Abstract
There is growing evidence regarding chest X-ray and computed tomography (CT) findings for coronavirus disease 2019 (COVID-19). At present, the role of lung ultrasonography (LUS) has yet to be explored. The main purpose of this study was to evaluate the correlation between LUS findings and chest CT in patients confirmed to have (positive reverse transcription polymerase chain reaction [RT-PCR]) or clinically highly suspected of having (dyspnea, fever, myasthenia, gastrointestinal symptoms, dry cough, ageusia or anosmia) COVID-19. This prospective study was carried out in the emergency department, where patients confirmed of having or clinically highly suspected of having COVID-19 were recruited and underwent chest CT and concurrent LUS exam. An experienced emergency department physician performed the LUS exam blind to the clinical history and results of the CT scan, which were reviewed by two radiologists in consensus for signs compatible with COVID-19 (bilateral ground-glass opacities in peripheral distribution). A compatible LUS exam was considered a bilateral pattern of B-lines, irregular pleural line and subpleural consolidations. Between March and April 2020, 51 patients were consecutively enrolled. The indication for CT was a negative or indeterminate RT-PCR test (49.0%) followed by suspicion of pulmonary embolism (41.2%). Radiologic signs compatible with COVID-19 were present in 37 patients (72.5%) on CT scan and 40 patients (78.4%) on LUS exam. The presence of LUS findings was correlated with a positive CT scan suggestive of COVID-19 (odds ratio: 13.3, 95% confidence interval: 4.5–39.6, p < 0.001) with a sensitivity of 100.0%, specificity of 78.6%, positive predictive value of 92.5% and negative predictive value of 100.0%. There was no missed diagnosis of COVID-19 with LUS compared with CT in our cohort. The correlation between LUS score and CT total severity score was good (intraclass correlation coefficient: 0.803, 95% confidence interval: 0.60–0.90, p < 0.001). LUS exhibited similar accuracy compared with chest CT in the detection of lung abnormalities in COVID-19 patients.
Key Words: Coronavirus disease 2019, COVID-19, Severe acute respiratory syndrome coronavirus 2, SARS-CoV-2 Lung ultrasonography, Chest computed tomography
Introduction
Coronavirus disease 2019 (COVID-19) is a highly contagious illness caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). On March 11, 2020, the World Health Organization declared a pandemic caused by SARS-CoV-2, with spread to more than 180 countries; 2,954,106 cases were confirmed and 205,398 deaths occurred (Johns Hopkins Coronavirus Resource Center 2020).
In this emergency, the ability to quickly confirm and characterize a suspected case is critical, as almost any emergency department (ED) will struggle to keep up with the increasing number of patients and the shortage of health resources. The main diagnostic method, reverse transcription polymerase chain reaction (RT-PCR) of the nucleic acid of SARS-CoV-2, has many limitations, such as low sensitivity and technical difficulties in performing the test (Ai et al. 2020).
There is growing evidence regarding the imaging findings of COVID-19. The most common form of radiographic presentation is the presence of a local or bilateral patchy shadowing infiltrate on chest X-ray, although with low sensitivity (absent in more than 40% of cases) (Guan et al. 2020). Computed tomography (CT) scan reveals with higher sensitivity ground glass opacities (GGOs) (Shi et al. 2020), which is why it has been proposed as the main imaging test, incorporated in different therapeutic and triage strategies since the outbreak started (Zhang et al. 2020).
The use of chest CT remains very limited because of some notable drawbacks. For mild illness, radiation exposure, overuse of health care resources or inability to get a CT scan seem to overshadow the need. In the critically ill, the transport of unstable patients and exposure of infected patients may also outweigh the clinical benefit. Therefore, we require alternative modalities to quickly characterize our patients.
Ultrasound machines are widely available; therefore, lung ultrasonography (LUS) can be performed in few minutes, in mild or even unstable patients and in different hospital settings (American College of Emergency Physicians 2017). Although there is ongoing debate about how it should be applied, there is a general consensus on its usefulness (Kruser et al. 2020; Walter et al. 2020). In this pandemic, the usefulness of LUS has been suggested in small case reports (Buonsenso et al. 2020a, 2020b; Soldati et al. 2020b). The presence of subpleural consolidations, an irregular pleural line and B-lines are highly suggestive of COVID-19 pneumonia (Kruser et al. 2020; Li et al. 2020).
In this global public health emergency, the evidence for the role of this technique as compared with chest CT is limited and needs to be further defined, to minimize the infectious risks.
Methods
This prospective study was carried out in the ED of an academic hospital in Spain. The study was conducted in accordance with the Declaration of Helsinki and was approved by the Research Ethics Committee of our University Hospital (PI-4089). Informed consent was obtained from each enrolled patient.
Patient selection
Patients admitted to the ED had RT-PCR-proven COVID-19 or negative or indeterminate RT-PCR but a clinically high suspicion of COVID-19 (dyspnea, fever, myasthenia, gastrointestinal symptoms, dry cough, ageusia or anosmia) that required chest CT for evaluation.
The main indication for CT was a negative or indeterminate RT-PCR but clinically high suspicion of COVID-19 or pulmonary embolism (PE).
We excluded patients <18 y and patients who refused to participate. A convenience sample of 51 patients who met these inclusion criteria were consecutively enrolled and prospectively studied.
Initial patient assessment
Initial evaluation of the patients included a medical history (demographic data, comorbidities, symptoms); physical exam (temperature, blood pressure, heart rate, respiratory rate, oxygen saturation); laboratory tests (hemogram, basic metabolic panel [glucose, electrolytes, kidney function, liver enzymes, etc.], lactate dehydrogenase, ferritin, interleukin-6, C-reactive protein [CRP] procalcitonin, coagulation [D-dimer, international normalized ratio, prothrombin time, fibrinogen]).
