spiro medium (blue) showing that Spiroplasma cells can partly be discriminated according to their SSC but not their FSC. (B) Dot plot of SSC-A versus 581-nm fluorescence (corresponding to tdTomato emission peak) of axenic BSH-H-spiro medium (blue), wild-type S. poulsonii culture (yellow), and transformed S. poulsonii culture (red) showing that transformants expressing tdTomato can be discriminated and sorted from the whole population. (C) Scheme of the FACS experimental design. (D) Histogram of tdTomato-positive cells by culture age without (blue) or with (pink) a FACS enrichment step.
Recolonization of flies by the transformant S. poulsonii culture.
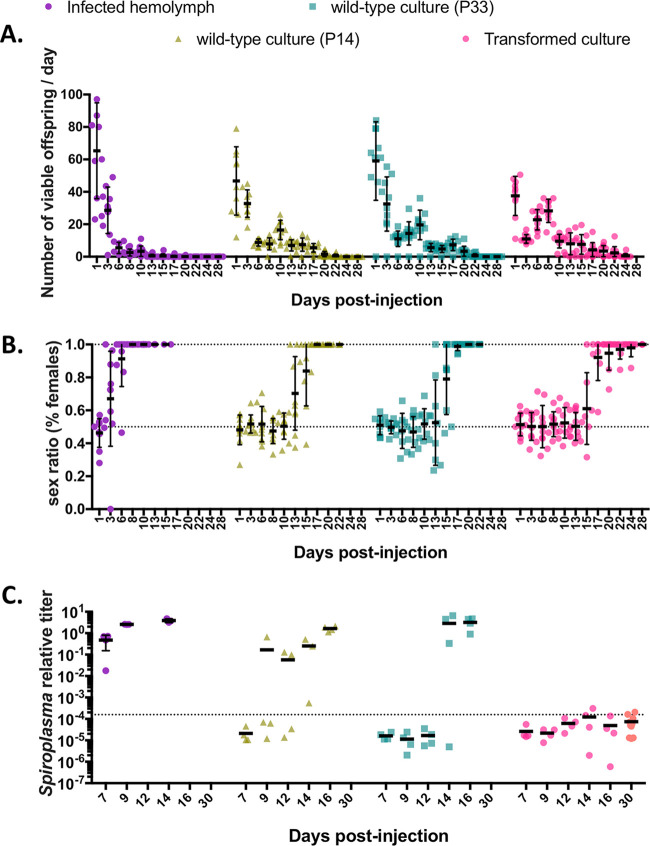
To pave the way for in vivo studies, we assessed the ability of the transformant culture to reestablish a stable symbiosis with Drosophila melanogaster. The objective was to verify whether transforming the culture leads to deleterious effects on critical symbiotic properties of the bacteria, most notably its ability to replicate in fly hemolymph and to cause embryonic male killing. We injected the FACS-enriched transformant culture into Oregon-R females as well as in vitro cultures of wild-type S. poulsonii and hemolymph of stably infected flies. We used two wild-type control cultures, one of an age similar to that of the transformant culture (P33, i.e., the 33th passage after extraction of the bacteria from fly hemolymph, approximately 1 year old) and one that was younger (P14, 14th passage after extraction of the bacteria from fly hemolymph, approximately 6 months old). The point was to compare putative fitness decrease due to the transformation (comparison of P33 versus transformed culture) to the fitness cost of in vitro adaptation (hemolymph versus P14 versus P33). We thus monitored the fertility, offspring sex ratio, and S. poulsonii titer of those flies over 1 month to compare the fitness of the three cultures in vivo (Fig. 4).
FIG 4.
Determination of fly colonization ability of transformant S. poulsonii culture. (A) Fertility of female flies as a function of time postinjection of infected hemolymph (hemolymph; purple circles), a 6-month-old wild-type in vitro culture (P14; triangles), a 12-month-old wild-type in vitro culture (P33; squares), and transformed culture paired to P33 (circles). (B) Sex ratio of the progeny of flies injected with infected hemolymph, P14, P33, or transformed culture, expressed as the percentage of females. Data in panels A and B are paired; each point represents a mother, and error bars indicate the mean ± SD. (C) qPCR quantification of S. poulsonii chromosomal copy number relative to the rps17 host copy number after injection of infected hemolymph, P14, P33, or transformed culture. The dotted line indicates the detection limit. Each dot represents a pool of 5 females; bars indicate the mean. The orange dots for the transformed culture data set indicate that these data were acquired from an independent experiment. Fully detailed statistical analysis of this figure is given in Table 1.
