Abstract
The global COVID-2019 pandemic has presented to the field of radiation oncology a management dilemma in providing evidence-based treatments to all cancer patients. There is a need for appropriate measures to be taken to reduce infectious spread between the medical healthcare providers and the patient population. Such times warrant resource prioritization and to continue treatment with best available evidence, thereby reducing the risk of COVID-2019 transmission in times where the workforce is reduced. There has been literature presented in different aspects related to providing safety measures, running of a radiation department and for the management of various cancer subsites. In this article, we present a comprehensive review for sustaining a radiation oncology department in times of the COVID-2019 pandemic.
Key words: COVID-2019, Pandemic, Radiation oncology, Evidence-based oncology
Introduction
The world, as we write this review, is facing a pandemic of catastrophic proportions in the form of Novel Coronavirus (COVID-2019) [1], first identified in Wuhan, in Hubei principality in China in December 2019 [2]. The pandemic hit the Indian healthcare scenario in the most unanticipated manner, with numbers in China and the European Union on the rise. While at the time of this writing the number of cases in India have not been overwhelming, healthcare workers and the healthcare system have tried to take swift actions in the hopes of preventing the devastation that has occurred in America and Europe. Public health workers believe that the pandemic will escalate and it will continue to last for a long time [3] Oncological institutes specifically, have been especially impacted as they address the management of their patients and devise plans to provide treatment options for already immunocompromised patients as they have shown to have a five-fold relative risk for severe manifestations compared to the general population [4]. The literature from various oncological institutes and societies has paved the way for a better understanding of the management of the disease. Various institutions have described their experiences in individual publications. Here we attempt to present a comprehensive review of the literature published related to the handling of radiation oncology setups and the management of various cancer subsites during the COVID-2019 pandemic.
Methodology
We performed a PubMed search with the following MesH terms: Coronavirus, COVID-2019 AND Oncology, Coronavirus AND radiation therapy, Coronavirus AND radiation oncology along with the articles published in the various oncology societies through google search and the individual websites. A total of 19 articles were found to be suitable for the review. The focus was on the best available practice in terms of radiation therapy in institutes that addressed the medical professional working protocols, guidance for individual disease sites and safety measures while handling this patient population.
COVID-19 specific guidelines
Prevention or mitigation of transmission forms the basic priority while managing patients, especially those with a diagnosis of cancer and providing optimum supportive measures. The guidelines mentioned in the literature can be divided into various priority levels or tiers of management. The priority levels as defined by Fillipi et al are illustrated in Fig. 1 [5].
Fig. 1.
Priority levels in COVID management in Radiatherepy centers.
Other specific measures
Dinh et al [6], present additional guidelines in terms of patient care, mitigation of disease transmission and training of residents in radiation oncology as well as the healthcare staff. These recommendations are enumerated below:
-
1.
Employees who can perform duties remotely (eg, research coordinators, research residents, administrative staff, and some medical physics staff) should be instructed to work from home.
-
2.
Meetings should be limited to five persons or less, with at least 6 feet of distance between any two individuals.
-
3.
Tumor boards should be transitioned to virtual conferencing, except for small meetings with five persons or less, with exceptions made only for a tumor board in which in-person coordination is felt indispensable to patient care.
-
4.
New patient consultations for treatment of indolent or benign conditions should be deferred at the discretion of the radiation oncologist.
-
5.
Routine follow-ups should be offered to patients via telephone or postponed.
-
6.
To conserve PPE, institute policies limiting the number of providers required to come into direct contact with patients with suspected or confirmed COVID-19.
-
7.
Immobilization devices such as VacLoc bags should be individually disinfected and wrapped in a plastic bag that is sealed and changed after each daily use.
-
8.
Treatment tables and positioning aides should be extensively disinfected between patients.
-
9.
Active breathing controller (ABC, Elekta Inc.) should not be used in suspected or confirmed cases and abdominal compression should be used instead.
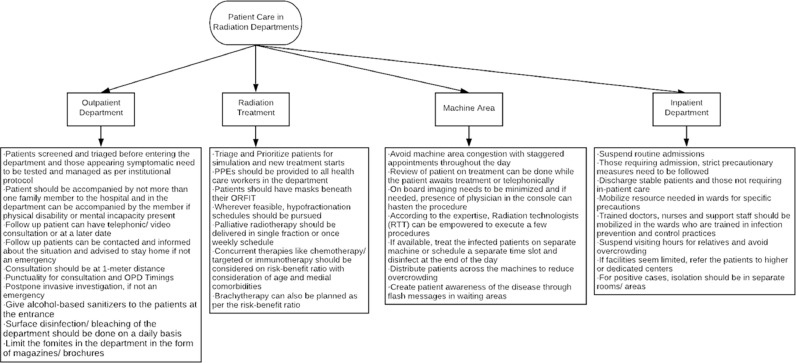
Also, in correspondence from Tata Memorial Hospital, Mumbai by Mummadi, et al, elaborated on the nuances and finer details associated with running a radiation oncology department in times of a pandemic. The salient features about the patient care in times of COVID-2019 while running the radiation oncology department, have been highlighted in Fig. 2 .
Fig. 2.
Patient Care in Radiation Oncology department in COVID times.
Treatment priority according to disease site
Oncological decisions have been evidence-based and level I evidence has been chased while delivering optimum treatment. However, pandemics like the COVID-2019 provides a dilemma and an ethical challenge because of limited resources and elevated risks of infections in treating with evidence-based practice. Such times demand appropriate treatment decisions that require a careful balance of patient benefits and associated risks. This warrants the need to look into phase II trials, prospective evidence and also some retrospective series. The harm associated with COVID infection in cancer patients has been presented by a simple model by Simcock, et al [7] If a patient has a 5% risk of infection and a 10% risk of death from infection there may be a 0.5% mortality through exposure and attendance for radiotherapy. If the patient is young and healthy with a 5% risk of infection and a 1% risk of death, then there is a 0.05% mortality from COVID-19. The use of chemotherapy in combination with radiotherapy is likely to significantly increase the risk of morbidity and mortality from synchronous COVID-19 infection. The various disease sites have been discussed with the look into the available literature.
Central Nervous System (CNS)
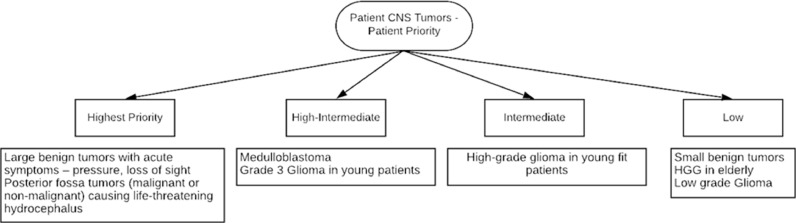
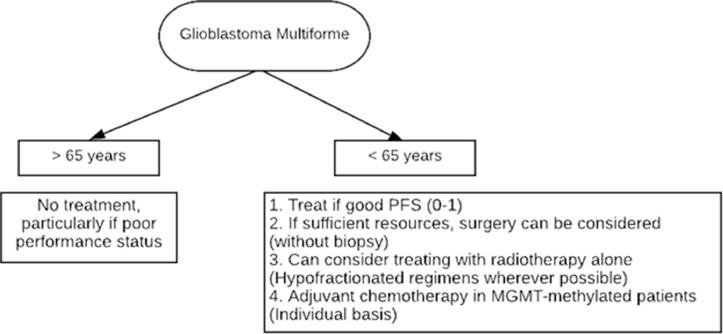
Available guidelines in the wake of the pandemic coming from the various neuro-oncology societies have been limited. The available literature in view of disease mitigation and appropriate treatment approaches are presented in Table 1 and the priority given to the management of CNS tumor is shown in Figs. 3 and 4 .
Table 1.
