The anticaries effects and microecological regulation effects of the antimicrobial peptide GH12 were evaluated systematically in vitro and in vivo. GH12 inhibited the cariogenic virulence of dental plaque without overintervening in the microbial ecology of healthy individuals in vitro. GH12 regulated the microbial ecology of dental plaque to a certain extent in vivo under cariogenic conditions, increased the proportion of commensal bacteria, and decreased the abundance of caries-associated bacteria. GH12 significantly suppressed the incidence and severity of dental caries in vivo. This study thus describes an alternative antimicrobial therapy for dental caries.
KEYWORDS: antimicrobial peptides, biofilms, dental caries, dental plaque, microbiota
ABSTRACT
Due to the complex microecology and microenvironment of dental plaque, novel caries prevention strategies require modulating the microbial communities ecologically and reducing the cariogenic properties effectively. Antimicrobial peptide GH12 reduced the lactic acid production and exopolysaccharide (EPS) synthesis of a Streptococcus mutans biofilm and a three-species biofilm in vitro in previous studies. However, the anticaries effects and microecological effects of GH12 remained to be investigated in a complex biofilm model in vitro and an animal caries model in vivo. In the present study, GH12 at 64 mg/liter showed the most effective inhibition of lactic acid production, EPS synthesis, pH decline, and biofilm integrity of human dental plaque-derived multispecies biofilms in vitro, and GH12 at 64 mg/liter was therefore chosen for use in subsequent in vitro and in vivo assays. When treated with 64-mg/liter GH12, the dental plaque-derived multispecies biofilms sampled from healthy volunteers maintained its microbial diversity and showed a microbial community structure similar to that of the control group. In the rat caries model with a caries-promoting diet, 64-mg/liter GH12 regulated the microbiota of dental plaque, in which the abundance of caries-associated bacteria was decreased and the abundance of commensal bacteria was increased. In addition, 64-mg/liter GH12 significantly reduced the caries scores of sulcal and smooth surface caries in all locations. In conclusion, GH12 inhibited the cariogenic properties of dental plaque without perturbing the dental plaque microbiota of healthy individuals and GH12 regulated the dysbiotic microbial ecology and arrested caries development under cariogenic conditions.
IMPORTANCE The anticaries effects and microecological regulation effects of the antimicrobial peptide GH12 were evaluated systematically in vitro and in vivo. GH12 inhibited the cariogenic virulence of dental plaque without overintervening in the microbial ecology of healthy individuals in vitro. GH12 regulated the microbial ecology of dental plaque to a certain extent in vivo under cariogenic conditions, increased the proportion of commensal bacteria, and decreased the abundance of caries-associated bacteria. GH12 significantly suppressed the incidence and severity of dental caries in vivo. This study thus describes an alternative antimicrobial therapy for dental caries.
INTRODUCTION
Dental caries is one of the most prevalent infectious diseases worldwide, causing a heavy burden on economies and public health (1, 2). The ecological plaque hypothesis has been widely accepted as the means of microbial pathogenesis in dental caries, indicating that caries development is a consequence of dietary sugar-driven biofilm assembly and localized acidification, which cause the dysbiosis of the microbial community and the disequilibrium of enamel mineral homeostasis (3, 4). High-carbohydrate diets enhance the accumulation of exopolysaccharides (EPS) and increase the proportion of acidogenic-aciduric bacteria within dental plaque (5). The plaque subsequently becomes dominated by acidogenic and aciduric organisms, and these microorganisms interact with each other in a dynamic way to form a cariogenic biofilm, which further promotes extracellular matrix synthesis and microenvironmental acidification (6, 7). The resultant acidic pH may eliminate acid-sensitive species and favor the acidogenic-aciduric microbiota, which decrease microbial diversity and lead to enamel demineralization and caries formation.
Dental plaque possesses physical and biochemical properties that help enhance adhesion strength, defend against external aggression, promote microbial interactions, and maintain an acidic microenvironment (4). The complex microecology and the microenvironment of dental plaque pose obstacles to the development of effective anticaries agents. Conventional antimicrobials, such as chlorhexidine, eradicate commensal bacteria and pathogenic bacteria indiscriminately, which disrupts the microbial ecology and results in adverse impacts on oral health (8). A novel small molecule could disperse Streptococcus mutans biofilms while preserving the oral microbiome (9). A mucolytic compound could inhibit oral biofilm formation and bacterial aggregation while preserving biofilm ecology (10). The novel antimicrobial strategies for caries prevention emphasize the preservation of the bacterial ecology of the biofilm while reducing its cariogenic properties. A study of a precision-guided antimicrobial peptide (AMP) showed that it could suppress a three-species biofilm and regulate the microbiome of a planktonic multispecies bacterial community (11, 12), which demonstrated that AMPs with antibacterial effects and multimodal mechanisms could serve as adjuncts in the control of dental caries (13). However, the efficacy of AMPs against a complex multispecies biofilm in vitro and in vivo remained to be further investigated.
In previous studies, GH12, a cationic amphipathic α-helical AMP with good stability, low cytotoxicity, and strong antimicrobial activity, was designed (14, 15). GH12 killed S. mutans in preformed biofilms and reduced the cariogenic properties of S. mutans by inhibiting various virulence factors, such as water-insoluble glucan synthesis and lactic acid production (16). In addition, GH12 inhibited the cariogenic properties of a three-species biofilm and shifted the microbial composition to a healthier condition by suppressing S. mutans and favoring commensal streptococci (17). Furthermore, short-term treatments with 8-mg/liter GH12 reduced the incidence and severity of caries in the rat caries model, and its effects on reducing the caries scores rivaled those of NaF and chlorhexidine (18). However, the anticaries effects and the microecological effects of GH12 remained to be further investigated in vitro and in vivo to evaluate its potential as an ecological caries prevention strategy.
The objectives of this study were (i) to determine the effects of GH12 on the cariogenic properties of human dental plaque-derived multispecies biofilms in vitro, (ii) to explore its effects on the microbiota of human dental plaque-derived biofilms in vitro, and (iii) to further evaluate the anticaries effects and microecological regulation effects of GH12 in an animal caries model. The anticaries effects and ecological regulation effects of the AMP were systematically evaluated in vitro and in vivo, which set a foundation for the practical application of GH12 for the treatment of dental caries.
RESULTS
GH12 inhibited the cariogenic properties of human dental plaque-derived multispecies biofilms.
