Abstract
Purpose
Cardiovascular diseases (CVDs), including ischemic heart disease, stroke, and heart failure, are well-established late effects of therapy in survivors of childhood and young adult (< 40 years at diagnosis) cancers; less is known regarding CVD in long-term survivors of adult-onset (≥ 40 years) cancer.
Methods
A retrospective cohort study design was used to describe the magnitude of CVD risk in 36,232 ≥ 2-year survivors of adult-onset cancer compared with matched (age, sex, and residential ZIP code) noncancer controls (n = 73,545) within a large integrated managed care organization. Multivariable regression was used to examine the impact of cardiovascular risk factors (CVRFs; hypertension, diabetes, dyslipidemia) on long-term CVD risk in cancer survivors.
Results
Survivors of multiple myeloma (incidence rate ratio [IRR], 1.70; P < .01), carcinoma of the lung/bronchus (IRR, 1.58; P < .01), non-Hodgkin lymphoma (IRR, 1.41; P < .01), and breast cancer (IRR, 1.13; P < .01) had significantly higher CVD risk when compared with noncancer controls. Conversely, prostate cancer survivors had a lower CVD risk (IRR, 0.89; P < .01) compared with controls. Cancer survivors with two or more CVRFs had the highest risk of CVD when compared with noncancer controls with less than two CVRFs (IRR, 1.83 to 2.59; P < .01). Eight-year overall survival was significantly worse among cancer survivors who developed CVD (60%) when compared with cancer survivors without CVD (81%; P < .01).
Conclusion
The magnitude of subsequent CVD risk varies according to cancer subtype and by the presence of CVRFs. Overall survival in survivors who develop CVD is poor, emphasizing the need for targeted prevention strategies for individuals at highest risk of developing CVD.
INTRODUCTION
There are an estimated 14 million cancer survivors living in the United States today, and this number is expected to reach 19 million by 2024.1-3 Among these cancer survivors, two thirds will have survived more than 5 years beyond their cancer diagnosis, and two of every five survivors will be considered a 10-year survivor, contributing to a growing population of aging cancer survivors.1-3 In the general US population, cardiovascular disease (CVD; ischemic heart disease, stroke, or heart failure) is a leading cause of morbidity and mortality, and cardiovascular risk factors (CVRFs; diabetes, hypertension, dyslipidemia) are well-established modifiers of disease risk.4 Research in childhood5 and young adult (< 40 years of age at diagnosis)5,6 cancer survivors has found a substantially higher risk of CVD when compared with the general population; this is largely attributable to exposure to cardiotoxic therapies (eg, anthracyclines, chest radiation) at a young age and emergence of new CVRFs later in life.5,6 Less is known regarding the magnitude of CVD risk in individuals diagnosed with cancer at an older age (≥ 40 years), a population that accounts for 95% of new cancer diagnoses in the United States and has a high prevalence of CVRFs.1,2
Studies examining cardiovascular outcomes in survivors of adult-onset cancers in the United States have, for the most part, focused on adverse events reported on clinical trials,7-10 which may not be generalizable to the large proportion of patients with cancer treated in the community, or have used public administrative databases focused specifically on patients ≥ 65 years old.11-14 Importantly, studies have often described short-term risk as defined by less than 3 years9,15-17 and have not included noncancer comparisons.7,18-21 To address these limitations, we used a retrospective cohort study design to define the risk of CVD in a large population of cancer survivors and controls who were members of Kaiser Permanente Southern California (KPSC), which is an integrated managed care organization that provides comprehensive primary and subspecialty care to over 3.6 million individuals who are broadly representative of the residents in southern California.22
METHODS
Study Population
The KPSC SEER-affiliated cancer registry was used to identify potentially eligible members for the cancer survivor cohort. Eligibility criteria included the following: diagnosis of cancer at KPSC between 2000 and 2007; age 40 years or older at cancer diagnosis; and survived for at least 2 years (index date) after cancer diagnosis. Cancer survivors were excluded (Data Supplement) if they had less than 1 year of KPSC membership before index date; were diagnosed with a rare (< 1% prevalence) primary cancer, or were diagnosed with another cancer before the index date. In addition, because we intended to describe the incidence of late-occurring CVD after completion of therapy, individuals who had developed CVD before the index date were also excluded.
KPSC members without a history of cancer were individually matched (2:1) to cancer survivors by age, sex, and residential ZIP code. Within these matching strata, controls were randomly sampled from the pool of KPSC members who met the following eligibility criteria: had to have at least 1 year of KPSC membership before the corresponding index year for matched cancer survivors, survived and remained a KPSC member until or beyond the index date (ie, 2 years after), and had no history of CVD at the corresponding index date. Control participants were sampled without replacement.
Study participants were observed until the diagnosis of CVD, death, or termination of KPSC membership, whichever came first; date of last follow-up was December 31, 2012. This study was approved by KPSC’s Institutional Review Board, which waived the requirement for informed consent.