Chest CT data collection
Non-contrast chest CT scans were obtained using multidetector CT (SOMATOM go.Up, Siemens Healthliners, Erlangen, Germany). Scanning was performed with the patient in the supine position and at end inspiration. The scans were acquired and reconstructed as axial images using the following parameters: 1.5-mm section thickness, 0.7-mm interval, 130 kVp. A low-dose protocol was implemented with an average CT dose index volume (CTDIvol) of 2 mGy
Our routine protocol for patients suspected of having a PE was multidetector pulmonary CT angiography using an 80-slice multi-detector CT (Prime SP Aquileon, Canon Medical Systems, Tustin, CA, USA) after intravenous injection of 70 mL of iodinated contrast agent (Iomeron 400 mg I/mL) at a flow rate of 4 mL/s, followed by a 25-mL saline flush. The automatic bolus-tracking technique had the region of interest positioned at the level of the main pulmonary artery with a trigger threshold of 120 HU. CT scan settings were 120 kVp, 1-mm section thickness, 0.5-mm interval, CTDIvol 4 mGy.
Blinded to the clinical information, two radiologist trainees with 2–4 y of experience (S.A.F. and R.A.G.) reviewed all images independently under the supervision of a senior radiologist with more than 10 y of experience (M.M.d.G). Each of the five lung lobes was assessed for percentage of the lobar involvement and classified as none (0%), minimal (1%–25%), mild (26%–50%), moderate (51%–75%) or severe (76%–100%), with the corresponding score of 0, 1, 2, 3 or 4. The CT Total Severity Score (TSS) was obtained by summing the five lobe scores (range: 0–20) (Li et al. 2020).
The images were interpreted using the lung and mediastinum window settings. The CT images were assessed, according to a standardized protocol, for the presence and distribution of the following abnormalities: ground-glass opacities (GGOs), defined as hazy areas of increased attenuation without obscuration of the underlying vascular markings; interlobular septal thickening and intralobular septal line; crazy paving; consolidations, such as parenchymal opacities obscuring underlying vessels; and other non-typical findings for COVID-19 pneumonia (pleural effusion, centrilobular, perilymphatic or random distributed nodules, tree in bud, etc.).
We considered a compatible COVID-19 pneumonia if multilobar or patchy GGOs, with or without interlobular septal thickening (crazy paving) or consolidation, were present.
We also classified chest CT patterns according to the three main phenotypes recently proposed (Robba et al. 2020): (i) multiple, focal, possibly overperfused ground-glass opacities; (ii) inhomogeneously distributed atelectasis and peribronchial opacities; (iii) a patchy, acute respiratory disease syndrome (ARDS)-like pattern. Chest X-rays obtained during episodes were recorded and analyzed.
Ultrasound data collection
An emergency physician (Y.T.C) with longstanding experience in LUS (an experienced sonologist based on the American College of Emergency Physicians’ [2020] ultrasonographic guidelines who had performed more than 10 ultrasound exams per week and had 5 y of experience in performing and interpreting point of care ultrasound) performed all ultrasound exams.
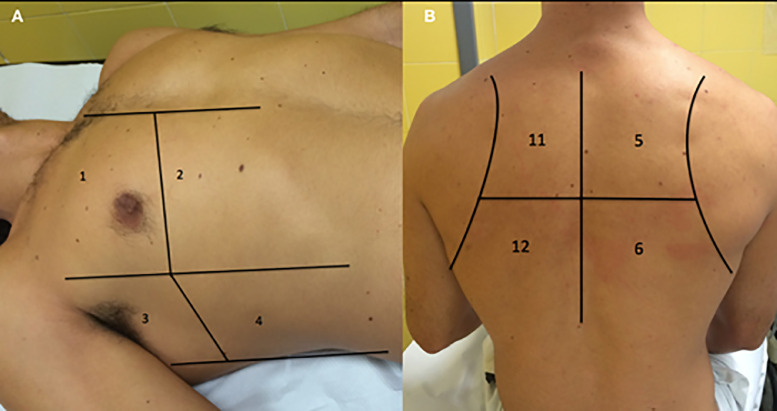
Participants underwent an LUS exam according to a 12-zone protocol (Soummer et al. 2012) (Fig. 1 ). Each intercostal space of the upper and lower parts of the anterior, lateral and posterior regions of the left and right chest wall were carefully examined, and findings (pleural effusions, confluent and isolated B-lines, irregular pleural line, consolidations) were recorded (Cantinotti et al. 2020; Volpicelli et al. 2020). For each of the 12 zones, a score from 0–3 was given depending on the finding: irregular or isolated B-lines (1 point), confluent B-lines (2 points), consolidations or pleural effusion (3 points). The total LUS score was calculated by summing the scores of all 12 zones (range of possible scores: 0–36).
Fig. 1.
The 12 zones of the chest. (A) 1 and 2, right anterior; 3 and 4, right lateral; 7 and 8, left anterior, not represented; 9 and 10, left lateral, not represented. (B) 5 and 6, right posterior; 11 and 12, left posterior.
A compatible LUS exam was considered a bilateral pattern of B-lines, isolated or confluent, irregular pleural line and subpleural consolidations.
The examinations were performed using a Butterfly IQ (Butterfly Network, Guilford, CT, USA), a hand-held ultrasound system fitted with a curvilinear array transducer (1.5–4.5 MHz) and lung pre-set.
The physician was blinded to the patient's past medical history, vital signs, symptoms, laboratory measurements and CT scan results.
Outcome measures and definitions
The main purpose of this study was to evaluate the correlation between LUS findings and chest CT in COVID-19 patients (Fig. 2, Fig. 3 ).
