Abstract
Sensing of pathogens by specialized receptors is the hallmark of the innate immunity. Innate immune response also mounts a defense response against various allergens and pollutants including particulate matter present in the atmosphere. Air pollution has been included as the top threat to global health declared by WHO which aims to cover more than three billion people against health emergencies from 2019 to 2023. Particulate matter (PM), one of the major components of air pollution, is a significant risk factor for many human diseases and its adverse effects include morbidity and premature deaths throughout the world. Several clinical and epidemiological studies have identified a key link between the PM existence and the prevalence of respiratory and inflammatory disorders. However, the underlying molecular mechanism is not well understood. Here, we investigated the influence of air pollutant, PM10 (particles with aerodynamic diameter less than 10 μm) during RNA virus infections using Highly Pathogenic Avian Influenza (HPAI) – H5N1 virus. We thus characterized the transcriptomic profile of lung epithelial cell line, A549 treated with PM10 prior to H5N1infection, which is known to cause severe lung damage and respiratory disease. We found that PM10 enhances vulnerability (by cellular damage) and regulates virus infectivity to enhance overall pathogenic burden in the lung cells. Additionally, the transcriptomic profile highlights the connection of host factors related to various metabolic pathways and immune responses which were dysregulated during virus infection. Collectively, our findings suggest a strong link between the prevalence of respiratory illness and its association with the air quality.
Keywords: Air pollution, PM10, Viral infection, Anti-viral innate immunity, Metabolic pathways genes
Graphical abstract
Highlights
-
•
Air pollution skews innate immunity during RNA virus (Influenza virus) infection.
-
•
Particulate matter (PM10) suppresses anti-viral innate immunity.
-
•
PM10 enhances Influenza virus replication via metabolic pathway genes modulation.
-
•
PM10 enhances the severity of respiratory tract viral infection.
Particulate matter (PM10) enhances severity of influenza virus infection through skewing innate immunity via modulation of metabolic pathways-related genes.
1. Introduction
Alarming rate of air pollution has been shown to be linked with enhanced morbidity and subsequent mortality rate which affected the global health and economy (Anderson et al., 2012; Cohen et al., 2017; Katsouyanni et al., 2009; Krewski et al., 2009; Landrigan et al., 2018; Lelieveld et al., 2015; Liu et al., 2019a; Romieu et al., 2012; Wong et al., 2008). One of the major components of air pollution is the particulate matter (PM) which are a complex mixture of particles of varied sizes and physiochemical properties, derived from both the natural and anthropogenic sources. Pollution by ambient PM has been reported to cause 2.9 million deaths and 69.7 million disability-adjusted life-years in 2013, according to a systematic analysis for the Global Burden of Disease Study (Collaborators et al., 2015). These particles are divided into three main categories on the basis of their diameter: coarse particles, or PM10, (with an aerodynamic diameter between 10 and 2.5 μm); fine particles, or PM2.5, (with diameters <2.5 μm); and ultrafine particles, or PM0.1, (with diameters <0.1 μm) (Deng et al., 2019; Idani et al., 2018).
PM10 is generally deposited in the upper respiratory tract and found to be linked with many diseases other than respiratory damage like cardiovascular diseases (Dastoorpoor et al., 2020; Dastoorpoor et al., 2019; Momtazan et al., 2019), various types of cancers (Chu et al., 2019; Consonni et al., 2018; Hamra et al., 2014; Li and Gao, 2014; Reyes-Zarate et al., 2016), tuberculosis (Rivas-Santiago et al., 2015; Sarkar et al., 2019), Asthma (Donaldson et al., 2000; Saygin et al., 2017; Tecer et al., 2008). A large cohort epidemiology study of 39,054 participants showed that an increase in 10 μg/m3 PM10 concentration is associated with the increase in 3.4%–6% of lung cancer mortality (Chen et al., 2016).