Injecting S. poulsonii into flies tends to decrease their fertility, which is assumed to reflect a slowed oogenesis, possibly due to the bacteria hijacking the metabolism of the host. This effect disappears in subsequent generations as the host and symbiont adapt one to another. The effect of injecting cultured S. poulsonii on fly fertility was stronger upon infected hemolymph injection than upon culture injection, likely because the bacterial titer in the injected hemolymph is higher than that in the cultures. Although statistically different, the effects of the P14 and the P33 injections were similar (Fig. 4A and Table 1): after a brief decrease after injection of the transformant culture, likely because of residual tetracycline in the culture medium, the overall fertility decay speed was lower than that of P14- or P33-injected flies (rate constant K = 0.103, 0.189, and 0.199, respectively) (Table 1). This indicates a lower adverse effect of the transformant culture on fly fertility than of P14 and P33 cultures.
TABLE 1.
| Analysis and parameter | Model parameters |
Pairwise comparisons |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Y0 | K | Half-life | S | Hill slope | df | R2 | Absolute sum of squares | Comparison of fits | F | P value | |
| Fertility analysisb | |||||||||||
| Hemolymph | 101.2 | 0.4341 | 1.597 | 118 | 0.8056 | 10,073 | Hemolymph vs P14 | 21.01 | <0.0001 | ||
| P14 | 54.9 | 0.1892 | 3.663 | 118 | 0.7362 | 7,491 | P14 vs P33 | 4.017 | 0.0193 | ||
| P33 | 67.11 | 0.1995 | 3.474 | 118 | 7.7131 | 12,144 | P33 vs TrCult | 20.55 | <0.0001 | ||
| TrCult | 35.08 | 0.103 | 6.731 | 108 | 0.546 | 8,108 | P14 vs TrCult | 11.38 | <0.0001 | ||
| Sex ratio analysisc | |||||||||||
| Hemolymph | 3.972 | 0.4138 | 51 | 0.6682 | 1.129 | Hemolymph vs P14 | 115.6 | <0.0001 | |||
| P14 | 13.69 | 0.3502 | 84 | 0.7691 | 1.156 | P14 vs P33 | 8.146 | 0.0049 | |||
| P33 | 14.8 | 0.7105 | 79 | 0.7601 | 1.197 | P33 vs TrCult | 6.004 | 0.003 | |||
| TrCult | 15.86 | 0.6314 | 98 | 0.8064 | 1.025 | P14 vs TrCult | 33.9 | <0.0001 | |||
Y0, span; df, degrees of freedom; R2, coefficient of determination; TrCult, transformant culture.
The model was a one-phase exponential decay model. The equation was as follows: offspring = (Y0 − plateau) × exp(−K × days) + plateau. The plateau was fixed at 0.
The model was a four-parameter logistic model. The equation was as follows: % of females = bottom + (top − bottom)/{1 + 10[(S − days) × Hill slope]}. The top value was fixed at 1, and the bottom value was fixed at 0.5.
Male killing, quantified by the proportion of females in the offspring of injected flies, appeared at various times postinfection depending on the injected bacteria. The injection of infected hemolymph led to a 100% female population within less than 10 days. A 75% female population was achieved 13.69 days following P14 injection, 14.8 days following P33 injection, and 15.86 days following transformant culture injection, and 100% male killing was achieved after 17, 20, and 28 days, respectively (Fig. 4B and Table 1). Injected bacteria could be detected inside the flies by qPCR in some of the samples after 9 days for P14 injection and after 14 days for P33 injection and in all samples after 16 days for both P14 and P33 injections. The bacterial titer reached a level comparable to that observed in flies that have received an injection of infected hemolymph. However, the bacterial titer remained below the qPCR detection limit at all times for flies that had received an injection of S. poulsonii from the transformant culture (Fig. 4C). Taken together, the male killing and qPCR data suggest that an aging in vitro culture has a decreased ability to recolonize flies and cause male killing and that undergoing transformation and FACS amplifies this fitness cost.
DISCUSSION
In this study, we report the transformation of the Drosophila facultative endosymbiont S. poulsonii. We validated the usability of the S. citri pE plasmid replication system, the TetM resistance marker, and the tdTomato fluorescent marker in S. poulsonii. Using antibiotic selection and FACS, we were able to enrich to a detectable level the population of tdTomato-expressing bacteria.
