Abstract
Since the WHO declared COVID-19 a pandemic, a great effort has been made to understand this serious disease. Thousands of studies are being devoted to understanding its epidemiology, its molecular characteristics, its mechanisms, and the clinical evolution of this viral infection. However, little has been published on its pathogenesis and the host response mechanisms in the progress of the disease. Therefore, we propose a hypothesis based on strong scientific documentation, associating oxidative stress with changes found in patients with COVID-19, such as its participation in the amplification and perpetuation of the cytokine storm, coagulopathy, and cell hypoxia. Finally, we suggest a therapeutic strategy to reduce oxidative stress using antioxidants, NF-κB inhibitors, Nrf2 activators, and iron complexing agents. We believe that this hypothesis can guide new studies and therapeutic strategies on this topic.
Keywords: COVID-19, Nrf-2, NF-κB, Sepsis, Hypoxia, Antioxidants
Introduction
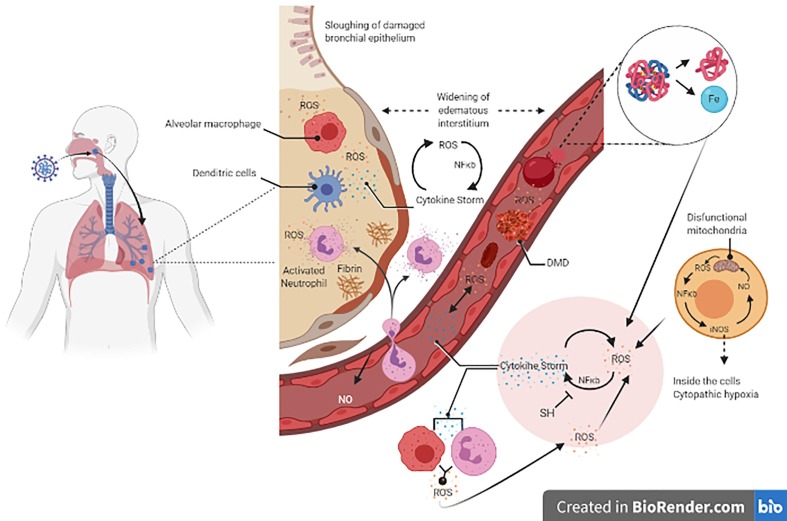
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1] has emerged recently as a new challenge for the medical sciences. Although coronavirus disease 2019, COVID-19, primarily causes respiratory disease, its pathogenesis is still poorly understood. COVID-19 was considered a pandemic by the World Health Organization on March 11, 2020, because it was widely spread around the globe and presented a high rate of human-to-human transmission. It has been reported that COVID-19 progression involves initial symptoms of mild chills and a dry cough, followed by fever, fatigue, and shortness of breath with dyspnea, and, in more severe cases, hypoxemia with oxygen saturation under 85%.[2] Several signs and symptoms presented by patients with COVID-19 have been described, from asymptomatic patients with a mild cold, to important changes in clinical and laboratory examinations, which can progress to severe organ failure and death. The changes observed include decreased total lymphocytes, prolonged prothrombin time, elevated lactate dehydrogenase, cellular immune deficiency, coagulation activation, myocardial, hepatic, and renal injury [3], [4] and endotheliitis in the lung, heart and small bowel [5], [6] (Fig. 1. ).
Fig. 1.
After the virus enters the airways, its replication occurs, and the immune innate response begins with the activation of macrophage and dendritic cells via Toll-like and NOD receptors against the production of inflammatory cytokines and reactive oxygen species (ROS). The consequent spread to the blood has two consequences: 1) erythrocytes are damaged by ROS and other inflammatory mechanisms leading to the generation of heme and free iron; and 2) activated macrophages and neutrophils produce respiratory bursts generating superoxide radicals and H2O2 leading to oxidative stress. Oxidative stress plus free iron converts soluble plasma fibrinogen into abnormal fibrin clots in the form of dense matted deposits (DMD resistant to the enzymatic degradation; blood clots), leading to microthrombosis in the vascular system and the pulmonary microcirculation. The cytokine storm occurs through the upregulation of cytokine expression via NF-κB. After this scenario is established, the cytokine storm induces oxidative stress via macrophage and neutrophil respiratory burst activity, and oxidative stress induces the cytokine storm. This cycle provokes serious tissue damage independent of the virus. In addition, mitochondria produce ROS, which increases iNOS expression via NF-κB and, consequently, NO formation. NO induces dysfunctional mitochondria that, in turn, results in cytopathic hypoxia. Moreover, the virus inhibits Nrf2, responsible for the increase in enzymatic antioxidants, establishing the oxidative stress. Overall, low hemoglobin-carrier, high lung proteinaceous exudate leads to pulmonary hypoxia, cytopathic hypoxia and endothelium damage, and disseminated coagulation results in multiple organ collapse.
