Abstract
Worldwide, clinical data remain the gold standard for disease surveillance and tracking. However, such data are limited due to factors such as reporting bias and inability to track asymptomatic disease carriers. Disease agents are excreted in the urine and feces of infected individuals regardless of disease symptom severity. Wastewater surveillance – that is, monitoring disease via human effluent – represents a valuable complement to clinical approaches. Because wastewater is relatively inexpensive and easy to collect and can be monitored at different levels of population aggregation as needed, wastewater surveillance can offer a real-time, cost-effective view of a community's health that is independent of biases associated with case-reporting. For SARS-CoV-2 and other disease-causing agents we envision an aggregate wastewater-monitoring system at the level of a wastewater treatment plant and exploratory or confirmatory monitoring of the sewerage system at the neighborhood scale to identify or confirm clusters of infection or assess impact of control measures where transmission has been established. Implementation will require constructing a framework with collaborating government agencies, public or private utilities, and civil society organizations for appropriate use of data collected from wastewater, identification of an appropriate scale of sample collection and aggregation to balance privacy concerns and risk of stigmatization with public health preservation, and consideration of the social implications of wastewater surveillance.
Keywords: Wastewater surveillance, SARS-CoV-2, Health management, Fecal-oral transmission, Data privacy
Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is responsible for causing the deadly respiratory disease termed Corona Virus Disease-19 (COVID-19), a global pandemic that has sickened over 12.4 million people and caused more than 559,000 deaths to date (Source: New York Times, July 11, 2020). Coronaviruses are enveloped, single stranded RNA viruses 60 to 220 nm in size that display helical symmetry with spike proteins on the surface. SARS-CoV-2 shares about 79% similarity in genome sequence with the SARS-CoV, which caused the first coronavirus epidemic in 2003 claiming 8000 lives (Lu et al., 2020). SARS-CoV-2 is a highly contagious virus and spreads through respiratory droplets, direct contact with infected individuals and contaminated surfaces (WHO 2020). Concerns about other possible exposure routes include via aerosols and fecal-oral transmission. A significant proportion of individuals infected with SARS-CoV-2 exhibit mild or no symptoms (Gandhi et al., 2020), and symptom onset is preceded by an asymptomatic stage contributing to its spread. Asymptomatic carriers can carry virus in stool but test negative in nasopharyngeal samples (Jiang et al., 2020). Tools are urgently needed to track the number of infected individuals in a community, without biases introduced by clinical case reporting. Multiple recent reports indicate the utility of wastewater-based surveillance of SARS-CoV-2 for estimating population prevalence and as an early warning for impending outbreaks (e.g. Medema et al., 2020; Ahmed et al., 2020a; Randazzo et al., 2020; Wu et al., 2020a, see also database of newly released pre-prints and peer-reviewed publications as described by Bivins et al., 2020). In this article, we discuss the importance of monitoring untreated wastewater in sewerage (the pipes, pumps and force mains that help collect and convey domestic wastewater from its source to the treatment plant) and at the intake of municipal wastewater treatment plants (WWTPs) to provide an early warning of SARS-CoV-2 outbreaks, and local tracing of infection clusters. Next, we consider implications of virus detection in untreated wastewater and how such data could be used in epidemiology and decision making while considering societal implications.
2. Infection and transmission pathways
SARS-CoV-2 spreads through the respiratory route. Similar to SARS-CoV, the virus initiates infection by binding of the spike (S) proteins to angiotensin converting enzyme 2 (ACE2) receptors on host cells (Li et al., 2005; Lan et al., 2020). Subsequently, viral RNA enters the cytoplasm and takes control of the cellular machinery to replicate itself. After inhalation of the virus, infection of alveolar cells in bronchi and lungs leads to fluid accumulation and onset of severe acute respiratory syndrome (SARS) in COVID-19 patients. Release of the virus into air occurs in the form of respiratory droplets when an infected person coughs, sneezes or talks. The contaminated droplets can land, and persist for some time, on different surfaces, and contact with them can lead to infection.