Management of Various malignancy sites during COVID-19 – Part 1
|
GUIDELINES FOR CNS TUMORS 1. GBM in >60 yrs, methylated status → Temozolomide alone [8], [9] 2. Asymptomatic Meningioma Grade 1-2 → Omit Radiotherapy 3. Asymptomatic arteriovenous malformation (AVM) → Omit radiotherapy 4. Grade 3 glioma: • 1p19q co-deleted (anaplastic oligodendroglioma) → Can consider delay in radiotherapy and chemotherapy for 4-6 months • Non-co-deleted (anaplastic astrocytoma) → Deliver radiation therapy and delay chemotherapy for 4-6 months; Post RT imaging at 3 months 5. Hypofractionated regimens in RT: • 40 Gy/15 fractions • 30 Gy/6 fractions 6. Consider delaying the control MRI appointments in asymptomatic, long-term survivors of less malignant brain tumors, e.g., meningiomas, schwannomas. |
|
GUIDELINES FOR HEAD AND NECK MALIGNANCIES Curative treatment – High priority patients 1. Hypofractionated radiotherapy can be considered • 65 Gy in 30 fractions can be considered • 55 Gy in 20 fractions over 4 weeks [10] 2. Concurrent chemotherapy use can be restricted to patients < 60 years as the benefit decreases with advancing age [11] 3. Accelerated fractionation without chemotherapy (6 fractions per week) / Simultaneous integrated boost approach [12] |
| Adjuvant treatment 1. Consider omitting adjuvant chemotherapy 2. Patient with R0 resection and minor risk factor – can consider omitting radiation therapy |
| Palliative treatment 1. Deliver only when benefits outweigh the risk 2. Consider short fractionation schedules: • 25 Gy in 5 fractions • 20 Gy in 5 fractions • 30 Gy in 6 fractions • IMRT over 2 weeks • Single 8 Gy fraction [13], [14] 3. Do not start or delay palliative chemotherapy/ immunotherapy |
| Other Considerations: 1. Patients with laryngectomy / tracheostomy should be treated only if proper protective measures are available as maximum aerosol dispersion is present in such cases 2. Omit cisplatin-based induction chemotherapy [15] 3. Delay post-operative RT in patients with salivary gland tumors until 12 weeks after surgery. Time factor is not strictly linked to adverse effect in these cases [16] |
|
GUIDELINES FOR BREAST CANCER: DIFFERENT PRESENTATIONS OF LOCALIZED CARCINOMA OF THE BREAST AND REASONABLE OPTIONS TO DE-ESCALATE TREATMENT | |
|---|---|
| Presentation | Recommendation |
| Ductal carcinoma in situ (DCIS) | • Good risk → Omit RT. If planned, can delay up to 12 weeks [17], [18] • Others → Can consider omission. If planned, it can be delayed up to 12 weeks [19] |
| Early breast cancer (EBC) | • Age >70 yrs, post breast-conserving surgery (BCS) - T1, N0, ER+(receiving ET), margins clear → May omit RT [20] • Age >65yrs, ER+, N0, T1/T2 (up to 3 cms), clear margins; grade 3 or LVI [21] • Young premenopausal women → Proceed with RT as scheduled. • EBC post BCS: If RT without chemotherapy is planned → can be delayed up to 20 weeks post BCS [22] |
| Locally advanced breast cancer (LABC) | • LABC → Proceed with RT as scheduled |
| Good risk DCIS: | • Low/intermediate grade, <2.5 cms, margin >3 mm. → If omission/delay in RT is planned for ER/PR+ EBC/DCIS, endocrine therapy can be initiated immediately |
|
Boost dose recommendations: | ||
|---|---|---|
| Disease type | DCIS | Early breast cancer (EBC) |
| Recommendation |
May be omitted Less than 2% benefit in Ipsilateral breast tumor recurrence rate at 10 years, less than 4% benefit at 15 years. [23], [24] Caution in women less than 40 years (significant benefit) |
• May be omitted in elderly (>60 years) → Improves local control, no impact on OS, Largest benefit in young women • Hypofractionate • SIB (simultaneous integrated boost) or concomitant boost - daily or weekly can be incorporated to decrease treatment time • 5.2 Gy single fraction boost → May be considered after ultra-hypofractionated regimens [25] |
|
Prioritizing radiation services in breast cancer during COVID-2019 | ||
|---|---|---|
| Tier 1 (continue radiation) | Tier 2 (short delay acceptable) | Tier 3 (omit) |
| • Inflammatory breast cancer • Residual nodal disease after NACT • N2 disease (4 or more nodes) • Recurrent disease • Node positive TNBC • Extensive LVI |
• ER+ disease with N1a nodes (1-3 nodes) • Node negative TNBC • Pathological N0 post NACT • LVI (not otherwise specified) |
• Early stage ER+ breast cancer especially in elderly • DCIS |
Fig. 3.
Patient Priority based management in CNS Tumors.
Fig. 4.
Algorithm for management decisions for GBM.
Head and neck malignancies
Radiation therapy with concurrent chemotherapy forms the mainstay in the majority of head and neck malignancies with a tendency towards improved survival and providing the option of organ preservation. In India, head and neck malignancies form the major bulk of disease and hence management options need to be meticulous. As with other subsites, a multi-disciplinary board needs to weigh the risk-benefit ratio and chose the best available modality suitable for the patient without severely compromising their oncological outcome. The available recommendations for head and neck malignancies are enumerated in Table 1.
Breast cancer
Radiation therapy for patients with breast cancer contributes to a significant proportion of the workload in any radiation oncology department. Knowledge about priorities and exercising these to decrease unnecessary patient visits will significantly impact radiation facility resources. In the present resource strained COVID-19 pandemic situation, it is important to prioritize patients and consider low-risk situations where delaying/de-escalating or omitting treatment may not be so detrimental yet save resources for other more pressing indications. Table 1. illustrates the best available evidence for the management of various stages of breast cancer and indicates the techniques that can be used for boost delivery.
Fractionation
Hypo-fractionated radiation therapy is widely accepted for whole breast radiation therapy. 42.5 Gy in 16 fractions or 40 Gy in 15 fractions are commonly utilized regimens. Especially in the present pandemic situation, 50 Gy in 25 fractions should be deferred. Newer, still shortened and accelerated regimens are finding their place. There is 3 years toxicity data showing equivalence in toxicity for 28.5 Gy in 5 fractions (once a week) as compared to the 15 fractions regimens and acute toxicity results for 26 Gy in 5 daily fractions.
Accelerated partial breast irradiation
Accelerated partial breast irradiation (APBI) involves partial breast irradiation (tumor cavity with adequate margin), delivered in an accelerated regimen over 1-2 weeks. It has been an acceptable treatment option for more than a decade now. APBI can be offered to women with infiltrating ductal carcinoma over 60 years, tumor size up to 2 cm, node-negative, ER+, no Lymphovascular Stromal Invasion (LVSI), and clear margins. Target volume includes the surgical cavity with a margin. APBI can be delivered through balloon brachytherapy, interstitial brachytherapy, and external beam radiation therapy. However, in the present COVID-19 pandemic, it is advisable to do using external beam radiation therapy using 3DCRT or Intensity Modulated Radiation Therapy/Volumated arc Therapy (IMRT/VMAT) as brachytherapy would require additional resources and also would lead to unnecessary exposure and hospital stay. It may also increase the risk of transmission during intubations or upper endoscopic procedures and necessitate increased PPE while they are in short supply. Regimens include 30 Gy in 5 fractions every other day (Florence regimen), 38.5 Gy in 10 fractions, twice daily and 40 Gy in 10 daily fractions, amongst some others. In the present COVID-19 pandemic, a shift to APBI for eligible patients will drastically relieve the radiation service resources and there are institutions and countries releasing documents in its favor amidst the pandemic [26].
Post-mastectomy and regional nodal radiation therapy
There is an ongoing trial investigating the omission of post-mastectomy chest wall and regional nodal radiation in patients with cT1-3 N1 disease who have ypN0 status post neoadjuvant chemotherapy and surgery. However, until the results are published, the omission is not recommended outside of a clinical trial. Postmastectomy chest wall radiation can be delivered with hypofractionation in 15 fractions with non- inferior outcomes and similar toxicity [27]. There is also preliminary literature suggesting hypofractionated 15–16 fraction regimens for regional nodes. There is evidence building up even for the 5 fractionated regimen. However, with the current literature, 15 fractions are safe.
Cardiac sparing methods
Several radiation oncology centers use cardiac sparing deep inspiratory breath-hold techniques especially in left breast cancers for better cardiac sparing. There are advised caution against the use of active breathing control devices that involve the use of a mouthpiece as that involves a risk of viral transmission as the major mode of transmission is coming into contact with secretion droplets. Techniques that involve voluntary breath-hold to achieve the desired effect are recommended. Treatment in a prone position can be an option to reduce cardiac dose, though it can be a little cumbersome procedure.
Miscellaneous
Intra-operative radiation therapy can be an option as this will obviate any further need for visiting the hospital for radiation therapy. It is delivered over 20-45 minutes to the tumor bed. The surface of the bed typically receives 20 Gy that attenuates to 5–7 Gy at 1 cm depth. There are studies to support its use. Although not adopted universally in practice, it might be a very reasonable option, if available, in the COVID-19 pandemic where reduced hospital visits and social distancing is an important strategy to prevent/reduce infection.
Prioritizing of radiation therapy
Braunstein et al from MSKCC [28] has proposed an informative tier system for prioritizing radiation services in breast cancer patients during this COVID-19 pandemic. The details are shown in Table 1.
Lung cancer
In the time of the COVID-19 Pandemic, lung cancer poses a unique challenge as both the cancer and the virus predominantly affect the lung parenchyma causing damage and respiratory symptoms. While cough and breathlessness are common symptoms of lung cancer, COVID-19 infections also present with dry cough and breathlessness commonly. As the pulmonary reserve may be affected by cancer, with the superseding risk of COVID-19 infection capable of producing severe manifestations in them, patient selection for treatment is key. Also, sequential treatments over concurrent treatments may be preferred keeping in mind the long-term goal of successful treatment completion. Management as per the stage of the disease is discussed in Table 2 .
Table 2.