Although the mechanisms involved in these changes are not known, observation by the naked eye of the lungs of patients with SARS-CoV-2 showed pathological characteristics of exudative proteinaceous lesion, while microscopic examination showed inflammatory lymphocytic infiltrates [7]. Biopsy and post-mortem studies show diffuse alveolar damage with the formation of hyaline membranes, mononuclear cells and macrophages infiltrating the air spaces, and diffuse thickening of the alveolar wall. In addition, spleen atrophy, hilar lymph node necrosis, focal hemorrhage in the kidney and, enlarged liver with inflammatory cell infiltration constitute considerable worsening during the disease [2], [8]. Some patients have poor outcomes in COVID-19 that mostly correlates with clinical and laboratory features of a cytokine storm. Moreover, hypercoagulation and hypoxia in multiple organs are the worst scenario. Oxidative stress by reactive oxygen species (ROS) is related to all the main changes observed in other inflammatory and infectious diseases and could be the connecting point that unites all these events. Our study focused on the host’s response to viral infection, emphasizing oxidative stress rather than the virus’s mechanisms of aggression.
The cytokine storm and oxidative stress
Many studies have related the cytokine storm to the deterioration in the patient’s condition with COVID-19 infection and it is considered a major factor in the development of acute respiratory distress syndrome and multiple organ dysfunction [2], [9], [10], [11], [12], [13], [14]. A previous study demonstrated that influenza infection induced expression of cytokines via activation of pattern recognition receptors, including the Toll-like receptors TLR3, TLR7, and TLR8, retinoic acid-inducible gene I, and the NOD-like receptor family members in lung epithelial cells, macrophages, and dendritic cells [4], [15]. It is possible that the innate immune response in COVID-19 infection follows the same pathways. The inflammasome is an important element in the cytokine storm [16] and studies have shown that ROS is a potent ligand and direct mediator for triggering NLRP3 (NOD-like receptors P3) inflammasome. Additionally, TLR and NLR ligands enhanced NF-κB driven transcriptional levels of NLRP3. NF-κB, in turn, is activated by ROS, thus direct or indirectly, inflammasome is increased by ROS [17], [18], [19]. Besides ROS, H2O2 mainly activates NF-κB to produce inflammatory cytokines [20], [21]. Hyperproduction of IL-6, TNFα, IL-1β, interferon-gamma inducible protein 10, granulocyte-colony stimulating factor, monocyte chemoattractant, macrophage inflammatory proteins 1-α and elevated blood ferritin are also observed in COVID-19 patients [16]. Similar to influenza infection, COVID-19 infection appears to have an initial pulmonary phase that consists of virus replication and inflammation, with the involvement of ROS as a direct and indirect NLRP3 inflammasome activator, and subsequent blood dissemination, possibly associated with the adaptive immune response to oxidative stress as a mechanism of systemic injury. It is important to understand the mechanism by which SARS-CoV-2 induces pulmonary and systemic injury, and whether the virus is solely responsible or whether there are other synergistic mechanisms that sustain and aggravate the tissue damage observed in this disease.