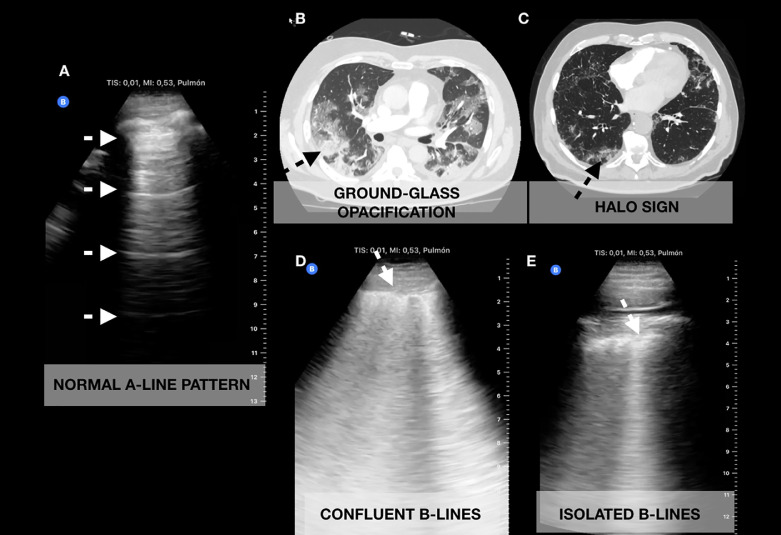
Fig. 2.
Correlation of chest computed tomography (CT) with lung ultrasonography (LUS) images obtained with a curvilinear probe. (A) Normal A-line pattern on LUS. (B) Ground-glass opacification correlating with (D) confluent B-lines. (C) Halo sign correlating with (E) isolated B-lines.
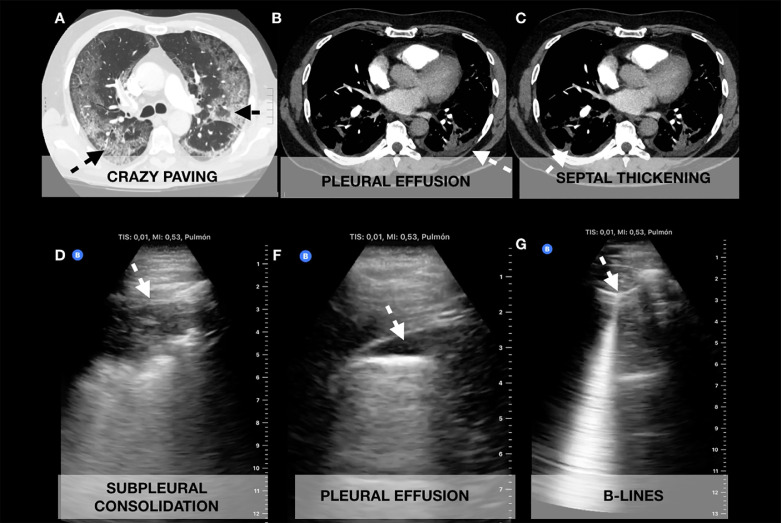
Fig. 3.
Correlation of chest computed tomography (CT) with lung ultrasonography (LUS) images obtained with a curvilinear probe. (A) Crazy paving correlating with (D) subpleural consolidation. (B) Small pleural effusion seen in CT and (F) LUS. (C) Septal thickening correlating with (G) isolated B-lines.
We defined a confirmed case in patients with a positive RT-PCR test and clinically highly suspicion and patients with dyspnea, fever, myasthenia, gastrointestinal symptoms, dry cough and ageusia or anosmia but negative RT-PCR.
Statistical analysis
Baseline characteristics are expressed as the mean and standard deviation (SD) for continuous variables and count and proportion for categorical variables. Quantitative parameters were compared using a Mann–Whitney test for continuous variables and the χ2 or Fisher exact test for categorical variables.
Cohen's kappa (κ) test was used to compare abnormal chest CT findings with abnormal LUS and chest X-ray findings. The intraclass correlation coefficients (ICC) was used to assess the degree of agreement between LUS score and CT TSS. An ICC <0.50 was considered poor, that from 0.50–0.75 moderate, that from 0.75–0.90 good and that from 0.90–1 excellent. The diagnostic performance of LUS compared with the RT-PCR test in detecting CT scan abnormalities was evaluated through receiver operating characteristic (ROC) curve analysis.
The correlations between continuous variables were tested using Spearman's ρ for categorical variables. The sample size for correlation was calculated to detect a 20% difference between LUS and CT findings, assuming a 95% confidence interval (CI) and power of 80%.
Mean values were reported along with 95% CIs. Statistical significance was set at p < 0.05.
Statistical analyses were conducted with SPSS Software v20.0 (IBM, Armonk, NY, USA).
Results
Fifty-one patients were consecutively enrolled between March and April 2020 (summarized in Table 1 ). The mean age was 61.4 y (SD - 17.7). At the end of the first week of follow-up, 34 patients were admitted to the hospital (66.7%), 4 were admitted to an intensive care unit (ICU, 7.8%), 6 patients had died (11.8%) and 17 were discharged home (33.3%).
Table 1.