Data Collection
Medical information on cancer survivors and controls was obtained using KPSC’s electronic health records system, which accounts for all inpatient and outpatient encounters for its members; this includes membership characteristics, laboratory tests, clinical diagnoses, and medical procedures, linkable through a unique member identifier. Individuals were coded as having CVD if they had two or more outpatient diagnoses on separate days or one inpatient diagnosis or if CVD was the primary cause of death. The International Classification of Diseases (ICD) 9th revision (ICD-9; inpatient and outpatient diagnoses) and 10th revision (ICD-10; death as a result of CVD) codes were used to identify the composite primary outcome of CVD, which included ischemic heart disease (ICD-9 410-414, V45.81/82, 00.66, 36; ICD-10 I20-25), stroke (ICD-9 430-435, 00.61-00.65, 38.1, 39.74; ICD-10 I60-79), and cardiomyopathy/heart failure (ICD-9 425, 428, V42.1, 37.51; ICD-10 I30-I52; Data Supplement). Covariates hypothesized to influence risk of CVD were captured from medical records and KPSC’s cancer registry, including demographics characteristics (age, sex, race/ethnicity), cancer characteristics (site/histology, stage), presence of conventional CVRFs (diabetes, hypertension, dyslipidemia), and other risk factors such as smoking and overweight/obesity. Information on race/ethnicity was missing in 11.5% of noncancer controls; missing information was imputed using the Bayesian Improved Surname Geocoding method.23,24
Diabetes was defined per an algorithm developed by KPSC’s case management system, which uses a combination of ICD-9 codes (250, 357.2, 362.0, 366.41, and 648.0), laboratory test results, and prescription medications (Data Supplement). Hypertension and dyslipidemia were captured using a combination of ICD-9 codes (401 to 405, 272.0 to 272.4) and documentation of receipt of medications for these conditions (Data Supplement). An individual was noted to have multiple CVRFs if they had two or more of the following: hypertension, diabetes, or dyslipidemia. Information on smoking (ever/never smoking at index date) and overweight/obesity relied on ICD-9 codes (smoking: 305.1, V15.82, V68.9, V65.42; overweight/obesity: 278, V85.0 to V85.4) and KPSC’s internal social history and vital sign tables, respectively.25 Date and cause of death were obtained from KPSC’s membership records, state death files, and national Social Security death files.
Statistical Analysis
Cancer survivor versus noncancer controls.
Descriptive statistics for demographics, follow-up time, and prevalence of CVRFs were generated for cancer survivors and the noncancer controls. Cumulative incidence of CVD was calculated taking into consideration competing risk of non-CVD–related death.26 Gray’s test was used to compare cancer survivors and the noncancer controls. Incidence rates for CVD (overall and by CVD subtype [cardiomyopathy/heart failure, ischemic heart disease, stroke]) were calculated using person-time at risk. Crude incidence rate ratio (IRR) was derived by dividing the CVD incidence rate for cancer survivors by the rate for noncancer controls. Multivariable time-dependent Poisson regression was used to derive adjusted IRR, accounting for age, sex, race/ethnicity, diabetes (yes v no), hypertension (yes v no), dyslipidemia (yes v no), smoking (ever v never), and overweight/obesity (yes v no); diabetes, hypertension, and dyslipidemia were adjusted in a time-dependent manner. Bonferroni adjustment was performed to account for multiple comparisons, and P < .003 (.05/15) was considered statistically significant. The following additional analyses were considered exploratory: examination of risk by CVD subtype; risk by cancer stage (survivors with nonhematologic malignancies; see Data Supplement for rationale); and examination of the interaction between CVRFs and cancer history. For the latter, we created a multivariable regression model that included the following categories: noncancer control with less than two CVRFs (referent group), cancer survivor with less than two CVRFs, noncancer control with two or more CVRFs, and cancer survivor with two or more CVRFs.
Survival analysis.
Kaplan-Meier survival curves were generated for cancer survivors and noncancer controls, stratified by CVD status. Among cancer survivors, multivariable time-dependent Cox regression analyses were performed to examine the risk of death among individuals with and without CVD, adjusting for age, sex, race/ethnicity, and cancer stage (model 1). These analyses were further adjusted for CVRFs, overweight/obesity, and smoking history (model 2). All analyses were performed using SAS Version 9.3 (SAS Institute, Cary, NC).
RESULTS
The clinical characteristics of cancer survivors (n = 36,232) and the noncancer controls (n = 73,545) are listed in Table 1. For cancer survivors, median age at diagnosis was 60 years (range 40 to 96 years), and the most common diagnoses were prostate cancer (30.2%), breast cancer (28.8%), colon cancer (7.2%), and melanoma (6.0%). As of December 31, 2012, median follow-up from index date for the cancer survivor cohort was 4.4 years (range, 0 to 8 years), providing 162,487 person-years of follow-up. Among 5-year cancer survivors, 5-year KPSC membership retention rate was 81%; for 10-year survivors, the 10-year retention rate was 70%. The median number of consecutive years of KPSC membership before the index date was 13.1 years (range, 1 to 60 years).
Table 1.
Study Participant Characteristics
| Characteristic | Cancer Survivors* (N = 36,232) | Noncancer Controls* (N = 73,545) | P |
|---|---|---|---|
| Age at diagnosis, years | — | ||
| Median | 60 | 60 | |
| Range | 40.0-96.0 | 40.0-96.0 | |
| Sex, No. (%) | — | ||
| Female | 19,055 (52.6) | 39,225 (53.3) | |
| Male | 17,177 (47.41) | 34,320 (46.7) | |
| Race/ethnicity, No. (%) | < .01 | ||
| Non-Hispanic white | 23,395 (64.6) | 35,087 (47.7) | |
| Hispanic | 5,063 (14.0) | 15,234 (20.8) | |
| Black | 4,683 (12.9) | 7,626 (10.4) | |
| Asian/Pacific Islander | 3,019 (8.3) | 6,379 (8.7) | |
| Other/unknown | 72 (0.2) | 9,129 (12.4) | |
| Follow-up time from index date, years | < .01 | ||
| Median | 4.4 | 4.5 | |
| Range | 0-8.0 | 0-8.0 | |
| Hypertension, No. (%)† | < .01 | ||
| No | 12,344 (34.1) | 29,790 (40.5) | |
| Yes | 23,888 (65.9) | 43,755 (59.5) | |
| Diabetes, No. (%)† | < .01 | ||
| No | 27,745 (76.6) | 57,719 (78.5) | |
| Yes | 8,487 (23.4) | 15,826 (21.5) | |
| Dyslipidemia, No. (%)† | < .01 | ||
| No | 15,257 (42.1) | 32,408 (44.1) | |
| Yes | 20,975 (57.9) | 41,137 (55.9) | |
| Overweight/obese, No. (%)† | < .01 | ||
| No | 20,502 (56.6) | 47,497 (64.6) | |
| Yes | 15,730 (43.4) | 26,048 (35.4) | |
| Smoking, No. (%)† | < .01 | ||
| Never | 24,368 (67.3) | 58,045 (78.9) | |
| Ever | 11,864 (32.7) | 15,500 (21.1) | |
| Cancer type, No. (%) | |||
| Bladder | 509 (1.4) | — | — |
| Breast | 10,429 (28.8) | — | — |
| Chronic lymphocytic leukemia | 519 (1.4) | — | — |
| Colon | 2,601 (7.2) | — | — |
| Kidney | 1,009 (2.8) | — | — |
| Lung and bronchus | 1,195 (3.3) | — | — |
| Melanoma | 2,186 (6.0) | — | — |
| Multiple myeloma | 394 (1.1) | — | — |
| Non-Hodgkin lymphoma | 1,524 (4.2) | — | — |
| Ovarian | 814 (2.3) | — | — |
| Prostate | 10,932 (30.2) | — | — |
| Rectum/rectosigmoid | 1,299 (3.6) | — | — |
| Thyroid | 1,060 (2.9) | — | — |
| Uterus | 1,761 (4.9) | — | — |
Group matching criteria: age (yearly), sex, and ZIP code.