Hematological findings and oxidative stress
During the disease progression, patients with COVID-19 show disseminated intravascular coagulopathy, characterized by prominent elevation of fibrin/fibrinogen degradation products (D-dimer) [12], [13], [23] which can translocate to extravascular space and damage the cells in the tissue [24]
Hematological disorders have also been reported as inflammation biomarkers. For hemoglobin levels, a meta-analysis study of 1,210 COVID-19 patients showed significant lower hemoglobin levels of 7.1 g/L or even 5.9 g/L in severe cases [24]. In another study, besides presenting decreased hemoglobin, patients with coronavirus pneumonia presented elevated serum ferritin, erythrocyte sedimentation rate, lactate dehydrogenase activity, and acidosis [16]. In addition to the inflammation markers, the disseminated intravascular coagulation contributes to the establishment of organ failure due to hypoxia [25], [26]. Treatment of 27 coronavirus patients with heparin promoted recovery in all of them [27], contributing to the proposal of a treatment protocol to be applied to hospitalized patients. Another observation made about patients with severe COVID-19 progression is that they developed typical clinical manifestations of shock, characteristic of the evolution to viral sepsis [4]. It has been shown that free hemoglobin and heme is augmented in the blood of patients with sepsis due to hemolysis [23]. Although several mechanisms involved in hemolysis have been described in viruses infections, there are a number of studies showing that ROS play a role in damaging the membrane of erythrocytes [23], [28]. The production of high ROS levels in septic patients leads to alterations in the erythrocyte membrane that, in turn, induces phagocytosis in macrophages and neutrophils, perpetuating a cycle with further production of ROS [29]. This may contribute to increased free hemoglobin and low levels of oxygen transport to the tissues [29], [30]. Free hemoglobin is dissociated, generating heme, which then is degraded by heme oxygenase (OH-1) to produce free iron; both heme and free iron are harmful to cells [30]. A murine study of a sepsis model by cecal ligation and puncture (CLP) showed increased total body free iron up to three times higher when compared to the control [31]. In addition, administration of apoferritin (ferritin that is not combined with iron) and the induction of the expression of ferritin heavy chain, decreases CLP mice mortality [32]. Reduced oxygen saturation leads to superoxide radical and H2O2 generation by mitochondria [33], [34], [35]. Superoxide anion radical reduces iron (III) to iron (II), which, in the presence of H2O2, also generated by phagocytic cells in the inflammatory environment, produces hydroxyl radicals (•OH), an extremally toxic agent that promotes the formation of cell membrane lipid peroxides and protein oxidation, triggering cell death by apoptosis and/or necrosis [36]. It has also been demonstrated that iron III induces blood coagulation [37] via hydroxyl radicals. Hydroxyl radicals convert soluble plasma fibrinogen into abnormal fibrin clots in the form of dense matted deposits resistant to enzymatic degradation [37], [38].
We believe that once started, all these events occur independent of the causative agent, worsening the patient’s condition.
Hypoxia and oxidative stress
Disseminated intravascular coagulopathy, sepsis, and decreased oxygen transport to the tissues are some of the main elements presented by COVID-19 patients [22]. Moreover, free heme in the blood decreases circulating nitric oxide (NO), further worsening the ischemia of the organs [24], [39], [40]. Despite hypoxemia, pulmonary compliance is preserved [27], indicating a possible mitochondrial impairment. Mitochondrial inability to produce ATP [41] has been described in experimental and human sepsis in normal oxygen saturation and tension. This points to mitochondria dysfunction as a crucial role for sepsis pathogenesis [41]. The impairment of mitochondria leads to cytopathic hypoxia [40], [41], [42] that results in oxygen partial reduction with ROS generation and reduced energy production.
In this regard, hypoxia that accompanies sepsis is known to generate superoxide, H2O2, and other reactive species mainly by the mitochondrial respiratory chain [40], [41], [43], [44]. Hydrogen peroxide triggers the expression of many genes that upregulate pro-inflammatory cytokines, such as IL-1, IL-6, and TNFα, and inducible nitric oxide synthase (iNOS) via activation of the NF-κB pathway [20], [21], [22]. In a cyclic manner, these pro-inflammatory cytokines activate macrophages, neutrophils, endothelium cells via NADPH oxidase (NOx) to produce more superoxide and H2O2 [20]. Further, superoxide radical reacts with NO produced by iNOS that is expressed via the NF-κB pathway, yielding peroxynitrite. Both peroxynitrite and NO are toxic to mitochondria [44]. This may explain the inability of the mitochondria to utilize oxygen despite normal tissue oxygen saturation [41]. This interplay between ROS and cytokines generates a self-sustaining cycle between cytokine storm and oxidative stress production that eventually leads to multiorgan failure in patients with COVID-19 who progress to sepsis and shock (Fig. 1).