Ingestion is a second potential exposure route for viral pathogens (Koopmans et al., 2002); however, transmission through contaminated food or the fecal-oral route has not been established for SARS-CoV-2 (Ding and Liang, 2020). ACE2 receptors are also present in intestinal cells (Hamming et al., 2004) and expressed in the ileum and colon at comparable levels to the lung (Zhang et al., 2020). Intestinal infection is indicated by several lines of evidence including SARS-CoV-2 infection of intestinal cells (Lamers et al., 2020), detection of viral RNA in stool samples of COVID-19 patients (Xu et al., 2020), and reports of intestinal ailments including diarrhea in some COVID-19 patients. For example, in several early studies 10 - 32% of patients reported gastrointestinal symptoms while diarrhea was at a prevalence of 2 - 12% (Chen et al., 2020; Cholankeril et al., 2020; Wang et al., 2020a). The incidence of diarrhea among COVID-19 patients ranged from 2 to 50% across twenty-four studies reviewed by D'Amico et al. (2020), with an overall incidence of 10.4% from a pooled analysis. Importantly, the virus has also been detected in several fecal sample cultures providing limited evidence of infectious virus in human stool (Wang et al., 2020b; Xiao et al., 2020). Whether infection of the intestine with SARS-CoV-2 implicates ingestion as an exposure route remains an open question as this observation could also be explained by secondary infection, for example, due to systemic spread after respiratory infection or swallowing virus-laden respiratory secretions (Ding and Liang, 2020).
One striking observation is that shedding of viral RNA via the digestive system appeared to last longer than via the respiratory tract (Xu et al., 2020). Fecal samples of 41 (55%) out of 74 patients tested positive for SARS-CoV-2 RNA up to 27.9 days from the first sign of symptoms compared to 16.7 days for respiratory tract samples (Wu et al., 2020c). In contrast to other RNA viruses previously studied, SARS-CoV-2 can remain intact under highly acidic conditions (Chin et al., 2020). Hence shedding of viral RNA through feces may be common and widespread among COVID-19 patients, where many infected people may be asymptomatic (Gandhi et al., 2020) and excreting viral RNA not captured by clinical reports. Further studies with a large set of COVID-19 patients and subjects showing no symptoms are needed to assess the full extent of digestive tract ailments and shedding of viral RNA and/or infectious virus via feces. The viability of SARS-CoV-2 in wastewater has not yet been established, but new methods like capsid integrity quantitative PCR are likely to make cultivation-independent assessment of viable coronaviruses possible in the near future (Leifels et al., 2020). While the significance of respiratory and fomite-based transmission of SARS-CoV-2 is clear, understanding the extent of fecal-oral virus transmission remains an open question. However, it has been clearly established that SARS-CoV-2 RNA is present in feces from infected individuals and enters wastewater streams, consistent with recovery of SARS-CoV-2 genetic signatures from wastewater systems around the world (Kitajima et al., 2020).
The presence of SARS-CoV-2 in wastewater may represent a potential health risk, if the virus remains infectious in wastewater and individuals are exposed via aerosol inhalation or ingestion. Several different coronavirus types can be cultured from wastewater for a few days (Gundy et al., 2009; Naddeo and Liu 2020). Nevertheless, at the time of this article, the authors are not aware of any reports indicating significant COVID-19 disease clusters among wastewater utility workers, suggesting that monitoring of sewerage and wastewater treatment plants (WWTPs) will not introduce significant risk to the community (CDC, 2020). However long-term observations are needed to support this tentative conclusion. Owing to its novelty, unanswered questions and concerns remain with respect to the pathogenesis of infection and transmission routes of SARS-CoV-2. Additional studies on the viability and infectivity of SARS-CoV-2 in feces and wastewater are required for an accurate understanding of transmission in the environment.
3. The case for sustained observations of wastewater systems
Early work suggests detection and quantification of SARS-CoV-2 in wastewater tracks the prevalence of circulating virus and disease dynamics in a community. Dutch scientists reported the presence of SARS-CoV-2 in influent wastewater of six out of seven cities with known clinical cases and at a major international airport prior to widespread case reporting (Lodder and de Roda Husman 2020; Medema et al., 2020). Similarly, in Boston, USA, viral titers at a WWTP were higher than expected from clinical cases, based on conservative estimates of viral loading in feces and wastewater flow (Wu et al., 2020a), while in Southeast Queensland, Australia, Monte Carlo simulations based on measured RNA copies in wastewater suggested viral prevalence was consistent with clinical cases (Ahmed et al., 2020a). Viral dynamics over several weeks in Paris (Wurtzer et al., 2020) and Spain (Randazzo et al., 2020) tracked the rise of levels of circulating virus that paralleled a rise in clinical cases.
These studies demonstrate that it is possible to quantify the circulation of SARS-CoV-2 viral RNA in environmental settings; hence, sustained observations of wastewater can act as sentinels for public health authorities. In many countries, testing is limited to those with severe symptoms, persons with travel history to COVID-19 affected regions, or those who have had close contact with positive cases. As asymptomatic and mildly symptomatic cases are underreported, wastewater surveillance provides information on the full cross-section of the community including infected persons with mild or no symptoms but who are contagious, as was likely the case in the Boston study (Wu et al., 2020a, 2020b). These individuals would be missed during clinical testing. Wastewater surveillance may thus provide complementary information to clinical reporting, especially when testing is limited.