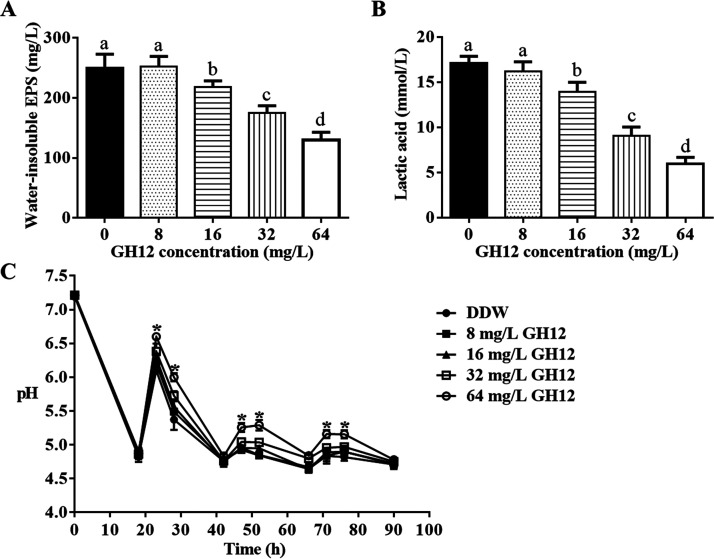
As shown in Fig. 1A and B, GH12 showed a concentration-dependent inhibition of acid production and water-insoluble EPS synthesis of human dental plaque-derived multispecies biofilms in vitro. Short-term treatments with 8-mg/liter GH12 showed no inhibitory effects on these two cariogenic virulence factors (P > 0.05). GH12 at more than 16 mg/liter significantly suppressed lactic acid production and the synthesis of water-insoluble EPS by the dental plaque-derived multispecies biofilms, and 64-mg/liter GH12 showed the most effective inhibitory effects (P < 0.05). After short-term treatments with 64-mg/liter GH12, lactic acid production was reduced by 65.11% and water-insoluble EPS synthesis was decreased by 48.00%. As shown in Fig. 1C, GH12 at concentrations of 16 mg/liter or less did not prevent the acidification of the culture medium effectively, while GH12 at 32 mg/liter and 64 mg/liter slowed the pH decline. Though GH12 at 32 mg/liter maintained the medium pH at 5.0 at 47 h and 52 h, the pH values were not statistically significantly different from those for the groups treated with the lower concentrations. The pH values for the group treated with 64-mg/liter GH12 were significantly higher than those for every other group at 23 h, 28 h, 47 h, 52 h, 71 h, and 76 h (P < 0.05), and 64-mg/liter GH12 maintained the medium pH above 5.0 even at 71 h and 76 h. GH12 at 64 mg/liter showed the most effective inhibition of the acidification ability of the biofilms.
FIG 1.
Effects of GH12 on the cariogenic properties of the human dental plaque-derived multispecies biofilms. (A) Quantification of water-insoluble EPS synthesis. (B) Quantification of lactic acid production. (C) pH of the culture medium. All the experiments were repeated at least three times. All values are presented as the mean ± SD. Statistical analyses were performed using one-way ANOVA and Tukey’s HSD test. Different superscript letters indicate significant differences (P < 0.05). *, the pH values in the group treated with 64-mg/liter GH12 were significantly different from those in every other group (P < 0.05).
As shown in Fig. 2, 8-mg/liter GH12 and 16-mg/liter GH12 had no impact on the structure or the integrity of the dental plaque-derived multispecies biofilms. When treated with 32-mg/liter GH12, a slight reduction in the extracellular matrix was observed. GH12 at 64 mg/liter changed the morphology and structure of the biofilms most effectively. When the biofilms of the various groups were compared, the multispecies biofilms of the group treated with 64-mg/liter GH12 were loose, flat, and porous, whereas those of the other groups showed a dense network of bacteria surrounded by a mass of extracellular matrix.
FIG 2.
Representative SEM images of biofilms treated with DDW and GH12.
Because GH12 at 64 mg/liter showed the most effective inhibition of the cariogenic properties of the human dental plaque-derived multispecies biofilms, 64-mg/liter GH12 was chosen for subsequent in vitro and in vivo evaluations.
GH12 did not perturb the microbiota of human dental plaque-derived multispecies biofilms from healthy volunteers.
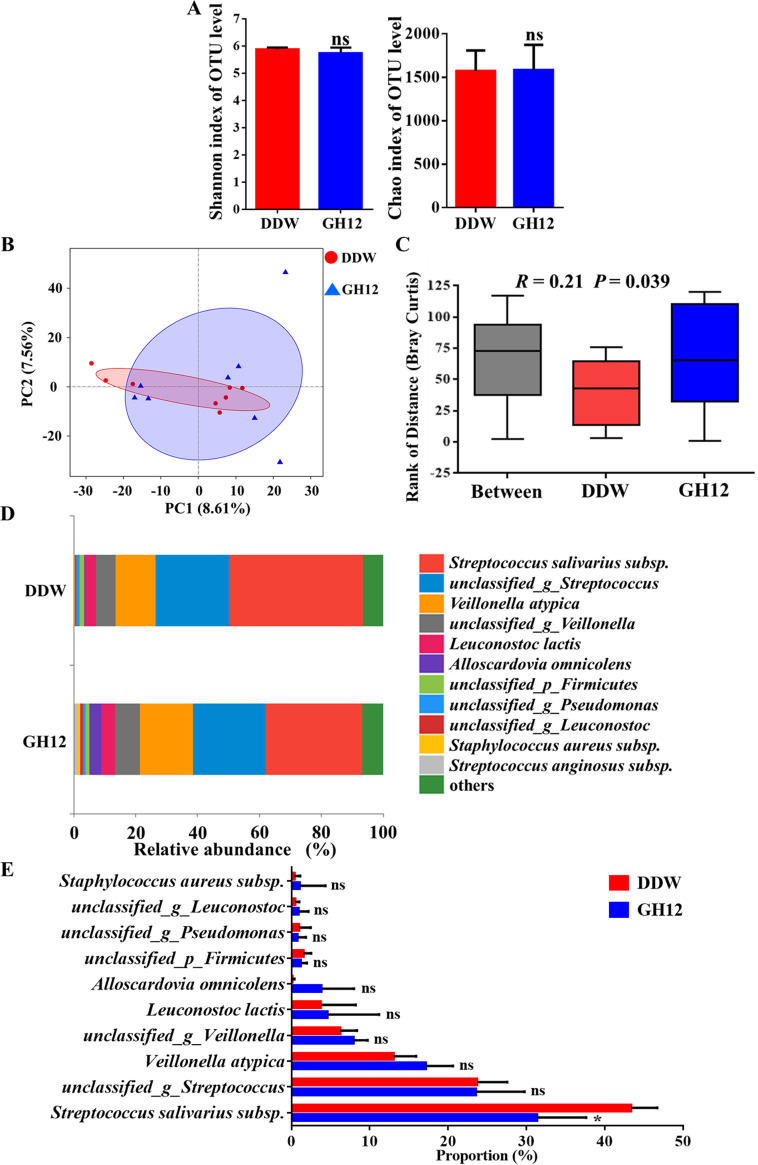
Alpha diversity was measured using the Shannon index and the Chao index at the operational taxonomic unit (OTU) level (Fig. 3A). These indices are estimators of community diversity and community richness, respectively. The richness index and the diversity index of the bacterial communities of the human dental plaque-derived multispecies biofilms in the group treated with GH12 (the GH12 group) were not significantly affected (P > 0.05), which indicated that biofilms treated with GH12 maintained their ecological diversity. The beta diversity of the two groups was analyzed using a principal-component analysis (PCA) plot at the OTU level to investigate microbial community differences. As shown in Fig. 3B, the samples from the GH12 group and the distilled deionized water (DDW)-treated group (the DDW group) clustered coherently in the PCA plot, suggesting that the two groups possessed similar microbial community structures. As shown in Fig. 3C, the level of between-group differences was similar to that of the within-group differences in the analysis of similarities (ANOSIM) box plot (R = 0.21, P = 0.039) (19). Figure 3D visually illustrates the relative abundance of the bacterial communities at the species level. The microbial composition of the GH12 group was similar to that of the DDW group. In a further comparison by the Wilcoxon rank-sum test (Fig. 3E), among the top 10 most abundant species, only the abundance of Streptococcus salivarius subspecies was significantly reduced in the GH12 group (P < 0.05), and no significant differences in the abundances of the other species were observed between the DDW group and the GH12 group (P > 0.05). The findings presented above indicate that, after the treatment with 64-mg/liter GH12, human dental plaque-derived multispecies biofilms maintained the ecological diversity of the DDW group and possessed a microbial community structure similar to that of the DDW group.