Management of different stages / presentations of various cancers during a pandemic
| LUNG CANCER | |
|---|---|
| Disease category | Recommendation in COVID-19 pandemic |
| NSCLC, T1/2N0M0, medically inoperable; peripheral[29], [30] | • SBRT 30-34 Gy in single fraction (T1 N0M0) |
| • 54 Gy in 3 fractions in 1.5 weeks (Eligibility includes T1, 2 (<5 cms), T3 <5 cms, chest wall involvement positive, no mediastinal or bronchial tree invasion) • 48 Gy in 4 fractions, daily RT |
|
| NSCL, T1/2N0M0, medically inoperable, central[31] | • 60 Gy in 8 daily fractions • 70 Gy in 10 daily fractions • 50 Gy in 5 daily fractions |
| Stage I-IIIB tumor, operated | • Short delay in radiation if R0 resection |
| Stage III, Locally advanced NSCLC[32] | • 55 Gy in 20 fractions with concurrent/sequential chemotherapy • 60 Gy in 15-20 fractions • (RT alone) |
| NSCLC, advanced- inoperable, large for curative RT[33] | • Palliative RT → 1-2 fractions of 8 to 10 Gy/fraction (weekly if 2; may be supported with sequential chemotherapy: |
| SCLC, localized[34], [35] | • 40-42 Gy in 15 daily fractions |
| SCLC, Extensive[36], [37], [38] | • Omit Prophylactic cranial irradiation. • ESTRO COVID-19 pandemic guideline also recommends - Consider omission of consolidation thoracic radiotherapy in extensive stage disease |
|
CERVICAL CANCER | |||
|---|---|---|---|
| Site of Cancer | Preferred treatment | EBRT | Brachytherapy |
| Stages (FIGO 2019) IB3, IIA2-IIIC2 and early IVA (focal infiltration of bladder or rectum: 1 × 1 cm) | • Radical: Radiotherapy (EBRT) + concurrent chemotherapy [39], [40] | • 50.4 Gy / 28 fractions (preferred for bulkier or node positive) with 3DCRT • 45 Gy / 25 fractions with SIB to gross nodes • 55-62.5 Gy / 25 fractions) with IMRT [41], [42] |
• Intracavitary HDR brachytherapy 3 fractions (EQD2 of at least 85 Gy to point. A) • If theatre/anaesthesia limitations present, then consider EBRT boost (18 Gy in 10 fractions) |
| IA1, IA2, IB1, IB2, IIA1 | • Adjuvant: EBRT + conc. CT for high risk patients (Sedlis criteria + positive nodes / parametria / margin) | • 45 Gy / 25 fractions with IMRT; if resource constraints, 3DCRT [43] | • Vault brachytherapy 6 Gy x 2 fractions where indicated |
| IVA (frank bladder or rectal infiltration) or IVB | Palliative • Pain • Bleeding |
• 8 Gy single fraction or 20 Gy in 5 fractions [44], [45] | • No |
|
ENDOMETRIAL CANCER | |||
|---|---|---|---|
| Site of Cancer | Preferred treatment | EBRT | Brachytherapy |
| Stages IA Gr 1-Gr 3 and IB Gr 1-2 | • Observation only | • No | • Vault brachytherapy if positive margins, suboptimal surgery (defer as long as possible) |
| Stages IB Gr 3, Stage II | • EBRT 8-12 weeks post surgery | • 45 Gy in 25 fractions (IMRT preferred) [47] | • Vault brachytherapy [can consider only brachytherapy (no EBRT) in Stage IB G3 and Stage II, G1 and G2 with no high-risk features] |
| Stage IIIA-IIIC | • Systemic therapy and / or EBRT 6-8 weeks post-op | • 45 Gy in 25 Fr (IMRT preferred) [48] | • Vault brachytherapy [49] |
| Stage IVB | • Only systemic therapy • Palliative: i)Pain ii) Bleeding |
• 8 Gy single fraction • 20 Gy / 5 fractions [50] |
• No |
| All stages | • Radical: EBRT + chemotherapy considered on a case by case basis (those unable to have surgery) [51] | • EBRT (IMRT) + brachytherapy should deliver an EQD2 D90 of 65 Gy to the uterus, cervix and upper vagina • Only brachytherapy should deliver at EQD2 D90 of 48 Gy |
• Alone or in combination with EBRT |
|
VULVAR AND VAGINAL CANCERS | |||
|---|---|---|---|
| Site of cancer or presentation | Preferred treatment in a Pandemic | EBRT | Brachytherapy |
| Vulva, radical | • EBRT + chemotherapy [52], [53] | • 45 Gy / 25 fractions followed by 18-20 Gy / 9-10 fractions to gross disease (IMRT or VMAT only) • SIB to primary and nodes (preferred to reduce overall time) |
• Best to avoid in resource-limited setting |
| Vulva, adjuvant | • Adjuvant: Groin and pelvic EBRT (U/L or B/L) in high risk features (mentioned in text) | • 45 Gy / 25 fractions [54], [55] | • No |
| Vulva, palliative | • Palliative [56]: i) Pain ii) Bleeding |
• 8 Gy single fraction • 20 Gy / 5 fractions |
• No |
| Vagina, upper vagina | • Treat as cervix cancer | • Yes | • Yes |
| Vagina, lower vagina | • Treat as vulvar cancer | • Yes | • Yes |
|
PROSTATE CANCER | ||||
|---|---|---|---|---|
| Disease Stage | Preferred treatment | ADT (before RT) | EBRT | Brachytherapy |
| Very low/low risk | • Active surveillance and repeat PSA after 6 months | • No | • No [59] | • No |
| Favourable Intermediate risk | • Active surveillance and repeat PSA after 3-6 months | • No | • Delay until safe [59] | • Delay until safe |
| Unfavourable Intermediate risk | • ADT + RT | • 4-6 months (up to 8 months also if safe) [60] | • Modest hypofractionation (60 Gy/20 fractions) • Ultra hypo-fractionated regimens: - 42.7 Gy/7 fractions every other day - 36 Gy/6 fractions/6 weeks. • 5 fraction stereotactic body radiotherapy (SBRT) (most preferred) if good planning and delivery possible [61], [62], [63], [64] |
• Delay until safe |
| High/very high risk | • ADT + RT | • 2-4 months | • Delay or avoid | |
| N+ | • ADT + RT • [65] |
• 2-4 months | Not recommended | |
| Adjuvant RT | • Early salvage (consider strongly) over adjuvant RT ± ADT [66], [67] | • 4-6 months | • Standard (33-35 fractions) • Hypofractionation (60 Gy/20 fractions) if high risk features on HPR Note: Attempt to defer RT in all cases with use of ADT] |
• - |
| Oligometastatic | • ADT + RT [68] | • 2-3 months | • SABR though popular has less survival benefit (1 fraction or 3 fractions) Note: Needs to be discussed in resource limited setting and deferred as much as possible |
• - |
| Low volume M1 | • ADT + prostate- directed therapy [69], [70] | • 4-6 months | • 5 or 6 fractions Note: Needs to be discussed in resource limited setting and deferred as much as possible |
• - |
| GI MALIGNANCIES |
|
Esophageal malignancies 1. If neo-adjuvant chemoradiotherapy considered → 40 Gy in 15 fractions with concurrent chemotherapy (carboplatin and paclitaxel) [74] 2. Induction chemotherapy can result in increased in immunosuppression adding to the risk of infection 3. Definitive chemoradiation therapy can be considered as the best curative option with carboplatin and taxol based chemotherapy because of lower toxicity [75]. If tumor 5 cm in length → 50 Gy in 16 fractions and if up to 10 cm → 50-55 Gy in 20 fractions [76] 4. Consider delaying adjuvant therapy for up to 12 weeks |
|
Locally advanced unresectable pancreatic cancers 1. Hypofractionated radiation therapy (45 Gy in 15 fractions) with concurrent capecitabine 2. A hypofractionated regimen of radiation therapy (25-35 Gy in 5 fractions) [77] 3. Radiation therapy needs to be avoided if the direct invasion of bowel and stomach is observed |
|
Rectal cancer 1. Long course treatment for threatening margins should be converted to short course treatments (avoid in young, fit patients) [78] 2. Avoid short course if significant pelvic disease present 3. 25 Gy/ 5 daily fractions [79] 4. Surgery can be delayed up to 8 weeks [80] |
|
SOFT TISSUE SARCOMA 1. Standard pre-operative radiotherapy needs to be discontinued during the pandemic 2. Consider surgery as the first line for local management 3. Role of protracted radiotherapy regimens: if pre-operative radiation is being considered → 25 Gy in 5 fractions can be considered if the disease is not close to critical structures followed by surgery after 1-2 weeks [97] 4. If radiation therapy facilities are restricted due to COVID-2019, the treatment can be deferred and started within a reasonable time frame and if no local recurrences have developed 5. In some patients, hypofractionated dose schedules of 40-45 Gy in 15-20 fractions and 36 Gy in 6 once weekly fractions can be considered, except in young patients due to increased late radiotherapy related toxicities [98] 6. In other forms of sarcoma → . Radiation therapy can be deferred in view of restricted radiation therapy delivery and individual case-based decisions need to be taken |
|
UROTHELIAL MALIGNANCIES 1. For T2-T4 N0M0 disease - Radical radiation therapy with 55 Gy in 20 fractions can be considered with weekly gemcitabine [99] 2. Palliative radiation therapy schedules: • 21 Gy in 3 fractions [100] • 36 Gy in 6 fractions given weekly [101] • 8-10 Gy in single fraction for bleeding or local symptom control |
|
PEDIATRIC MALIGNANCIES 1. CNS tumors including medulloblastoma, grade 2/3 ependymoma, embryonal CNS tumors, intracranial germ cell tumors, atypical teratoid/ rhabdoid tumor → Delay in radiation therapy can lead to poor survival outcomes and need to be treated on priority basis 2. Similarly, total body irradiation, retinoblastoma, nasopharynx and head and neck malignancies require priority for treatment with radiation therapy 3. Adjuvant therapy can be considered as a priority in cases where tumors present with an aggressive histopathology or residual disease which could lead to deteriorating symptoms |