At index date.
Cancer survivors, compared with controls, were significantly more likely to have hypertension (65.9% v 59.5%, respectively; P < .01), diabetes (23.4% v 21.5%, respectively; P < .01), and dyslipidemia (57.9% v 55.9%, respectively; P < .01); be overweight/obese (43.4% v 35.4%, respectively; P < .01); and have a history of smoking (32.7% v 21.1%, respectively; P < .01; Table 1).
CVD Risk: Cancer Survivors Versus Noncancer Controls
Multivariable regression analysis found a greater risk of CVD in survivors of multiple myeloma (IRR, 1.70; P < .01), carcinoma of the lung/bronchus (IRR, 1.58; P < .01), non-Hodgkin lymphoma (IRR, 1.41; P < .01), ovarian cancer (IRR, 1.41; P = .02), kidney cancer (IRR, 1.24; P = .03), and breast cancer (IRR, 1.13; P < .01) when compared with noncancer controls (Table 2). The increased risk in survivors of ovarian and kidney cancer was not considered statistically significant after adjusting for multiple comparisons (Table 2). Cumulative incidence curves mirrored the findings from the multivariable regression analysis, with significantly increased 8-year incidence of CVD among survivors of multiple myeloma, non-Hodgkin lymphoma, kidney cancer, and breast cancer (Data Supplement). In exploratory analysis, presence of multiple (two or more) CVRFs was associated with the highest risk of CVD among cancer survivors, when compared with noncancer controls with less than two CVRFs (IRR, 1.83 to 2.59; P < .01; Table 3).
Table 2.
CVD Incidence Rates by Cancer Type for Cancer Survivors and Comparison Controls and Calculated Crude and Adjusted (age, sex, race/ethnicity, CVRFs, overweight/obesity, smoking history) IRR
| Cancer Type | Cancer Survivors | Comparison Cohort | Crude IRR | Adjusted IRR | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. With CVD | No. of Person-Years | Incidence Rate* | No. With CVD | No. of Person-Years | Incidence Rate* | IRR | 95% CI | P | IRR | 95% CI | P | |
| Overall | 5,026 | 162,487.13 | 30.93 | 9,795 | 339,488.42 | 28.85 | 1.07 | 1.04 to 1.11 | < .01 | 1.02 | 0.99 to 1.06 | .17 |
| Bladder | 88 | 2,087.05 | 42.16 | 224 | 5,085.33 | 44.05 | 0.96 | 0.75 to 1.22 | .73 | 0.82 | 0.63 to 1.07 | .14 |
| Breast | 1,287 | 49,136.35 | 26.19 | 2,196 | 100,738.05 | 21.80 | 1.20 | 1.12 to 1.29 | < .01 | 1.13 | 1.06 to 1.22 | < .01† |
| Chronic lymphocytic leukemia | 92 | 2,152.52 | 42.74 | 168 | 4,670.90 | 35.97 | 1.19 | 0.92 to 1.53 | .18 | 1.13 | 0.87 to 1.47 | .34 |
| Colon | 345 | 10,667.78 | 32.34 | 823 | 24,942.41 | 33.00 | 0.98 | 0.86 to 1.11 | .75 | 0.93 | 0.82 to 1.06 | .30 |
| Kidney | 156 | 4,356.44 | 35.81 | 284 | 10,454.74 | 27.16 | 1.32 | 1.08 to 1.60 | .01 | 1.24 | 1.02 to 1.51 | .03 |
| Lung and bronchus | 218 | 3,437.07 | 63.43 | 478 | 12,728.58 | 37.55 | 1.69 | 1.44 to 1.98 | < .01 | 1.58 | 1.30 to 1.90 | < .01† |
| Melanoma | 245 | 9,770.25 | 25.08 | 543 | 20,342.80 | 26.69 | 0.94 | 0.81 to 1.09 | .42 | 0.94 | 0.80 to 1.09 | .40 |
| Multiple myeloma | 90 | 1,356.70 | 66.34 | 157 | 4,274.16 | 36.73 | 1.81 | 1.39 to 2.34 | < .01 | 1.70 | 1.31 to 2.21 | < .01† |
| Non-Hodgkin lymphoma | 256 | 6,490.77 | 39.44 | 437 | 15,247.46 | 28.66 | 1.38 | 1.18 to 1.61 | < .01 | 1.41 | 1.20 to 1.65 | < .01† |
| Ovary | 77 | 3,234.80 | 23.80 | 135 | 7,892.56 | 17.10 | 1.39 | 1.05 to 1.84 | .02 | 1.41 | 1.06 to 1.88 | .02 |
| Prostate | 1,774 | 51,008.24 | 34.78 | 3,520 | 94,507.31 | 37.25 | 0.93 | 0.88 to 0.99 | .02 | 0.89 | 0.84 to 0.95 | < .01† |
| Rectum/rectosigmoid | 145 | 5,390.93 | 26.90 | 304 | 11,577.31 | 26.26 | 1.02 | 0.84 to 1.25 | .82 | 0.94 | 0.77 to 1.15 | .55 |
| Thyroid | 66 | 5,192.31 | 12.71 | 153 | 10,043.91 | 15.23 | 0.83 | 0.62 to 1.11 | .22 | 0.82 | 0.61 to 1.10 | .19 |
| Uterus | 187 | 8,205.92 | 22.79 | 373 | 16,982.90 | 21.96 | 1.04 | 0.87 to 1.24 | .68 | 0.98 | 0.81 to 1.17 | .79 |
NOTE. Number of CVD cases in each category is listed.