Demographic and clinical characteristics of patients included on presentation (N = 51)
| Demographics | |
|---|---|
| Sex, female, N (%) | 23 (45.1%) |
| Age, y, mean (SD) | 61.4 (17.7) |
| Past medical history | |
| Cardiovascular disease | 14 (27.5%) |
| Pulmonary disease | 12 (23.5%) |
| Diabetes mellitus | 10 (19.6%) |
| Chronic kidney disease | 6 (11.8%) |
| Immunosuppression | 8 (15.8%) |
| Hypertension | 20 (39.2%) |
| Malignancy | 13 (25.5%) |
| Symptoms | |
| Dyspnea | 29 (56.9%) |
| Fever | 23 (45.1%) |
| Myasthenia | 22 (43.1%) |
| Gastrointestinal symptoms | 10 (19.6%) |
| Cough | 22 (43.1%) |
| Ageusia/anosmia | 4 (7.8%) |
| Onset of symptoms, d | 3.5 (5.6) |
| Physical exam | |
| Systolic blood pressure (mm Hg) | 123.8 (18.5) |
| Diastolic blood pressure (mm Hg) | 72.8 (13.1) |
| Heart rate (bpm) | 94.9 (17.3) |
| Temperature (°C) | 36.5 (1.1) |
| O2 saturation (%) | 93 (5) |
| Respiratory rate (rpm) | 14.3 (4.1) |
| Laboratory results | |
| White blood cells, × 109/L | 7.22 (3.3) |
| Lymphocytes, × 109/L | 1.27 (0.8) |
| Creatinine, mg/ | 0.93 (0.49) |
| Urea, mg/dL | 45.2 (25.6) |
| Alanine transaminase, U/L | 57.8 (128.5) |
| Lactate dehydrogenase, U/L | 382.5 (291.9) |
| D-Dimer, ng/mL | 6870.9 (14,324) |
| C-Reactive protein, mg/dL | 72.0 (103.1) |
| Troponin I, ng/mL | 296.4 (1,285.3) |
| NT-proBNP, pg/mL | 2963.0 (2,837.9) |
| Interleukin-6, pg/mL | 214.5 (351.8) |
| Ferritin, ng/mL | 873.6 (1,567.5) |
| SARS-CoV-2 (RT-PCR) test | 48 (94.1%) |
| Positive | 23 (47.9%) |
| Negative | 23 (47.9%) |
| Indeterminate | 2 (4.2%) |
| Follow-up | |
| Admission | 34 (66.7%) |
| Intensive care unit | 4 (7.8%) |
| Discharge | 17 (33.3%) |
| Mortality | 6 (11.8%) |
NT-ProBNP = N-terminal pro-brain natriuretic peptide; RT-PCR = reverse transcriptase polymerase chain reaction; SARS-CoV-2 = Severe acute respiratory syndrome coronavirus 2.
Values are expressed as N (%) or mean (standard deviation).
Approximately half of the patients (54.9%) had a chest X-ray, of which 33.2% were normal (Table 2 ). GGOs were present in 37 patients (72.5%), with peripheral or diffuse involvement, followed by septal thickening (18 patients, 35.2%). There were 2 patients with only central involvement on CT; one patient had a mild cardiac failure and another patient had viral bronchiolitis.
Table 2.
Imaging modalities (chest CT, lung ultrasonography and chest X-ray) findings of patients included
| Imaging modality | N (%) | ||
|---|---|---|---|
| Chest computed tomography (N =51) | |||
| COVID-19 suggestive | 37 (72.5) | ||
| Pleural thickening, % | 1 (2) | ||
| Ground-glass opacity | 37 (72.5) | ||
| Septal thickening, % | 18 (35.2) | ||
| Crazy paving, % | 10 (19.6) | ||
| Subpleural consolidation, % | 10 (19.6) | ||
| Pleural effusion, % | 12 (23.5) | ||
| COVID-19 phenotypes (N = 37) | |||
| Phenotype 1, % | 24 (47.1) | ||
| Phenotype 2, % | 12 (23.5) | ||
| Phenotype 3, % | 1 (2) | ||
| Distribution (N = 51) | |||
| Peripheral | 23 (45.1) | ||
| Diffuse | 7 (13.7) | ||
| Central and peripheral | 7 (13.7) | ||
| Central | 2 (3.9) | ||
| Normal | 12 (23.5) | ||
| CT total severity score, % | 7.48 (6.32) | ||
| Mild | 19 (37.3) | ||
| Moderate | 4 (7.8) | ||
| Severe | 14 (27.5) | ||
| CT pulmonary angiogram (N = 51) | 21 (41.2) | ||
| Pulmonary embolism | 7 (13.7) | ||
| Lung ultrasonography (N = 51) | |||
| COVID-19 suggestive, % | 40 (78.4) |
||
| Affected zone | IP/IBL | CBL | C |
| 1 (right upper anterior) | 9 | 4 | 8 |
| 2 (right lower anterior) | 12 | 9 | 3 |
| 3 (right upper lateral) | 10 | 10 | 4 |
| 4 (right upper lateral) | 14 | 10 | 3 |
| 5 (left upper anterior) | 11 | 4 | 6 |
| 6 (left lower anterior) | 9 | 4 | 5 |
| 7 (left upper lateral) | 8 | 8 | 6 |
| 8 (left lower lateral) | 13 | 9 | 2 |
| 9 (right upper posterior) | 8 | 6 | 9 |
| 10 (right lower posterior) | 13 | 5 | 19 |
| 11 (left upper posterior) | 7 | 5 | 6 |
| 12 (right lower posterior) | 13 | 5 | 18 |
| Right pleural effusion, % | 8 (15.7) | ||
| Left pleural effusion, % | 7 (13.7) | ||
| Pericardial effusion, % | 13 (25.5) | ||
| Lung score, mean (SD) | 10.6 (8.4) | ||
| Chest X-ray results, N = 28 | |||
| COVID-19 suggestive, % | 16 (57.1) | ||
| Ground-glass opacity, % | 12 (42.9) | ||
| Interstitial pattern, % | 13 (46.4) | ||
C = subpleural consolidation; CBL = confluent B-lines; COVID-19 = Coronavirus Disease 2019; CT = computed tomography; IBL = Isolated B-lines; IP = irregular pleural line; SD = standard deviation.