There is precedent for the integration of wastewater surveillance within a public health framework. In 1939, poliovirus was found in untreated wastewater in three North American cities, demonstrating that a disease causing agent could be detected in sewerage during an outbreak (Paul et al., 1940). Further, enteroviruses were monitored over a period of 100 months in the influent of a wastewater treatment plant in Milwaukee, Wisconsin, establishing that serotypes from wastewater reflected those of clinical cases (Sedmak et al. 2003). Wastewater-based surveillance has been implemented previously to track polio outbreaks in Israel and Egypt (Blomqvist et al., 2012; Brouwer et al., 2018; Kopel et al., 2014) and provide early warning of Norovirus and Hepatitis A outbreaks in Sweden (Berchenko et al., 2017; Hellmér et al., 2014; Tebbens et al., 2017). Lessons learned from experience with one virus can help inform strategies for dealing with emerging viruses, but approaches are not always transferable. For example, Poliovirus, Norovirus and Hepatitis A are non-enveloped viruses, while SARS-CoV-2 is enveloped, which may influence its ability to be recovered by established methods. Hence, it is advisable to compare different methods using surrogates like mouse hepatitis virus, a group 2 coronavirus, or actual samples (Ahmed et al., 2020b).
Sustained wastewater surveillance may also prove useful to track emergence of viral genotypes that differ in pathogenicity, symptomology, and possibly susceptibility to emerging treatment methodologies. As an RNA virus, SARS-CoV-2 undergoes high rates of mutations, compared to DNA based viruses and bacterial pathogens. Reports are beginning to emerge that suggest different levels of infectivity in SARS-CoV-2 genome variants (Yao et al. 2020). Analytical methods that allow viral sequence interrogation should be incorporated into wastewater-based surveillance to track the dynamics of viral genotypes. A confounding factor for extrapolating wastewater viral titers to population prevalence is if the various genotypes are excreted to a different extent due to differential pathophysiology. Strong knowledge sharing between the medical community and wastewater surveillance practitioners and an integrated approach will be crucial as the wastewater surveillance effort grows around the world.
4. Towards population-based health management
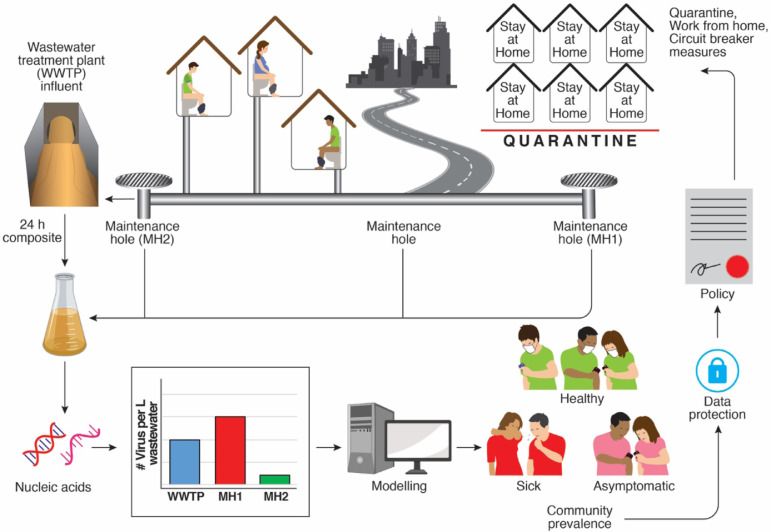
Influent to a WWTP represents aggregated wastewater from a region or community based on the catchment area and the density of the settlement. In regions with an existing infrastructure, it is possible to design sampling plans to include pump stations and maintenance holes at sentinel locations and areas. In areas without existing WWTP infrastructure, regional approaches may be implemented through sampling of sewerage from buildings that host a large cross-section of the public such as hospitals or government buildings. In the following sections we describe an integrated approach to wastewater surveillance as 1) a sentinel for the health of the community at large, and 2) a means to identify and manage disease clusters at a neighborhood or regional scale (Fig. 1 ).
Fig. 1.
