Abstract
Purpose
To describe the epidemiology and outcome of the first 100 COVID-19 cases in Taiwan.
Methods
We included the first 100 patients with laboratory-confirmed SARS-CoV-2 infection in Taiwan. Demographic, clinical, epidemiological and laboratory data were extracted from outbreak investigation reports and medical records.
Results
Illness onset of the 100 patients was during January 11 to March 16, 2020. Twenty-nine (29%) had at least one underlying condition and ten (10%) were asymptomatic. Seventy-one were imported, including four clusters. Twenty-nine were locally-acquired, including four clusters. The median days from onset to report was longer in locally-acquired cases (10 vs 3 days). Three patients died (case fatality rate 3%) and all of them had underlying conditions. As of May 13, 2020, 93 had been discharged in stable condition; the median hospital stay was 30 days (range, 10–79 days).
Conclusion
The first 100 cases of COVID-19 in Taiwan showed the persistent threat of imported cases from different countries. Even though sporadic locally-acquired disease has been identified, through contact investigation, isolation, quarantine and implementation of social distancing measures, the epidemic is contained to a manageable level with minimal local transmission.
Keywords: SARS-CoV-2, COVID-19, Taiwan, Epidemiology
Introduction
Since December 2019, an increasing number of cases of pneumonia had been reported in Wuhan, China. In the beginning, most cases reportedly had exposure to Huanan Seafood Wholesale Market. However, patients without exposure to the Market were also found. A novel coronavirus, later named Severe Acute Respiratory Virus Coronavirus-2 (SARS-CoV-2), was identified as the causative agent and the disease caused by SARS-CoV-2 was named as coronavirus infectious disease-2019 (COVID-19).
Because of geographic proximity between Taiwan and China, shared culture, and common language, approximately 400,000 Taiwanese work in China,1 plus another 10,000 study there. Having such close ties, Taiwan was expected to have importation of the disease from China early on. To prevent widespread transmission of the disease, Taiwan began onboard quarantine on December 31, 2019, for all flights coming in directly from Wuhan. Taiwan Centers for Disease Control (Taiwan CDC) laboratory set up protocol to test for SARS-CoV-2 so that by the time COVID-19 became a nationally reportable disease on January 15, 2020, Taiwan CDC was able to confirm the disease.
By mid-January, Thailand and Japan had identified cases of COVID-19 in persons who had traveled there from Wuhan.2 , 3 By the end of January 2020, the COVID-19 had been identified in 24 different countries, spanning Asia, Middle East, Europe, and North America.4 COVID-19 was declared a pandemic on March 11, 2020, by the World Health Organization. To prevent the spread of COVID-19 in the community, throughout the first three months of 2020, Taiwan slowly expanded its border quarantine efforts from Wuhan, to Hubei Province of China, to China, and later other countries with outbreaks. By March 20, 2020, all travelers from abroad must undergo 14 days of quarantine upon their arrival to Taiwan.5
The first case of SARS-CoV-2 infection in Taiwan was diagnosed on January 21, 2020. Here, we provide an analysis of the first 100 laboratory confirmed COVID-19 cases in Taiwan to describe the epidemiological characteristics and their outcome.
Materials and methods
Surveillance
When Taiwan CDC announced COVID-19 as a notifiable disease on January 15, 2020, the reporting criteria included clinical symptoms (fever and respiratory symptoms, or cough with dyspnea/respiratory difficulty, or radiologically/pathologically diagnosed pneumonia) as well as travel history to Wuhan, China, within 14 days of disease onset. The reporting criteria evolved to include all relevant travel exposure or contact history, and cover a wider spectrum of clinical illnesses. Since January 25, the reporting clinical criteria was modified as fever and/or any respiratory symptoms.