The most common finding and affected zones on LUS were subpleural consolidations on the posterior lower lobes (Table 2). The mean LUS score was 10.6 (SD = 8.4). The mean CT TSS was 7.48 (SD = 6.32). The LUS score correlated well with CT TSS (ICC = 0.803, 95% CI = 0.601–0.903, p < 0.001). The severity of lung lesions (Lu et al. 2020) in patients with COVID-19 evaluated by LUS and CT is outlined in Table 3 .
Table 3.
Severity of lung lesions in patients with Coronavirus Disease-19 assessed by lung ultrasonography and chest computed tomography
| Lung ultrasonography | Computed tomography |
Total | |||
|---|---|---|---|---|---|
| None | Mild | Moderate | Severe | ||
| None (LUS score 0) | 9 | 0 | 0 | 0 | 9 |
| Mild (LUS score 1–7) | 3 | 7 | 1 | 1 | 12 |
| Moderate (LUS score 8–18) | 1 | 11 | 1 | 5 | 18 |
| Severe (LUS score 19–36) | 1 | 1 | 2 | 8 | 12 |
| Total | 14 | 19 | 4 | 14 | 51 |
LUS = lung ultrasonography.
Age was moderately correlated with LUS score (ρ = 0.486, p < 0.001) but not with CT TSS (p = 0.247). Oxygen saturation (SO2) correlated more strongly with LUS score (ρ = –0.553, p < 0.001) than with CT TSS (ρ = –0.360, p = 0.043). Respiratory rate correlated more strongly with LUS score (ρ = 0.529, p < 0.001) than with CT TSS (ρ = 0.429, p = 0.020). As well, inflammatory markers such as CRP correlated better with LUS score (ρ = 0.600, p = 0.004) than with CT TSS (ρ = 0.479, p = 0.005).
Radiologic signs suggestive of or highly compatible with COVID-19 were present in 37 patients (72.5%) on CT scan and 40 patients (78.4%) on LUS exam. All 37 patients with abnormal findings on CT were correctly diagnosed with LUS (odds ratio = 13.333, 95% CI: 4.490–39.591, p < 0.001) with a sensitivity of 100.0%, a specificity of 78.6%, positive predictive value of 92.5% and negative predictive value of 100.0%.
Cohen's κ was run to determine if there was agreement between chest X-ray, LUS and chest CT scan in detecting COVID-19 abnormal lung findings. There was strong agreement between chest CT and LUS (κ = 0.842, 95% CI: 0.666–1.000, p < 0.001). Chest X-ray findings did not statistically significantly agree with CT scan results (κ = 0.205, p = 0.161). LUS results were weakly correlated with chest X-ray findings (κ = 0.276, 95% CI: 0.002–0.550, p = 0.034).
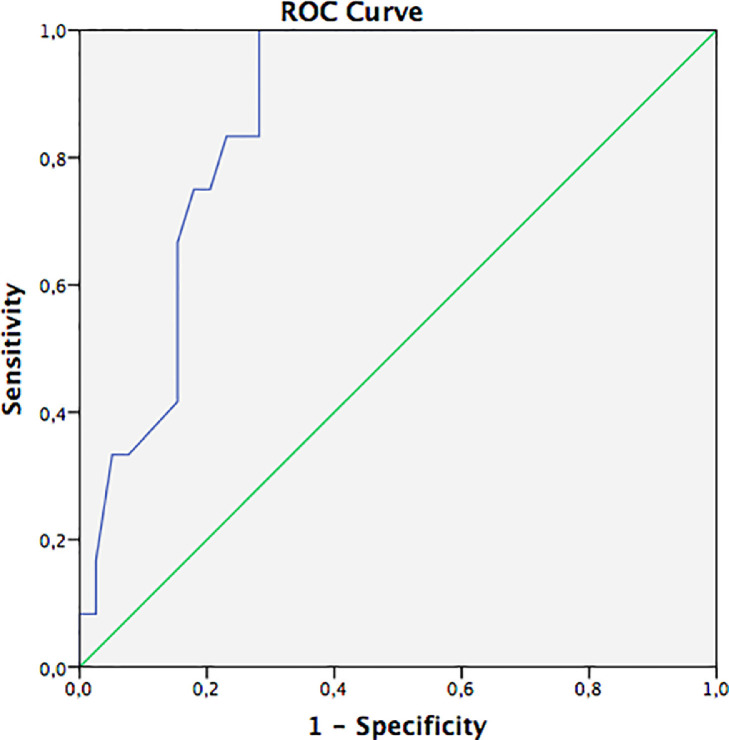
Most patients had chest CT phenotypes 1 (47.1%) and 2 (23.5%); only one patient had phenotype 3 (2%). Analysis of ROC curves (Fig. 4 ) revealed that the area under the curve (AUC) for LUS score was 86.4% (95% CI: 76.7%–96.2%, p < 0.001) in identifying phenotype 2. The cutoff value for LUS score of 9.5 had a sensitivity of 100% and specificity of 77.2% (p < 0.001).
Fig. 4.
Receiver operating characteristic (ROC) curve for lung ultrasonography (LUS) score, revealing an area under the curve (AUC) of 86.4% for detecting a computed tomography phenotype 2. Green line = reference line; blue line = LUS score.
Although, there were 3 patients with LUS findings compatible with COVID-19, 2 of the 3 were found to have a viral bronchiolitis and one had pulmonary metastatic disease.