FIG 3.
Effects of GH12 on the microbiota of the human dental plaque-derived multispecies biofilms. (A) Shannon index and Chao index at the OTU level. Values are presented as the mean ± SD (n = 8). Statistical analyses were performed using Student’s t test. ns, not significant (P > 0.05). (B) PCA at the OTU level (n = 8). (C) Rank of distance based on the Bray-Curtis distance between or within groups. Values are presented as the mean ± SD (n = 8). Statistical analyses were performed using ANOSIM. (D) Relative abundance of species-level taxa in the DDW group and the GH12 group. (E) Proportions of the top 10 most abundant species in the DDW group and the GH12 group. Values are presented as the mean ± SD (n = 8). Statistical analyses were performed using the Wilcoxon rank-sum test. *, P < 0.05; ns, not significant (P > 0.05); _p_, phylum; _g_, genus.
GH12 reduced the incidence and severity of dental caries in the rat caries model.
As shown in Table 1, GH12 at 64 mg/liter significantly decreased the incidence and severity of sulcal caries and smooth surface caries in all locations through the 5-week treatment. When treated with 64-mg/liter GH12, the scores of the enamel-only sulcal caries (P < 0.0001), slight dentinal sulcal caries (P < 0.05), and moderate dentinal sulcal caries (P < 0.05) were all significantly decreased, and the scores of smooth surface caries followed the same trend. Extensive sulcal dentinal caries and extensive smooth surface caries were not detected in the GH12 group. In addition, GH12 did not affect the ponderal growth of the rats, and all rats were in good health without any differences in weight or the rate of death (P > 0.05).
TABLE 1.
Keyes caries scores and weights of the rats
| Characteristica | Value for the following groupb: |
|
|---|---|---|
| DDW | GH12 | |
| Sulcal caries score | ||
| Enamel only | 38.60 ± 6.00 | 17.80 ± 4.4.85**** |
| Slight dentinal | 21.20 ± 4.92 | 12.80 ± 8.35* |
| Moderate dentinal | 6.00 ± 5.72 | 1.40 ± 2.37* |
| Extensive dentinal | 0.80 ± 0.97 | 0.0 ± 0.0* |
| Smooth surface caries score | ||
| Enamel only | 30.00 ± 6.51 | 12.80 ± 6.01**** |
| Slight dentinal | 6.00 ± 4.81 | 1.60 ± 2.15* |
| Moderate dentinal | 3.00 ± 2.41 | 0.60 ± 1.28* |
| Extensive dentinal | 1.60 ± 1.74 | 0.0 ± 0.0* |
| Wt (g) | 180.86 ± 19.22 | 179.62 ± 22.22NS |
Slight dentinal, less than 1/4 of the dentin affected; moderate dentinal, 1/4 to 3/4 of the dentin affected; extensive dentinal, more than 3/4 of the dentin affected.
Values are presented as the mean ± standard deviation (n = 10). Statistical analyses were performed using Student’s t test. *, P < 0.05; ****, P < 0.0001; NS, not significant (P > 0.05).
GH12 regulated the microbiota of dental plaque in the rat caries model in vivo.
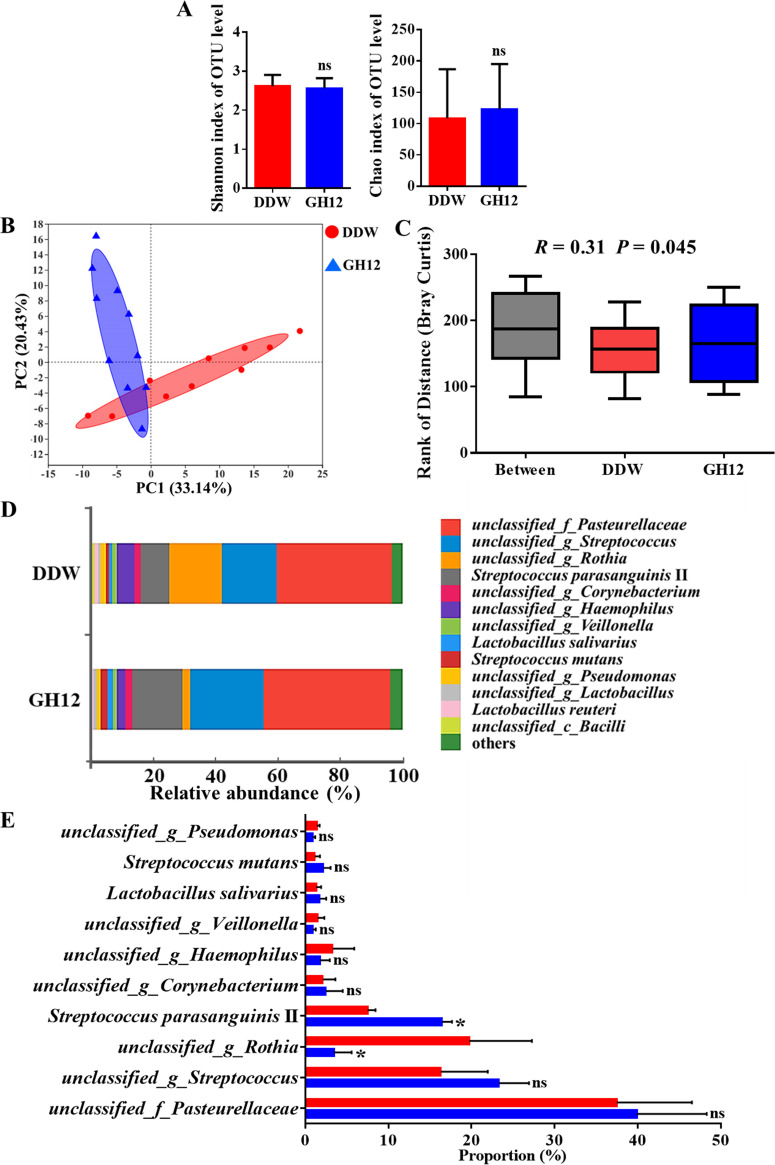
As shown in Fig. 4A, no significant differences in the Shannon index and the Chao index were found between the DDW group and the GH12 group (P > 0.05), which demonstrated that the alpha diversity was not impacted by GH12. The PCA plot at the OTU level showed that there was only a partial overlap between the microbial structure of the DDW group and that of the GH12 group (Fig. 4B). In addition, as shown in Fig. 4C, the between-group difference was slightly larger than the within-group difference in the ANOSIM box plot (R = 0.31, P = 0.045). The results presented above demonstrate that 64-mg/liter GH12 regulates the microbial community structure of dental plaque in the rat caries model to a certain extent while preserving the microecological diversity.
FIG 4.