Virus and oxidative stress
Several in vitro and in vivo studies have highlighted the strategy of some viruses to alter the redox balance of a cell in order to survive. Initiation of oxidative stress by virus infection was shown to play a key role in the activation of innate immunity via NF-κB generation of cytokines to fight off pathogenic microbes [45], [46], [47], [48]. Respiratory syncytial virus (RSV) infection has been shown to induce ROS production that, in turn, induced the expression of pro-inflammatory cytokines and innate immune defense [49], [50], [51]. Additionally, several viruses induce oxidative stress in order to facilitate their replication inside the cell [52]. As a way to control ROS levels, these viruses have evolved to acquire the ability to manipulate the Nrf2 pathway in their favor [52], [53], [54]. In some cases, viruses have the ability to suppress the Nrf2 pathway [54], [55]. For instance, RSV increases lipid peroxidation and decreases GSH in human alveolar type II-like epithelial cells and small airway epithelial cells and abrogate the activation of the Nrf2 pathway, resulting in a reduction in the expression of Nrf2 target genes, including hemoxigenase-1 (HO-1), superoxide dismutase 1 (SOD1), superoxide dismutase 3 (SOD3), glutathione S-transferase (GST), catalase (CAT), and glutathione peroxidase (GPx) [54]. At the same time, the Toll-like receptor and interferon (IFN) pathways are triggered by infection-induced oxidative stress to combat the virus infection [56], [57]. On other hand, defective redox balance by a host cell has been shown to contribute to viral pathogenesis resulting in massive induction of cell death provoked by oxidative stress [58]. Influenza virus was shown to induce apoptosis and cytotoxicity in alveolar epithelial cells that were accompanied by increased expression of caspase 1 and 3 and a proinflammatory cytokine, IL-8 [59]. This virus induces oxidative stress, but at the same time facilitates the nuclear translocation of Nrf2 with subsequent expression of HO-1, a protective enzyme against oxidative injury in the human alveolar epithelial cells [59]. These findings confirm the idea that oxidative stress is importantly involved in the success or failure of the response to virus infection.
Therapeutic strategies
Shaping a therapy for a disease whose pathogeny is unclear is a challenge. If oxidative stress is believed to be the link of every mechanism known for SARS-CoV-2 infection, the use of antioxidants, such as vitamin E, SH group donor (N-acetyl cysteine), iron complexing agents (deferoxamine), inhibitors of NF-κB, activators of Nrf2 (curcumin, resveratrol), and the use of specific pro-inflammatory cytokine inhibitors and anticoagulants could help the recovery of patients with COVID-19.
It is useful to emphasize that there are clinical and experimental studies showing the effectiveness of antioxidants in the treatment of many diseases. An in vitro sepsis model showed that vitamin E (α tocopherol), Trolox (soluble vitamin E), and motVit E (a polar derivative) reduced mitochondrial damage/dysfunction, NF-κB transcription molecule, and cytokine expression. MotVit E, which accumulates inside mitochondria, had a more efficient protective effect on genes linked to TLRs [60]. The treatment of animals with vitamin E in the model of acute sepsis also showed beneficial effects [61].
Triterpenoids (oltipraz (butylated hydroxyanisol), sulphoraphane and dimethyl fumarate, curcumin, silibinin, resveratrol) exert their antioxidant activity by activating Nrf2 and inhibiting NF-κB pathways [62]. High levels of blood peroxynitrite in 18 patients with sepsis were decreased using deferoxamine, an iron chelator used in patients with iron overload [29]. This indicates the potential application of this drug in iron complexing and peroxynitrite scavenging in patients with sepsis.
Glutathione (GSH) is the main antioxidant agent in mammalians and it is the most important antioxidant defense in the lungs [64]. When the balance between GSH/GSSG is disrupted by an increase in H2O2, a more oxidative environment oxidizes proteins that control the activation and localization of transcription factors, such as KEAP-1, which regulates Nrf2, and IκB, which regulates NF-κB [45]. Thereby, SH group donors, activators of Nrf2 and NF-κB inhibitors, through their regulator molecules (KEAP-1 and IκB, respectively), are potential therapeutic options for SARS-CoV-2 infection. A case report shows that this strategy successfully reverted the severe symptoms of COVID-19 patients [65]. Lipoic acid, also a SH donor, was used in a randomized study and reduced the mortality rate from 77.8% to 37.5% [66].