Abbreviations: CVD, cardiovascular disease; CVRF, cardiovascular risk factors; IRR, incidence rate ratio.
Per 1,000 person-years.
Considered to be statistically significant because P < .0033 (.05/15).
Table 3.
Calculated Crude and Adjusted (Age, Sex, Race/Ethnicity) CVD IRRs by Cancer Type and CVRF (Hypertension, Diabetes, Dyslipidemia) for Cancer Survivors and Comparison Controls
| Cancer Type | Crude IRR | Adjusted IRR | ||||
|---|---|---|---|---|---|---|
| IRR | 95% CI | P | IRR | 95% CI | P | |
| Breast | ||||||
| Noncancer comparison, < 2 CVRF | 1.00 (Ref) | — | 1.00 (Ref) | — | ||
| Cancer survivor, < 2 CVRF | 2.41 | 2.22 to 2.62 | < .01 | 1.79 | 1.64 to 1.95 | < .01 |
| Noncancer comparison, ≥ 2 CVRF | 1.30 | 1.19 to 1.43 | < .01 | 1.33 | 1.21 to 1.46 | < .01 |
| Cancer survivor, ≥ 2 CVRF | 2.52 | 2.28 to 2.78 | < .01 | 1.87 | 1.69 to 2.07 | < .01 |
| Kidney | ||||||
| Noncancer comparison, < 2 CVRF | 1.00 (Ref) | — | 1.00 (Ref) | — | ||
| Cancer survivor, < 2 CVRF | 1.53 | 1.20 to 1.93 | < .01 | 1.15 | 0.90 to 1.46 | .27 |
| Noncancer comparison, ≥ 2 CVRF | 1.01 | 0.75 to 1.36 | .96 | 1.08 | 0.80 to 1.46 | .61 |
| Cancer survivor, ≥ 2 CVRF | 2.19 | 1.71 to 2.80 | < .01 | 1.83 | 1.42 to 2.35 | < .01 |
| Lung and bronchus | ||||||
| Noncancer comparison, < 2 CVRF | 1.00 (Ref) | — | 1.00 (Ref) | — | ||
| Cancer survivor, < 2 CVRF | 1.74 | 1.45 to 2.08 | < .01 | 1.46 | 1.22 to 1.75 | < .01 |
| Noncancer comparison, ≥ 2 CVRF | 1.94 | 1.57 to 2.40 | < .01 | 2.04 | 1.64 to 2.52 | < .01 |
| Cancer survivor, ≥ 2 CVRF | 2.49 | 1.95 to 3.17 | < .01 | 2.29 | 1.79 to 2.92 | < .01 |
| Multiple myeloma | ||||||
| Noncancer comparison, < 2 CVRF | 1.00 (Ref) | — | 1.00 (Ref) | — | ||
| Cancer survivor, < 2 CVRF | 1.47 | 1.07 to 2.01 | .02 | 1.28 | 0.93 to 1.77 | .13 |
| Noncancer comparison, ≥ 2 CVRF | 1.70 | 1.17 to 2.47 | .01 | 1.74 | 1.20 to 2.53 | < .01 |
| Cancer survivor, ≥ 2 CVRF | 2.74 | 1.92 to 3.91 | < .01 | 2.59 | 1.81 to 3.71 | < .01 |
| Non-Hodgkin lymphoma | ||||||
| Noncancer comparison, < 2 CVRF | 1.00 (Ref) | — | 1.00 (Ref) | — | ||
| Cancer survivor, < 2 CVRF | 1.92 | 1.59 to 2.31 | < .01 | 1.45 | 1.20 to 1.75 | < .01 |
| Noncancer comparison, ≥ 2 CVRF | 1.42 | 1.15 to 1.75 | < .01 | 1.39 | 1.13 to 1.72 | < .01 |
| Cancer survivor, ≥ 2 CVRF | 2.62 | 2.09 to 3.28 | < .01 | 2.12 | 1.69 to 2.65 | < .01 |
| Ovary | ||||||
| Noncancer comparison, < 2 CVRF | 1.00 (Ref) | — | 1.00 (Ref) | — | ||
| Cancer survivor, < 2 CVRF | 2.11 | 1.49 to 2.98 | < .01 | 1.47 | 1.03 to 2.09 | .03 |
| Noncancer comparison, ≥ 2 CVRF | 1.36 | 0.94 to 1.96 | .11 | 1.54 | 1.06 to 2.23 | .02 |
| Cancer survivor, ≥ 2 CVRF | 2.83 | 1.90 to 4.22 | < .01 | 2.00 | 1.33 to 3.00 | < .01 |
Abbreviations: CVD, cardiovascular disease; CVRF, cardiovascular risk factors; IRR, incidence rate ratio; Ref, reference.
Prostate cancer survivors had a lower CVD risk (IRR, 0.89; P < .01), and survivors of bladder cancer, chronic lymphocytic leukemia, colon cancer, melanoma, rectum/rectosigmoid cancer, thyroid cancer, or uterine cancer were not at an increased risk of CVD (Table 2). Exploratory analysis by cancer stage at diagnosis revealed an incremental risk by stage among breast, kidney, carcinoma of lung/bronchus, and ovarian cancer survivors (Table 4). Among prostate cancer survivors, the overall decreased risk of CVD was abrogated in individuals with advanced (stage IV) disease (Table 4).
Table 4.