Microbiota analysis of the DDW group and the GH12 group in vivo. (A) The Shannon index and the Chao index at the OTU level. Values are presented as the mean ± SD (n = 10). Statistical analyses were performed using Student’s t test. ns, not significant (P > 0.05). (B) PCA at the OTU level. (C) Rank of distance based on the Bray-Curtis distance between or within groups. Values are presented as the mean ± SD (n = 10). Statistical analyses were performed using ANOSIM. (D) Relative abundance of species-level taxa in the DDW group and the GH12 group. (E) Proportions of the top 10 most abundant species in the DDW group and the GH12 group. Values are presented as the mean ± SD (n = 10). Statistical analyses were performed using the Wilcoxon rank-sum test. *, P < 0.05; ns, not significant (P > 0.05); _f_, family; _c_, class.
The bar plot in Fig. 4D visually illustrates the relative abundance of the bacterial species. The abundance of the species and the structure of the microbial communities of the DDW group and the GH12 group were different, which was consistent with the results of ANOSIM. Taxon-based analysis by the Wilcoxon rank-sum test was further used to compare the differences in the top 10 most abundant species between the two groups (Fig. 4E). The differences between the DDW group and the GH12 group demonstrated the ecological role of GH12 treatment in the development of dental caries under cariogenic conditions. The abundance of unclassified_g_Rothia (unclassified organisms of the genus Rothia) was much lower in the GH12 group than in the DDW group (P < 0.05), while the abundance of Streptococcus parasanguinis II was much higher in the GH12 group than in the DDW group (P < 0.05). No significant difference in the abundance of S. mutans was observed between the DDW group and the GH12 group (P > 0.05).
DISCUSSION
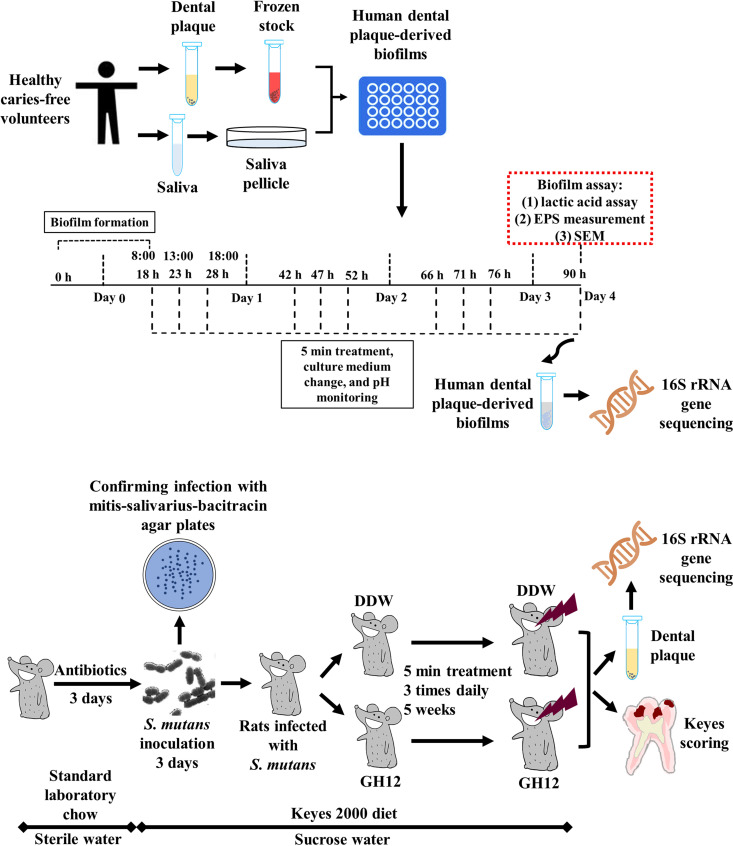
Although AMPs are promising alternatives to conventional antimicrobial strategies with the advantages of potent antimicrobial activities and pleiotropic bioactive functions (20), few studies have explored the effects of AMPs in vivo or on multispecies bacterial communities in vitro (11, 21). As shown in Fig. 5, multiple models were used to evaluate the efficacy of GH12 in this study. The dental plaque-derived multispecies biofilm model, representing the diversity (22) and the overall metabolic functionality (23) of the oral microbiome, provided an easy and ethically sound platform for evaluating the potential efficacy of antimicrobials on the cariogenic properties and microbial ecology of human dental plaque in vitro (24). The rat caries model and the Keyes system could provide detailed and comprehensive information about caries locations and severities, and they could be used to assess the in vivo efficacy of antimicrobials on caries prevention and their influence on the microbiota (9, 25). The short-term treatment better mimicked the topical application of anticaries agents in practical use. One of the novel aspects of this study was the systematic evaluation of the anticaries effects and the microecological regulation effects of GH12 in vitro and in vivo by the combined utilization of the above-described methods to explore its potential for ecological caries prevention.
FIG 5.
Schematic design of the present study. The anticaries effects and ecological regulation effects of the antimicrobial peptide GH12 were evaluated in both the complex biofilm model and the animal caries model.
An effective caries prevention method needs to be able to disrupt the biofilm matrix and protect the tooth surface from acid attack. Acid accumulation leads to reduced pH and demineralization. EPS enhances bacterial adhesion, provides mechanical stability, and protects biofilms from antimicrobials (5). In this study, 64-mg/liter GH12 significantly suppressed lactic acid production, water-insoluble EPS synthesis, and the acidification ability of the human dental plaque-derived multispecies biofilm and destroyed the scaffold structure of the biofilms (Fig. 1 and 2), which demonstrated that GH12 could suppress the cariogenic properties of dental plaque, prevent localized acidification, and make it easier to remove. In a previous study, 8-mg/liter GH12 suppressed the cariogenic properties of preformed S. mutans biofilms (18). However, in this study, GH12 at 8 mg/liter showed no inhibitory effects on the dental plaque-derived multispecies biofilms and only a high concentration of GH12 of 64 mg/liter significantly suppressed the cariogenic properties of the dental plaque-derived multispecies biofilms. The differences in these results suggest that dental plaque may show much stronger resistance to antimicrobial agents than simple biofilms and that the use of an S. mutans biofilm model alone is insufficient to evaluate the in vitro anticaries effects.
The results of PCA and ANOSIM in this study showed that there were two different trends of changes to the microbiota in the human dental plaque-derived multispecies biofilms (Fig. 3B and C) and in the plaque samples of the animal caries model (Fig. 4B and C). The profile for the DDW group was largely a subset of the profile for the GH12 group in the PCA plot of the human dental plaque-derived multispecies biofilms (Fig. 3B), while the profiles of the two groups in the rat caries model showed only a partial overlap (Fig. 4B). In ANOSIM, the R value represents the magnitude of the between-group difference and the P value is used to determine the significance of the between-group difference (19). The R value below 0.25 (Fig. 3C) suggested a strong overlap between the microbial communities of the DDW group and the GH12 group in the human dental plaque-derived multispecies biofilms, while the R value above 0.25 (Fig. 4C) was considered evidence of separation that indicated certain differences between the two groups in the rat caries model (19).