Numerous respiratory risk studies have shown the strong association of the PM with increased morbidity and mortality (Chen et al., 2017b; Dai et al., 2014; Daryanoosh et al., 2018; Goudarzi et al., 2018; Harrison and Yin, 2000; Idani et al., 2018; India State-Level Disease Burden Initiative Air Pollution, 2019; Landrigan et al., 2018; Lu et al., 2015; Samet et al., 2000; Valavanidis et al., 2008; Carugno et al., 2016; Lin et al., 2005). PM is readily associated with respiratory infections such as chronic obstructive pulmonary disease (COPD) (Khaefi et al., 2017; Ling and van Eeden, 2009; MacNee and Donaldson, 2003; Ni et al., 2015; Wen and Gao, 2018). The short-term exposure to PM including PM10 and PM2.5 was shown to be linked with disease which was demonstrated from a large-scale modelling study comprising the data from 652 cities across the globe (Liu et al., 2019a). Meanwhile, reduction in the PM10 levels as recommended by World Health Organisation (WHO) can increase the health benefits (Marzouni et al., 2017) and may decrease the mortality by 8%–9% in highly polluted cities (Yorifuji et al., 2015). WHO has recommended the standard permissible level of air contaminants but nearly 80% of the urban cities are well above the standard permissible level to cause severe health problems.
India recorded the highest increase in the air pollution related death among the most populated countries in 2015 compared to 1990, according to Lancet commission report (Landrigan et al., 2018). Annual PM concentration in most of the Indian cities were higher than the National Ambient Air Quality Standards of India. Several studies have attempted to understand the link between isolated PM from the heavily populated regions of India and associated health concerns in term of occurrence of disease (Jain et al., 2017; Khafaie et al., 2017; Manojkumar and Srimuruganandam, 2019; Sharma et al., 2018; Sharma et al., 2004; Sharma et al., 1998). However, there were very few studies which aim to explore the mechanistic action of PM, collected from Indian subcontinent.
PM has been shown to induce innate immune responses by the production of pro-inflammatory cytokines through activation of toll-like receptor signalling pathway (Bauer et al., 2012; Becker et al., 2002). It has been known that PM can modulate the level of cytokines, upon its exposure to the humans airways (Bengalli et al., 2013; Hirota et al., 2015; Tang et al., 2019). PM were also reported to be associated with the virus infections such as Respiratory Syncytial Virus (RSV) (Karr et al., 2009; Vandini et al., 2013), Influenza virus, Dengue virus (Carneiro et al., 2017), Measles virus (Chen et al., 2017a; Peng et al., 2020) and Rhinovirus (Proud et al., 2012). PM concentration has been shown to be linked with the increased number of infection rates in most of the virus-related outbreaks in the past two decades caused by RNA viruses, such as Severe Acute Respiratory Syndrome (SARS) – CoV in 2003 (Cui et al., 2003), Dengue outbreak (Massad et al., 2010), Swine flu H1N1 (Influenza) pandemic in 2009 (Morales et al., 2017), H5N2 Influenza outbreak in United States (US), (Zhao et al., 2019), Measles outbreak in 2019 (Peng et al., 2020) and the recent ongoing COVID-19 outbreak (Conticini et al., 2020; Dutheil et al., 2020b; Fattorini and Regoli, 2020; Sciomer et al., 2020; Setti et al., 2020a; Zhu et al., 2020). Many studies, based on the epidemiological and modelling suggest that PM caused health severity both in the urban and remote locations (Cohen et al., 2017; Idani et al., 2018; Karambelas et al., 2018; Landrigan et al., 2018). However, air pollutant-mediated lung infections and respiratory damage are poorly understood. Therefore, our study aims to investigate the PM-based modulation of innate immune responses which may shed light on the cause of severity and mortality during respiratory infections in virus outbreaks.
In this study, we hypothesize that PM10 modulates the innate immune system upon virus infection and promotes viral replication. To support this hypothesis, we aimed to observe the effect of PM10 during different RNA virus infections, including H5N1 flu virus, in the human lung epithelial cell line, A549 and performed the RNA-Sequencing to determine the pathways which were dysregulated by PM10 upon virus infection. Our results demonstrated that PM10 modulates the innate immune system associated pathway upon viral infection and significantly enhanced the viral replication of the RNA viruses like New-Castle Disease Virus (NDV), Influenza virus – H1N1 (PR8) and H5N1, via downregulation of innate immune responses and upregulation of several metabolic pathways-related genes, which collectively facilitates the infectivity of virus to cause enhanced respiratory illness.