Wastewater-based surveillance for population-based health management. The SARS-CoV-2 virus enters the sewerage system through fecal shedding. Wastewater sampled at maintenance holes or the treatment plant represents an aggregate and average of shed viral titers at the level of the neighborhood, region or municipality. A composite sample collected over 24 h captures the daily flux from the population and is purified from a defined volume to recover the RNA associated with wastewater-borne viral particles. The viral genomes are measured in the purified RNA sample using a molecular assay such as quantitative PCR and used to determine the number of viruses in the defined volume of sampled wastewater. Wastewater viral levels are then combined with wastewater flow rates and estimates of excreted viral load per toilet flush to model infection prevalence in the sampled community. Importantly, this estimate of prevalence reflects the excreted viral load from both sick and asymptomatic individuals and complements essential individual testing. As ongoing research refines these modeling approaches, it may become possible to gain actionable insights from viral levels in wastewater for implementing health-protective measures at the neighborhood or regional level. Concurrently, issues of privacy must be considered to ensure appropriate use of wastewater-based surveillance data, which holds the potential to become a new source of “big data” in our increasingly connected world.
4.1. Downstream sampling
The health of communities served by a centralized WWTP can be gauged by normalization of virus influent concentration per capita mass load using the daily flow and total population served. The WWTP influent collected by autosamplers may be calibrated to reflect the length of time wastewater travels in the sewerage and thus more accurately reflect a composite of the sampled population. If the number of confirmed clinical cases in the community is consistent with the predicted cases from wastewater-based surveillance, this signifies that clinical testing is adequate to describe the prevalence of the disease and that general population safety measures can be tightened or loosened by relying on clinical case reporting. However, if wastewater-based estimates of infection prevalence are significantly higher than indicated by clinical reporting, this provides an early warning of increased transmission potential, and policy makers may consider implementing more significant measures to protect public health.
4.2. Upstream sampling
While ‘downstream’ sampling (i.e., at the WWTP or other major points of wastewater confluence) offers the greatest level of population aggregation, it presents several problems, including highly variable waste travel times, signal degradation, and limited spatial resolution. By sampling multiple locations ‘upstream’, at the neighborhood level (e.g., at sewerage maintenance holes where flow aggregates at the scale of 2000–7000 individuals) the variability in waste travel-time is reduced, which allows for analysis of temporal dynamics and geographic distribution. A GIS-based sewerage map and flow rates would aid in the selection of sampling locations. A temporally resolved approach for collecting samples from the sewerage network more accurately tracks signatures of human health and activity (Matus et al., 2019) and can be used to calibrate viral prevalence on a per individual or per household level based on sampling frequency and intensity while preserving significant anonymity. A spatially resolved approach in sewerage has been deployed for tracking opioid related metabolites in the USA (Endo et al., 2020) and SARS-CoV-2 RNA in Boston and Singapore. In the Boston metropolitan area, SARS-CoV-2 titers in samples collected from maintenance holes correlated to neighborhood socioeconomic indicators, with less affluent areas showing higher titres, consistent with clinical case surveillance (Wu et al., 2020b). The Singapore National Environment Agency has integrated analysis of wastewater from sewerage maintenance holes with clinical surveillance to support monitoring and management of COVID-19 transmission among migrant workers living in dormitories, which represented the largest infection clusters in the nation (Yong, 2020a, Yong, 2020b). As viral RNA signals are detected from wastewater at a particular dormitory site, clinical testing for COVID-19 is carried out for the corresponding community, allowing screening for COVID-19 to be implemented in a more targeted manner to mitigate further transmission. Conversely, for areas with no detected COVID-19 cases, non-detection of SARS-CoV-2 RNA in the wastewater provides added assurance, given the potential for COVID-19 to be asymptomatic. A chief advantage of ‘upstream’ sampling is that spatial resolution supports assessment of disease incidence and prevalence within zones defined by sewerage networks, and affords better targeting and evaluation of interventions. Thus, ‘upstream’ surveillance for SARS-CoV-2 may facilitate more granular detection of SARS-CoV-2 in catchments with lower overall COVID-19 disease burden, to enable early warning, and may help inform decisions to advance or scale back social distancing and quarantine efforts while minimizing respective disruption or risk to the community at large.
5. Societal Implications
The implementation of wastewater-based disease tracking and surveillance may have unintended social consequences, and thus must be designed to mitigate them as much as possible. Digital surveillance tools developed in response to the COVID-19 pandemic provide several examples of unintended social outcomes including violation of individual privacy and introducing the potential for “mission creep,” i.e., use of data outside of the original purpose motivating collection. In South Korea, mobile phones received government alerts with patient details every time a new infectious case emerged, leading to doxxing and cyberbullying (Kim 2020), while Germany pivoted towards a decentralized approach to data storage in contact tracing apps after centralization faced stiff public opposition (Grüll 2020).