A confirmed case should meet the reporting criteria for COVID-19 and have respiratory specimen that tested positive by real-time reverse transcriptase-polymerase chain reaction (RT-PCR) or virus culture. To improve case detection, on February 12, Taiwan CDC started a screening program for COVID-19 to test persons who (I) had been tested negative for influenza but reported to have severe influenza with complications; (II) had specimens taken for upper respiratory disease surveillance; (III) were part of a cluster of influenza cases, or (IV) received a diagnosis of pneumonia but did not respond to empirical treatment.6 Retrospective testing of all specimens received during January 31 to February 11 from patients meeting the above criteria were also tested for SARS-CoV-2.
Imported cases were defined as patients who had history of international travel within 14 days of disease onset; imported-linked cases were defined as patients who had a history of close contact with an imported case within 14 days of disease onset. A locally-acquired case had no history of international travel or contact to an imported case within 14 days of disease onset. Detailed information including demographics, clinical and laboratory data was reported to the National Notifiable Disease Surveillance System. Cases with epidemiological linkages were classified as clusters.
Contact tracing
A thorough epidemiological investigation, including source determination and contact tracing, was conducted for each laboratory confirmed COVID-19 patient by the outbreak investigation team of Taiwan CDC and local health authorities. For each confirmed case, contacts during the 14 days prior to symptom onset until the patient was isolated were identified. A close contact was a person who had face-to-face contact with a confirmed case for more than 15 min while not wearing appropriate personal protection equipment (PPE). A household contact was a close contact who lived in the same household with the index case, while a family contact was a family member not living in the same household. All close contacts were required to undergo home quarantine for 14 days after last exposure. All other contacts were asked to self-monitor their health condition for 14 days after last exposure. If a contact developed fever or respiratory tract symptoms, respiratory tract specimens were taken and tested for SARS-CoV-2. All household contacts and some non-household close contacts with contact intensity comparable to household contacts, for example, having meal together with the index case, were tested even if they had no symptoms. For asymptomatic cases, onset date was defined as the date specimens were taken.
Case management and laboratory diagnosis
All confirmed cases of COVID-19 were immediately isolated in negative pressure or single-occupancy rooms in hospitals for further care. Clinical management was mainly supportive, but may also include antibiotics and/or anti-influenza medications as needed, according to the Interim Guideline for Clinical Management of SARS-CoV-2 Infection published by Taiwan CDC.7 Follow up respiratory tract specimens were collected to test for SARS-CoV-2. Two consecutive negative samples collected at least 24 h apart were required for patients to be de-isolated. Starting from February 28, 2020, three consecutive negative samples were required for de-isolation. Respiratory specimens were collected once every two to four days according to the Guideline, and the exact frequency of specimen collection was determined by clinicians. Patients fulfilling the criteria for de-isolation will be discharged if there is no need for clinical care, and the decision is up to the primary care physician.
Data collection and statistical analysis
Information on clinical presentation, underlying diseases and travel history of patients was collected during case investigation. Laboratory results within 48 h of admission were retrieved from medical records that were uploaded to the National Notifiable Disease Surveillance System. Based on the above information, the investigation team determined the clinical severity of each confirmed patient following the World Health Organization (WHO) interim guidance.8
Ethical approval
Data collection and analysis of cases were determined by the Taiwan Ministry of Health and Welfare to be part of a continuing public health outbreak response and were thus considered exempt from institutional review board approval.
Results
Demographics, clinical presentation and WHO classification (Table 1)
Table 1.
Characteristics of 100 COVID-19 cases in Taiwan.