2. Methods
2.1. Cell culture experiments
A549 human alveolar basal epithelial cells (Cell Repository, NCCS, India) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% Antibiotic-Antimycotic solution. DMEM, FBS and Antibiotic-Antimycotic solution were purchased from Invitrogen. Ambient particulate matter (PM) of coarse particle size PM10 was obtained from Dr. Gangamma S. laboratory which was collected and isolated from the selected monitoring stations geographically distributed across Bengaluru city, at NITK, Surathkal, Mangaluru, Karnataka. The A549 cells were seeded in 12 well culture plate at a concentration of 3 × 105/well overnight (37 °C, 5% CO2). Cells were treated with PM10 along with controls namely blank and/or LPS (100 ng) for 24 h prior to infection. Plasmids containing Firefly Luciferase gene under IFNβ (type- I interferon beta) and ISRE (interferon-sensitive response element) promoters, were obtained from Professor Shizuo Akira’s (Osaka University, Japan). All short hairpin (sh)- clones, were obtained from the whole RNAi human library for shRNA mediating silencing (Sigma, Aldrich) maintained at IISER, Bhopal, India.
2.2. Virus infection
Airborne PM10 treated A549 cells were infected with New-castle Disease Virus (NDV) – LaSota strain, Highly Pathogenic Avian Influenza virus (H5N1) - A/duck/India/02CA10/2011 strain and vaccine strain PR8 virus (H1N1) – A/PR8/H1N1 virus was generated using the eight plasmid reverse genetics system (Kumar et al., 2018), at respective multiplicity of infection as mentioned in the figures and/or figure legends. PM10 treated A549 cells were washed by 1X PBS (phosphate-buffered saline) solution and infected with appropriate RNA viruses in serum-free media as per the subsequent experiment then after 60 min, virus containing media was removed from the cells and cells were washed once with 1X PBS solution. Then cells were again supplemented with new PM10 containing DMEM media for 24 h. Samples were then harvested and forwarded for respective quantitative analysis.
2.3. Airborne particulate matter sampling method
Bengaluru is an inland city (12°58′ N, 77°34′) situated on the south-central part of India at a height over 900m above sea level. General sources of airborne PM in the city include vehicular emissions, industrial emissions and re-suspended road dust (ARAI, 2018; CPCB, 2020). Air samples were collected from six ambient air quality monitoring sites of Karnataka State Pollution Control Board (KSPCB). PM with aerodynamic diameter less than 10 μm was collected using high volume samplers (Poll tech, India). The time duration of sampling corresponds to 8 h. The samples were collected on quartz fibre filter paper (GE healthcare, India). The filter papers were de-pyrogenated and conditioned prior to sampling (Gangamma et al., 2011). To ensure contamination free sampling, field blanks were included in the samples. After sampling, filter papers were sealed in de-pyrogenated aluminium foil and transported to the laboratory. The samples were stored at −20 °C until further processing. PM on the filter was extracted into methanol. Further, methanol was purged and samples were reconstituted with dimethyl sulfoxide (DMSO) (Bach et al., 2015; Totlandsdal et al., 2014). Samples were pooled and used for further experiments.
2.4. Particulate matter (PM10) dose standardization
For all the preliminary experiments three different dosage form of PM10 was used in the ratios 1:1 (PM10: DMEM) – (50% of PM10 in DMEM), 0.25:1 (PM10: DMEM) - (25% of PM10 in DMEM) and 0.10:1 (PM10: DMEM) - (10% of PM10 in DMEM) named as PM(I), PM(II) and PM(III) respectively. And after the standardization through different experiments PM(I), that is 1:1 (PM10: DMEM) dosage of PM10 was used for subsequent experiments, labelled and/or described as PM.
2.5. Characterization of particulate matter (PM10) by scanning electron microscopy – Energy dispersive X-ray spectrometer (SEM-EDS) analysis
PM10 dissolved in appropriate solvents was installed on the metallic stabs in the form of droplets and dried overnight in the desiccator for complete solvent dry process. Samples were then loaded on the high-resolution field emission scanning electron microscope (SEM) (HR FESEM) from Zeiss, model name ULTRA Plus at IISER Bhopal for PM10 morphological analysis. Then chemical composition of the PM10 was elucidated by the Energy Dispersive X-ray Spectrometer (EDS) component of the scanning electron microscope.