|
THYROID 1. A delay in radioactive iodine treatment will not impact the prognosis in differentiated thyroid cancer (DTC) 2. Metastatic disease → Treatment decisions can be made based on the risk-benefit assessment on an individual basis |
|
NON-MALIGNANT LESION AND SKIN TUMORS 1. Suspend all treatment forms until the pandemic is over |
| PALLIATIVE RADIATION THERAPY |
|
Brain metastases 1. Keep the patient visit and duration of stay in the hospital as brief as possible 2. For metastases <10cc → consider single fraction treatment 3. If SRS is a feasible option, it can replace neurosurgical options where resources are deemed limited [81] 4. Postoperative: SRS to the cavity → 5 Gy in 7 fractions [82], [83], [84] 5. If life expectancy >3 months → 4 Gy in 5 fractions to whole brain [85] 6. Poor performance status → Best supportive care with review for the need of steroids [86] |
|
Spinal cord compression (SCC) 1. Assess for extent of spinal cord compression, presence or absence of spinal cord instability → 8 Gy in single fraction [87] |
|
Tumor bleeding 1. ‘Quad-Shot’ regimen → 3.7 Gy x 4 fractions twice daily, 3 weekly, 3 courses [88] 2. 4 Gy x 5 fractions given daily [89] 3. Single fraction of 8Gy [90] 4. Twice daily treatment is not recommended during a pandemic |
|
SVC syndrome 1. 8.5 Gy x 2 fractions, a week apart [91] 2. 4 Gy x 5 daily fractions [92] 3. 8-10 Gy in single fraction [93], [94], [95] |
|
Painful bone metastases 1. Consider all medical strategies and supportive care before offering radiation. 2. In patients with impending fracture, discuss the option of mechanical stabilization with orthopedic surgeon and interventional radiologist 3. 8 Gy single fraction [96] |
Additional points to be considered:
-
a)Breath hold devices are indicated in lung SBRT. As much as possible, avoid using ABC that requires a mouthpiece. Voluntary Deep Inspiration Breath Holding (DIBH) methods would be encouraged. ABC may be used for:
-
i.Re-irradiation cases where the anticipated toxicity will be reduced.
-
ii.Conventionally fractionated/hypofractionated or stereotactive ablative radiotherapy (SABR) plan where normal tissue constraints are not met without breath-hold technique.
-
i.
-
b)
Dose gradients recommended for stereotactive body radiation therapy (SBRT) have been given in RTOG 0915 and RTOG 0813 and these can be referred to the for the constraints described.
-
c)
Centers with prior experience delivering lung SABR should offer single fraction SABR.
-
d)
Tumors with movement of less than 1 cm on 4DCT imaging should be considered for SBRT.
Gynecological cancers
The following guidelines look into the potential adaptations to the evidence-based management in gynecological malignancies
Cervical cancer
External beam radiation therapy (EBRT):
-
•
Pelvic radiotherapy with concurrent chemotherapy (wherever indicated) is still the standard of care.
-
•
Both concurrent and systemic (cisplatin-based) chemotherapy should be used only if strongly indicated and depending on resource availability. It needs to be avoided altogether in patients over 70 years and with comorbidities.
-
•
Carboplatin should be avoided due to higher rates of pancytopenia.
-
•
Gross nodes should be addressed with a simultaneous rather than a sequential EBRT boost.
-
•
Less resource-intensive techniques like weekly cone beam computed tomography verification and liberal clinical target volume / internal target volume / planning target volume (CTV-ITV-PTV) margins may have to be accepted.
-
•
Avoid extended field EBRT as far as possible.
Brachytherapy:
-
•
Intrauterine brachytherapy is an essential part of the treatment for cervical cancer that cannot be omitted in the curative setting.
-
•
In resource-limited set-up, a reference to another center for brachytherapy (without severely affecting overall treatment time) is advised. When that is not feasible, consider the EBRT boost.
-
•
An expert panel should sit together to prioritize patients according to the potential benefit of brachytherapy depending on EBRT response, age, nodal involvement and overall treatment time. A virtual tumor board through online meetings can also help make decisions.
-
•
Another alternative is to deliver 2 or 3 fractions per insertion with a gap of at least 6 hours between fractions. In the absence of spinal or general anesthesia, small diameter applicators using a local anesthetic or mild sedation can be used for brachytherapy.
-
•
MRI-based planning may be done at least for the first fraction, especially in bulky residual disease after EBRT requiring interstitial needles. CT based planning is acceptable for all other cases.
-
•
Image-guided adaptive planning is preferred in gross residual disease cases at the time of brachytherapy. And simple point A based planning is good for low volume good responders.
Stage wise treatment recommendations are given in Table. 2
Endometrial cancer
External beam radiation therapy:
-
•
Endometrial cancer is typically a disease of postmenopausal, elderly women, a group that is most vulnerable during this coronavirus pandemic.
-
•
Surgery remains the mainstay of treatment for endometrial cancer. However, if most elective surgeries get canceled during the lockdown period, consider alternatives such as megestrol, medroxyprogesterone, or a levonorgestrel intrauterine device to allow surgery after a delay of a few weeks or months [46].
-
•
Radical radiotherapy (EBRT) is an alternative option, however, much inferior in results to surgery. Very rarely, brachytherapy alone is used for early-stage disease (using a Rotte applicator). Both of these are options only in severely resource-limited set-ups.
-
•
For very locally advanced disease inoperable or metastatic disease, consider chemotherapy with or without radiotherapy.
-
•
Adjuvant radiotherapy: It may be omitted in low and intermediate-risk Stage I cases and may be delayed in the high intermediate-risk category for up to 3 months. But the high-risk Stage I and Stage II onwards or high-risk features (positive margin, type 2 histology, residual disease at stump) may warrant adjuvant EBRT and/or vault brachytherapy.
Brachytherapy:
-
•
Vault Brachy: With EBRT: 6-8 Gy x 2 # weekly and without EBRT: 7 Gy x 3# weekly
Management of endometrial cancer as per the stage of the disease is shown in Table 2.
Vulvar cancer
-
•
This group of cancers is also common in elderly women making them a susceptible group for hospital acquired Covid 19 infection.
-
•
In view of reduced surgeries during a pandemic, radical radiotherapy becomes the treatment of choice in vulvar cancers.
-
•
Concurrent chemotherapy may be avoided in patients >70 years, poor PS, co-morbidities or immunocompromised.
-
•
Adjuvant radiotherapy should be given to patients with positive resection margins, residual disease or 2 or more lymph node involvement (or extra capsular spread). No concurrent chemotherapy should be given.
-
•
Radical or boost treatment with brachytherapy is not encouraged as theatres and anesthesia should be conserved for high volumes of cervix and endometrial brachytherapy.
Vaginal cancer
-
•
Upper vaginal cancer: Treat like cervix cancer
-
•
Lower vaginal cancer: Treat like vulvar cancer but followed by brachytherapy
Management of vulvar and vaginal cancers is shown in Table 2.
Genitourinary cancers
Prostate cancer
-
•
Compared to other cancers in India, prostate cancer is not a heavy burden on radiotherapy departments. Generally, it has a more favorable prognosis and is responsive to androgen deprivation therapy (ADT) which allows a safe delay of radical treatment for many months. This helps to reduce the overall burden on the system and staff considerably. Thus, it is possible to divert resources to more urgent needs during the COVID-19 pandemic.
-
•
Fiducial application and rectal spacer application must be considered only if performing SBRT.
-
•
If ADT is initiated consider 6-month depot injection, in which case radiotherapy can be delayed for up to 4–6 months (in low-risk cases, even for up to 8 months).
-
•
If ADT cannot be delivered (eg, patient refusal, cardiac toxicity) and there is a rapid Prostate Specific Antigen (PSA) doubling time (≤3 months) the benefits of starting RT must be weighed against COVID-19 exposure and subsequent morbidity and mortality especially in the aged with multiple co-morbidities or in the immunocompromised.
-
•
Hypofractionation with 5 fractions (preferred) or 20 fractions should be established as the new normal during this phase.
-
•
Brachytherapy is to be avoided as far as possible as the availability of theatres and anaesthesiologists are limitations.
-
•
If pelvic nodes are to be treated, opt for a hypofractionated schedule (60 Gy/20 Fr) with uninvolved nodes receiving 42-44 Gy in 20 fractions [57,58].