The main hurdle to this approach is the transformant selection. Actively selecting transformants is a crucial step, as our results show that a nonfluorescent population ends up taking over the culture 50 to 75 days posttransformation, even in the presence of tetracycline. We suspect the emergence of spontaneous tetracycline-resistant mutants; such a phenomenon has been reported several times with various Spiroplasma species and antibiotics, including a spontaneous S. citri mutant with resistance to up to 20 μg/ml of tetracycline (32, 33). S. poulsonii proved to be refractory to colony-based clone selection on solid medium and to liquid selection by limiting dilutions, as the culture crashes if diluted too much (27). Encouraging results were obtained by FACS of fluorescent individuals, although this technique led to an enrichment of transformants in the culture but not to a complete purification. Two main caveats could be identified with FACS: (i) the thickness of the bacteria (100 to 200 nm) is too low for most detectors to distinguish them from debris naturally present in the culture medium and (ii) nonfluorescent bacteria always get sorted, even with stringent sorting parameters, likely because they entangle themselves with fluorescent ones and get sorted along with them. Alternative approaches for clone selection thus need to be developed, e.g., the use of optical tweezers to select single fluorescent cells (34) or the use of multiple selection markers to overcome the emergence of spontaneously resistant bacteria. Electroporation is a method of choice for the transformation of pathogenic Spiroplasma species (28, 35), but other methods have been successfully used, such as the transduction of S. citri by a modified SpV4 virus (36, 37) or fusion-mediated plasmid transfers in S. floricola (38). These methods may represent more efficient alternatives, with a higher transformation rate that could facilitate selection.
The second hurdle is the establishment of a stable infection of the transformant culture in Drosophila. Our results show that the transformants lost their ability to stably colonize the fly, causing male killing but staying at titers below the detection limit. The comparison between P14 and P33 cultures indicates that the age of the culture negatively influences its ability to recolonize the fly, and by extension, we hypothesize that any selective pressure for in vitro adaptation (such as transformation and FACS selection) would decrease the probability of successful reinfection. This could be due to the production of tdTomato under the control of the strong and constitutive PspiralinB promoter, which is likely to be a burden for transformant bacteria. This hypothesis is supported by the detection by qPCR of a higher copy number of tdTomato after 90 days (Fig. 2B) despite a lower prevalence of fluorescent bacteria at 75 days (Fig. 3D). This could be an indication that some transformants mutated the tdTomato sequence or its promoter, thus gaining a selective advantage to take over the culture while not being selectable by fluorescence. This can be overcome by the use of inducible genetic promoters, such as the tetracycline-inducible promoter recently developed in S. citri (39). Yet, further investigation is needed to test this system in S. poulsonii and to find an alternative selection marker to tetM.
The characterization of selection markers (antibiotic resistance and fluorescence) and a replicative backbone suitable for S. poulsonii also opens the way to genomic engineering, for example knocking out genes of interest to better understand their function in endosymbiosis homeostasis, by leveraging the molecular techniques that were developed for other Mollicutes, such as pathogenic Spiroplasma or Mycoplasma species. Such techniques include the use of oriC plasmids and variants for gene disruption by homologous recombination, successfully used in S. citri (13, 40, 41), Mycoplasma species (42), and more recently in Mesoplasma florum (43). The homologous recombination ability of S. poulsonii was questioned because the major recombination protein coding genes recA and recF are pseudogenized and absent, respectively, from its genome (27, 44). However, recent observations of naturally occurring homologous recombination (45) suggest that such approaches might still be used in this model, even if the recombination rate is expected to be low, as observed in S. citri and other Mollicutes (12, 46). Eventually, techniques that do not require homologous recombination may be more efficient for S. poulsonii manipulation, including the recently developed CRISPR interference (CRISPRi) system (47), which has been used successfully in Mycoplasma pneumoniae (48) and can provide a gene knockdown solution using extrachromosomal plasmids.
In conclusion, we have overcome a first limitation to the genetic manipulation of an endosymbiotic bacterium by achieving its transformation. Future development should now focus on tools allowing for better selection of the transformant subpopulation and for successful recolonization of flies. Beyond fluorescent marking of the bacteria, the pSpo-tdTomato plasmid is, however, already a functional tool to overexpress genes and examine their effect on bacterial physiology in vitro and toward endosymbiosis balance in vivo. In endosymbiotic models, such a tool would, for example, allow the expression of fully functional copies of genes putatively lost under selective pressure of the endosymbiotic lifestyle, thus validating the role of such pressure in their erosion if complemented bacteria become pathogenic. Most notably in the case of the Drosophila-Spiroplasma symbiosis, this tool would allow functional characterization of the genes involved in vertical transmission, male killing, or protection against macroparasites, as well as the exploration of the fundamental biology of Spiroplasma species, such as their division by longitudinal scission (49) and their motility mechanism (50).
MATERIALS AND METHODS
Spiroplasma and Drosophila stocks.