| Patient characteristics | Number of cases or median (range) |
|---|---|
| Gender (Male: Female) | 44:56 |
| Age, years | 44 (11–88) |
| WHO classification | |
| Mild cases | 60 |
| Pneumonia | 31 |
| Severe pneumonia | 2 |
| Acute respiratory distress syndrome (ARDS) | 6 |
| Sepsis/septic shock | 1 |
| Initial symptoms | |
| Fever | 54 |
| Cough | 54 |
| Sore throat | 35 |
| Rhinorrhea | 27 |
| General weakness | 25 |
| Muscle pain | 14 |
| Diarrhea | 10 |
| Headache | 10 |
| Nausea/vomiting | 2 |
| Anomsia/dysgeusia | 8 |
| Asymptomatic | 10 |
| Underlying conditions | |
| Cardiovascular disease (including hypertension) | 17 |
| Diabetes mellitus | 8 |
| Chronic lung disease | 4 |
| Asthma | 3 |
| Obesity (Body mass index>30 kg/m2) | 3 |
| Renal disease | 1 |
| Neuromuscular disease | 1 |
| Malignancy | 1 |
| Immunodeficiency | 1 |
| Any of the above | 29 |
| Days from onset to reporta | |
| ≦ 3 days | 51 |
| 4–6 days | 16 |
| ≧ 7 days | 33 |
| Days from onset to lab diagnosisa | |
| ≦ 3 days | 41 |
| 4–6 days | 24 |
| ≧ 7 days | 35 |
For asymptomatic cases, onset date is defined as sampling date.
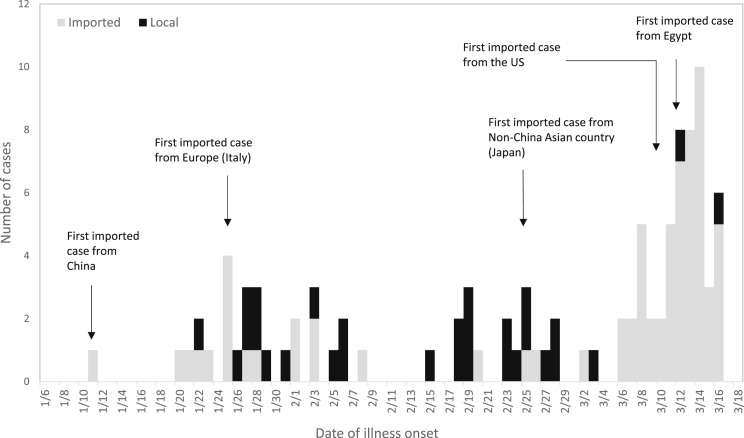
During January 21 to March 18, 2020, there were 17,935 cases reported and tested for SARS-CoV-2, and 100 patients were diagnosed with COVID-19, with symptom onset from January 11 to March 16, 2020 (Fig. 1 ). Forty-four were male. Median age was 44 years (range, 11–88). The most common initial symptoms were fever (54%), cough (54%) and sore throat (35%). Ten patients had diarrhea, but nausea and/or vomiting was infrequent (2%). Eight (8%) patients reported anosmia/hyposmia and/or dysgeusia. All 10 asymptomatic cases were household or close contacts of confirmed cases, and were diagnosed through contact investigation. Of the 29 patients who had at least one underlying condition, 17 had cardiovascular diseases (including hypertension), 8 had diabetes mellitus and 4 had chronic lung disease. There were 51 patients who were reported within 3 days of symptom onset, and 41 patients were diagnosed within 3 days of symptom onset (Table 1). Among the 56 patients with total white blood cell count and percentage of lymphocyte available, 18 (32.1%) had lymphopenia (<1000/mL). Among the 50 patients with C-reactive protein (CRP) levels available, 19 (38%) had CRP>1 mg/dL. Only five patients had D-dimer levels available, and two were elevated (590 and 1539 ng/mL, respectively). Renal and liver function tests mostly remained within normal range (Table 2 ).
Figure 1.
Epidemiological curve of first 100 COVID-19 cases in Taiwan.
Table 2.