-
•
Further stage-wise management is described in Table 2.
Also, note that in centers where brachytherapy of prostate is commonly done,
-
•
Convert all HDR monotherapy cases (2 implants) to HDR boost (single implant 15 Gy in 1 Fr) if the operation theatre resources permit. If not, convert to EBRT or commence ADT.
-
•
EBRT schedules that are due for HDR boosts (15 Gy in 1 Fr) can be converted to 37.5 Gy/15 fractions.
Seminoma: Rarely treated with RT. Even in Stage 1, favor surveillance [71]
Renal cell carcinoma
Primary and metastatic RCC are typically treated with SABR, mostly under the trial setting. All cases require thorough discussion. Defer cases where it is deemed possible to perform active surveillance. If treatment is warranted urgently, and suitable resources are available, a single fraction SABR may be given [72,73].
Gastrointestinal malignancies
The spectrum of GI malignancies encompasses various disease sites and the best available treatment options are discussed in Table 2.
Palliative radiation during pandemic
Palliative radiation has a crucial role in the prevention of serious morbidity, palliation of bothering symptoms and also in the setting of oncologic emergencies. Estimated benefits and prognosis should be communicated to the patients, including the risk-benefit analysis. Patients who are well prognosticated regarding their disease, avoid aggressive treatment near the end of life and usually opt for medical supportive care. The use of abbreviated RT courses may reduce hospital visits and therefore the risk of exposure.
Concerns associated with treatment
-
•
Radiation needs daily hospital visits, thus a higher risk of exposure to infection. Exposing fragile patients to this risk for palliative treatment needs risk-benefit analysis.
-
•
Patients with a life expectancy of days to weeks should be offered best supportive care.
-
•
Disease extent, palliative intent of treatment, available treatment options, the benefit of each option is to be explained to patients and care-givers.
-
•
Patients receiving palliative treatment usually need support for mobilization and positioning for different procedures. A higher number of caregivers is needed, with a higher risk of transmission. The number of visitors should be kept to a minimum.
-
•
Patients receiving palliative treatment are at a higher need for in-hospital admissions due to their limited mobility or continuous need for medical attention. They should be offered options that reduce their hospital stay or hospital visits.
-
•
Treatment options should be chosen after considering the benefit obtained from a particular modality, hospital stay required, along with the chances of contact with another individual.
Management of Oncologic Malignancies: The various oncological emergencies and palliative radiation therapy needs are covered in Table 2 and an algorithm for management decisions in palliative oncology have been depicted in Fig. 5 .
Fig. 5.
Algorithm for management decisions for palliative treatment during COVID-2019.
Prioritizing treatment options in miscellaneous sites: We have summarized the various subsites of oncology and the required management of malignancies which do not form the bulk of patient load in the regular radiation oncology department but when faced with, can be challenging to treat in a pandemic. Table 2 presents the details related to the treatment options and the available evidence.
Conclusion
Over the next few months, we as oncologists will see a major change in the management strategies as long as the pandemic lasts and along with the best available treatment options, counseling of the patient will also take center stage. It is vital to explain to patients the benefit of radiotherapy balanced against the accepted risk associated with contracting COVID-19 during the treatment period. These new protocols will have to be accepted as the “new normal” and set in place as there is a high likelihood that the pandemic may last for several months. We have attempted to bring together guidelines from all over the world under one roof to help clinicians make a well-planned decision related to radiation therapy. The guidelines are ever-evolving and the structured plans depicted in the article may change with potential developments in the future.
Conflict of Interest
None.
Disclosure
None of the authors have any disclosures to declare
References
- 1.Guan W., et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johns Hopkins University Center for Systems Science and Engineering (CSSE). Wuhan coronavirus (2019-nCoV) global cases
- 3.Fauci A.S., Lane H.C., Redfield R.R. COVID-19 - Navigating the Uncharted. N. Engl. J. Med. 2020 doi: 10.1056/NEJMe2002387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang W., et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. The Lancet Oncology. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Filippi A.R., et al. COVID-19 Outbreak in Northern Italy: First practical indications for radiotherapy departments. International Journal of Radiation Oncology • Biology • Physics. 2020 doi: 10.1016/j.ijrobp.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dinh T.K., et al. Radiation Therapy in King County, Washington During the COVID-19 Pandemic: Balancing patient care, Transmission mitigation and. Resident training; Advances in Radiation Oncology. March 2020 doi: 10.1016/j.adro.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simcock R., Thomas T.V., Mercy C.E., Filippi A.R., Katz M.A., Pereira I.J., Saeed H. COVID-19: Global Radiation Oncology's Targeted Response for Pandemic Preparedness. Clinical & Translational Radiation Oncology. 2020 doi: 10.1016/j.ctro.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malmström A., et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916–926. doi: 10.1016/S1470-2045(12)70265-6. [DOI] [PubMed] [Google Scholar]
- 9.Wick W., et al. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13(7):707–715. doi: 10.1016/S1470-2045(12)70164-X. [DOI] [PubMed] [Google Scholar]
- 10.Chan A.K., Sanghera P., Choo B.A. Hypofractionated accelerated radiotherapy with concurrent carboplatin for locally advanced squamous cell carcinoma of the head and neck. Clinical oncology. 2011;23:34–39. doi: 10.1016/j.clon.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 11.Pignon J.P., Le Maitre A., Maillard E., Bourhis J. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92(1):4–14. doi: 10.1016/j.radonc.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 12.Overgaard J., Hansen H.S., Specht L. Five compared with six fractions per week of conventional radiotherapy of squamous-cell carcinoma of head and neck: DAHANCA 6 and 7 randomised controlled trial. Lancet. 2003;362:933–940. doi: 10.1016/s0140-6736(03)14361-9. [DOI] [PubMed] [Google Scholar]
- 13.Porceddu S.V., et al. Hypofractionated radiotherapy for the palliation of advanced head and neck cancer in patients unsuitable for curative treatment–"Hypo Trial". Radiother Oncol. 2007;85(3):456–462. doi: 10.1016/j.radonc.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen N.T.A., et al. 0-7-21 hypofractionated palliative radiotherapy: an effective treatment for advanced head and neck cancers. The British Journal of Radiology. 2015;88(1049) doi: 10.1259/bjr.20140646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blanchard P., Bourhis J., Lacas B., Posner M.R., Vermorken J.B., Cruz Hernandez J.J., et al. Taxane-cisplatin-fluorouracil as induction chemotherapy in locally advanced head and neck cancers: an individual patient data meta-analysis of the meta-analysis of chemotherapy in head and neck cancer group. J Clin Oncol. 2013;31(23):2854–2860. doi: 10.1200/JCO.2012.47.7802. [DOI] [PubMed] [Google Scholar]
- 16.Terhaard C.H., Lubsen H., Rasch C.R., Levendag P.C., Kaanders H.H., Tjho-Heslinga R.E., et al. The role of radiotherapy in the treatment of malignant salivary gland tumors. Int J Radiat Oncol Biol Phys. 2005;61(1):103–111. doi: 10.1016/j.ijrobp.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 17.McCormick B., Winter K., Hudis C., Kuerer H.M., Rakovitch E., Smith B.L., Sneige N., Moughan J., Shah A., Germain I., Hartford A.C., Rashtian A., Walker E.M., Yuen A., Strom E.A., Wilcox J.L., Vallow L.A., Small W., Jr, Pu A.T., Kerlin K.…White J. RTOG 9804: a prospective randomized trial for good-risk ductal carcinoma in situ comparing radiotherapy with observation. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2015;33(7):709–715. doi: 10.1200/JCO.2014.57.9029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Correa C., McGale P., Taylor C., Wang Y., Clarke M., Davies C., Peto R., Bijker N., Solin L., Darby S. Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast. Journal of the National Cancer Institute. Monographs. 2010;2010(41):162–177. doi: 10.1093/jncimonographs/lgq039. Early Breast Cancer Trialists' Collaborative Group (EBCTCG) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shurell E., Olcese C., Patil S., McCormick B., Van Zee K.J., Pilewskie M.L. Delay in radiotherapy is associated with an increased risk of disease recurrence in women with ductal carcinoma in situ. Cancer. 2018;124(1):46–54. doi: 10.1002/cncr.30972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hughes K.S., Schnaper L.A., Bellon J.R., Cirrincione C.T., Berry D.A., McCormick B., Muss H.B., Smith B.L., Hudis C.A., Winer E.P., Wood W.C. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2013;31(19):2382–2387. doi: 10.1200/JCO.2012.45.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kunkler I.H., Williams L.J., Jack W.J., Cameron D.A., Dixon J.M., PRIME II investigators Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a randomised controlled trial. The Lancet. Oncology. 2015;16(3):266–273. doi: 10.1016/S1470-2045(14)71221-5. [DOI] [PubMed] [Google Scholar]