Sprioplasma poulsonii strain MSRO Ug-1 was cultured in BSK-H-spiro medium as described previously (27). The culture was maintained by passaging the cells every 2 weeks by a one-half or one-third dilution in fresh medium. Drosophila melanogaster Oregon-R (ORR) flies were kept at 25°C on standard cornmeal medium (35.28 g of cornmeal, 35.28 g of inactivated yeast, 3.72 g of agar, 36 ml of fruit juice, 2.9 ml of propionic acid, and 15.9 ml of Moldex for 600 ml of medium). Flies had been cured from Wolbachia years prior to this work (51).
Antibiogram.
Antibiotic stocks were prepared at 500× or 1,000× concentrations in water or ethanol according to the supplier’s instructions and diluted in BSK-H-spiro medium to the working concentrations shown in Fig. 1. S. poulsonii culture was diluted 1/10 in the antibiotic-containing medium and a “day 0” sample was immediately frozen. Cultures were allowed to grow for a week before a “day 7” sample was frozen. Samples were quantified using qPCR (see below). The number of divisions per week was calculated using the ΔΔCT method, comparing day 7 to day 0.
Plasmid construction.
The tdTomato coding sequence was copied for the pPLV22 vector and codon optimized for S. poulsonii usage using COOL (52). The spiralin B gene promoter sequence has been arbitrarily defined as the 200 bases upstream of the SpiB gene. The spiBpromoter-tdTomato cassette flanked by SphI and SalI restriction sites was produced by full synthesis by Genewiz, South Plainfield, NJ, USA. The cassette was then cloned into the SphI and SalI sites on pSci21NT (29) to generate pSpo-tdTomato.
Transformation.
S. poulsonii was transformed by electroporation according to a previously published protocol designed for S. citri (28). Briefly, 70 ml of growing culture (passaged for 5 days) was collected by centrifugation for 15 min at 14,000 × g at 18°C. The cells were resuspended in two aliquots of 2 ml of ice-cold 8 mM HEPES–280 mM saccharose (pH 7.4), centrifuged a second time, and resuspended in 400 μl of ice-cold buffer before being transferred to 4-mm electroporation cuvettes containing 20 μg of pSpo-tdTomato for a 20- to 30-min incubation on ice. Electroporation was done at 1,000 Ω, 3 μF, and 2.5 kV using a Gene Pulser Xcell (Bio-Rad). The cells were transferred to 3 ml of BSK-H-spiro medium at room temperature as swiftly as possible after the shock. After 3 h of incubation at 25°C in 10% O2 and 5% CO2, various volumes (20 to 200 μl) of the transformant were plated on solid BSK-H-spiro medium with 1.5% agarose and 4 μg/ml of tetracycline. The next day, the rest of the culture was collected by centrifugation for 30 min at 14,000 × g at 18°C and the cells were resuspended in BSK-H-spiro medium with 4 μg/ml of tetracycline. The transformant liquid culture and plates were then kept under the same conditions as the wild type but with tetracycline.
Photonic microscopy.
Microscopic assessment of S. poulsonii density in culture was done by mixing 1 μl of culture with 1 μl of Syto9 at 0.02 mM in phosphate-buffered saline (PBS) and observation by epifluorescence using a 488-nm excitation and 525- to 550-nm emission setup for Syto9 and a 561-nm excitation and 585- to 595-nm emission setup for tdTomato. For Movie S1 in the supplemental material, 0.5 μl of transformant culture was trapped between a 1% agarose pad and a coverslip to ensure a flat focal plane. Images were acquired every 50 ms at 561-nm excitation and 585- to 595-nm emission.
Bacterial quantification by qPCR.
Culture DNA was extracted by osmotic shock by heating at 95°C for 15 min a 10-μl culture sample diluted in 400 μl of distilled water. qPCR was performed using PowerUp SYBR green master mix (Thermo Fisher) according to the manufacturer’s protocol with 1 μl of DNA. Cycling was done at 60°C using primers hybridizing to the S. poulsonii 16S rRNA gene for S. poulsonii chromosome quantification (forward, 5′-TACATGCAAGTCGAACGGGG-3′; reverse, 5′-CTACTGCTGCCTCCCGTAG-3′) and primers hybridizing to tdTomato for plasmid quantification (forward, 5′-CCCGGGTAATTGAACCGGT-3′; reverse, 5′-AGATGGGCCCGTTATGCA-3′). Absolute quantification was performed using a standard curve made of a serially diluted PCR product of known concentration.
For assessment of fly infections, three samples of five flies were collected for each time point and DNA was extracted as previously described (51). The S. poulsonii titer relative to the host cell number was determined using the ΔΔCT method to quantify the abundance of S. poulsonii 16S rRNA gene copies relative to the host rps17 gene copies quantified with the following primers: forward, 5′-CACTCCCAGGTGCGTGGTAT-3′, and reverse, 5′-GGAGACGGCCGGGACGTAGT-3′. The detection limit was determined experimentally by performing the quantification on uninfected ORR females.