Certainly, a different approach is on the table, one where we seek new drug strategies directly targeting the host response to viral aggression, rather than the SARS-CoV-2 virus, in which antioxidants can exert their action by breaking the cytokine storm oxidative stress cycle, decreasing hematological damage and reducing cytopathic hypoxia.
Conclusion
It appears that oxidative stress plays a role in the pathogenesis of COVID-19, perpetuates the cytokine storm cycle, blood clotting mechanism, and exacerbates hypoxia. Taken together, this evidence indicates an important participation of oxidative stress in the pathogenesis of viral infection in all direct tissue injury, including mitochondrial dysfunction, and in the signaling of the process (Fig. 1).
Further, it indicates an interplay between oxidative stress and the cytokine storm as a mechanism that sustains and worsens the tissue injury, which terminates in hypoxia and organ failure. Moreover, it reveals that the SARS-CoV-2 virus may interfere with the equilibrium between the NF-κb transcription molecule involved in the expression of cytokines and Nrf2 activation, responsible for the expression of antioxidant enzymes (yet to be proved conclusively). We are aware of the importance of the role of oxidative stress in the pathogenesis of viral infection and sepsis, however, its participation in COVID-19 disease remains unclear [63]. We believe that there is crosstalk between the cytokine storm and oxidative stress. This crosstalk may play an important role in the severity of symptoms presented by patients with COVID-19 infection. Thus, it is reasonable to propose a pathogenesis model of coronavirus syndrome with primary lung injury and late hematological, tissue hypoxemia (cytopathic hypoxemia), and mitochondrial dysfunction due to the involvement of oxidative stress, which is supported by robust experimental evidence presented by the scientific literature. Thereby, we aim to contribute to a rational therapeutic decision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mehy.2020.110102.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Gorbalenya A.E., Baker S.C., Baric R.S. Severe acute respiratory syndrome-related coronavirus – the species and its viruses, a statement of the Coronavirus Study Group. Nat Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu Z., Shi L., Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D., Hu B., Hu C. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2019;2020 doi: 10.1001/JAMA2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hui Li, Liang Liu, Dingyu Zhang, Jiuyang Xu, Huaping Dai, Nan Tang, Xiao Su, Bin Cao. SARS-CoV-2 and viral sepsis: observations and hypotheses. www.thelancet.com Published online April 17; 2020. doi:10.1016/S0140- 6736(20)30920-X. [DOI] [PMC free article] [PubMed]
- 5.Varga Z., Flammer A.J., Steiger P. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020 doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonetti P.O., Lerman L.O., Lerman A. Endothelial dysfunction – a marker of atherosclerotic risk. Arterioscl Throm Vas. 2003;23:168–175. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- 7.Qian L., Rongshuai W., Guoqiang Q. A report on the general observation of a 2019 novel coronavirus autopsy. J Forens Sci. 2020;36:1–3. [Google Scholar]
- 8.Tian S., Hu W., Niu L., Liu H., Xu H., Xiao S.-Y. novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol. 2019;2020 doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chousterman B.G., Swirski F.K., Weber G.F. Cytokine storm and sepsis disease pathogenesis. Seminars Immunopathol. 2017;39(5):517–528. doi: 10.1007/s00281-017-0639-8. [DOI] [PubMed] [Google Scholar]
- 10.Shimabukuro-Vornhagen Alexander, Gödel Philipp, Subklewe Marion, Stemmler Hans Joachim, Schlößer Hans Anton, Schlaak Max, Kochanek Matthias, Böll Boris, von Bergwelt-Baildon Michael S. Cytokine release syndrome. J. Immunotherapy Cancer. 2018;6(1) doi: 10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wan S., Yi Q., Fan S. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP) medRxiv. 2020 doi: 10.1016/j.jinf.2020.03.037. [DOI] [Google Scholar]