CVD Incidence Rates by Stage for Select Cancer Diagnoses and Comparison Cohort and Calculated Crude and Adjusted (Age, Sex, Race/Ethnicity, CVRFs, Overweight/Obesity, Smoking History) IRRs
| Cancer Type and Stage | Cancer Survivors | Comparison Cohort | Crude IRR | Adjusted IRR | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. With CVD | Person-Years | Incidence Rate* | No. With CVD | No. of Person-Years | Incidence Rate* | IRR | 95% CI | P | IRR | 95% CI | P | |
| Breast | ||||||||||||
| I | 610 | 24,394.09 | 25.01 | 2,196 | 100,738.05 | 21.80 | 1.15 | 1.05 to 1.25 | < .01 | 1.00 | 0.91 to 1.09 | .97 |
| II | 518 | 19,823.11 | 26.13 | 2,196 | 100,738.05 | 21.80 | 1.20 | 1.09 to 1.32 | < .01 | 1.21 | 1.10 to 1.34 | < .01 |
| III | 102 | 3,927.28 | 25.97 | 2,196 | 100,738.05 | 21.80 | 1.19 | 0.98 to 1.45 | .08 | 1.33 | 1.09 to 1.63 | .01 |
| IV | 40 | 586.50 | 68.20 | 2,196 | 100,738.05 | 21.80 | 3.13 | 2.28 to 4.3 | < .01 | 3.30 | 2.32 to 4.7 | < .01 |
| Kidney | ||||||||||||
| I/II/III | 141 | 4,133.15 | 34.11 | 284 | 10,454.74 | 27.16 | 1.26 | 1.03 to 1.54 | .03 | 1.19 | 0.97 to 1.46 | .1 |
| IV | 12 | 133.22 | 90.08 | 284 | 10,454.74 | 27.16 | 3.31 | 1.82 to 6.05 | < .01 | 3.36 | 1.86 to 6.05 | < .01 |
| Lung and bronchus | ||||||||||||
| I/II | 117 | 1,954.08 | 59.87 | 478 | 12,728.58 | 37.55 | 1.59 | 1.3 to 1.95 | < .01 | 1.39 | 1.10 to 1.75 | .01 |
| III/IV | 94 | 1,268.1 | 74.13 | 478 | 12,728.58 | 37.55 | 1.97 | 1.58 to 2.47 | < .01 | 2.01 | 1.56 to 2.6 | < .01 |
| Ovary | ||||||||||||
| I | 22 | 1,332.02 | 16.52 | 135 | 7,892.56 | 17.10 | 0.97 | 0.62 to 1.51 | .88 | 1.11 | 0.72 to 1.71 | .63 |
| II/III/IV | 51 | 1,455.13 | 35.05 | 135 | 7,892.56 | 17.10 | 2.05 | 1.48 to 2.83 | < .01 | 1.78 | 1.26 to 2.5 | < .01 |
| Prostate | ||||||||||||
| I/II | 1,546 | 44,482.45 | 34.76 | 3,520 | 94,507.31 | 37.25 | 0.93 | 0.88 to 0.99 | .02 | 0.89 | 0.84 to 0.95 | < .01 |
| III | 118 | 4,277.95 | 27.58 | 3,520 | 94,507.31 | 37.25 | 0.74 | 0.62 to 0.89 | < .01 | 0.79 | 0.66 to 0.95 | .01 |
| IV | 57 | 1,306.32 | 43.63 | 3,520 | 94,507.31 | 37.25 | 1.17 | 0.89 to 1.53 | .25 | 1.03 | 0.78 to 1.35 | .86 |
Abbreviations: CVD, cardiovascular disease; CVRF, cardiovascular risk factors; IRR, incidence rate ratio.
Per 1,000 person-years.
Exploratory analysis by CVD subtype revealed a significantly increased risk of cardiomyopathy/heart failure among survivors of breast cancer, carcinoma of the lung/bronchus, chronic lymphocytic leukemia, multiple myeloma, and non-Hodgkin lymphoma (IRR, 1.35 to 2.52; P < .05; Data Supplement). There was an increased risk of ischemic heart disease in survivors of multiple myeloma and non-Hodgkin lymphoma (IRR, 1.35 to 1.54; P < .01) and a decreased risk among prostate cancer survivors (IRR, 0.86; P < .01; Data Supplement). The risk for stroke was increased in survivors of ovarian cancer and carcinoma of the lung/bronchus (IRR, 1.64 to 1.85; P < .01) and was decreased among prostate cancer survivors (IRR, 0.87; P < .01; Data Supplement).
Overall Survival After CVD Diagnosis
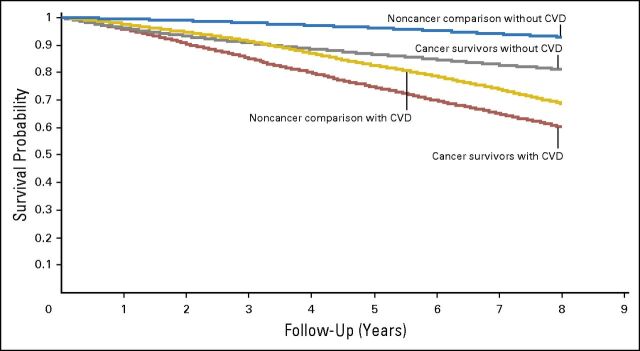
Five- and 8-year overall survival was significantly worse among cancer survivors who developed CVD (75% and 60%, respectively) when compared with cancer survivors without CVD (87% and 81%, respectively). Similar trends were observed among noncancer controls (P < .01; Fig 1). Among cancer survivors, the vast majority of subsequent deaths were a result of cancer (70.5%), followed by CVD (8.8%), neurologic disorders (4.1%), and pulmonary disorders (3.5%). Multivariable regression analysis adjusting for age, sex, race/ethnicity, and cancer stage revealed a 3.78-fold risk of all-cause mortality in cancer survivors who developed CVD when compared with those without CVD (IRR, 3.78; 95% CI, 3.55 to 4.01; P < .01). This risk remained elevated after additional adjustment for CVRFs, overweight/obesity, and smoking history (IRR, 1.65; 95% CI, 1.55 to 1.75; P < .01). Subanalysis by cancer type revealed poorer survival rates among those who developed CVD across nearly all cancer survivor populations (Data Supplement).