Laboratory results of 100 COVID-19 cases in Taiwan.
| Laboratory results within 48 h of admission (number of cases with data available) | Median (interquartile range)/Number (%) of patients |
|---|---|
| Total white blood cell count (103/uL) (n = 62) | 5.7 (4.2–6.9) |
| <4 | 12 (19.4) |
| 4-10 | 48 (77.4) |
| >10 | 2 (3.2) |
| Lymphocyte count (103/uL) (n = 56) | 1.2 (0.9–1.9) |
| <1 | 18 (32.1) |
| Hemoglobin (g/dL) (n = 60) | 14.3 (13.2–14.8) |
| <10 | 2 (3.3) |
| Platelet count (103/uL) (n = 60) | 208.5 (174.8–266.0) |
| <100 | 2 (3.3) |
| Blood urea nitrogen (mg/dL) (n = 40) | 14.1 (11.0–15.0) |
| >20 | 2 (5.0) |
| Serum creatinine (mg/dL) (n = 58) | 0.8 (0.6–0.9) |
| >1.3 | 3 (5.2) |
| Aspartate aminotransferase, AST (U/L) (n = 45) | 25.0 (19.0–33.8) |
| >40 | 8 (17.8) |
| Alanine aminotransferase, ALT (U/L) (n = 43) | 23.0 (15.0–31.0) |
| >40 | 5 (11.6) |
| C-reactive protein, CRP (mg/dL) (n = 50) | 0.6 (0.2–1.5) |
| >1 | 19 (38.0) |
| Lactate dehydrogenase, LDH (U/L) (n = 27) | 218.0 (150.0–265.0) |
| >245 | 10 (37.0) |
| Creatine kinase, CK (U/L) (n = 15) | 78.0 (45.0–165.0) |
| >185 | 3 (20.0) |
| D-dimer (ng/mL) (n = 5) | 238.0 (190.0–1064.5) |
| >500 | 2 (40.0) |
| Procalcitonin (ng/mL) (n = 9) | 0.058 (0.046–0.115) |
| >0.5 | 0 (0) |
Epidemiological features
Among the 100 cases, 71 were imported, with 12 having been in China, including 11 from Wuhan and 1 from Macau. Thirty-one had traveled to Europe, including Spain (n = 5), Italy (n = 4), Germany (n = 4), France (n = 4), the United Kingdom (n = 4), Austria (n = 3), the Netherlands (n = 2), and one of each from Iceland, Greece, Ireland, Switzerland and the Czech Republic. There were 13 cases from a tour group that had traveled to Turkey. Six cases had traveled to Asian countries other than China, including the Philippines (n = 3), Japan (n = 2) and Indonesia (n = 1). Four had traveled to Egypt, four to the United States and one was from the Diamond Princess cruise ship. In addition to the cluster from Turkey, there were 3 more imported clusters: 3 cases were classmates in Spain; 2 were in the same travel group to Egypt, and 4 were family members who had traveled to Italy together.
Of the 29 locally-acquired cases, there were 5 imported-linked cases and 1 isolated locally-acquired case; the other 23 cases could be classified into 4 clusters. After extensive contact tracing, none of the 4 index cases had contacted people with recent travel history or respiratory symptoms. We failed to identify the infection source but additional cases were found. Cluster sizes ranged from 3 to 9 cases, as described below:
Cluster A (5 cases): The index patient was diagnosed by retrospective COVID-19 screening because he reported to have severe influenza with complications but tested negative for influenza. Two household and two non-household family contacts were diagnosed.
Cluster B (3 cases): The index patient was tested for SARS-CoV-2 because she reported to have severe influenza with complications but tested negative for influenza. After the index patient was diagnosed, 1 household and 1 non-household family contact were diagnosed.
Cluster C (6 cases): The index patient was tested for SARS-CoV-2 because he received a diagnosis of pneumonia but did not respond to empirical treatment. After the index patient was diagnosed, 5 household contacts were diagnosed.
Cluster D (9 cases): The index patient was tested for SARS-CoV-2 because she received a diagnosis of pneumonia but did not respond to empirical treatment. After the index patient was diagnosed, a hospital outbreak was found, involving 3 healthcare workers, 2 household contacts, 1 patient on the same ward, 1 family member of another patient, and 1 janitor.