- 12.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J., Li S., Liu J. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. medRxiv. 2020 doi: 10.1101/2020.02.16.20023671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwasaki A., Pillai P.S. Innate immunity to influenza virus infection. Nat Rev Immunol. 2014;14:315–328. doi: 10.1038/nri3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehta P., McAuley D.F., Brown M. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:28. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braciale T.J., Sun J., Kim T.S. Regulating the adaptive immune response to respiratory virus infection. Nature Rev Immunol. 2012;12:295–305. doi: 10.1038/nri3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muralidharan S., Mandrekar P. Cellular stress response and innate immune signaling: integrating pathways in host defense and inflammation. J Leukoc Biol. 2013;94(6):1167–1184. doi: 10.1189/jlb.0313153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y., Zhou Z., Min W. Mitochondria, oxidative stress and innate immunity. Front. Physiol. 2018;18(9):1487. doi: 10.3389/fphys.2018.01487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nanduri J., Yuan G., Kumar G.K. Transcriptional responses to intermittent hypoxia. Respir. Physiol. Neurobiol. 2008;164:277–281. doi: 10.1016/j.resp.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takada Y., Mukhopadhyay A., Kundu G.C. Hydrogen peroxide activates NF-ΚB through tyrosine phosphorylation of IκBα and serine phosphorylation of p65. J Biol Chem. 2003;278(26):24233–24241. doi: 10.1074/jbc.M212389200. [DOI] [PubMed] [Google Scholar]
- 22.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;27 doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Effenberger-Neidnicht K., Hartmann M. Mechanisms of hemolysis during sepsis. Inflammation. 2018;41:5. doi: 10.1007/s10753-018-0810-y. [DOI] [PubMed] [Google Scholar]
- 24.Lippi G., Mattiuzzi C. Hemoglobin value may be decreased in patients with severe coronavirus disease 2019. Hematol Transfus Cell Ther. 2020 doi: 10.1016/J.HTCT.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yao X.H., Li T.Y., He Z.C. A pathological report of three COVID-19 cases by minimally invasive autopsies. Zhonghua Bing Li Xue Za Zhi. 2020;49:E009. doi: 10.3760/cma.j.cn112151-20200312-00193. [DOI] [PubMed] [Google Scholar]
- 26.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D.A.M.P.J., Kant K.M., Kaptein F.H.J., van Paassen J., Stals M.A.M., Huisman M.V., Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thrombosis Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Negri EM, Piloto BM, Morinaga LK, et al. Heparin therapy improving hypoxia in COVID-19 patients-a case series. medRxiv doi:10.1101/2020.04.15.20067017. [DOI] [PMC free article] [PubMed]
- 28.Simao A.N.C., Suzukawa A.A., Casado M.F. Genistein abrogates pre- hemolytic and oxidative stress damage induced by 2,2V-Azobis (Amidinopropane) Life Sci. 2006;78:1202–1210. doi: 10.1016/j.lfs.2005.06.047. [DOI] [PubMed] [Google Scholar]
- 29.Oliveira Y.P.A., Pontes-de-Carvalho L.C., Couto R.D. Oxidative stress in sepsis. Possible production of free radicals through an erythrocyte-mediated positive feedback mechanism. Braz J Infect Dis. 2017;21(1):19–26. doi: 10.1016/j.bjid.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vasconcellos L.R.C., Dutra F.F., Siqueira M.S. Protein aggregation as a cellular response to oxidative stress induced by heme and iron. PNAS. 2016;113(47):E7474–E7482. doi: 10.1073/pnas.1608928113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fenga P., Ma L., Xua F., Gou X. In vivo bioluminescence imaging of labile iron pools in a murine model of sepsis with a highly selective probe. Talanta. 2019;203:29–33. doi: 10.1016/j.talanta.2019.05.017. [DOI] [PubMed] [Google Scholar]
- 32.Weis S., Carlos A.R., Moita M.R. Metabolic adaptation establishes disease tolerance to sepsis. Cell. 2017;169:1263–1275. doi: 10.1016/j.cell.2017.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prauchner C.A. Oxidative stress in sepsis: Pathophysiological implications justifying antioxidant co-therapy. Burns. 2017;43(3):471–485. doi: 10.1016/j.burns.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 34.Raffaella Trentadue, Fiore Flavio, Fabrizia Massaro, Francesco Papa, Arcangela Iuso, Salvatore Scacco. Induction of mitochondrial dysfunction and oxidative stress in human fibroblast cultures exposed to serum from septic patients. Life Sci. 2012;91:237–243. doi: 10.1016/j.lfs.2012.06.041. [DOI] [PubMed] [Google Scholar]