- 22.Olivotto I.A., Lesperance M.L., Truong P.T., Nichol A., Berrang T., Tyldesley S., Germain F., Speers C., Wai E., Holloway C., Kwan W., Kennecke H. Intervals longer than 20 weeks from breast-conserving surgery to radiation therapy are associated with inferior outcome for women with early-stage breast cancer who are not receiving chemotherapy. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27(1):16–23. doi: 10.1200/JCO.2008.18.1891. [DOI] [PubMed] [Google Scholar]
- 23.Moran M.S., Zhao Y., Ma S., Kirova Y., Fourquet A., Chen P., Hoffman K., Hunt K., Wong J., Halasz L.M., Freedman G., Prosnitz R., Jr, Yassa M., Nguyen D., Hijal T., Haffty B.G., Wai E.S., Truong P.T. Association of Radiotherapy Boost for Ductal Carcinoma In Situ With Local Control After Whole-Breast Radiotherapy. JAMA oncology. 2017;3(8):1060–1068. doi: 10.1001/jamaoncol.2016.6948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yerushalmi R, Sulkes A, Mishaeli M, Neumann A, Dinerman M, Sulkes J, Rizel S, Yarom N, Gutman H, Fenig E. Neoplasma. 2006;53(6):507–510. [PubMed] [Google Scholar]
- 25.Bartelink H., Maingon P., Poortmans P., Weltens C., Fourquet A., Jager J., Schinagl D., Oei B., Rodenhuis C., Horiot J.C., Struikmans H., Van Limbergen E., Kirova Y., Elkhuizen P., Bongartz R., Miralbell R., Morgan D., Dubois J.B., Remouchamps V., Mirimanoff R.O.…European Organisation for Research and Treatment of Cancer Radiation Oncology and Breast Cancer Groups Whole-breast irradiation with or without a boost for patients treated with breast-conserving surgery for early breast cancer: 20-year follow-up of a randomised phase 3 trial. The Lancet. Oncology. 2015;16(1):47–56. doi: 10.1016/S1470-2045(14)71156-8. [DOI] [PubMed] [Google Scholar]
- 26.Rashadan Al, et al. Adapting Radiotherapy Treatments for Breast Cancer Patients during the COVID-19 Pandemic: HypoFractionation and Accelerated Partial Breast Irradiation to Address World Health Organization Recommendations. Adv in RadOnc. March 2020 [Google Scholar]
- 27.Wang S.L., Fang H., Song Y.W., Wang W.H., Hu C., Liu Y.P., Jin J., Liu X.F., Yu Z.H., Ren H., Li N., Lu N.N., Tang Y., Tang Y., Qi S.N., Sun G.Y., Peng R., Li S., Chen B., Yang Y.…Li Y.X. Hypofractionated versus conventional fractionated postmastectomy radiotherapy for patients with high-risk breast cancer: a randomised, non-inferiority, open-label, phase 3 trial. The Lancet. Oncology. 2019;20(3):352–360. doi: 10.1016/S1470-2045(18)30813-1. [DOI] [PubMed] [Google Scholar]
- 28.Braunstein L.Z., Thor M., Flynn J., Cost Z., Wilgucki M., Rosenbaum S., Zhang Z., Gillespie E., McCormick B., Khan A., Ho A., Cahlon O., Deasy J.O., Powell S.N. Daily Fractionation of External Beam Accelerated Partial Breast Irradiation to 40 Gy Is Well Tolerated and Locally Effective. International journal of radiation oncology, biology, physics. 2019;104(4):859–866. doi: 10.1016/j.ijrobp.2019.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Videtic G.M., Paulus R., Singh A.K., et al. Long-term follow-up on NRG oncology RTOG 0915 (NCCTG N0927): a randomized phase 2 study comparing 2 stereotactic body radiation therapy schedules for medically inoperable patients with stage i peripheral non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2019;103:1077–1084. doi: 10.1016/j.ijrobp.2018.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Timmerman R., Hu C., Michalsky J., et al. Long-term results of RTOG 0236: A phase II trial of stereotactic body radiation therapy (SBRT) in the treatment of patients with medically inoperable stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2014;90(Suppl. 1):S30. [Google Scholar]
- 31.Kimura T., Nagata Y., Harada H., et al. Phase I study of stereotactic body radiation therapy for centrally located stage IA non-small cell lung cancer (JROSG10-1) Int J Clin Oncol. 2017;22:849–856. doi: 10.1007/s10147-017-1125-y. [DOI] [PubMed] [Google Scholar]; Chang J.Y., Li Q.Q., Xu Q.Y., et al. Stereotactic ablative radiation therapy for centrally located early stage or isolated parenchymal recurrences of non-small cell lung cancer: how to fly in a "no fly zone". Int J Radiat Oncol Biol Phys. 2014;88:1120–1128. doi: 10.1016/j.ijrobp.2014.01.022. [DOI] [PubMed] [Google Scholar]; Chaudhuri A.A., Tang C., Binkley M.S., et al. Stereotactic ablative radiotherapy (SABR) for treatment of central and ultra-central lung tumors. Lung Cancer. 2015;89:50–56. doi: 10.1016/j.lungcan.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 32.Maguire J., Khan I., McMenemin R., et al. SOCCAR: A randomised phase II trial comparing sequential versus concurrent chemotherapy and radical hypofractionated radiotherapy in patients with inoperable stage III Non-Small Cell Lung Cancer and good performance status. Eur J Cancer. 2014;50:2939–2949. doi: 10.1016/j.ejca.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 33.Jeremic B., Fidarova E., Sharma V., et al. The International Atomic Energy Agency (IAEA) randomized trial of palliative treatment of incurable locally advanced non small cell lung cancer (NSCLC) using radiotherapy (RT) and chemotherapy (CHT) in limited resource setting. Radiother Oncol. 2015;116:21–26. doi: 10.1016/j.radonc.2015.06.017. [DOI] [PubMed] [Google Scholar]; A Medical Research Council (MRC) randomised trial of palliative radiotherapy with two fractions or a single fraction in patients with inoperable non-small-cell lung cancer (NSCLC) and poor performance status. Medical Research Council Lung Cancer Working Party. Br J Cancer. 1992;65(6):934–941. doi: 10.1038/bjc.1992.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giuliani M.E., Atallah S., Sun A., Bezjak A., Le L.W., Brade A., Cho J., Leighl N.B., Shepherd F.A., Hope A.J. Clinical outcomes of extensive stage small cell lung carcinoma patients treated with consolidative thoracic RT. Clin Lung Cancer. 2011;12:375–379. doi: 10.1016/j.cllc.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 35.Sculier J.P., et al. A phase III randomised study of concomitant induction radiochemotherapy testing two modalities of radiosensitisation by cisplatin (standard versus daily) for limited small-cell lung cancer. Ann Oncol. 2008 doi: 10.1093/annonc/mdn354. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi T., Yamanaka T., Seto T., et al. Prophylactic cranial irradiation versus observation in patients with extensive-disease small-cell lung cancer: a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2017:18. doi: 10.1016/S1470-2045(17)30230-9. [DOI] [PubMed] [Google Scholar]
- 37.Murray N., et al. Importance of timing for thoracic irradiation in the combined modality treatment of limited-stage small-cell lung cancer. The National Cancer Institute of Canada clinical trials group. J Clin Oncol. 1993 doi: 10.1200/JCO.1993.11.2.336. [DOI] [PubMed] [Google Scholar]
- 38.Rusthoven C.G., Kavanagh B.D. Prophylactic Cranial Irradiation (PCI) versus Active MRI Surveillance for Small Cell Lung Cancer: The Case for Equipoise. Journal of Thoracic Oncology. 2017 doi: 10.1016/j.jtho.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 39.Shrivastava S., Mahantshetty U., Engineer R., et al. Cisplatin chemoradiotherapy vs radiotherapy in FIGO stage IIIB squamous cell carcinoma of the uterine cervix a randomized clinical trial. JAMA Oncol. 2018;4(4):506–513. doi: 10.1001/jamaoncol.2017.5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vale C., Tierney J.F., Stewart L.A., et al. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: A systematic review and meta-analysis of individual patient data from 18 randomized trials. J Clin Oncol. 2008;26(35):5802–5812. doi: 10.1200/JCO.2008.16.4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vargo J.A., Kim H., Choi S., et al. Extended field intensity modulated radiation therapy with concomitant boost for lymph node-positive cervical cancer: Analysis of regional control and recurrence patterns in the positron emission tomography/computed tomography era. Int J Radiat Oncol Biol Phys. 2014;90(5):1091–1098. doi: 10.1016/j.ijrobp.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 42.Lazzari R., Riva G., Augugliaro M., et al. Intensity modulated radiation therapy boost in locally-advanced cervical cancer in the absence of brachytherapy. Int J Gynecol Cancer. March 2020 doi: 10.1136/ijgc-2019-000735. ijgc-2019-000735. [DOI] [PubMed] [Google Scholar]