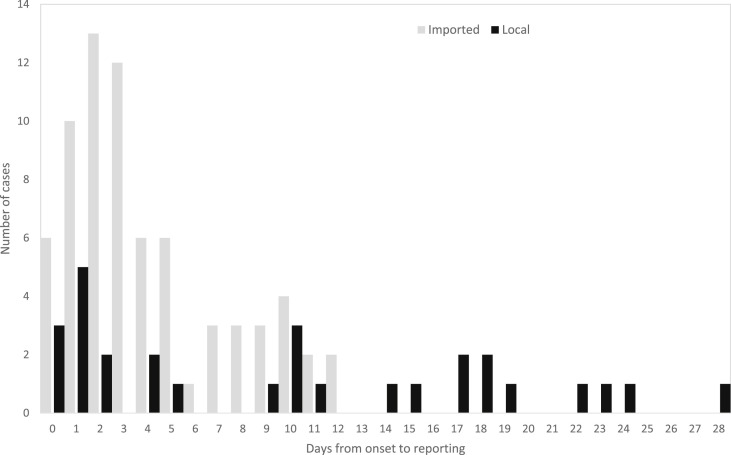
Fifty-seven percent (41/71) of the 71 imported cases were reported within 3 days of symptoms onset (median 3 days, range 0–12) (Fig. 2 ). However, only 34% (10/29) of the locally-acquired cases were reported within 3 days of symptoms onset (median 10 days, range 0–28). Thirty-eight percent (11/29) of locally-acquired cases were reported more than 14 days after symptom onset. The 4 index cases of clusters A to D were diagnosed 19, 28, 18 and 10 days after symptom onset.
Figure 2.
Distribution of days from symptom onset to reporting of 100 COVID-19 cases in Taiwan, by source of infection.
Patient outcome
As of May 13, 2020, there were 93 patients who had been discharged in stable condition; the median hospital stay was 30 days (range, 10–79 days). Eight patients received mechanical ventilation, including 1 with severe pneumonia, 6 with acute respiratory distress syndrome (ARDS) and 1 with septic shock. Among patients with medication records available, 4 had used hydroxychloroquine, 2 had used hydroxychloroquine plus azithromycin, and 1 had used lopinavir-ritonavir.
Three patients died (case fatality rate 3%). The ages of the cases that died were 56, 62 and 87 years; 1 was female. All had multiple underlying conditions including diabetes mellitus, cardiovascular or renal diseases; invasive ventilator support was used for 12–30 days. One patient had used extra-corporeal membrane oxygenation (ECMO) for 14 days. One patient had restricted lung function upon discharge.
Among the 10 asymptomatic cases, 6 had been discharged after being hospitalized for a median of 23 days (range, 14–46); 4 remained hospitalized because of persistent positive test results for SARS-CoV-2.
Discussion
During January 21 to March 18, 2020, there were 100 cases of COVID-19 confirmed in Taiwan. Taiwan had implemented multiple measures to contain SARS-CoV-2 infection. As of April 9, 2020, there were more than 1.4 million confirmed cases of COVID-19 worldwide; on the other hand, during the same period, Taiwan had only 379 confirmed cases and 5 deaths. With a population of 23 million, the incidence of laboratory confirmed COVID-19 in Taiwan (16.4/million population) was among the lowest in developed countries, and Taiwan remained in the containment phase of the COVID-19 response. We share our experience during the early stage of this epidemic.
Because of different surveillance and enrollment criteria, distribution of clinical presentations varied in reports from different countries. Reported percentage of patients with fever ranged between 43 and 98%.9 , 10 Because fever had been removed as an essential clinical criterion for reporting COVID-19 since January 25, 2020, it is not surprising that not 100% of our patients had fever. During the Severe Acute Respiratory Syndrome (SARS) outbreak in 2003, fever screening was used widely to identify cases, because nearly all patients with SARS had fever as initial presentation.11 Furthermore, the viral shedding from respiratory specimens of SARS patients peaked at 6–11 days after onset of illness. Low rate of viral shedding in the first few days of illness meant that early isolation measures would be effective.12 In 2003, Taiwan was able to identify SARS at international ports and prevent community spread by adopting fever screening at various locations. Such effects led to the continued use of fever screening since 2003. However, knowing that only 54% of the COVID-19 patients in Taiwan had fever initially, the high level of viral shedding early in the disease course and the possibility of pre-symptomatic transmission, fever screening should not be the only method used to identify cases.13 In fact, the effectiveness of widespread adoption of fever screening in public places for the current COVID-19 outbreak should be re-evaluated.