- 35.Koskenkorva-Frank T.S., Weiss G., Koppenol W.H. The complex interplay of iron metabolism, reactive oxygen species, and reactive nitrogen species: Insights into the potential of various iron therapies to induce oxidative and nitrosative stress. Free Radic Biol Med. 2013;65:1174–1194. doi: 10.1016/j.freeradbiomed.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 36.Jerzy J., Philip L., Pretorius E., Skrzypczak-Jankun E., Lipinski B. Unusual clotting dynamics of plasma supplemented with iron (III) Int J Mol Med. 2014;33:367–372. doi: 10.3892/ijmm.2013.1585. [DOI] [PubMed] [Google Scholar]
- 37.Pretorius E., Bester J., Vermeulen N. Oxidation inhibits iron-induced blood coagulation. Current Drug. 2013;14(1):13–19. doi: 10.2174/1389450111314010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schaer D.J., Buehler P.W., Alayash A.I. Hemolysis and free hemoglobin revisited: exploring hemoglobin and hemin scavenger as a novel class of therapeutic proteins. Blood. 2013;121:1276–1284. doi: 10.1182/blood-2012-11-451229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weinberg J.A., Barnum S.R., Patel R.P. Red blood cell age and potentiation of transfusion-related pathology in trauma patients. Transfusion. 2011;51:867–873. doi: 10.1111/j.1537-2995.2011.03098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mantzarlis K., Tsolaki V., Zakynthinos E. Role of oxidative stress and mitochondrial dysfunction in sepsis and potential therapies. Ox Med Cell Long. 2017 doi: 10.1155/2017/5985209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fink M.P. “Bench-to-bedside review”: cytopathic hypoxia. Crit Care. 2002;6(6):491–499. doi: 10.1186/cc1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ottolenghi S., Zulueta A., Caretti A. Iron and sphingolipids as common players of (Mal)adaptation to hypoxia in pulmonary diseases. Int J Mol Sci. 2020;21:307. doi: 10.3390/ijms21010307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takeda K., Shimada Y., Amano M. Plasma lipid peroxides and alpha- tocopherol in critically ill patients. Crit Care Med. 1984;12:11. doi: 10.1097/00003246-198411000-00007. [DOI] [PubMed] [Google Scholar]
- 44.Ademowo O.S., Dias H.K.I., Burton D.G.A. Lipid (per) oxidation in mitochondria: an emerging target in the ageing process? Biogerontol. 2017;18:859–879. doi: 10.1007/s10522-017-9710-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Winterbourn C.C., Hampton M.B. Thiol chemistry and specificity in redox signaling. Free Rad Biol Med. 2008;45:549–561. doi: 10.1016/j.freeradbiomed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 46.Day R.M., Suzuki Y.J. Cell proliferation, reactive oxygen and cellular glutathione. Dose Resp. 2005;3:3. doi: 10.2203/dose-response.003.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Narayanan A., Amaya M., Voss K. Reactive oxygen species activate NFκB (p65) and p53 and induce apoptosis in RVFV infected liver cells. Virology. 2014;449:270–286. doi: 10.1016/j.virol.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 48.Kim H.J., Kim C.H., Ryu J.H. Reactive oxygen species induce antiviral innate immune response through IFN-λ regulation in human nasal epithelial cells. Am J Resp Cell Mol Biol. 2013;49(5):855–865. doi: 10.1165/rcmb.2013-0003OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu T., Castro S., Brasier A.R. Reactive oxygen species mediate virus induced STAT activation: role of tyrosine phosphatases. J Biol Chem. 2004;279(4):2461–2469. doi: 10.1074/jbc.M307251200. [DOI] [PubMed] [Google Scholar]
- 50.Casola A., Burger N., Liu T. Oxidant tone regulates RANTES gene expression in airway epithelial cells infected with respiratory syncytial virus role in viral-induced interferon regulatory factor activation. J Biol Chem. 2001;276(23):19715–19722. doi: 10.1074/jbc.M101526200. [DOI] [PubMed] [Google Scholar]