Moreover, surveillance in its many forms (closed-circuit television, geolocational mobile phone tracking, and monitoring transportation, social media usage and financial transaction) has been shown to exacerbate and compound existing social inequalities, where underprivileged groups are heavily monitored (Hier and Greenberg 2010; Monahan 2017). News reports indicate that outside Wuhan, SARS-CoV-2 travelled the world through socioeconomically privileged carriers. Yet, the pandemic has shown how a nation's social and socioeconomic disparities increase disease burdens for those in precarious circumstances shaped by race, age and income (Bibbins-Domingo 2020). Wastewater disease monitoring could add to surveillance already faced by these groups.
Integrated wastewater surveillance programs must be developed to address both privacy and inequality concerns. For example, wastewater monitoring can be designed to collect aggregate and not granular household-level data. In addition, upstream sampling locations for general surveillance should be selected to not reinforce inequalities and to reflect demographics consistent with existing socioeconomic variability. Otherwise, vulnerable populations may become subject to over-surveillance and restrictive policies that may accompany it. Targeted surveillance of high-risk communities should be disclosed, and coordinated with availability of interventions such as clinical testing and treatment to reduce disease burden. In some communities access to sewerage is restricted to those with higher means who would then disproportionately reap the public health benefits of centralized monitoring. Most importantly, the success of surveillance requires public legitimacy and trust of such measures. Otherwise, regulatory authorities may witness public resistance in creative and unusual methods. For example, some individuals already wary of government surveillance may install outhouses or toilet technology to avoid data capture. Resultant management strategies based on wastewater data would be blind to risk introduced by those with the means to “opt-out” of wastewater surveillance. Integrated wastewater surveillance must not be unilaterally undertaken by regulatory agencies and private industry but should substantively include diverse representatives of the public interest.
Conclusions
We posit that wastewater surveillance for infectious diseases at the level of sewerage and treatment plant should be a top priority of public health at the local, national and international level with the aim of acquiring actionable community-level information needed to navigate pandemics like COVID-19:
-
•
The viral load of SARS-CoV-2 in wastewater represents the sum of both asymptomatic and symptomatic shedding, and thus indicating viral circulation in a community at any level.
-
•
Wastewater surveillance is an economical and scalable companion to essential individual testing.
-
•
As a measure of changes in viral circulation, it is especially useful to guide public health decision makers.
-
•
The success of surveillance requires public legitimacy and trust of such measures. It should not compound social inequalities, and test populations should reflect demographics consistent with existing socioeconomic variability.
Declaration of Competing Interest
Eric Alm is scientific advisor and shareholder of BioBot Analytics. The other authors declare no conflict of interest.
Acknowledgements
This research is supported by the National Research Foundation, Prime Minister's Office, Singapore under its Campus for Research Excellence and Technological Enterprise (CREATE) program, by the Singapore National Environment Agency, and by the Singapore Ministry of Education and National Research Foundation through a RCE award to Singapore Centre for Environmental Life Sciences Engineering (SCELSE). VBR is funded by Agencia Nacional de Promoción de la Investigación, el Desarrollo Tecnológico y la Innovación, Argentina through grant #IP COVID 19–785. The authors thank Ivan Chin Hin Tan for graphical assistance.
References