Our case series included outbreaks from different places and situations, including exposure among household and non-household family members, classmates, tour groups and hospitals. According to a previous study from Taiwan CDC, household contacts have the highest secondary clinical attack rate.14 We found that interaction between members of the same tour group during the trip were akin to households in contact intensity, and they had similar risk of secondary attack. In SARS and Middle East respiratory syndrome (MERS) epidemics, hospital outbreaks involving healthcare workers led to loss of workforce and had a huge negative impact on the public health and healthcare systems.15 , 16 Among the first 100 cases of COVID-19, we had a hospital outbreak involving 9 cases. The outbreak was controlled after the involved ward was shut down and all cases and contacts were isolated. After the SARS outbreak in 2003, Taiwan put special emphasis on infection control measures in hospitals.17 Proper use of PPE and implementation of infection control practices may have helped in reducing SARS-CoV-2 transmission before the disease was diagnosed, leading to the low secondary clinical attack rate among healthcare workers and limited the size of the hospital outbreak.
Because of the geographical proximity and the frequent exchanges between Taiwan and China, we identified the first imported case from China nine days after the genetic sequence of SARS-CoV-2 was made public.18 It is not surprising that the first 13 cases in Taiwan were all imported or epidemiologically-linked to cases from China. Cases with travel history in Italy was reported as early as February 6, one week after Italy reported its first 2 cases, and before the establishment of COVID-19 transmission was recognized in Italy.19 The first cluster of cases with travel history to Egypt was diagnosed in mid-March, while Egypt had less than 100 cases.20 Sharing information on cases with travel history abroad with the countries of possible disease acquisition or exposure is crucial in disease control. Such notification may prompt countries to strengthen surveillance and possibly identify local transmission. Information sharing also may foster collaboration between countries in outbreak investigation.21
Compared to imported cases, locally-acquired cases took longer time to be diagnosed (10 days vs. 3 days). The difference in the timing of the reporting and diagnosis between imported and locally-acquired cases is of concern. Because SARS-CoV-2 is highly transmissible in the early phase of infection,13 , 14 early diagnosis and isolation of patients is crucial for disease containment. Multiple policies, including 14-day home quarantine for all travelers arriving from COVID-19 risk areas, declaration of health upon arrival at international ports and integration of recent travel history to the centralized database of National Health Insurance, all contributed to the timely isolation and detection of imported cases by clinicians and public health officials.6
While imported cases did not cause outbreaks, locally-acquired cases led to the finding of clusters up to 9 cases. Our experience showed that because imported cases could be quickly identified, secondary cases were usually limited to household contacts when the index case was imported. On the contrary, the outbreak size was larger if the index case had locally-acquired disease and the infection source could not be found. Because of aggressive contact tracing and isolation, the locally-acquired disease outbreaks did not cause sustained transmission. Name-based rationing system securing affordable mask supply to the public since February, announcement of Guidelines for Large-Scale Public Gatherings by Taiwan CDC in March, and social distancing measures in April may have all helped to reduce transmission risk.22, 23, 24 To prevent the establishment of COVID-19 in the community, Taiwan CDC changed the reporting criteria to include all patients with pneumonia of unknown etiology on February 16, 2020. Furthermore, all patients can be reported and tested when COVID-19 is suspected by clinicians. However, because COVID-19 was, and still is, a largely imported disease,25 to diagnose the disease, clinicians must have heightened awareness and suspicion of the disease when seeing patients who have no history of international travel.