- 51.Lee Y.H., Lai C.L., Hsieh S.H. Influenza A virus induction of oxidative stress and MMP-9 is associated with severe lung pathology in a mouse model. Virus Res. 2013;178(2):411–422. doi: 10.1016/j.virusres.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 52.Gjyshi O., Bottero V., Veettil M.V. Kaposi’s sarcoma-associated herpesvirus induces Nrf 2 during de novo infection of endothelial cells to create a microenvironment conducive to infection. PLoS Pathog. 2014;10:10. doi: 10.1371/journal.ppat.1004460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu B., Fang M., He Z. Hepatitis B virus stimulates G6PD expression through HBx-mediated Nrf 2 activation. Cell Death Dis. 2015;6:11. doi: 10.1038/cddis.2015.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hosakote Y.M., Jantzi P.D., Esham D.L. Viral-mediated inhibition of antioxidant enzymes contributes to the pathogenesis of severe respiratory syncytial virus bronchiolitis. Am J Resp Crit Care Med. 2011;183(11):1550–1560. doi: 10.1164/rccm.201010-1755OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Komaravelli N., Ansar M., Garofalo R.P. Respiratory syncytial virus induces NRF2 degradation through a promyelocytic leukemia protein–ring finger protein 4 dependent pathway. Free Rad Biol Med. 2017;113:494–504. doi: 10.1016/j.freeradbiomed.2017.10.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Imai Y., Kuba K., Neely G.G. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133(2):235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cho H.Y., Imani F., Miller-DeGraff L. Antiviral activity of Nrf 2 in a murine model of respiratory syncytial virus disease. Am J Resp Crit Care Med. 2009;179(2):138–150. doi: 10.1164/rccm.200804-535OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Garofalo R.P., Kolli D., Casola A. Respiratory syncytial virus infection: mechanisms of redox control and novel therapeutic opportunities. Antiox Redox Sign. 2013;18(2):186–217. doi: 10.1089/ars.2011.4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kosmider B., Messier E.M., Janssen W.J. Nrf 2 protects human alveolar epithelial cells against injury induced by influenza A virus. Resp Res. 2012;13(1):43. doi: 10.1186/1465-9921-13-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Minter B.E., Lowes D.A., Nigel R. Diferential effects of MitoVitE, α-tocopherol and trolox on oxidative stress, mitochondrial function and inflammatory signaling pathways in endothelial cells cultured under conditions mimicking sepsis. Antioxidants. 2020;9:195. doi: 10.3390/antiox9030195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vícto V.M., Esplugues J.V., Hernández-Mijares A., Rocha M. Oxidative stress and mitochondrial dysfunction in sepsis: a potential therapy with mitochondria-targeted antioxidants infectious disorders. Drug Targets. 2009;9:376–389. doi: 10.2174/187152609788922519. [DOI] [PubMed] [Google Scholar]
- 62.Panieri E., Buha A., Telkoparan-Akillilar P. Potential applications of NRF2 modulators in cancer therapy. Antioxidants. 2020;9:193. doi: 10.3390/antiox9030193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Delgado-Roche Livan, Mesta Fernando. Oxidative stress as key player in severe acute respiratory syndrome coronavirus (SARS-CoV) infection. Arch Med Res. 2020;51(5):384–387. doi: 10.1016/j.arcmed.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cantin A.M., North S.L., Hubbard R.C. Normal alveolar epithelial lining fluid contains high levels of glutathione. J. Appl. Physiol. 1987;63(1):152–157. doi: 10.1152/jappl.1987.63.1.152. [DOI] [PubMed] [Google Scholar]
- 65.Horowitz R.I., Freeman P.R., Bruzzese J. Efficacy of glutathione therapy in relieving dyspnea associated with COVID-19 pneumonia: a report of 2 cases. Resp Med Case Reports. 2020 doi: 10.1016/j.rmcr.2020.101063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhong Ming, Sun Aijun, Xiao Ting, Yao Ge, LingSang Xia Zheng, Zhang Jinyan, Jin Xuejuan, Lei Xu, Yang Wenlong, Wang Peng, Kai Hu, Zhang Dingyu, Ge Junbo. A Randomized, Single-blind, Group sequential, Active- controlled Study to evaluate the clinical efficacy and safety of α-Lipoic acid for critically ill patients with coronavirus disease 2019 (COVID-19) MedRxiv. 2019 doi: 10.1101/2020.04.15.20066266. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.