FACS.
Fluorescence-activated cell sorting (FACS) was performed on a BD FACSAria II (BD Biosciences) on undiluted cultures. Gating was determined empirically using axenic medium and wild-type culture to distinguish cells from debris and tdTomato-positive from tdTomato-negative cells, respectively, and fluorescent cells were gated for singlets (tdTomato peak height versus tdTomato peak area). FACS was carried out at the FCCF facility of École Polytechnique Fédérale de Lausanne (EPFL, Lausanne, Switzerland).
TEM.
Three-week-old BSK-H-spiro plates with 1.5% agar were chosen for their color change from red to orange, indicating an acidification of the medium. Plates were submerged overnight with 2% paraformaldehyde and 2.5% glutaraldehyde. A piece of medium 1 mm by 1 mm by 1 mm was cut, fixed again for 2 h, and rinsed once with PBS and three times in 0.1 M cacodylate buffer and then postfixed with 1% osmium tetroxide and 1.5% potassium ferrocyanide solution in 0.1 M cacodylate buffer for 40 min at room temperature, followed by 1% osmium tetroxide solution in 0.1 M cacodylate buffer for 40 min at room temperature. The samples were then treated with 1% uranyl acetate in water for 40 min at room temperature. Dehydration of the piece was performed in an ascending series of ethanol concentrations, and then the samples were embedded in Durcupan (Fluka). The medium piece was cut at random positions (close to the surface or inside the gel) with a Leica ultramicrotome and observed on a FEI Tecnai Spirit transmission electron microscope (TEM) at 120 kV. TEM was carried out at the BioEM facility of EPFL (Lausanne, Switzerland).
Fly colonization.
Eighteen nanoliters of plateaued culture or hemolymph of 2- to 3-day-old infected female flies was injected into the thorax of 2- to 3-day-old ORR female flies using a Nanoject II (Drummond). S. poulsonii cultures make plateaus at a given density threshold (regardless of culture age) and can continue living at this density for several weeks, allowing for reproducibility between injections. The cultures were checked by microscopy prior to injection to confirm the similar bacterial densities between conditions. Injected females were mated the next day, kept at 25°C, and transferred to new vials every 2 to 3 days. The number of adult progeny emerging from each vial was counted and normalized by the number of days of egg laying to estimate the fertility per day, and the numbers of males and females were counted separately to estimate the sex ratio.
Statistical analyses and replication scheme.
The transformation of S. poulsonii and detection of positive transformants by microscopy were achieved at least four independent times, and extensive characterization by qPCR and FACS was performed on one of the transformants. Fly injection experiments were performed two independent times with 10 technical replicates. Fertility data were analyzed by fitting a one-phase exponential decay model with a plateau at zero and by comparing the fits between conditions using the F test. Sex ratio data were analyzed by fitting a four-parameter logistic model with the top and bottom values fixed at 1 and 0.5, respectively, and by comparing the fits between conditions using the F test. Models for each data type were chosen based on empirical trials and comparison of their goodness of fit through R2 values. Fully detailed statistical analyses and values are shown in Table 1.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Colette Saillard for the gift of pSci21NT and to the staff of the UMR1332 “Biologie du Fruit et Pathologie,” INRAE Bordeaux, France, for their insights on S. citri. We also thank André Mozes and the FCCF facility of EPFL for the FACS, Stéphanie Clerc-Rosset and the BioEM facility of EPFL for electron microscopy, Alexandre Persat and Alice Cont for their help with live microscopy for Movie S1 in the supplemental material, and Samuel Rommelaere for critically reviewing the manuscript.
F.M. and B.L. designed the project. F.M., F.S., and C.J. performed the experiments. The first draft was written by F.M. and edited by B.L. All authors read and approved the final manuscript.