This study has limitations. First, at the time of writing this manuscript, some of the contacts were still under self health-monitoring or isolation and we do not know if they would become cases. Therefore, the number of clusters and cases involved may change over time. Second, we do not have treatment details for all patients, so we are unable to evaluate the effectiveness of treatment. Third, viral load or culture results of clinical samples is not available. Fourth, baseline laboratory data are not available for all patients.
In conclusion, the first 100 cases of COVID-19 in Taiwan showed that even though sporadic locally-acquired disease has been identified, the disease is mainly imported by international travelers. Through contact investigation, isolation, quarantine and implementation of social distancing measures, the epidemic is contained to a manageable level with minimal local transmission. To prevent widespread community transmission, continued vigilance in diagnosing locally-acquired cases are needed.
Declaration of Competing Interest
None.
Acknowledgments
We thank all the medical personnel for taking care of COVID-19 patients. We acknowledge the dedicated work of public health staff in conducting case investigation.
Taiwan COVID-19 outbreak investigation team: Wan-Chin Chen, Angela Song-En Huang, Chia-Ping Su, Tsung-Pei Tsou, Pin-Hui Lee, Pei-Chun Chan, Hao-Hsin Wu, Shih-Tse Huang, Wei-Ju Su, Ying-Shih Su, Hsin-Yi Wei, Meng-Yu Chen, Pei-Yuan Wu, Kung-Chin Wang, Huai-Te Tsai, Hsin-Chun Lee, Min-Nan Hung.
Contributor Information
the Taiwan COVID-19 Outbreak Investigation Team:
Wan-Chin Chen, Angela Song-En Huang, Chia-Ping Su, Tsung-Pei Tsou, Pin-Hui Lee, Pei-Chun Chan, Hao-Hsin Wu, Shih-Tse Huang, Wei-Ju Su, Ying-Shih Su, Hsin-Yi Wei, Meng-Yu Chen, Pei-Yuan Wu, Kung-Chin Wang, Huai-Te Tsai, Hsin-Chun Lee, and Min-Nan Hung
References
- 1.National Statistics R.O.C.(Taiwan) 2018. Number of Taiwanese citizens working overseas.https://www.stat.gov.tw/ct.asp?xItem=44935&ctNode=6395&mp=4 [Google Scholar]
- 2.World Health Organisation . World Health Organization; Geneva, Switzerland: 2020. Novel coronavirus- Thailand (ex-China)https://www.who.int/csr/don/14-january-2020-novel-coronavirus-thailand/en/ [Google Scholar]
- 3.World Health Organisation . World Health Organization; Geneva, Switzerland: 2020. Novel coronavirus- Japan (ex-China)https://www.who.int/csr/don/17-january-2020-novel-coronavirus-japan-ex-china/en/ [Google Scholar]
- 4.World Health Organization . World Health Organization; Geneva, Switzerland: 2020. COVID-19 dashboard.https://covid19.who.int/ [Google Scholar]
- 5.Taiwan Centers for Disease Control . 2020. CECC raises travel notice for all countries to Level 3: warning; advises against all nonessential travel.https://www.cdc.gov.tw/En/Bulletin/Detail/qaBvfAzLOukWMMGyNubKWw?typeid=158 [Google Scholar]
- 6.Lin C., Braund W.E., Auerbach J., Chou J.H., Teng J.H., Tu P. Policy decisions and use of information technology to fight 2019 novel coronavirus disease, Taiwan. Emerg Infect Dis. 2020;26(7) doi: 10.3201/eid2607.200574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taiwan Centers for Disease Control . 2020. Interim Guidelines for clinical management of SARS-CoV-2 infection.https://www.cdc.gov.tw/En/File/Get/_Dv_q75ZjLcNeRvlnrPgUg [Google Scholar]
- 8.World Health Organization . World Health Organization; Geneva, Switzerland: 2020. Clinical management of severe acute respiratory infection when COVID-19 is suspected.https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected [Google Scholar]