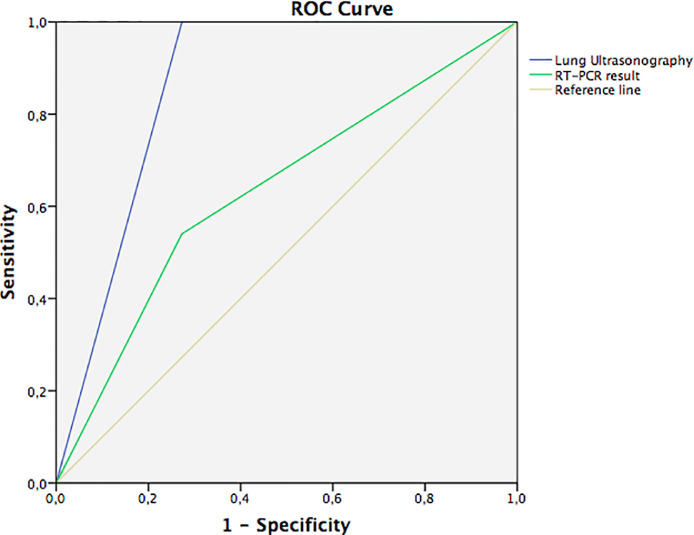
Analysis of ROC curves (Fig. 5 ) revealed that the AUC for LUS (86.4%, 95% CI: 70.2%–100%, p < 0.001) was better than that for RT-PCR (63.4%; 95% CI: 45.0%–81.8%, p = 0.181) for detection of CT abnormalities.
Fig. 5.
Green line: Receiver operating characteristic (ROC) curve for reverse transcription polymerase chain reaction test; area under the curve (AUC) = 63.4%. Blue line: Lung ultrasonography (LUS) exam; area under the curve (AUC) = 86.4% for detecting computed tomography abnormalities.
Therefore, there was no missed diagnoses of COVID-19 with LUS compared with CT in our cohort.
Discussion
We observed an excellent correlation between CT and LUS. All abnormal CT findings were detected on LUS; therefore, no abnormal CT findings were labeled as normal on LUS. In other words, with this technique, the proportion of false-negative rates is really low, which in this pandemic key to avoiding additional infections.
There is growing literature regarding the challenges faced in diagnosis of COVID-19 patients (Shi et al. 2020; Zhang et al. 2020). The positive rate of RT-PCR has been quantified as 63% in nasal swabs and 32% in pharyngeal swabs (Wang et al. 2020), similar to our results; we obtained a positive rate of only 47.9%. Because of these limitations, diagnostic imaging plays a key role in the management of these patients.
A study of 1049 patients undergoing chest CT scan and RT-PCR testing determined that CT abnormalities had high sensitivity for diagnosis of COVID-19 patients (Ai et al. 2020), suggesting that CT scan should be considered as a screening tool, especially in epidemic areas with high pre-test probability. Therefore, in many centers CT scans have replaced chest X-rays. However, the use of CT scan in the ED has many limitations, such as radiation exposure, especially for mild illness, low availability and contraindication to its use in unstable patients. Also, we found that the proportion of normal chest CT scans was relatively high (27.4%), but similar to previous reports (30.8%) (Li et al. 2020).
Preliminary reports in the COVID-19 era suggest that LUS findings correlate with CT scan results (Peng et al. 2020; Poggiali et al. 2020). These reports have characterized LUS findings in COVID-19 patients. Moreover, Soldati et al. (2020a) proposed a standardized approach to performing LUS in these patients, including a 14-zone technique and a scoring system to quantify severity of lung involvement. Although we agree there should be consensus on the LUS exam method, the 12-zone technique has been used more extensively and is validated (Cantinotti et al. 2020).
There have been reports regarding cardiac damage ranges from 7.2%–14% in COVID-19 patients (Huang et al. 2020). In our study, we found one young patient with a normal LUS exam, which prompted a sonographic focused cardiovascular assessment, revealing left ventricular dysfunction and pericardial effusion, resulting in immediate adjustment of therapy. His chest CT scan was unremarkable.
There were 2 patients who did not have the typical findings for COVID-19 (central distribution): one patient had acute bronchiolitis and the other patient had a decompensated heart failure episode. Although both had mainly central involvement on CT, they both also had some degree of peripheral involvement of the lungs, which was detected on LUS and misidentified as findings suspicious for COVID-19. This is one of the main limitations of LUS (Volpicelli et al. 2012), low specificity, as the findings might overlap with those for other lung disease etiologies, such as viral illnesses, pulmonary infarction and metastatic disease. The same limitation may apply to CT scans, which can misidentify other viral pneumonias as COVID-19. However, in this pandemic, positive LUS or CT features, even in the presence of a negative RT-PCR test, can still be highly suggestive of COVID-19 infection.
There are several advantages of performing LUS over CT scan, particularly for specific populations, such as pregnant women (Buonsenso et al. 2020c; Inchingolo 2020) and children (De Rose 2020; Musolino 2020), especially when LUS is performed with portable handheld ultrasound devices, which provide an inexpensive, accessible, portable, user-friendly and easy-to-disinfect method for assessing progression of cardiopulmonary pathology in patients with COVID-19 pneumonia. Moreover, it avoids transport of the patient with suspected COVID-19 to radiology (exposing other patients or health care providers).
In our study we found a high correlation between LUS findings and chest CT abnormalities suggestive of lung involvement caused by SARS-CoV-2 infection. As previously reported (Zhang et al. 2020), most of the patients had a predominance of peripheral involvement of both lungs (92.5%, 37 of 40 patients with abnormal CT findings), which can be reached with ultrasound. Notably, the LUS score was better correlated than the CT TSS with demographic data (age), physical exam (respiratory rate, SO2) and other established biomarkers (CRP) with proven utility in this setting. This higher correlation should be interpreted cautiously, although it might suggest that a 12-zone LUS score, with representation of posterior lobes in at least half of the zones (in comparison to a 5-lobe division, with representation of posterior lobes in 2 zones) better reflects the physiologic state of the patient.
Chest CT features differ among patients, making it possible to establish distinct phenotypes that might guide therapy and ventilator settings (Robba et al. 2020). In phenotype 1, there is high pulmonary compliance and severe hypoxemia; these patients may benefit from use of low to moderate positive end-expiratory pressure (PEEP) and are likely to respond well to inhaled nitric oxide. In phenotype 2, moderate to high PEEP, as well as prone positioning, may help recruit collapsed areas. Phenotype 3 resembles typical ARDS and should be managed as such. In our study, we found that a cutoff lung score of 9.5 had high sensitivity and specificity for detecting phenotype 2.