We declare no competing interests.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Douglas AE. 2016. How multi-partner endosymbioses function. Nat Rev Microbiol 14:731–743. doi: 10.1038/nrmicro.2016.151. [DOI] [PubMed] [Google Scholar]
- 2.Moran NA, McCutcheon JP, Nakabachi A. 2008. Genomics and evolution of heritable bacterial symbionts. Annu Rev Genet 42:165–190. doi: 10.1146/annurev.genet.41.110306.130119. [DOI] [PubMed] [Google Scholar]
- 3.Kikuchi Y. 2009. Endosymbiotic bacteria in insects: their diversity and culturability. Microbes Environ 24:195–204. doi: 10.1264/jsme2.me09140s. [DOI] [PubMed] [Google Scholar]
- 4.Dale C, Beeton M, Harbison C, Jones T, Pontes M. 2006. Isolation, pure culture, and characterization of “Candidatus Arsenophonus arthropodicus,” an intracellular secondary endosymbiont from the hippoboscid louse fly Pseudolynchia canariensis. Appl Environ Microbiol 72:2997–3004. doi: 10.1128/AEM.72.4.2997-3004.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sabri A, Leroy P, Haubruge E, Hance T, Frère I, Destain J, Thonart P. 2011. Isolation, pure culture and characterization of Serratia symbiotica sp. nov., the R-type of secondary endosymbiont of the black bean aphid Aphis fabae. Int J Syst Evol Microbiol 61:2081–2088. doi: 10.1099/ijs.0.024133-0. [DOI] [PubMed] [Google Scholar]
- 6.Brandt JW, Chevignon G, Oliver KM, Strand MR. 2017. Culture of an aphid heritable symbiont demonstrates its direct role in defence against parasitoids. Proc Biol Sci 284:20171925. doi: 10.1098/rspb.2017.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Vooght L, Caljon G, Stijlemans B, De Baetselier P, Coosemans M, Van Den Abbeele J. 2012. Expression and extracellular release of a functional anti-trypanosome Nanobody in Sodalis glossinidius, a bacterial symbiont of the tsetse fly. Microb Cell Fact 11:23. doi: 10.1186/1475-2859-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Vooght L, Van Keer S, Van Den Abbeele J. 2018. Towards improving tsetse fly paratransgenesis: stable colonization of Glossina morsitans morsitans with genetically modified Sodalis. BMC Microbiol 18:165. doi: 10.1186/s12866-018-1282-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trachtenberg S. 1998. Mollicutes—wall-less bacteria with internal cytoskeletons. J Struct Biol 124:244–256. doi: 10.1006/jsbi.1998.4063. [DOI] [PubMed] [Google Scholar]
- 10.Gasparich GE. 2002. Spiroplasmas: evolution, adaptation and diversity. Front Biosci 7:d619–640. doi: 10.2741/gaspari. [DOI] [PubMed] [Google Scholar]
- 11.Gasparich GE. 2010. Spiroplasmas and phytoplasmas: microbes associated with plant hosts. Biologicals 38:193–203. doi: 10.1016/j.biologicals.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Renaudin J. 2002. Extrachromosomal elements and gene transfer, p 347–370. In Razin S, Herrmann R (ed), Molecular biology and pathogenicity of mycoplasmas. Springer US, Boston, MA. [Google Scholar]
- 13.Duret S, Danet J-L, Garnier M, Renaudin J. 1999. Gene disruption through homologous recombination in Spiroplasma citri: an scm1-disrupted motility mutant is pathogenic. J Bacteriol 181:7449–7456. doi: 10.1128/JB.181.24.7449-7456.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duret S, Berho N, Danet JL, Garnier M, Renaudin J. 2003. Spiralin is not essential for helicity, motility, or pathogenicity but is required for efficient transmission of Spiroplasma citri by its leafhopper vector Circulifer haematoceps. Appl Environ Microbiol 69:6225–6234. doi: 10.1128/aem.69.10.6225-6234.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.André A, Maucourt M, Moing A, Rolin D, Renaudin J. 2005. Sugar import and phytopathogenicity of Spiroplasma citri: glucose and fructose play distinct roles. Mol Plant Microbe Interact 18:33–42. doi: 10.1094/MPMI-18-0033. [DOI] [PubMed] [Google Scholar]
- 16.Clark TB, Whitcomb RF, Tully JG, Mouches C, Saillard C, Bové JM, Wroblewski H, Carle P, Rose DL, Henegar RB, Williamson DL. 1985. Spiroplasma melliferum, a new species from the honeybee (Apis mellifera). Int J Syst Evol Microbiol 35:296–308. doi: 10.1099/00207713-35-3-296. [DOI] [Google Scholar]
- 17.Wang W, Wen B, Gasparich GE, Zhu N, Rong L, Chen J, Xu Z, Wang W. 2004. A spiroplasma associated with tremor disease in the Chinese mitten crab (Eriocheir sinensis). Microbiology 150:3035–3040. doi: 10.1099/mic.0.26664-0. [DOI] [PubMed] [Google Scholar]