A healthy oral ecosystem may shift toward dysbiosis under an overintervention with external factors, and more and more studies have emphasized the importance of preserving the diversity and stability of the oral microbiota (10, 26). In this study, the microbiota of the dental plaque-derived multispecies biofilm was dominated by streptococci, which was a pattern similar to that seen in previous studies (22, 23), which demonstrated the successful establishment of the dental plaque-derived multispecies biofilms from healthy caries-free volunteers. The ideal antimicrobial approach is to suppress the cariogenic properties of dental plaque without causing microecological dysbiosis. GH12 at 64 mg/liter did not reduce the microbial diversity of the human dental plaque-derived multispecies biofilm (Fig. 3A). Admittedly, the relative abundance of S. salivarius decreased in the GH12 group (Fig. 3E), probably because of the potent antibacterial activity of GH12 against the bacteria (14). The role of S. salivarius in dental caries is controversial. Some studies consider S. salivarius bacteria to be commensal and probiotic (27), while others believe that S. salivarius is involved in dental caries (28, 29). However, GH12 did not affect the dominant proportion of S. salivarius, and other abundant species were not impacted (Fig. 3D and E). In addition, after treatment with 64-mg/liter GH12, the human dental plaque-derived multispecies biofilms still possessed a microbial community structure similar to that of the DDW group (Fig. 3B and C). Overall, GH12 did not perturb the microbiome of the human dental plaque-derived multispecies biofilms from the healthy volunteers.
Use of the rat caries model with exposure to caries-promoting diets helped with exploration of the ecological role of GH12 in a cariogenic environment. In the animal study, the incidence and severity of dental caries were significantly reduced when the animals were treated with 64-mg/liter GH12 (Table 1), and there were certain differences between the GH12 group and the DDW group in the microbiota of the rat dental plaque (Fig. 4B and C), which suggested that GH12 protected teeth from cariogenic factors and regulated the microbiota of dental plaque to a certain extent under conditions of exposure to a caries-promoting diet. An interesting point to note is that the relative abundance of S. mutans, which has been considered the principal pathogen of dental caries, did not differ between the GH12 group and the DDW group (Fig. 4E). Although S. mutans has been used as an essential pathogen to induce dental caries in animal studies, more recent molecular analyses have revealed that the proportion of S. mutans bacteria is not strictly linearly correlated with dental caries (30). Cariogenic bacteria can frequently be detected in healthy individuals (31). S. mutans is not the only determinant of dental caries, as multiple microorganisms act collectively to contribute to the development of dental caries under the influence of host and dietary factors (31). S. parasanguinis II was much more abundant and Rothia was much less abundant in the GH12 group than in the DDW group, which could result in a less cariogenic microbiota. S. parasanguinis II antagonizes S. mutans via arginine metabolism and H2O2 production, which inhibit the activity of S. mutans, prevent the acidification of biofilms, and decrease the risk for dental caries (32). S. parasanguinis II may play a crucial role in the promotion of stable, health-associated oral biofilm communities by moderating the plaque pH and interfering with the growth and virulence of caries pathogens (33). Rothia is commonly associated with dental caries and can help with the later colonization of other cariogenic bacteria (34, 35). Species of Rothia can often be identified as bacteria enriched in individuals with dental caries (35, 36). These results imply a promising ecological effect of GH12, in which GH12 regulates the microbial ecology of the dental plaque under exposure to a cariogenic diet, increases the proportion of commensal and probiotic bacteria, and reduces the abundance of caries-associated bacteria.
Although GH12 at 8 mg/liter was previously found to prevent caries development (18), GH12 at 64 mg/liter showed a greater inhibition of the incidence and the severity of dental caries in this study. In the previous study, GH12 at 8 mg/liter reduced the sulcal caries scores by approximately 20% and the smooth surface caries scores by approximately 55% (18). In this study, GH12 at 64 mg/liter reduced the sulcal caries scores by approximately 55% and the smooth surface caries by approximately 60%. GH12 at 64 mg/liter showed much better effects on sulcal caries than 8-mg/liter GH12, and this high concentration did not show negative effects on the rats’ health in this study (Table 1) or the growth of human gingival fibroblasts in previous studies (14, 15). However, the effects of GH12 at 64 mg/liter on smooth surface caries were similar to those at the low concentration, which indicates that, in addition to the concentration-dependent inhibitory effects on dental plaque biofilms, GH12 may also possess certain anticaries activities working at both high and low concentrations. Therefore, the potential of GH12 is worth further investigation.
Although the dental plaque-derived multispecies biofilm and the rat caries model were used to mimic dental plaque and for evaluation of the practical therapeutic application of GH12, they cannot entirely represent the effects of GH12 in the human oral cavity, and further studies should therefore be conducted to evaluate the effects of GH12. The saliva microbiota should also be investigated to further explore the ecological effects of GH12. The use of dental plaque from subjects with caries active in culture medium containing high and low concentrations of sucrose would provide valuable information on the role of GH12 in taming the cariogenic microbiota. In addition, a scrambled peptide will be used as a negative control in further studies to determine the effects of the sequence on antimicrobial activity and to further explore the features of GH12.
In summary, GH12 inhibited the cariogenic properties of dental plaque without perturbing the microbiota of healthy individuals, and GH12 regulated the dysbiotic microbial ecology of dental plaque to a certain extent under cariogenic conditions and arrested caries development. Treatment with GH12 possesses the potential to be a novel approach to dental caries control.
MATERIALS AND METHODS
Peptides, chemicals, assay kits, and bacterial strains.
GH12 (Gly-Leu-Leu-Trp-His-Leu-Leu-His-His-Leu-Leu-His-NH2) was synthesized and identified by GL Biochem (Shanghai, China) (15). GH12 was dissolved in sterilized distilled deionized water (DDW) and stored at −20°C. Unless otherwise stated, chemicals and assay kits were purchased from Hopebio (Qingdao, China). S. mutans UA159, used for the rat caries model, was obtained from the State Key Laboratory of Oral Diseases of Sichuan University. S. mutans was grown in brain heart infusion broth (Oxoid, UK) anaerobically (85% N2, 10% H2, 5% CO2) at 37°C. SHI medium (37) was used for preparing a frozen stock and cultivating dental plaque-derived multispecies biofilms, which contained 10-g/liter proteose peptone, 5-g/liter Trypticase peptone (BD Difco, USA), 5-g/liter yeast extract (Oxoid, UK), 2.5-g/liter KCl, 5-g/liter sucrose, 5-mg/liter hemin, 1-mg/liter vitamin K, 0.06-g/liter urea, 0.174-g/liter arginine, 2.5-g/liter mucin (type III; porcine, gastric; Sigma-Aldrich, USA), 5% sheep blood (Baoxin, Chengdu, China), and 10-mg/liter N-acetylmuramic acid (Sigma-Aldrich, USA).
Human dental plaque sampling and frozen stock preparation.
Human dental plaque was collected from five healthy caries-free volunteers (3 males and 2 females) aged 22 to 24 years old with the approval of the Institutional Review Board of the West China Hospital of Stomatology (approval number WCHSIRB-D-2018-109). None of the subjects was taking any medications, and the subjects were not under treatment for any systemic disease or oral disease. The subjects were asked to refrain from food or drink overnight. Before oral hygiene measures in the morning, dental plaque from the supragingival regions of the first molars was sampled using oral swabs, and the samples were pooled to prepare a frozen stock (11, 23). As described previously (11), 1 ml pooled sample was seeded into 5 ml SHI medium and incubated anaerobically at 37°C for 24 h. After centrifugation (14,000 × g, 3 min) and resuspension into fresh SHI medium with 20% glycerol, the stocks were stored at −80°C.
Cultivation of human dental plaque-derived multispecies biofilms.