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O'Brien J.W., Choi P.M., Kitajima M., Simpson S.L., Li J. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020 doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Bertsch P.M., Bivins A., Bibby K., Farkas K., Gathercole A., Haramoto E., Gyawali P., Korajkic A., McMinn B.R., Mueller J.F., Simpson S.L., Smith W.J.M., Symonds E.M., Thomas K.V., Verhagen R., Kitajima M. Comparison of virus concentration methods for the RT-qPCR-based recovery of murine hepatitis virus, a surrogate for SARS-CoV-2 from untreated wastewater. Sci. Total Environ. 2020;739 doi: 10.1016/j.scitotenv.2020.139960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berchenko Y., Manor Y., Freedman L.S., Kaliner E., Grotto I., Mendelson E., Huppert A. Estimation of polio infection prevalence from environmental surveillance data. Sci. Transl. Med. 2017;9(383):eaaf6786. doi: 10.1126/scitranslmed.aaf6786. [DOI] [PubMed] [Google Scholar]
- Bibbins-Domingo K. This time must be different: disparities during the COVID-19 pandemic. Ann. Intern. Med. 2020 doi: 10.7326/M20-2247. Published online 28 April 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bivins A., North D., Ahmad A., Ahmed W., Alm E., Been F., Bhattacharya P., Bijlsma L., Boehm A.B., Brown J., Buttiglieri G., Calabro V., Carducci A., Castiglioni S., Cetecioglu Gurol Z., Chakraborty S., Costa F., Curcio S., de los Reyes F.L., Delgado Vela J., Farkas K., Fernandez-Casi X., Gerba C., Gerrity D., Girones R., Gonzalez R., Haramoto E., Harris A., Holden P.A., Islam M.T., Jones D.L., Kasprzyk-Hordern B., Kitajima M., Kotlarz N., Kumar M., Kuroda K., La Rosa G., Malpei F., Mautus M., McLellan S.L., Medema G., Meschke J.S., Mueller J., Newton R.J., Nilsson D., Noble R.T., van Nuijs A., Peccia J., Perkins T.A., Pickering A.J., Rose J., Sanchez G., Smith A., Stadler L., Stauber C., Thomas K., van der Voorn T., Wigginton K., Zhu K., Bibby K. Wastewater-based epidemiology: global collaborative to maximize contributions in the fight against COVID-19. Environ. Sci. Technol. 2020 doi: 10.1021/acs.est.0c02388. [DOI] [PubMed] [Google Scholar]
- Blomqvist S., El Bassioni L., El Maamoon Nasr E.M., Paananen A., Kaijalainen S., Asghar H., de Gourville E., Roivainen M. Detection of imported wild polioviruses and of vaccine-derived polioviruses by environmental surveillance in Egypt. Appl. Environ. 2012;78(15):5406–5409. doi: 10.1128/AEM.00491-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer A.F., Eisenberg J.N., Pomeroy C.D., Shulman L.M., Hindiyeh M., Manor Y., Grotto I., Koopman J.S., Eisenberg M.C. Epidemiology of the silent polio outbreak in Rahat, Israel, based on modeling of environmental surveillance data. Proc. Natl. Acad. Sci. 2018;115(45):E10625–E10633. doi: 10.1073/pnas.1808798115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC, 2020. Information for sanitation and wastewater workers on COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/community/sanitation-wastewater-workers.html.
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin A.W.H., Chu J.T.S., Perera M.R.A., Hui K.P.Y., Yen H.-.L., Chan M.C.W., Peiris M., Poon L.L.M. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microb. 2020 doi: 10.1016/S2666-5247(20)30003-3. Published online 02 April 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholankeril G., Podboy A., Aivaliotis V.I., Tarlow B., Pham E.A., Spencer S., Kim D., Hsing A., Ahmed A. High prevalence of concurrent gastrointestinal manifestations in patients with SARS-CoV-2: early experience from California. Gastroenterol.. Articles in press. 2020 doi: 10.1053/j.gastro.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amico F., Baumgart D.C., Danese S., Peyrin-Biroulet L. Diarrhea during COVID-19 Infection: pathogenesis, epidemiology, prevention, and management. Clin. Gastroenterol. Hepatol. 2020;18:1663–1672. doi: 10.1016/j.cgh.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S., Liang T.J. Is SARS-CoV-2 also an enteric pathogen with potential fecal-oral transmission: a COVID-19 virological and clinical review. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo N., Ghaeli N., Duvallet C., Foppe K., Erickson T.B., Matus M., Chai P.R. Rapid assessment of opioid exposure and treatment in cities through robotic collection and chemical analysis of wastewater. J. Med. Toxicol. 2020;16(2):195–203. doi: 10.1007/s13181-019-00756-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi M., Yokoe D.S., Havlir D.V. Asymptomatic transmission, the Achilles’ heel of current strategies to control Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMe2009758. Published online. April 24, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grüll, P., 2020. German government presents ‘best coronavirus tracing app worldwide’ (S. Lawton Trans.) [WWW Document]. EURACTIV. [https://www.euractiv.com/section/digital/news/german-government-presents-best-coronavirus-tracing-app-worldwide/(accessed 6.24.20).