Therefore, this technique could be more easily replaced with LUS as it would be more accessible during the pandemic, especially as artificial intelligence algorithms to easily recognize COVID-19-related pathology and telemedicine programs are developed.
Strengths
To our knowledge, this is the first study evaluating the correlation of LUS with CT scan, with diagnostic and prognostic implications. We evaluated the radiologic burden (CT and LUS) with respect to clinical manifestations, laboratory results and outcomes.
Limitations
There are several limitations to consider. The main limitation is that LUS in general has poor specificity; its findings overlap with those for other pneumonia etiologies or incidental chronic findings (e.g., chronic heart failure or pulmonary fibrosis). In epidemic areas, however, positive LUS features, even with negative RT-PCR or chest X-ray, can still be highly suggestive of COVID-19 infection. Thus, the results from this study provide an opportunity to further investigate the use of ultrasound in various settings and clinical scenarios, when the prevalence and incidence of COVID-19 infection decreases.
Another limitation is that selection bias might have occurred. The expert sonographer performed all ultrasound scans on a consecutive sample selected based on his availability (during his working hours), which limits the generalizability of our results. This was mitigated by the variable schedule and changing shifts, unpredictable a priori (in continuous care). Additionally, false-negative ultrasound or CT results might be obtained in the initial stage of the disease, before lung involvement; consequently, imaging techniques should be considered a complement to RT-PCR and laboratory tests.
We did not correlate the LUS or CT findings with patient outcomes. Moreover, the study was not powered to evaluate the performance of a diagnostic strategy based on LUS exam; therefore, for this purpose, the study can only be considered hypothesis generating. Thus, the results from this study furnish an opportunity to further investigate the use of ultrasound in different settings and clinical scenarios, especially in follow-up.
We want to share our study findings, given the urgent need for different strategies to better manage COVID-19 patients and diminish the spread of SARS-CoV-2 and its prognosis in the current pandemic context. As the shortage of resources constitutes an undeniable public health threat, we consider LUS to be a potential solution, and recommend that it should be performed as first-line and follow-up imaging tests for COVID-19 patients.
Conclusions
LUS had accuracy similar to that of chest CT in detecting lung abnormalities in COVID-19 patients. In this pandemic, as the shortage of resources constitutes an undeniable public health threat, LUS can play a strategic role that has the potential to affect the management of these patients.
Acknowledgments
Acknowledgments
This research received no external funding.
Conflict of interest disclosure
The authors have declared no conflicts of interest.
References
- Ai T., Yang Z., Hou H., Zhan C., Chen C., Lv W., Tao Q., Sun Z., Xia L. Correlation of Chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: A report of 1014 cases. Radiology. 2020;296 doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American College of Emergency Physicians Ultrasound guidelines: Emergency, point-of-care and clinical ultrasound guidelines in medicine. Ann Emerg Med. 2017;69:e27–e54. doi: 10.1016/j.annemergmed.2016.08.457. [DOI] [PubMed] [Google Scholar]
- Buonsenso D., Pata D., Chiaretti A. COVID-19 outbreak: Less stethoscope, more ultrasound. Lancet Respir Med. 2020;8:e27. doi: 10.1016/S2213-2600(20)30120-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonsenso D., Piano A., Raffaelli F., Bonadia N., Donati K.D.G., Franceschi F. Point-of-care lung ultrasound findings in novel coronavirus disease-19 pnemoniae: A case report and potential applications during COVID-19 outbreak. Eur Rev Med Pharmacol Sci. 2020;24:2776–2780. doi: 10.26355/eurrev_202003_20549. [DOI] [PubMed] [Google Scholar]
- Buonsenso D., Raffaelli F., Tamburrini E., Biasucci D.G., Salvi S., Smargiassi A., Inchingolo R., Scambia G., Lanzone A., Testa A.C., Moro F. Clinical role of lung ultrasound for the diagnosis and monitoring of COVID-19 pneumonia in pregnant women. Ultrasound Obstet Gynecol. 2020;56:106–109. doi: 10.1002/uog.22055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantinotti M., Giordano R., Scalese M., Marchese P., Franchi E., Viacava C., Molinaro S., Assanta N., Koestenberger M., Kutty S., Gargani L., Ait-Ali L. Prognostic value of a new lung ultrasound score to predict intensive care unit stay in pediatric cardiac surgery. Ann Thorac Surg. 2020;109:178–184. doi: 10.1016/j.athoracsur.2019.06.057. [DOI] [PubMed] [Google Scholar]
- De Rose C., Inchingolo R., Smargiassi A., Zampino G., Valentini P., Buonsenso D. How to perform pediatric lung ultrasound examinations in the time of COVID‐19 [e-pub ahead of print] J Ultrasound Med. 2020 doi: 10.1002/jum.15306. Accessed May 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W., Ni Z., Hu Y., Liang W., Ou C., He J., Liu L., Shan H., Lei C., Hui D., Du B., Li L., Zeng G., Yuen K., Chen R., Tang C., Wang T., Chen P., Xiang J., Li S., Wang J., Liang Z., Peng Y., Wei L., Liu Y., Hu Y., Peng P., Wang J., Liu J., Chen Z., Li G., Zheng Z., Qiu S., Luo J., Ye C., Zhu S., Zhong N., China Medical Treatment Expert Group for Covid-19 Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan F., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inchingolo R., Smargiassi A., Moro F., Buonsenso D., Salvi S., Del Giacomo P., Scoppettuolo G., Demi L., Soldati G., Testa A.C. The diagnosis of pneumonia in a pregnant woman with COVID-19 using maternal lung ultrasound. Am J Obstet Gynecol. 2020;20:30463–30468. doi: 10.1016/j.ajog.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns Hopkins Coronavirus Resource Center. Available at:htps://coronavirus.jhu.edu/map.html. Accessed 26 April 2020.