- 18.Mateos M, Castrezana SJ, Nankivell BJ, Estes AM, Markow TA, Moran NA. 2006. Heritable endosymbionts of Drosophila. Genetics 174:363–376. doi: 10.1534/genetics.106.058818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herren JK, Paredes JC, Schüpfer F, Lemaitre B. 2013. Vertical transmission of a Drosophila endosymbiont via cooption of the yolk transport and internalization machinery. mBio 4:e00532-12. doi: 10.1128/mBio.00532-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harumoto T, Lemaitre B. 2018. Male-killing toxin in a bacterial symbiont of Drosophila. Nature 557:252–255. doi: 10.1038/s41586-018-0086-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engelstädter J, Hurst G. 2009. The ecology and evolution of microbes that manipulate host reproduction. Annu Rev Ecol Evol Syst 40:127–149. doi: 10.1146/annurev.ecolsys.110308.120206. [DOI] [Google Scholar]
- 22.Xie J, Butler S, Sanchez G, Mateos M. 2014. Male killing Spiroplasma protects Drosophila melanogaster against two parasitoid wasps. Heredity (Edinb) 112:399–408. doi: 10.1038/hdy.2013.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamilton PT, Peng F, Boulanger MJ, Perlman SJ. 2016. A ribosome-inactivating protein in a Drosophila defensive symbiont. Proc Natl Acad Sci U S A 113:350–355. doi: 10.1073/pnas.1518648113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ballinger MJ, Perlman SJ. 2019. The defensive Spiroplasma. Curr Opin Insect Sci 32:36–41. doi: 10.1016/j.cois.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Paredes JC, Herren JK, Schüpfer F, Lemaitre B. 2016. The role of lipid competition for endosymbiont-mediated protection against parasitoid wasps in Drosophila. mBio 7:e01006-16. doi: 10.1128/mBio.01006-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ballinger MJ, Perlman SJ. 2017. Generality of toxins in defensive symbiosis: ribosome-inactivating proteins and defense against parasitic wasps in Drosophila. PLoS Pathog 13:e1006431. doi: 10.1371/journal.ppat.1006431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masson F, Calderon Copete S, Schüpfer F, Garcia-Arraez G, Lemaitre B. 2018. In vitro culture of the insect endosymbiont Spiroplasma poulsonii highlights bacterial genes involved in host-symbiont interaction. mBio 9:e00024-18. doi: 10.1128/mBio.00024-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stamburski C, Renaudin J, Bove JM. 1991. First step toward a virus-derived vector for gene cloning and expression in spiroplasmas, organisms which read UGA as a tryptophan codon: synthesis of chloramphenicol acetyltransferase in Spiroplasma citri. J Bacteriol 173:2225–2230. doi: 10.1128/jb.173.7.2225-2230.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Breton M, Duret S, Arricau-Bouvery N, Béven L, Renaudin J. 2008. Characterizing the replication and stability regions of Spiroplasma citri plasmids identifies a novel replication protein and expands the genetic toolbox for plant-pathogenic spiroplasmas. Microbiology 154:3232–3244. doi: 10.1099/mic.0.2008/019562-0. [DOI] [PubMed] [Google Scholar]
- 30.Saillard C, Carle P, Duret-Nurbel S, Henri R, Killiny N, Carrère S, Gouzy J, Bové J-M, Renaudin J, Foissac X. 2008. The abundant extrachromosomal DNA content of the Spiroplasma citri GII3-3X genome. BMC Genomics 9:195. doi: 10.1186/1471-2164-9-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Renaudin J, Pascarel MC, Saillard C, Chevalier C, Bové J-M. 1986. Chez les spiroplasmes le codon UGA n’est pas non-sens et semble coder pour le tryptophane. C R Acad Sci Paris Série III 303:539–540. [Google Scholar]
- 32.Liao CH, Chen TA. 1981. In vitro susceptibility and resistance of two spiroplasmas to antibiotics. Phytopathology 71:442–445. doi: 10.1094/Phyto-71-442. [DOI] [Google Scholar]
- 33.Mutaqin K, Comer JL, Wayadande AC, Melcher U, Fletcher J. 2011. Selection and characterization of Spiroplasma citri mutants by random transposome mutagenesis. Can J Microbiol 57:525–532. doi: 10.1139/w11-026. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Z, Kimkes TEP, Heinemann M. 2019. Manipulating rod-shaped bacteria with optical tweezers. Sci Rep 9:19086. doi: 10.1038/s41598-019-55657-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Terahara N, Tulum I, Miyata M. 2017. Transformation of crustacean pathogenic bacterium Spiroplasma eriocheiris and expression of yellow fluorescent protein. Biochem Biophys Res Commun 487:488–493. doi: 10.1016/j.bbrc.2017.03.144. [DOI] [PubMed] [Google Scholar]