- 43.Peters W.A., Liu P.Y., Barrett R.J., et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18(8):1606–1613. doi: 10.1200/JCO.2000.18.8.1606. [DOI] [PubMed] [Google Scholar]
- 44.Mishra S.K., Laskar S., Muckaden M.A., Mohindra P., Shrivastava S.K., Dinshaw K.A. Monthly palliative pelvic radiotherapy in advanced carcinoma of uterine cervix. J Cancer Res Ther. 2005;1(4):208–212. doi: 10.4103/0973-1482.19588. [DOI] [PubMed] [Google Scholar]
- 45.Kim D.H., Lee J.H., Ki Y.K., et al. Short-course palliative radiotherapy for uterine cervical cancer. Radiat Oncol J. 2013;31(4):216–221. doi: 10.3857/roj.2013.31.4.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baker J., Obermair A., Gebski V., Janda M. Efficacy of oral or intrauterine device-delivered progestin in patients with complex endometrial hyperplasia with atypia or early endometrial adenocarcinoma: A meta-analysis and systematic review of the literature. Gynecol Oncol. 2012;125(1):263–270. doi: 10.1016/j.ygyno.2011.11.043. [DOI] [PubMed] [Google Scholar]
- 47.Klopp A.H., Yeung A.R., Deshmukh S., et al. Patient-reported toxicity during pelvic intensity-modulated radiation therapy: NRG oncology-RTOG 1203. J Clin Oncol. 2018;36(24):2538–2544. doi: 10.1200/JCO.2017.77.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Boer S.M., Powell M.E., Mileshkin L., et al. Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial. Lancet Oncol. 2019;20(9):1273–1285. doi: 10.1016/S1470-2045(19)30395-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nout R.A., Smit V.T.H.B.M., Putter H., et al. Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): an open-label, non-inferiority, randomised trial. Lancet. 2010;375(9717):816–823. doi: 10.1016/S0140-6736(09)62163-2. [DOI] [PubMed] [Google Scholar]
- 50.Yan J., Milosevic M., Fyles A., Manchul L., Kelly V., Levin W. A Hypofractionated Radiotherapy Regimen (0-7-21) for Advanced Gynaecological Cancer Patients. Clin Oncol. 2011;23(7):476–481. doi: 10.1016/j.clon.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 51.Van der Steen-Banasik E., Christiaens M., Shash E., et al. Systemic review: Radiation therapy alone in medical non-operable endometrial carcinoma. Eur J Cancer. 2016;65:172–181. doi: 10.1016/j.ejca.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 52.Han S.C., Kim D.H., Higgins S.A., Carcangiu M.L., Kacinski B.M. Chemoradiation as primary or adjuvant treatment for locally advanced carcinoma of the vulva. Int J Radiat Oncol Biol Phys. 2000;47(5):1235–1244. doi: 10.1016/s0360-3016(00)00569-1. [DOI] [PubMed] [Google Scholar]
- 53.Natesan D., Hong J.C., Foote J., Sosa J.A., Havrilesky L., Chino J. Primary Versus Preoperative Radiation for Locally Advanced Vulvar Cancer. Int J Gynecol Cancer. 2017;27(4):794. doi: 10.1097/IGC.0000000000000938. LP - 804. [DOI] [PubMed] [Google Scholar]
- 54.Mahner S., Jueckstock J., Hilpert F., et al. Adjuvant therapy in lymph node-positive vulvar cancer: The AGO-CaRE-1 study. J Natl Cancer Inst. 2015;107(3) doi: 10.1093/jnci/dju426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ignatov T., Eggemann H., Burger E., Costa S.D., Ignatov A. Adjuvant radiotherapy for vulvar cancer with close or positive surgical margins. J Cancer Res Clin Oncol. 2016;142(2):489–495. doi: 10.1007/s00432-015-2060-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yan J., Milosevic M., Fyles A., Manchul L., Kelly V., Levin W. A Hypofractionated Radiotherapy Regimen (0-7-21) for Advanced Gynaecological Cancer Patients. Clin Oncol. 2011;23(7):476–481. doi: 10.1016/j.clon.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 57.Pommier P., et al. Is there a role for pelvic irradiation in localized prostate adenocarcinoma? Update of the long-term survival results of the GETUG-01 randomized study. Int J Radiat Oncol Biol Phys. 2016;96(4):759–769. doi: 10.1016/j.ijrobp.2016.06.2455. [DOI] [PubMed] [Google Scholar]
- 58.Pommier P., et al. Is there a role for pelvic irradiation in localized prostate adenocarcinoma? Preliminary results of GETUG-01. J Clin Oncol. 2007;25(34):5366–5373. doi: 10.1200/JCO.2006.10.5171. [DOI] [PubMed] [Google Scholar]; Supiot S., et al. Hypofractionated radiotherapy in prostate cancer. Cancer Radiother. 2013;17(5–6):349–354. doi: 10.1016/j.canrad.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 59.Hamdy F.C., Donovan J.L., Lane J.A., et al. 10-Year Outcomes After Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N Engl J Med. 2016;375(15):1415–1424. doi: 10.1056/NEJMoa1606220. [DOI] [PubMed] [Google Scholar]
- 60.Crook J., Ludgate C., Malone S., et al. Report of a multicenter Canadian phase III randomized trial of 3 months vs. 8 months neoadjuvant androgen deprivation before standard-dose radiotherapy for clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2004;60(1):15–23. doi: 10.1016/j.ijrobp.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 61.Dearnaley D., Syndikus I., Mossop H., et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17(8):1047–1060. doi: 10.1016/S1470-2045(16)30102-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Catton C.N., Lukka H., Gu C.-S., et al. JOURNAL OF CLINICAL ONCOLOGY Randomized Trial of a Hypofractionated Radiation Regimen for the Treatment of Localized Prostate Cancer. J Clin Oncol. 2017;35(17):1884–1890. doi: 10.1200/JCO.2016.71.7397. [DOI] [PubMed] [Google Scholar]
- 63.Widmark A., Gunnlaugsson A., Beckman L., et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. Lancet. 2019;394(10196):385–395. doi: 10.1016/S0140-6736(19)31131-6. [DOI] [PubMed] [Google Scholar]
- 64.Brand D.H., Tree A.C., Ostler P., et al. Intensity-modulated fractionated radiotherapy versus stereotactic body radiotherapy for prostate cancer (PACE-B): acute toxicity findings from an international, randomised, open-label, phase 3, non-inferiority trial. Lancet Oncol. 2019;20(11):1531–1543. doi: 10.1016/S1470-2045(19)30569-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mallick I., Das A., Arunsingh M. Moderately Hypofractionated Radiotherapy in Node-positive Prostate Cancer. Clin Oncol. 2019;31(4):260–264. doi: 10.1016/j.clon.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 66.Parker C., Clarke N.W., Cook A., et al. Timing of radiotherapy (RT) after radical prostatectomy (RP): First results from the RADICALS RT randomised controlled trial (RCT) [ NCT00541047] Ann Oncol. 2019;30(October):v883–v884. [Google Scholar]
- 67.Pearse M., Fraser-Browne C., Davis I.D., et al. A phase III trial to investigate the timing of radiotherapy for prostate cancer with high-risk features: Background and rationale of the Radiotherapy - Adjuvant Versus Early Salvage (RAVES) trial. BJU Int. 2014;113(SUPPL. 2):7–12. doi: 10.1111/bju.12623. [DOI] [PubMed] [Google Scholar]
- 68.Ost P. It Ain't Over Till the Fat Lady Sings: The POPSTAR Trial. Eur Urol. 2018;74(4):463–464. doi: 10.1016/j.eururo.2018.06.031. [DOI] [PubMed] [Google Scholar]
- 69.Parker C.C., James N.D., Brawley C.D., et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet. 2018;392(10162):2353–2366. doi: 10.1016/S0140-6736(18)32486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boevé L.M.S., Hulshof M.C.C.M., Vis A.N., et al. Effect on Survival of Androgen Deprivation Therapy Alone Compared to Androgen Deprivation Therapy Combined with Concurrent Radiation Therapy to the Prostate in Patients with Primary Bone Metastatic Prostate Cancer in a Prospective Randomised Clinical Trial: Data from the HORRAD Trial. Eur Urol. 2019;75(3):410–418. doi: 10.1016/j.eururo.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 71.Cohn-Cedermark G., Stahl O., Tandstad T. Surveillance vs. adjuvant therapy of clinical stage I testicular tumors - a review and the SWENOTECA experience. Andrology. 2015;3(1):102–110. doi: 10.1111/andr.280. or single dose Carboplatin. (Oliver RTD, Mason MD. [DOI] [PubMed] [Google Scholar]; Oliver R.T.D., Mason M.D., Mead G.M., et al. Radiotherapy versus single-dose carboplatin in adjuvant treatment of stage I seminoma: A randomised trial. Lancet. 2005;366(9482):293–300. doi: 10.1016/S0140-6736(05)66984-X. [DOI] [PubMed] [Google Scholar]
- 72.Siva S., Louie A.V., Warner A., et al. Pooled analysis of stereotactic ablative radiotherapy for primary renal cell carcinoma: A report from the International Radiosurgery Oncology Consortium for Kidney (IROCK) Cancer. 2018;124(5):934–942. doi: 10.1002/cncr.31156. [DOI] [PubMed] [Google Scholar]