Dental plaque-derived multispecies biofilms were established with SHI medium containing 0.5% sucrose (22). The saliva used for pellicle formation was collected from five volunteers with approval (approval number WCHSIRB-D-2018-109, Institutional Review Board of the West China Hospital of Stomatology). The saliva was sterilized with filters (pore size, 0.22 μm; ultra-low-binding protein filter; Millipore, USA). Briefly, 100 μl saliva was added into 24-well plates to allow saliva pellicle growth at 37°C for 2 h, after which 10 μl stock and 1 ml medium were added. The cultivation and treatment of the dental plaque-derived multispecies biofilms are detailed in Fig. 5. The plates were incubated at 37°C anaerobically for 18 h to allow biofilm formation. The preformed biofilms were treated with 1 ml of DDW or GH12 for 5 min three times daily at 18 h, 23 h, 28 h, 42 h, 47 h, 52 h, 66 h, 71 h, 76 h, and 90 h. After each treatment, the biofilms were washed with phosphate-buffered saline (PBS) and fresh SHI medium was added. The used culture medium was collected for pH measurement. After the 10th and final treatment, the 90-h-old biofilms were subsequently used for the lactic acid assay, water-insoluble EPS measurement, observation by scanning electron microscopy (SEM), and 16S rRNA gene sequencing.
Lactic acid assay.
Lactic acid levels were measured as described previously (38). The biofilms were rinsed with PBS, and 1 ml buffered peptone water containing 0.2% sucrose was added. After incubation at 37°C anaerobically for 2 h, the supernatants were tested with a lactate assay kit (catalog number A019-2; Jiancheng, Nanjing, China). The absorbance at 570 nm was detected, and lactate concentrations were calculated using a standard curve.
Water-insoluble EPS measurement.
The water-insoluble EPS level was measured using the anthrone method (39). Biofilms were collected in PBS. After centrifugation (8,000 rpm, 5 min), the precipitates were washed with sterile distilled water and resuspended in 1 ml of 0.4 M NaOH. After centrifugation, 200 μl supernatant was mixed with 600 μl the anthrone reagent at 95°C for 6 min. The absorbance at 625 nm was detected, and the concentrations were calculated with a standard curve.
pH measurement.
The pH values were measured at every time point when the medium was changed (Fig. 5). The initial pH of the medium was adjusted to 7.2. At the time of medium change, the replaced medium was collected and the pH was tested using an Orion Dual Star pH benchtop meter (Thermo Scientific, USA).
Observations by SEM.
Observations by SEM were conducted as described previously (15). The dental plaque-derived multispecies biofilms were cultivated on saliva-coated coverslips in 24-well plates, and the cultivation and treatment were performed as described above. The biofilms were rinsed with PBS, fixed in 2.5% glutaraldehyde overnight, and serially dehydrated in ethanol for 30 min in the presence of increasing concentrations (35%, 50%, 75%, 2 × 90%, and 2 × 100%) (40). Samples were dried to the critical point, sputter coated, and observed using a scanning electron microscope (Inspect F; FEI, The Netherlands) at 20.0 kV.
DNA isolation and 16S rRNA gene sequencing.
Genomic DNA of the human dental plaque-derived multispecies biofilms was isolated using a QIAamp DNA minikit (catalog number 51304; Qiagen, Germany). Samples were prepared for 16S rRNA gene amplification (V1 to V3 region) and sequencing at Majorbio (Shanghai, China) with an MiSeq 300 PE sequencer (Illumina MiSeq System) using primers described previously (primers 27F [5′-AGAGTTTGATCCTGGCTCAG-3′] and 534R [5′-ATTACCGCGGCTGCTGG-3′]) (11). Both groups contained two biofilm samples. The experiment was independently repeated four times. A total of eight samples in each group were prepared (n = 8). Bioinformatics analyses were performed by use of the mothur and QIIME (version 2.0) programs. Operational taxonomic unit (OTU) clustering was performed using the Usearch program. Sequences were aligned and taxonomically assigned by use of the sequences in the Silva database. To avoid biases due to different sequencing depths, OTU tables were rarefied to the lowest number of sequences per sample. Analyses were performed on the I-Sanger cloud platform. For alpha diversity, the Shannon index and the Chao index were calculated at the OTU level. For beta diversity, principal-component analysis (PCA) was performed at the OTU level, and analysis of similarities (ANOSIM) based on the Bray-Curtis distance was used to examine the community differences (24). The average relative abundances of the species were shown with bar plots. Differences in the relative abundance between the two groups at the species level were evaluated using the Wilcoxon rank-sum test (41).
Rat caries model.
The animal experiment was conducted with approval (approval number WCHSIRB-D-2017-133) and followed the national guidelines for the requirements of environmental and housing facilities for laboratory animals (GB14925-2010 guidelines) and the ARRIVE guidelines (42). The animal experiment was performed with a modified rat caries model (18). Twenty male specific-pathogen-free Sprague-Dawley rats aged 17 days were purchased from the Animal Experimental Centre of Sichuan University. The rats were treated with antibiotics (by use of a diet containing 0.1% ampicillin, 0.1% chloramphenicol, and 0.1% carbenicillin and water with 4,000 U/ml penicillin) for 3 days (43), during which they were offered standard laboratory chow (Trophic, Nantong, China) and sterile distilled water ad libitum (44). The rats were then infected orally with S. mutans UA159 for 3 days, and the infection was confirmed by oral swabbing and culturing on mitis-salivarius-bacitracin agar plates (45, 46). The infected animals were randomly assigned to one of two groups: the DDW group and the GH12 group. They were treated with DDW or GH12 topically using a camel hair brush for 5 min three times daily for 5 weeks. The rats were fed the cariogenic Keyes 2000 diet (Trophic, Nantong, China) and 5% sucrose water ad libitum during the inoculation of S. mutans and during the 5-week treatment (18). The rats’ weights were recorded to monitor for signs of toxicity.
Rat dental plaque sampling and 16S rRNA gene sequencing.
Immediately following the 5-week treatment, dental plaque was sampled from the supragingival regions of the rats in the DDW group and the GH12 group (n = 10 per group) by oral swabbing while the rats were under intraperitoneal anesthesia with chloral hydrate. The swab samples were collected and placed in sterile Eppendorf tubes, which were stored on dry ice, after which genomic DNA was extracted and purified from the swabs with a QIAamp DNA minikit (catalog number 51304; Qiagen, Germany) (47). The resulting DNA samples were sent to Majorbio (Shanghai, China) for 16S rRNA gene sequencing. The process of 16S rRNA gene sequencing was the same as that used for the human dental plaque-derived multispecies biofilms. For alpha diversity, the Shannon index and the Chao index were calculated at the OTU level. For beta diversity, PCA at the OTU level and ANOSIM were conducted. The average relative abundances of the species were shown with bar plots. Differences in the relative abundances between the two groups at the species level were calculated using the Wilcoxon rank-sum test (41).
Keyes scoring.
The rats were euthanized by CO2 asphyxiation, and the jaws were surgically removed and aseptically dissected. All jaws were fixed and stained with 0.4% murexide for 12 h. The main sulcus of each tooth was exposed by a mesiodistal sagittal hemisection. The sulcus and smooth surfaces were used for caries scoring using the Keyes system (48).
Statistical analysis.
The alpha diversity indices, the Keyes scores, and the rats’ weights were analyzed by Student’s t test. Lactic acid production, water-insoluble EPS synthesis, and pH measurements were analyzed by one-way analysis of variance (ANOVA) and Tukey’s honestly significant difference (HSD) test. The data were statistically analyzed by GraphPad Prism (version 6) software (GraphPad Software, San Diego, CA, USA), and differences were considered significant when the P value was <0.05.
Accession number(s).