- Kruser J.M., Schmidt G.A., Kory P.D. COUNTERPOINT: Should the use of diagnostic point-of-care ultrasound in patient care require hospital privileging/credentialing? Chest. 2020;157:498–500. doi: 10.1016/j.chest.2019.10.037. [DOI] [PubMed] [Google Scholar]
- Li K., Fang Y., Li W., Pan C., Qin P., Zhong Y., Liu X., Huang M., Liao Y., Li S. CT image visual quantitative evaluation and clinical classification of coronavirus disease (COVID-19) Eur Radiol. 2020;30:4407–4416. doi: 10.1007/s00330-020-06817-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W., Zhang S., Chen B., Chen J., Xian J., Lin Y., Shan H., Su Z.Z. A clinical study of noninvasive assessment of lung lesions in patients with coronavirus disease-19 (COVID-19) by bedside ultrasound. Ultraschall Med. 2020;41:300–307. doi: 10.1055/a-1154-8795. [DOI] [PubMed] [Google Scholar]
- Musolino A.M., Supino M.C., Buonsenso D., Ferro V., Valentini P., Magistrelli A., Lombardi M.H., Romani L., D'Argenio P., Campana A., on behalf of the ROMULUS COVID Team Lung ultrasound in children with COVID-19: Preliminary findings. Ultrasound Med Biol. 2020;46:2094–2098. doi: 10.1016/j.ultrasmedbio.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Q.Y., Wang X.T., Zhang L.N., Chinese Critical Care Ultrasound Study Group (CCUSG) Findings of lung ultrasonography of novel corona virus pneumonia during the 2019–2020 epidemic. Intensive Care Med. 2020;46:849–850. doi: 10.1007/s00134-020-05996-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggiali E., Dacrema A., Bastoni D., Tinelli V., Demichele E., Mateo Ramos P., Marcianò T., Silva M., Vercelli A., Magnacavallo A. Can lung US help critical care clinicians in the early diagnosis of novel coronavirus (COVID-19) pneumonia? Radiology. 2020;296:E55–E64. doi: 10.1148/radiol.2020200847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robba C., Battaglini D., Ball L., Patroniti N., Loconte M., Brunetti I., Vena A., Giacobbe D., Bassetti M., Rocco P.R.M., Pelosi P. Distinct phenotypes require distinct respiratory management strategies in severe COVID-19. Resp Physiol Neurobiol. 2020;279 doi: 10.1016/j.resp.2020.103455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Han X., Jiang N., Cao Y., Alwalid O., Gu J., Fan Y., Zheng C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: A descriptive study. Lancet Infect Dis. 2020;20:425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldati G., Smargiassi A., Inchingolo R., Buonsenso D., Perrone T., Briganti D.F., Perlini S., Torri E., Mariani A., Mossolani E., Tursi F., Mento F., Demi L. Proposal for international standardization of the use of lung ultrasound for patients with COVID-19: A simple, quantitative, reproducible method. J Ultrasound Med. 2020;39:1413–1419. doi: 10.1002/jum.15285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldati G., Smargiassi A., Inchingolo R., Buonsenso D., Perrone T., Briganti D.F., Perlini S., Torri E., Mariani A., Mossolani E., Tursi F., Mento F., Demi L. Is there a role for lung ultrasound during the COVID-19 pandemic? Clin Letter J Ultrasound Med. 2020;39:1459–1462. doi: 10.1002/jum.15284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soummer A., Perbet S., Brisson H., Arbelot C., Constantin J.M., Lu Q., Rouby J.J., Lung Ultrasound Study Group Ultrasound assessment of lung aeration loss during a successful weaning trial predicts postextubation distress. Crit Care Med. 2012;40:2064–2072. doi: 10.1097/CCM.0b013e31824e68ae. [DOI] [PubMed] [Google Scholar]
- Volpicelli G., Elbarbary M., Blaivas M., Lichtenstein D.A., Mathis G., Kirkpatrick A., Melniker L., Gargani L., Noble V., Via G., Dean A., Tsung J., Soldati G., Copetti R., Bouhemad B., Reissig A., Agricola E., Rouby J., Arbelot C., Liteplo A., Sargsyan A., Silva F., Hoppmann R., Breitkreutz R., Seibel A., Neri L., Storti E., Petrovic T., International Liaison Committee on Lung Ultrasound (ILC-LUS) for the International Consensus Conference on Lung Ultrasound (ICC-LUS) International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38:577–591. doi: 10.1007/s00134-012-2513-4. [DOI] [PubMed] [Google Scholar]
- Volpicelli G., Lamorte A., Villén T. What's new in lung ultrasound during the COVID-19 pandemic. Intensive Care Med. 2020;46:1445–1448. doi: 10.1007/s00134-020-06048-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter J.M., Satterwhite L., Lyn-Kew K.E. POINT: Should the use of diagnostic point-of-care ultrasound in patient care require hospital privileging/credentialing? Yes. Chest. 2020;157:496–498. doi: 10.1016/j.chest.2019.10.041. [DOI] [PubMed] [Google Scholar]
- Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., Tan W. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Ouyang H., Fu L., Wang S., Han J., Huang K., Jia M., Song Q., Fu Z. CT features of SARS-CoV-2 pneumonia according to clinical presentation: A retrospective analysis of 120 consecutive patients from Wuhan City [e-pub ahead of print] Eur Radiol. 2020 doi: 10.1007/s00330-020-06854-1. Accessed April 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]