- 73.Meyer J.J., Foster R.D., Lev-Cohain N., et al. A Phase I Dose-Escalation Trial of Single-Fraction Stereotactic Radiation Therapy for Liver Metastases. Ann Surg Oncol. 2016;23(1):218–224. doi: 10.1245/s10434-015-4579-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Walsh T.N., Noonan N., Hollywood D., Kelly A., Keeling N., Hennessy T.P.J. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med. 1996;335(7):462–467. doi: 10.1056/NEJM199608153350702. [DOI] [PubMed] [Google Scholar]
- 75.Crosby T., Hurt C.N., Falk S., Gollins S., Staffurth J., Ray, et al. Long-term results and recurrence patterns from SCOPE-1: a phase II/III randomized trial of definitive chemoradiotherapy +/- cetuximab in oesophageal cancer. Br J Cancer. 2017;116(6):709–716. doi: 10.1038/bjc.2017.21. March 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jones C.M., Spencer K., Hitchen C., Pelly T., Wood B., Hatfield P., Crellin A., Sebag-Montefiore D., Goody R., Crosby T., Radhakrishna Hypofractionated Radiotherapy in Oesophageal Cancer for Patients Unfit for Systemic Therapy: A Retrospective Single-Centre Analysis. Clin Oncol (R Coll Radiol) 2019;31:356–364. doi: 10.1016/j.clon.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 77.Pollom E.L., Alagappan M., von Eyben R., Kunz P.L., Fisher G.A., Ford J.A., Poultsides G.A., Visser B.C., Norton J.A., Kamaya A., Cox V.L., Columbo L.A., Koong A.C., Chang D.T. Single- versus multifraction stereotactic body radiation therapy for pancreatic adenocarcinoma: outcomes and toxicity. International journal of radiation oncology, biology, physics. 2014;90(4):918–925. doi: 10.1016/j.ijrobp.2014.06.066. [DOI] [PubMed] [Google Scholar]
- 78.Zhou Z.R., Liu S.X., Zhang T.S., Chen L.X., Xia J., Hu Z.D., Li B. Short-course preoperative radiotherapy with immediate surgery versus long-course chemoradiation with delayed surgery in the treatment of rectal cancer: a systematic review and meta-analysis. Surgical oncology. 2014;23(4):211–221. doi: 10.1016/j.suronc.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 79.Liu S.X., Zhou Z.R., Chen L.X., Yang Y.J., Hu Z.D., Zhang T.S. Short-course Versus Long-course Preoperative Radiotherapy plus Delayed Surgery in the Treatment of Rectal Cancer: a Meta-analysis. Asian Pacific journal of cancer prevention: APJCP. 2015;16(14):5755–5762. doi: 10.7314/apjcp.2015.16.14.5755. [DOI] [PubMed] [Google Scholar]
- 80.Erlandsson J., Holm T., Pettersson D., Berglund Å., Cedermark B., Radu C., Johansson H., Machado M., Hjern F., Hallböök O., Syk I., Glimelius B., Martling A. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): a multicentre, randomised, non-blinded, phase 3, non-inferiority trial. The Lancet. Oncology. 2017;18(3):336–346. doi: 10.1016/S1470-2045(17)30086-4. [DOI] [PubMed] [Google Scholar]
- 81.Kocher M., et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29(2):134–141. doi: 10.1200/JCO.2010.30.1655. 38. [DOI] [PMC free article] [PubMed] [Google Scholar]; Yamamoto M., et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol. 2014;15(4):387–395. doi: 10.1016/S1470-2045(14)70061-0. [DOI] [PubMed] [Google Scholar]
- 82.Brown P.D., et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA. 2016;316(4):401–409. doi: 10.1001/jama.2016.9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mahajan A., et al. Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single-Centre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18(8):1040–1048. doi: 10.1016/S1470-2045(17)30414-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sahgal A., et al. Stereotactic radiosurgery alone for multiple brain metastases? A review of clinical and technical issues. Neuro-Oncology. 2017;19(suppl_2):ii2–ii15. doi: 10.1093/neuonc/nox001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Borgelt B., et al. Ultra-rapid high dose irradiation schedules for the palliation of brain metastases: final results of the first two studies by the radiation therapy oncology group. Int J Radiat Oncol Biol Phys. 1981;7(12):1633–1638. doi: 10.1016/0360-3016(81)90184-x. [DOI] [PubMed] [Google Scholar]
- 86.Mulvenna P., et al. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): results from a phase 3, non-inferiority, randomised trial. Lancet. 2016;388(10055):2004–2014. doi: 10.1016/S0140-6736(16)30825-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chow E., et al. Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol. 2007;25(11):1423–1436. doi: 10.1200/JCO.2006.09.5281. [DOI] [PubMed] [Google Scholar]
- 88.Lok B.H., Jiang G., Gutiontov S., Lanning R.M., Sridhara S., Sherman E.J. Palliative head and neck radiotherapy with the RTOG 8502 regimen for incurable primary or metastatic cancers. Oral Oncol. 2015 doi: 10.1016/j.oraloncology.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Spanos W.J., Jr, Wasserman T., Meoz R, et al. Palliation of advanced pelvic malignant disease with large fraction pelvic radiation and misonidazole: final report of RTOG phase I/II study. Int J Radiat Oncol Biol Phys. 1987;13:1479–1482. doi: 10.1016/0360-3016(87)90314-2. [DOI] [PubMed] [Google Scholar]
- 90.Sapienza L.G., et al. Short-course palliative radiation therapy leads to excellent bleeding control: a single Centre retrospective study. Clin Transl Radiat Oncol. 2019;14:40–46. doi: 10.1016/j.ctro.2018.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sundstrøm S., Bremnes R., Aasebø U., et al. Hypofractionated palliative radiotherapy (17 Gy per two fractions) in advanced non-small-cell lung carcinoma is comparable to standard fractionation for symptom control and survival: a national phase III trial. J Clin Oncol. 2004;22:801–810. doi: 10.1200/JCO.2004.06.123. [DOI] [PubMed] [Google Scholar]
- 92.Senkus-Konefka E., Dziadziuszko R., Bednaruk-Młyński E., et al. A prospective, randomised study to compare two palliative radiotherapy schedules for non-small-cell lung cancer (NSCLC) Br J Cancer. 2005;92:1038–1045. doi: 10.1038/sj.bjc.6602477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jeremic B., et al. The International Atomic Energy Agency (IAEA) randomized trial of palliative treatment of incurable locally advanced non small cell lung cancer (NSCLC) using radiotherapy (RT) and chemotherapy (CHT) in limited resource setting. Radiother Oncol. 2015;116(1):21–26. doi: 10.1016/j.radonc.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 94.Rathod S., et al. Quality of Life Outcomes in a Phase 3 Randomized Trial of Optimization of Treatment of Advanced Non–small Cell Lung Cancer Using Radiation Therapy and Chemotherapy: IAEA Multicentric Randomized Phase 3 Study ( NCT00864331) International Journal of Radiation Oncology • Biology • Physics. 2017;99(2):S103. [Google Scholar]
- 95.Report to the Medical Research Council by its Lung Cancer Working, P. Inoperable non-small-cell lung cancer (NSCLC): a Medical Research Council randomised trial of palliative radiotherapy with two fractions or ten fractions. British Journal of Cancer. 1991;63(2):265–270. doi: 10.1038/bjc.1991.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sze W.M. Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy – a systematic review of the randomised trials. Cochrane Database Syst Rev. 2004;2 doi: 10.1002/14651858.CD004721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kosela-Paterczyk H., Szacht M., Morysinski T., Lugowska I., Dziewirski W., Falkowski S. Preoperative hypofractionated radiotherapy in the treatment of localized soft tissue sarcomas. Eur J Surg Oncol. 2014;40(12):1641–1647. doi: 10.1016/j.ejso.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 98.Ashby M.A., Ago C.T., Harmer C.L. Hypofractionated radiotherapy for sarcomas. Int J Radiat Oncol Biol Phys. 1986;12:13–17. doi: 10.1016/0360-3016(86)90409-8. [DOI] [PubMed] [Google Scholar]
- 99.Choudhury A., Swindell R., Logue J.P., Elliott P.A., Livsey J.E., Wise M., et al. Phase II study of conformal hypofractionated radiotherapy with concurrent gemcitabine in muscle-invasive bladder cancer. J Clin Oncol. 2011;29(6):733–738. doi: 10.1200/JCO.2010.31.5721. [DOI] [PubMed] [Google Scholar]
- 100.Duchesne G.M., Bolger J.J., Griffiths G.O., Trevor Roberts J., Graham J.D., Hoskin P.J., Fossa S.D., Uscinska B.M., Parmar M.K. A randomized trial of hypofractionated schedules of palliative radiotherapy in the management of bladder carcinoma: Results of medical research council trial ba09. Int J Radiat Oncol Biol Phys. 2000;47(2):379–388. doi: 10.1016/s0360-3016(00)00430-2. [DOI] [PubMed] [Google Scholar]
- 101.Hafeez S., McDonald F., Lalondrelle S., McNair H., Warren-Oseni K., Jones K., et al. Clinical outcomes of image guided adaptive hypofractionated weekly radiation therapy for bladder cancer in patients unsuitable for radical treatment. Int J Radiat Oncol Biol Phys. 2017;98:115–122. doi: 10.1016/j.ijrobp.2017.01.239. [DOI] [PMC free article] [PubMed] [Google Scholar]