The 16S rRNA gene sequences are available in the Sequence Read Archive of NCBI (BioProject PRJNA606440).
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (grants 81771062 and 81970931), the National Innovation and Entrepreneurship Training Program (grant C2020109411), and the Innovation Sparking Program of Sichuan University.
We declare no other potential conflicts of interest with respect to the authorship and/or publication of this article.
Wentao Jiang and Yufei Wang contributed to conceptualization, data curation, formal analysis, investigation, methodology, validation, and writing (original draft); Junyuan Luo, Xiangshu Chen, Yuhao Zeng, Xinwei Li, and Zening Feng contributed to investigation and validation; Linglin Zhang contributed to conceptualization, supervision, and writing (review and editing). We all gave final approval and agreed to be accountable for all aspects of the work.
REFERENCES
- 1.Zemaitiene M, Grigalauskiene R, Vasiliauskiene I, Saldunaite K, Razmiene J, Slabsinskiene E. 2016. Prevalence and severity of dental caries among 18-year-old Lithuanian adolescents. Medicina (Kaunas) 52:54–60. doi: 10.1016/j.medici.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Edelstein BL. 2006. The dental caries pandemic and disparities problem. BMC Oral Health 6:S2. doi: 10.1186/1472-6831-6-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takahashi N, Nyvad B. 2011. The role of bacteria in the caries process: ecological perspectives. J Dental Res 90:294–303. doi: 10.1177/0022034510379602. [DOI] [PubMed] [Google Scholar]
- 4.Bowen WH, Burne RA, Wu H, Koo H. 2018. Oral biofilms: pathogens, matrix, and polymicrobial interactions in microenvironments. Trends Microbiol 26:229–242. doi: 10.1016/j.tim.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y, Ren Z, Hwang G, Koo H. 2018. Therapeutic strategies targeting cariogenic biofilm microenvironment. Adv Dent Res 29:86–92. doi: 10.1177/0022034517736497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gross EL, Beall CJ, Kutsch SR, Firestone ND, Leys EJ, Griffen AL. 2012. Beyond Streptococcus mutans: dental caries onset linked to multiple species by 16S rRNA community analysis. PLoS One 7:e47722. doi: 10.1371/journal.pone.0047722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johansson I, Witkowska E, Kaveh B, Holgerson PL, Tanner A. 2016. The microbiome in populations with a low and high prevalence of caries. J Dent Res 95:80–86. doi: 10.1177/0022034515609554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuang X, Chen V, Xu X. 2018. Novel approaches to the control of oral microbial biofilms. Biomed Res Int 2018:6498932. doi: 10.1155/2018/6498932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia SS, Blackledge MS, Michalek S, Su L, Ptacek T, Eipers P, Morrow C, Lefkowitz EJ, Melander C, Wu H. 2017. Targeting of Streptococcus mutans biofilms by a novel small molecule prevents dental caries and preserves the oral microbiome. J Dent Res 96:807–814. doi: 10.1177/0022034517698096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rasmussen K, Nikrad J, Reilly C, Li Y, Jones RS. 2016. N-Acetyl-l-cysteine effects on multi-species oral biofilm formation and bacterial ecology. Lett Appl Microbiol 62:30–38. doi: 10.1111/lam.12513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo L, McLean JS, Yang Y, Eckert R, Kaplan CW, Kyme P, Sheikh O, Varnum B, Lux R, Shi W, He X. 2015. Precision-guided antimicrobial peptide as a targeted modulator of human microbial ecology. Proc Natl Acad Sci U S A 112:7569–7574. doi: 10.1073/pnas.1506207112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eckert R, He J, Yarbrough DK, Qi F, Anderson MH, Shi W. 2006. Targeted killing of Streptococcus mutans by a pheromone-guided “smart” antimicrobial peptide. Antimicrob Agents Chemother 50:3651–3657. doi: 10.1128/AAC.00622-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mai S, Mauger MT, Niu LN, Barnes JB, Kao S, Bergeron BE, Ling JQ, Tay FR. 2017. Potential applications of antimicrobial peptides and their mimics in combating caries and pulpal infections. Acta Biomater 49:16–35. doi: 10.1016/j.actbio.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 14.Tu H, Fan Y, Lv X, Han S, Zhou X, Zhang L. 2016. Activity of synthetic antimicrobial peptide GH12 against oral streptococci. Caries Res 50:48–61. doi: 10.1159/000442898. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Fan Y, Zhou Z, Tu H, Ren Q, Wang X, Ding L, Zhou X, Zhang L. 2017. De novo synthetic short antimicrobial peptides against cariogenic bacteria. Arch Oral Biol 80:41–50. doi: 10.1016/j.archoralbio.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Wang X, Jiang W, Wang K, Luo J, Li W, Zhou X, Zhang L. 2018. Antimicrobial peptide GH12 suppresses cariogenic virulence factors of Streptococcus mutans. J Oral Microbiol 10:1442089. doi: 10.1080/20002297.2018.1442089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang W, Wang Y, Luo J, Li X, Zhou X, Li W, Zhang L. 2018. Effects of antimicrobial peptide GH12 on the cariogenic properties and composition of a cariogenic multispecies biofilm. Appl Environ Microbiol 84:e01423-18. doi: 10.1128/AEM.01423-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Zeng Y, Wang Y, Li H, Yu S, Jiang W, Li Y, Zhang L. 2019. Antimicrobial peptide GH12 targets Streptococcus mutans to arrest caries development in rats. J Oral Microbiol 11:1549921. doi: 10.1080/20002297.2018.1549921. [DOI] [Google Scholar]
- 19.McCord AI, Chapman CA, Weny G, Tumukunde A, Hyeroba D, Klotz K, Koblings AS, Mbora DN, Cregger M, White BA, Leigh SR, Goldberg TL. 2014. Fecal microbiomes of non-human primates in western Uganda reveal species-specific communities largely resistant to habitat perturbation. Am J Primatol 76:347–354. doi: 10.1002/ajp.22238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mai S, Zhao Y, Song Q. 2016. Chemoselective acylation of benzimidazoles with phenylacetic acids under different Cu catalysts to give fused five-membered N-heterocycles or tertiary amides. Org Biomol Chem 14:8685–8690. doi: 10.1039/c6ob01167e. [DOI] [PubMed] [Google Scholar]
- 21.Sullivan R, Santarpia P, Lavender S, Gittins E, Liu Z, Anderson MH, He J, Shi W, Eckert R. 2011. Clinical efficacy of a specifically targeted antimicrobial peptide mouth rinse: targeted elimination of Streptococcus mutans and prevention of demineralization. Caries Res 45:415–428. doi: 10.1159/000330510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edlund A, Yang Y, Hall AP, Guo L, Lux R, He X, Nelson KE, Nealson KH, Yooseph S, Shi W, McLean JS. 2013. An in vitro biofilm model system maintaining a highly reproducible species and metabolic diversity approaching that of the human oral microbiome. Microbiome 1:25. doi: 10.1186/2049-2618-1-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agnello M, Cen L, Tran NC, Shi W, McLean JS, He X. 2017. Arginine improves pH homeostasis via metabolism and microbiome modulation. J Dent Res 96:924–930. doi: 10.1177/0022034517707512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng X, He J, Wang L, Zhou S, Peng X, Huang S, Zheng L, Cheng L, Hao Y, Li J, Xu J, Xu X, Zhou X. 2017. Ecological effect of arginine on oral microbiota. Sci Rep 7:7206. doi: 10.1038/s41598-017-07042-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bowen WH. 2013. Rodent model in caries research. Odontology 101:9–14. doi: 10.1007/s10266-012-0091-0. [DOI] [PubMed] [Google Scholar]