- 9.Guan W.J., Zhong N.S. Clinical characteristics of Covid-19 in China. Reply. N Engl J Med. 2020;382(19):1861–1862. doi: 10.1056/NEJMc2005203. [DOI] [PubMed] [Google Scholar]
- 10.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peiris J.S., Yuen K.Y., Osterhaus A.D., Stohr K. The severe acute respiratory syndrome. N Engl J Med. 2003;349(25):2431–2441. doi: 10.1056/NEJMra032498. [DOI] [PubMed] [Google Scholar]
- 12.Cheng P.K., Wong D.A., Tong L.K., Ip S.M., Lo A.C., Lau C.S. Viral shedding patterns of coronavirus in patients with probable severe acute respiratory syndrome. Lancet. 2004;363(9422):1699–1700. doi: 10.1016/S0140-6736(04)16255-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He X., Lau E.H.Y., Wu P., Deng X., Wang J., Hao X. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26:672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 14.Cheng H.Y., Jian S.W., Liu D.P., Ng T.C., Huang W.T., Lin H.H. Contact tracing assessment of COVID-19 transmission dynamics in Taiwan and risk at different exposure periods before and after symptom onset. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Memish Z.A., Perlman S., Van Kerkhove M.D., Zumla A. Middle East respiratory syndrome. Lancet. 2020;395(10229):1063–1077. doi: 10.1016/S0140-6736(19)33221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsieh Y.H., Chen C.W., Hsu S.B. SARS outbreak, Taiwan, 2003. Emerg Infect Dis. 2004;10(2):201–206. doi: 10.3201/eid1002.030515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yen M.Y., Schwartz J., Chen S.Y., King C.C., Yang G.Y., Hsueh P.R. Interrupting COVID-19 transmission by implementing enhanced traffic control bundling: implications for global prevention and control efforts. J Microbiol Immunol Infect. 2020;53(3):377–380. doi: 10.1016/j.jmii.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization . World Health Organization; Geneva, Switzerland: 2020. WHO timeline- COVID-19.https://www.who.int/news-room/detail/08-04-2020-who-timeline---covid-19 [Google Scholar]
- 19.World Health Organization . World Health Organization; Geneva, Switzerland: 2020. WHO COVID-19 dashboard- Italy.https://covid19.who.int/region/euro/country/it [Google Scholar]
- 20.World Health Organization . World Health Organization; Geneva, Switzerland: 2020. WHO COVID-19 dashboard- Egypt.https://covid19.who.int/region/emro/country/eg [Google Scholar]
- 21.Olsen S.J., Chen M.Y., Liu Y.L., Witschi M., Ardoin A., Calba C. Early introduction of severe acute respiratory syndrome coronavirus 2 into Europe. Emerg Infect Dis. 2020;26(7) doi: 10.3201/eid2607.200359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taiwan Centers for Disease Control . 2020. Name-based rationing system for purchases of masks to be launched on February 6; public to buy masks with their (NHI) cards.https://www.cdc.gov.tw/En/Bulletin/Detail/ZlJrIunqRjM49LIBn8p6eA?typeid=158 [Accessed June 15th 2020] [Google Scholar]
- 23.Taiwan Centers for Disease Control . 2020. CECC issues Guidelines for Large-Scale Public Gatherings to prevent widespread community transmissions.https://www.cdc.gov.tw/En/Bulletin/Detail/yAhL46r86lz1uIi4r2DqSQ?typeid=158 [Google Scholar]
- 24.Taiwan Centers for Disease Control . 2020. CECC announces social distancing measures for COVID-19.https://www.cdc.gov.tw/En/Bulletin/Detail/kM0jm-IqLwNBeT6chKk_wg?typeid=158 [Google Scholar]
- 25.Taiwan Centers for Disease Control . 2020. 2019-nCoV Taiwan.https://sites.google.com/cdc.gov.tw/2019-ncov/taiwan [Google Scholar]