Fig 1.
All-cause mortality in cancer survivors and noncancer comparison cohort by cardiovascular disease (CVD) status.
DISCUSSION
In this community-based, retrospective cohort study of 36,236 2-year cancer survivors, we found an increased risk (IRR, 1.70 to 1.13) of CVD (ischemic heart disease, stroke, or cardiomyopathy/heart failure) in patients diagnosed with breast, lung/bronchus, multiple myeloma, and non-Hodgkin lymphoma, when compared with age-, sex-, and ZIP code–matched noncancer controls. Interestingly, long-term survivors of prostate cancer had a lower CVD risk (IRR, 0.89), and there was no increase in risk among survivors of bladder, colon, melanoma, rectum/rectosigmoid, thyroid, and uterine cancers. Moreover, cancer survivors who developed CVD had significantly worse all-cause mortality when compared with cancer survivors without CVD. The findings from the current study speak to the importance of strategies to improve cardiovascular health in at-risk survivors long after completion of cancer therapy.
For the current study, we relied on diagnosis/procedures routinely recorded in a large integrative health care system that includes members who are broadly representative of the residents in southern California.22 Health-related outcomes were captured from a variety of health care delivery settings (inpatient and outpatient, primary and subspecialty care). Importantly, cancer survivors included in the current study continued to receive their primary and subspecialty care at KPSC well beyond their cancer diagnosis (5- and 10-year retention rates, 81% and 70%, respectively), providing us with reliable estimates of long-term CVD risk. This is in contrast to previously published reports from clinical trials7-10 or SEER-Medicare databases11-14 that may not be as representative of patients receiving cancer care in the United States. In the current study, cancer survivors with CVD had significantly worse survival when compared with cancer survivors without CVD, a difference that remained significant after adjustment for cancer stage, demographics (age, sex, race/ethnicity), and cardiovascular risk factors. Although the reasons for these findings are not clear, previous studies have shown that presence of CVD can markedly diminish treatment options or planned duration of therapy at the time of cancer recurrence.27-29 Additional studies are needed to examine whether early screening and treatment of asymptomatic CVD may prevent the onset of clinically overt CVD with its attendant morbidity and mortality.
CVD in cancer survivors is likely to be multifactorial, involving therapeutic exposures that may compromise the cardiovascular system, as well as comorbidities and lifestyle factors that may increase long-term CVD risk.30,31 This is reflected by the varying magnitudes of risk by cancer type as well as by CVD subtype observed in our study. With regard to therapeutic exposures, anticancer agents such as anthracycline chemotherapy, radiation to the heart and related blood vessels, and targeted therapies such as anti–human epidermal growth factor receptor 2 therapy (ie, trastuzumab) are established risk factors for CVD in breast cancer and lymphoma survivors.30-32 However, there is a paucity of this information for survivors of ovarian cancer, multiple myeloma, carcinoma of the lung/bronchus, and kidney cancer. Although this analysis did not include treatment information, the association between cancer stage and subsequent CVD risk, coupled with the lack of association with surgery alone, suggests a role for therapeutic exposures in the development of CVD. It is important to note that our analyses were considered exploratory and that additional work is needed to examine the potential impact of established or emerging (eg, kinase inhibitors, targeted therapies) therapeutic exposures on long-term CVD risk.
Our findings in the prostate cancer population are admittedly counterintuitive. There is considerable debate regarding the risk of CVD as a result of androgen-deprivation therapy (ADT) in prostate cancer survivors.33 ADT is typically reserved for patients with advanced-stage disease and can result in metabolic aberrations during treatment such as insulin sensitivity and changes in lipid profiles.33 A recent population-based study15 examining the risk and timing of CVD after ADT found a nearly two-fold risk of CVD 6 months after initiation of ADT when compared with the general population. However, this risk subsided with time, and there was no reported increase in risk beyond 2 years. Our study was limited to ≥ 2-year prostate cancer survivors, supporting the lack of observed increase in late CVD risk. However, we found a lower risk of CVD in patients with low-stage disease, potentially representing a subset of individuals who are more likely to undergo routine screening for early detection of prostate cancer and hence more likely to be engaged with the health care system for preventive care.34
In the current study, cancer survivors were significantly more likely to have CVRFs such as hypertension, diabetes, and dyslipidemia when compared with matched controls. Overall, survivors who developed multiple CVRFs were at an especially high risk of developing CVD. The findings from the current study are consistent with a number of cohort studies of childhood cancer5,35 and adult hematopoietic stem-cell transplantation survivors36,37 that have highlighted the modifying role of these conditions on CVD risk. For the current study, it is important to note that the higher prevalence of CVRFs did not fully explain the increased incidence of CVD among cancer survivors because these conditions were included in the multivariable regression analyses that examined CVD risk. Additional studies are needed to examine the complex interaction between cancer treatment, lifestyle behaviors, and comorbidities on long-term CVD risk. Information obtained from these studies may help set the stage for the development of predictive models for identifying high-risk individuals for targeted surveillance, as well as aggressive management of CVRFs.
The limitations of observational studies using electronic health records such as ours cannot be ignored. Studies have shown that ICD-9 coding for CVD among patients with cancer may include a substantial proportion of false-positive diagnoses (positive predictive values, 69% to 81%),38,39 attributed to increased screening for CVD in at-risk patients. However, screening for CVD is likely to occur before initiation of treatment and continue through the immediate post-treatment period. For the current study, we were interested in describing the risk of new-onset CVD occurring well-beyond the immediate post-treatment period. As such, individuals who had a history of CVD at the time of their cancer diagnosis or within 2 years of their diagnosis were excluded from the study. Recognizing the potential for screening bias in cancer survivors, we limited our case definition of CVD to clinically overt disease (ischemic heart disease, stroke, heart failure/cardiomyopathy) and excluded diagnoses (ie, valvular disease, asymptomatic atherosclerosis, arrhythmia) that may be detected during routine screening in asymptomatic individuals. In the current study, we did not find an increased risk of CVD across all cancer types, and there was a lower risk among prostate cancer survivors, suggesting that screening bias likely did not account for all the differences between cancer survivors and the noncancer comparison cohort.