- Gundy P.M., Gerba C.P., Pepper I.L. Survival of coronaviruses in water and wastewater. Food Environ. Virol. 2009;1(1):10. doi: 10.1007/s12560-008-9001-6. [DOI] [Google Scholar]
- Hamming I., Timens W., Bulthuis M., Lely A., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmér M., Paxéus N., Magnius L., Enache L., Arnholm B., Johansson A., Bergström T., Norder H. Detection of pathogenic viruses in sewage provided early warnings of hepatitis A virus and norovirus outbreaks. Appl. Environ. Microbiol. 2014;80(21):6771–6781. doi: 10.1128/AEM.01981-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hier S.P., Greenberg J. Vol. 2009. Vancouver: UBC Press; 2010. (Surveillance: power, problems, and Politics). Print. [Google Scholar]
- Jiang, X., Luo, M., Zou, Z., Wang, X., Chen, C. and Qiu, J., 2020. Asymptomatic SARS‐CoV‐2 infected case with viral detection positive in stool but negative in nasopharyngeal samples lasts for 42 days. J. Med. Virol. (Accepted author manuscript). 10.1002/jmv.25941 [DOI] [PMC free article] [PubMed]
- Kim M.S. The New Yorker; 2020. Seoul's Radical Experiment in Digital Contact Tracing.https://www.newyorker.com/news/news-desk/seouls-radical-experiment-in-digital-contact-tracing Published online April 17, 2020. [Google Scholar]
- Kitajima, M., Ahmed, W., Bibby, K., Carducci, A., Gerba, C.P., Hamilton, K.A., Haramoto, E. and Rose, J.B., 2020. SARS-CoV-2 in wastewater: state of the knowledge and research needs. Sci. Total Environ.139076. 10.1016/j.scitotenv.2020.139076. [DOI] [PMC free article] [PubMed]
- Koopmans M., von Bonsdorff C.-.H., Vinjé J., de Medici D., Monroe S. Foodborne viruses. FEMS Microbiol Rev. 2002;26(2):187–205. doi: 10.1111/j.1574-6976.2002.tb00610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopel E., Kaliner E., Grotto I. Lessons from a public health emergency—importation of wild poliovirus to Israel. N. Engl. J. Med. 2014;371(11):981–983. doi: 10.1056/NEJMp1406250. [DOI] [PubMed] [Google Scholar]
- Lamers, M.M., Beumer, J., van der Vaart, J., Knoops, K., Puschhof, J., Breugem, T.I., Ravelli, R.B., van Schayck, J.P., Mykytyn, A.Z. and Duimel, H.Q., 2020. SARS-CoV-2 productively infects human gut enterocytes. Science. Published online 1 May 2020. 10.1126/science.abc1669. [DOI] [PMC free article] [PubMed]
- Lan, J., Ge, J., Yu, J., Shan, S., Zhou, H., Fan, S., Zhang, Q., Shi, X., Wang, Q., Zhang, L. and Wang, X., 2020. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature.Published online 30 March 2020. 10.1038/s41586-020-2180-5. [DOI] [PubMed]
- Leifels, M., Cheng D., Sozzi, E., Shoults, D.C., Wuertz S., Mongkolsuk S., Sirikanchana, K., 2020. Capsid integrity quantitative PCR to determine virus infectivity in environmental and food applications – a systematic review. medRxiv preprint doi: 10.1101/2020.05.08.20095364 [DOI] [PMC free article] [PubMed]
- Li F., Li W., Farzan M., Harrison S.C. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science. 2005;309(5742):1864–1868. doi: 10.1126/science.1116480. [DOI] [PubMed] [Google Scholar]
- Lodder, W. and de Roda Husman, A.M., 2020. SARS-CoV-2 in wastewater: potential health risk, but also data source. Lancet Gastroenterol. Corresp.. Published online. April 01, 2020. DOI: 10.1016/S2468-1253(20)30087-X. [DOI] [PMC free article] [PubMed]
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus, M., Duvallet, C., Soule, M.K., Kearney, S.M., Endo, N., Ghaeli, N., Brito, I., Ratti, C., Kujawinski, E.B. and Alm, E.J., 2019. 24-hour multi-omics analysis of residential sewage reflects human activity and informs public health. BioRxiv preprint, 728022. 10.1101/728022. [DOI]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-Coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in The Netherlands. Environ. Sci. Technol. Lett. 2020 doi: 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- Monahan T. Regulating belonging: surveillance, inequality, and the cultural production of abjection. J. Cult. Econ. 2017;10(2):191–206. doi: 10.1080/17530350.2016.1273843. [DOI] [Google Scholar]
- Naddeo V., Liu H. Editorial perspectives: 2019 novel coronavirus (SARS-CoV-2): what is its fate in urban water cycle and how can the water research community respond? Environ. Sci. Water Res. 2020;6(5):1213–1216. doi: 10.1039/D0EW90015J. [DOI] [Google Scholar]
- Paul J.R., Trask J.D., Gard S.I. Poliomyelitic virus in urban sewage. J. Exp. Med. 1940;71(6):765–777. doi: 10.1084/jem.71.6.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo, S., Cuevas-Ferrando, E., Sanjuan, R., Domingo-Calap, P. and Sanchez, G., 2020. Metropolitan wastewater analysis for COVID-19 epidemiological surveillance. medRxiv preprint,, 2020.2004.2023.20076679. 10.1101/2020.04.23.20076679. [DOI] [PMC free article] [PubMed]
- Sedmak G., Bina D., MacDonald J. Assessment of an enterovirus sewage surveillance system by comparison of clinical isolates with sewage isolates from Milwaukee, Wisconsin, collected august 1994 to december 2002. Appl. Environ. Microbiol. 2003;69:7181–7187. doi: 10.1128/aem.69.12.7181-7187.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebbens R.J.D., Zimmermann M., Pallansch M.A., Thompson K.M. Insights from a systematic search for information on designs, costs, and effectiveness of poliovirus environmental surveillance systems. Food Environ. Virol. 2017;9(4):361–382. doi: 10.1007/s12560-017-9314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. J. Am. Med. Assoc. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, W., Xu, Y., Gao, R., Lu, R., Han, K., Wu, G. and Tan, W., 2020b. Detection of SARS-CoV-2 in different types of clinical specimens. J. Am. Med. Assoc. Published online March 11, 2020. 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed]
- WHO, 2020. Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations. Scientific brief.29 March 2020, World Health Organization.