- 36.Bove JM, Candresse T, Mouches C, Renaudin J, Saillard C. 1984. Spiroplasmas and the transfer of genetic material by transformation and transfection. Isr J Med Sci 20:836–839. [PubMed] [Google Scholar]
- 37.Pascarel-Devilder M-C, Renaudin J, Bove JM. 1986. The spiroplasma virus 4 replicative form cloned in Escherichia coli transfects spiroplasmas. Virology 151:390–393. doi: 10.1016/0042-6822(86)90060-7. [DOI] [PubMed] [Google Scholar]
- 38.Salman M, Tarshis M, Rottem S. 1992. Fusion-mediated transfer of plasmids into Spiroplasma floricola cells. J Bacteriol 174:4410–4415. doi: 10.1128/jb.174.13.4410-4415.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Breton M, Sagné E, Duret S, Béven L, Citti C, Renaudin J. 2010. First report of a tetracycline-inducible gene expression system for mollicutes. Microbiology 156:198–205. doi: 10.1099/mic.0.034074-0. [DOI] [PubMed] [Google Scholar]
- 40.Lartigue C, Duret S, Garnier M, Renaudin J. 2002. New plasmid vectors for specific gene targeting in Spiroplasma citri. Plasmid 48:149–159. doi: 10.1016/s0147-619x(02)00121-x. [DOI] [PubMed] [Google Scholar]
- 41.Duret S, André A, Renaudin J. 2005. Specific gene targeting in Spiroplasma citri: improved vectors and production of unmarked mutations using site-specific recombination. Microbiology 151:2793–2803. doi: 10.1099/mic.0.28123-0. [DOI] [PubMed] [Google Scholar]
- 42.Cordova CMM, Lartigue C, Sirand-Pugnet P, Renaudin J, Cunha RAF, Blanchard A. 2002. Identification of the origin of replication of the Mycoplasma pulmonis chromosome and its use in oriC replicative plasmids. J Bacteriol 184:5426–5435. doi: 10.1128/jb.184.19.5426-5435.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matteau D, Pepin M-E, Baby V, Gauthier S, Arango Giraldo M, Knight TF, Rodrigue S. 2017. Development of oriC-based plasmids for Mesoplasma florum. Appl Environ Microbiol 83:e03374-16. doi: 10.1128/AEM.03374-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paredes JC, Herren JK, Schüpfer F, Marin R, Claverol S, Kuo C-H, Lemaitre B, Béven L. 2015. Genome sequence of the Drosophila melanogaster male-killing Spiroplasma strain MSRO endosymbiont. mBio 6:e02437-14. doi: 10.1128/mBio.02437-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Masson F, Calderon-Copete S, Schüpfer F, Vigneron A, Rommelaere S, Garcia-Arraez MG, Paredes JC, Lemaitre B. 2020. Blind killing of both male and female Drosophila embryos by a natural variant of the endosymbiotic bacterium Spiroplasma poulsonii. Cell Microbiol 22:e13156. doi: 10.1111/cmi.13156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marais A, Bove JM, Renaudin J. 1996. Characterization of the recA gene regions of Spiroplasma citri and Spiroplasma melliferum. J Bacteriol 178:7003–7009. doi: 10.1128/jb.178.23.7003-7009.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qi LS, Larson MH, Gilbert LA, Doudna JA, Weissman JS, Arkin AP, Lim WA. 2013. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 152:1173–1183. doi: 10.1016/j.cell.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mariscal AM, Kakizawa S, Hsu JY, Tanaka K, González-González L, Broto A, Querol E, Lluch-Senar M, Piñero-Lambea C, Sun L, Weyman PD, Wise KS, Merryman C, Tse G, Moore AJ, Hutchison CA, Smith HO, Tomita M, Venter JC, Glass JI, Piñol J, Suzuki Y. 2018. Tuning gene activity by inducible and targeted regulation of gene expression in minimal bacterial cells. ACS Synth Biol 7:1538–1552. doi: 10.1021/acssynbio.8b00028. [DOI] [PubMed] [Google Scholar]
- 49.Ramond E, Maclachlan C, Clerc-Rosset S, Knott GW, Lemaitre B. 2016. Cell division by longitudinal scission in the insect endosymbiont Spiroplasma poulsonii. mBio 7:e00881-16. doi: 10.1128/mBio.00881-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shaevitz JW, Lee JY, Fletcher DA. 2005. Spiroplasma swim by a processive change in body helicity. Cell 122:941–945. doi: 10.1016/j.cell.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 51.Herren JK, Lemaitre B. 2011. Spiroplasma and host immunity: activation of humoral immune responses increases endosymbiont load and susceptibility to certain Gram-negative bacterial pathogens in Drosophila melanogaster. Cell Microbiol 13:1385–1396. doi: 10.1111/j.1462-5822.2011.01627.x. [DOI] [PubMed] [Google Scholar]
- 52.Chin JX, Chung B-S, Lee D-Y. 2014. Codon Optimization OnLine (COOL): a web-based multi-objective optimization platform for synthetic gene design. Bioinformatics 30:2210–2212. doi: 10.1093/bioinformatics/btu192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.