In summary, although we found an increased risk of subsequent CVD in a substantial proportion of cancer survivors, the magnitude of this risk varied by cancer subtype as well as by the presence of potentially modifiable risk factors. Outcomes after onset of CVD in long-term cancer survivors seem to be especially poor, emphasizing the need for additional studies to characterize the treatment-specific associations with long-term CVD risk as well as the effect of therapeutic strategies to mitigate this risk.
Footnotes
Presented in part at the 56th Annual Meeting of the American Society of Hematology, San Francisco, CA, December 6-9, 2014.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Saro H. Armenian, Smita Bhatia, Chun Chao
Financial support: Saro H. Armenian
Administrative support: Saro H. Armenian
Provision of study materials or patients: Saro H. Armenian
Collection and assembly of data: Saro H. Armenian, Lanfang Xu, Chun Chao
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Cardiovascular Disease Among Survivors of Adult-Onset Cancer: A Community-Based Retrospective Cohort Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Saro H. Armenian
No relationship to disclose
Lanfang Xu
Consulting or Advisory Role: MedHealth Statistical Consulting
Bonnie Ky
Consulting or Advisory Role: Roche, Bristol-Myers Squibb
Research Funding: Pfizer
Patents, Royalties, Other Intellectual Property: Have a patent on the use of neuregulin-1b as a biomarker in heart failure
Canlan Sun
No relationship to disclose
Leonardo T. Farol
No relationship to disclose
Sumanta Kumar Pal
Honoraria: Novartis, Medivation
Consulting or Advisory Role: Pfizer, Novartis, Aveo, Myriad Pharmaceuticals
Research Funding: Medivation
Pamela S. Douglas
Stock or Other Ownership: CardioDx (I), Omicia (I) , Pappas Ventures (I), Alere (I)
Honoraria: Elsevier, UpToDate
Consulting or Advisory Role: US Diagnostic Services (I), Omicia (I), CardioDx (I), Interleukin Genetics (I), Pappas Ventures (I), TGen (I), Third Point (I), Genome Magazine Editor at large (I), Q-CROC/PMPC/PreThera (I)
Research Funding: Abiomed (Inst), Bristol-Myers Squibb (Inst), Gilead Sciences (Inst), Edwards Lifesciences (Inst), HeartFlow ResMed (Inst), Roche (Inst), Novartis (Inst), Stealth Peptides (Inst), Merck (Inst)
Smita Bhatia
No relationship to disclose
Chun Chao
Employment: Southern California Permanente Medical Group
Research Funding: Amgen (Inst), Merck (Inst)
REFERENCES
- 1.de Moor JS Mariotto AB Parry C, etal: Cancer survivors in the United States: Prevalence across the survivorship trajectory and implications for care Cancer Epidemiol Biomarkers Prev 22:561–570,2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mariotto AB Yabroff KR Shao Y, etal: Projections of the cost of cancer care in the United States: 2010-2020 J Natl Cancer Inst 103:117–128,2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeSantis CE Lin CC Mariotto AB, etal: Cancer treatment and survivorship statistics, 2014 CA Cancer J Clin 64:252–271,2014 [DOI] [PubMed] [Google Scholar]
- 4.Alagona P, Jr, Ahmad TA: Cardiovascular disease risk assessment and prevention: Current guidelines and limitations Med Clin North Am 99:711–731,2015 [DOI] [PubMed] [Google Scholar]
- 5.Lipshultz SE Adams MJ Colan SD, etal: Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: Pathophysiology, course, monitoring, management, prevention, and research directions: A scientific statement from the American Heart Association Circulation 128:1927–1995,2013. [Erratum: Circulation 128:e394, 2013] [DOI] [PubMed] [Google Scholar]
- 6.Rugbjerg K Mellemkjaer L Boice JD, etal: Cardiovascular disease in survivors of adolescent and young adult cancer: A Danish cohort study, 1943-2009 J Natl Cancer Inst 106:dju110,2014 [DOI] [PubMed] [Google Scholar]
- 7.Ganz PA Hussey MA Moinpour CM, etal: Late cardiac effects of adjuvant chemotherapy in breast cancer survivors treated on Southwest Oncology Group protocol s8897 J Clin Oncol 26:1223–1230,2008 [DOI] [PubMed] [Google Scholar]
- 8.Swain SM, Whaley FS, Ewer MS: Congestive heart failure in patients treated with doxorubicin: A retrospective analysis of three trials Cancer 97:2869–2879,2003 [DOI] [PubMed] [Google Scholar]
- 9.Perez EA Suman VJ Davidson NE, etal: Cardiac safety analysis of doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab in the North Central Cancer Treatment Group N9831 adjuvant breast cancer trial J Clin Oncol 26:1231–1238,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seidman A Hudis C Pierri MK, etal: Cardiac dysfunction in the trastuzumab clinical trials experience J Clin Oncol 20:1215–1221,2002 [DOI] [PubMed] [Google Scholar]
- 11.Hull MC Morris CG Pepine CJ, etal: Valvular dysfunction and carotid, subclavian, and coronary artery disease in survivors of Hodgkin lymphoma treated with radiation therapy JAMA 290:2831–2837,2003 [DOI] [PubMed] [Google Scholar]
- 12.Chavez-MacGregor M Zhang N Buchholz TA, etal: Trastuzumab-related cardiotoxicity among older patients with breast cancer J Clin Oncol 31:4222–4228,2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hershman DL McBride RB Eisenberger A, etal: Doxorubicin, cardiac risk factors, and cardiac toxicity in elderly patients with diffuse B-cell non-Hodgkin’s lymphoma J Clin Oncol 26:3159–3165,2008 [DOI] [PubMed] [Google Scholar]
- 14.Doyle JJ Neugut AI Jacobson JS, etal: Chemotherapy and cardiotoxicity in older breast cancer patients: A population-based study J Clin Oncol 23:8597–8605,2005 [DOI] [PubMed] [Google Scholar]