- Wu, F., Xiao, A., Zhang, J., Gu, X., Lee, W.L., Kauffman, K., Hanage, W., Matus, M., Ghaeli, N., Endo, N., Duvallet, C., Moniz, K., Erickson, T., Chai, P., Thompson, J. and Alm, E., 2020a. SARS-CoV-2 titers in wastewater are higher than expected from clinically confirmed cases. medRxiv preprint, 2020.2004.2005.20051540. 10.1101/2020.04.05.20051540. [DOI] [PMC free article] [PubMed]
- Wu, F., Xiao, A., Zhang, J., Moniz, K., Endo, N., Armas, F., Bonneau, R., Brown, M.A., Bushman, M., Chai, P., Duvallet, C., Erickson, T., Foppe, K., Ghaeli, N., Gu, X., Hanage, W., Huang, K.H., Lee, W.L., Matus, M., McElroy, K.A., Nagler, J., Rhode, S.F. Santillana, M., Tucker, J.A., Wuertz, S., Zhao, S., Thompson, J. and Alm, E., 2020b. SARS-CoV-2 titers in wastewater foreshadow dynamics and clinical presentation of new COVID-19 cases. medRxiv preprint, 2020.06.15.20117747v1. https://www.medrxiv.org/content/10.1101/2020.06.15.20117747v1.
- Wu Y., Guo C., Tang L., Hong Z., Zhou J., Dong X., Yin H., Xiao Q., Tang Y., Qu X. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol. 2020;5:434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtzer, S., Marechal, V., Mouchel, J.M., 2020. Time course quantitative detection of SARS-CoV-2 in Parisian wastewaters correlates with COVID-19 confirmed cases. medRxiv preprint, 2020.04.12.20062679. 10.1101/2020.04.12.20062679. [DOI]
- Xiao, F., Sun, J., Xu, Y., Li, F., Huang, X., Li, H., Zhao, J., Huang, J., Zhao, J., 2020. Infectious SARS-CoV-2 in feces of patient with severe COVID-19. Emerg. Infect. Dis. J.26. 10.3201/eid2608.200681. [DOI] [PMC free article] [PubMed]
- Xu Y., Li X., Zhu B., Liang H., Fang C., Gong Y., Guo Q., Sun X., Zhao D., Shen J. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat. Med. 2020;26(4):502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, H.-.P., Lu, X., Chen, Q., Xu, K., Chen, Y., Cheng, L., Liu, F., Wu, Z., Wu, H. and Jin, C., 2020. Patient-derived mutations impact pathogenicity of SARS-CoV-2. medRxiv preprint. 10.1101/2020.04.14.20060160. [DOI]
- Yong, Clement. NEA-led programme at migrant worker dorms could detect spread of coronavirus through wastewater testing. The Straits Times. Jun 19, 2020a. https://www.straitstimes.com/singapore/nea-led-programme-at-migrant-worker-dorms-could-detect-spread-of-coronavirus-through.
- Yong Clement. NEA monitoring wastewater in bid to give dorms the virus all-clear. The Straits Times. 2020 https://www.straitstimes.com/singapore/nea-monitoring-wastewater-in-bid-to-give-dorms-the-virus-all-clear Jun 23. [Google Scholar]
- Zhang H., Kang Z., Gong H., Xu D., Wang J., Li Zhixiu, Li Zifu, Cui X., Xiao J., Zhan J., Meng T., Zhou W., Liu J., Xu H. Digestive system is a potential route of COVID-19: an analysis of single-cell coexpression pattern of key proteins in viral entry process. Gut. 2020;69:1010–1018. doi: 10.1136/gutjnl-2020-320953. [DOI] [Google Scholar]