- 26.Zaura E, Brandt BW, Prodan A, Teixeira de Mattos MJ, Imangaliyev S, Kool J, Buijs MJ, Jagers FL, Hennequin-Hoenderdos NL, Slot DE, Nicu EA, Lagerweij MD, Janus MM, Fernandez-Gutierrez MM, Levin E, Krom BP, Brand HS, Veerman EC, Kleerebezem M, Loos BG, van der Weijden GA, Crielaard W, Keijser BJ. 2017. On the ecosystemic network of saliva in healthy young adults. ISME J 11:1218–1231. doi: 10.1038/ismej.2016.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poorni S, Srinivasan MR, Nivedhitha MS. 2019. Probiotic Streptococcus strains in caries prevention: a systematic review. J Conserv Dent 22:123–128. doi: 10.4103/JCD.JCD_505_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prashant GM, Chandu GN, Murulikrishna KS, Shafiulla MD. 2007. The effect of mango and neem extract on four organisms causing dental caries: Streptococcus mutans, Streptococcus salivavius, Streptococcus mitis, and Streptococcus sanguis: an in vitro study. Indian J Dent Res 18:148–151. doi: 10.4103/0970-9290.35822. [DOI] [PubMed] [Google Scholar]
- 29.Kressirer CA, Chen T, Lake Harriman K, Frias-Lopez J, Dewhirst FE, Tavares MA, Tanner AC. 2018. Functional profiles of coronal and dentin caries in children. J Oral Microbiol 10:1495976. doi: 10.1080/20002297.2018.1495976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eriksson L, Lif Holgerson P, Esberg A, Johansson I. 2018. Microbial complexes and caries in 17-year-olds with and without Streptococcus mutans. J Dent Res 97:275–282. doi: 10.1177/0022034517731758. [DOI] [PubMed] [Google Scholar]
- 31.Simon-Soro A, Mira A. 2015. Solving the etiology of dental caries. Trends Microbiol 23:76–82. doi: 10.1016/j.tim.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 32.Huang X, Browngardt CM, Jiang M, Ahn SJ, Burne RA, Nascimento MM. 2018. Diversity in antagonistic interactions between commensal oral streptococci and Streptococcus mutans. Caries Res 52:88–101. doi: 10.1159/000479091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang X, Palmer SR, Ahn SJ, Richards VP, Williams ML, Nascimento MM, Burne RA. 2016. A highly arginolytic Streptococcus species that potently antagonizes Streptococcus mutans. Appl Environ Microbiol 82:2187–2201. doi: 10.1128/AEM.03887-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsuzukibashi O, Uchibori S, Kobayashi T, Umezawa K, Mashimo C, Nambu T, Saito M, Hashizume-Takizawa T, Ochiai T. 2017. Isolation and identification methods of Rothia species in oral cavities. J Microbiol Methods 134:21–26. doi: 10.1016/j.mimet.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 35.Heller D, Helmerhorst EJ, Gower AC, Siqueira WL, Paster BJ, Oppenheim FG. 2016. Microbial diversity in the early in vivo-formed dental biofilm. Appl Environ Microbiol 82:1881–1888. doi: 10.1128/AEM.03984-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vieira AR, Hiller NL, Powell E, Kim LH, Spirk T, Modesto A, Kreft R. 2019. Profiling microorganisms in whole saliva of children with and without dental caries. Clin Exp Dent Res 5:438–446. doi: 10.1002/cre2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tian Y, He X, Torralba M, Yooseph S, Nelson KE, Lux R, McLean JS, Yu G, Shi W. 2010. Using DGGE profiling to develop a novel culture medium suitable for oral microbial communities. Mol Oral Microbiol 25:357–367. doi: 10.1111/j.2041-1014.2010.00585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng L, Weir MD, Xu HH, Kraigsley AM, Lin NJ, Lin-Gibson S, Zhou X. 2012. Antibacterial and physical properties of calcium-phosphate and calcium-fluoride nanocomposites with chlorhexidine. Dent Mater 28:573–583. doi: 10.1016/j.dental.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koo H, Hayacibara MF, Schobel BD, Cury JA, Rosalen PL, Park YK, Vacca-Smith AM, Bowen WH. 2003. Inhibition of Streptococcus mutans biofilm accumulation and polysaccharide production by apigenin and tt-farnesol. J Antimicrob Chemother 52:782–789. doi: 10.1093/jac/dkg449. [DOI] [PubMed] [Google Scholar]
- 40.Weber K, Delben J, Bromage TG, Duarte S. 2014. Comparison of SEM and VPSEM imaging techniques with respect to Streptococcus mutans biofilm topography. FEMS Microbiol Lett 350:175–179. doi: 10.1111/1574-6968.12334. [DOI] [PubMed] [Google Scholar]
- 41.Santigli E, Trajanoski S, Eberhard K, Klug B. 2016. Sampling modification effects in the subgingival microbiome profile of healthy children. Front Microbiol 7:2142. doi: 10.3389/fmicb.2016.02142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. 2010. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. J Pharmacol Pharmacother 1:94–99. doi: 10.4103/0976-500X.72351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang H, Bi Y, Shang X, Wang M, Linden SB, Li Y, Li Y, Nelson DC, Wei H. 2016. Antibiofilm activities of a novel chimeolysin against Streptococcus mutans under physiological and cariogenic conditions. Antimicrob Agents Chemother 60:7436–7443. doi: 10.1128/AAC.01872-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu J, Miao C, Tian Z, Li J, Zhang C, Yang D. 2018. The effect of chemically modified tetracycline-3 on the progression of dental caries in rats. Caries Res 52:297–302. doi: 10.1159/000481412. [DOI] [PubMed] [Google Scholar]
- 45.Koo H, Pearson SK, Scott-Anne K, Abranches J, Cury JA, Rosalen PL, Park YK, Marquis RE, Bowen WH. 2002. Effects of apigenin and tt-farnesol on glucosyltransferase activity, biofilm viability and caries development in rats. Oral Microbiol Immunol 17:337–343. doi: 10.1034/j.1399-302X.2002.170602.x. [DOI] [PubMed] [Google Scholar]
- 46.Saito T, Ueda O, Teramoto S, Oguchi H, Yanagisawa S, Miyazawa HJ. 2007. Bacteriological evaluation of mutans streptococci using modified mitis-salivarius-bacitracin (MSB) agar medium in primary dentition period. Pediatr Dent J 17:53–57. doi: 10.1016/S0917-2394(07)70095-2. [DOI] [Google Scholar]
- 47.Jia X, Jia L, Mo L, Yuan S, Zheng X, He J, Chen V, Guo Q, Zheng L, Yuan Q, Xu X, Zhou X. 2019. Berberine ameliorates periodontal bone loss by regulating gut microbiota. J Dent Res 98:107–116. doi: 10.1177/0022034518797275. [DOI] [PubMed] [Google Scholar]
- 48.Keyes PH. 1958. Dental caries in the molar teeth of rats. II. A method for diagnosing and scoring several types of lesions simultaneously. J Dent Res 37:1088–1099. doi: 10.1177/00220345580370060901. [DOI] [PubMed] [Google Scholar]