- 15.O’Farrell S Garmo H Holmberg L, etal: Risk and timing of cardiovascular disease after androgen-deprivation therapy in men with prostate cancer J Clin Oncol 33:1243–1251,2015 [DOI] [PubMed] [Google Scholar]
- 16.Limat S Demesmay K Voillat L, etal: Early cardiotoxicity of the CHOP regimen in aggressive non-Hodgkin’s lymphoma Ann Oncol 14:277–281,2003 [DOI] [PubMed] [Google Scholar]
- 17.Baldini E Prochilo T Salvadori B, etal: Multicenter randomized phase III trial of epirubicin plus paclitaxel vs epirubicin followed by paclitaxel in metastatic breast cancer patients: Focus on cardiac safety Br J Cancer 91:45–49,2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boerman LM Berendsen AJ van der Meer P, etal: Long-term follow-up for cardiovascular disease after chemotherapy and/or radiotherapy for breast cancer in an unselected population Support Care Cancer 22:1949–1958,2014 [DOI] [PubMed] [Google Scholar]
- 19.Ryberg M Nielsen D Cortese G, etal: New insight into epirubicin cardiac toxicity: Competing risks analysis of 1097 breast cancer patients J Natl Cancer Inst 100:1058–1067,2008 [DOI] [PubMed] [Google Scholar]
- 20.Hequet O Le QH Moullet I, etal: Subclinical late cardiomyopathy after doxorubicin therapy for lymphoma in adults J Clin Oncol 22:1864–1871,2004 [DOI] [PubMed] [Google Scholar]
- 21.Guarneri V Lenihan DJ Valero V, etal: Long-term cardiac tolerability of trastuzumab in metastatic breast cancer: The M.D. Anderson Cancer Center experience J Clin Oncol 24:4107–4115,2006 [DOI] [PubMed] [Google Scholar]
- 22.Koebnick C Langer-Gould AM Gould MK, etal: Sociodemographic characteristics of members of a large, integrated health care system: Comparison with US Census Bureau data Perm J 16:37–41,2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elliott MN Fremont A Morrison PA, etal: A new method for estimating race/ethnicity and associated disparities where administrative records lack self-reported race/ethnicity Health Serv Res 43:1722–1736,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Derose SF Contreras R Coleman KJ, etal: Race and ethnicity data quality and imputation using U.S. Census data in an integrated health system: The Kaiser Permanente Southern California experience Med Care Res Rev 70:330–345,2013 [DOI] [PubMed] [Google Scholar]
- 25.Chen LH Quinn V Xu L, etal: The accuracy and trends of smoking history documentation in electronic medical records in a large managed care organization Subst Use Misuse 48:731–742,2013 [DOI] [PubMed] [Google Scholar]
- 26.Gray RJ: A class of k-sample tests for comparing the cumulative incidence of a competing risk Ann Stat 16:1141–1154,1988 [Google Scholar]
- 27.Tanvetyanon T Padhya T McCaffrey J, etal: Prognostic factors for survival after salvage reirradiation of head and neck cancer J Clin Oncol 27:1983–1991,2009 [DOI] [PubMed] [Google Scholar]
- 28.Nanda A Chen MH Braccioforte MH, etal: Hormonal therapy use for prostate cancer and mortality in men with coronary artery disease-induced congestive heart failure or myocardial infarction JAMA 302:866–873,2009 [DOI] [PubMed] [Google Scholar]
- 29.Wang SY Long JB Hurria A, etal: Cardiovascular events, early discontinuation of trastuzumab, and their impact on survival Breast Cancer Res Treat 146:411–419,2014 [DOI] [PubMed] [Google Scholar]
- 30.Yeh ET Tong AT Lenihan DJ, etal: Cardiovascular complications of cancer therapy: Diagnosis, pathogenesis, and management Circulation 109:3122–3131,2004 [DOI] [PubMed] [Google Scholar]
- 31.Lenihan DJ, Cardinale DM: Late cardiac effects of cancer treatment J Clin Oncol 30:3657–3664,2012 [DOI] [PubMed] [Google Scholar]
- 32.Accordino MK, Neugut AI, Hershman DL: Cardiac effects of anticancer therapy in the elderly J Clin Oncol 32:2654–2661,2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basaria S: Cardiovascular disease associated with androgen-deprivation therapy: Time to give it due respect J Clin Oncol 33:1232–1234,2015 [DOI] [PubMed] [Google Scholar]
- 34.Hughes E Kilmer G Li Y, etalCenters for Disease Control and Prevention (CDC) : Surveillance for certain health behaviors among states and selected local areas - United States, 2008 MMWR Surveill Summ 59:1–221,2010 [PubMed] [Google Scholar]
- 35.Armstrong GT Oeffinger KC Chen Y, etal: Modifiable risk factors and major cardiac events among adult survivors of childhood cancer J Clin Oncol 31:3673–3680,2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chow EJ Baker KS Flowers ME, etal: Influence of metabolic traits and lifestyle factors on cardiovascular disease after hematopoietic cell transplantation Biol Blood Marrow Transplant 18:S226–S227,2012. (suppl) [Google Scholar]
- 37.Armenian SH, Chow EJ: Cardiovascular disease in survivors of hematopoietic cell transplantation Cancer 120:469–479,2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bowles EJ Wellman R Feigelson HS, etalPharmacovigilance Study Team : Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: A retrospective cohort study J Natl Cancer Inst 104:1293–1305,2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allen LA Yood MU Wagner EH, etalPharmacovigilance Research Group : Performance of claims-based algorithms for identifying heart failure and cardiomyopathy among patients diagnosed with breast cancer Med Care 52:e30–e38,2014 [DOI] [PMC free article] [PubMed] [Google Scholar]