2.6. Quantitative real-time reverse transcription PCR – qRT-PCR analysis
Total RNA was extracted from the three and/or two independent experiments as mentioned in the figure legends with the Trizol reagent (Ambion/Invitrogen) and used to synthesize cDNA with the iScript cDNA Synthesis Kit (BioRad, Hercules, CA, USA) according to the manufacturer’s protocol. Gene expression was measured by quantitative real-time polymerase chain reaction (PCR) using gene-specific primers and SYBR Green (Biorad, Hercules, CA, USA). cDNA obtained from each experiment was analysed in triplicate. Ct (cycle threshold) values – that determines the abundance and expression of the transcript within the cells was obtained for calculation, to determine the fold change or relative expression of a particular transcript. Ct levels are inversely proportional to the amount of target transcript in the sample (i.e. the lower the Ct level the greater is the amount of target transcript in the sample. The formula and concept on which complete calculation of obtaining fold change/relative expression is based, defines as the difference in the Ct values (ΔCt) for selected genes/transcripts, among the treated (PM10 and virus infected) and non-treated (control/mock) group, calculated by: 2˄-ΔΔCt as the final fold change. The 18S gene was used as a reference control for normalization of Ct (critical threshold) values, obtained after qRT-pCR (of 40 cycles each), of the genes to determine the fold change of the selected gene/mRNA transcript in the treated group with respect to the control/mock group of the experiment. Real time quantification was done using StepOne Plus Real time PCR Systems by Applied BioSystems (Foster City, CA, USA).
2.7. Promoter activity estimation by luciferase reporter assay
A549 cells (5 × 104) were seeded into a 12-well plate and transiently transfected with 50 ng of the transfection control pRL-TK plasmid (Renilla luciferase containing thymidine kinase promoter plasmid) and 200 ng of the luciferase reporter plasmid (Firefly luciferase containing plasmid) of IFNβ and ISRE promoters. After 18 h cells were treated with PM10 at the different dosage as explained in the PM10 dose standardization method section (PM(I), PM(II) and PM(III)) and blank as a control for 24 h. Then after cells were infected with NDV (MOI 2) for 24 h. The cells were lysed at 24 h after final infection, and finally the luciferase activity in total cell lysates was measured with Glomax (Promega, Madison, WI, USA).
2.8. Protein estimation of cytokines by enzyme-linked immunosorbent assay (ELISA)
A549 cells were treated with PM10 at the different dosage as explained in the PM10 dose standardization method section (PM(I), PM(II) and PM(III) and blank as a control after 24 h of seeding. The culture media were harvested at 36 h after PM10 treatment and were analysed by specific ELISA kits (Becton Dickinson) according to the manufacturer’s instructions to determine the amounts of IL6 that were secreted by the cells.
2.9. Cell counting by trypan blue assay to estimate dead cells
A549 cells were seeded and after 24 h treated with PM10 and blank for 24 h before NDV infection. Cell supernatant were collected after 36 h of infection, mixed with trypan blue dye (Sigma) in the ratio 1:1. The mixture then used for counting the dead cells under the microscope.
2.10. Viral quantification of GFP signals by microscopy
A549 cells were seeded along with cover slips in low confluency and next day treated with PM10 at a dosage of 1:1 [PM: DMEM] for 24 h prior to virus infection. Cells were then infected with NDV-GFP (3 MOI – multiplicity of infection 3) in serum free media for 1 h. After infection cells were again supplemented with complete media and treated with PM10 at a dosage of 1:1(PM10: DMEM) for 24 h at 37 °C, 5% CO2. Cells were then washed twice with PBS for 5 min and fixed in 4% PFA (paraformaldehyde) for 20 min again washed in PBS and incubated with DAPI (20 mg/ml) for 30 min at room temperature and finally washed thrice with PBS. Cover slips then containing cells were carefully mounted on to the glass slides using Fluoroshield (Sigma) as mounting media. Slide was then kept for few hours for drying before imaging. Images were visualised at 40X with Apotome – AXIO fluorescence microscope by Zeiss. Image analysis (intensity of GFP and GPF positive cells) was done by using Image J software (Fiji).
2.11. Next generation sequencing (NGS) analysis for transcriptomic profiling
Total RNA was extracted using TRIzol reagent (Ambion/Invitrogen) and assessed for quality. The RNA-Seq paired end libraries were prepared from the QC passed RNA samples using Illumina Trueseq stranded mRNA sample prep kit. Libraries were sequenced using NextSeq500 with a read length (2 × 75 bp), by Eurofins Genomic India Private Limited, India. The Raw reads were assessed for quality using FastQC (Andrews et al., 2010). The filtering of reads and the removal of adapters were performed using the tool Trimmomatic (Bolger et al., 2014). Approximately 18 million base pair reads were mapped to the human transcriptome (hg38), using Kallisto (Bray et al., 2016) and the abundance of the assembled coding transcriptome were projected as transcripts per million (TPM). The transcripts level abundance counts were converted into gene-level abundance counts using the R package, Tximport (Soneson et al., 2015). Differential expression analysis was performed using Limma package (Ritchie et al., 2015) implemented in Biojupies tool (Torre et al., 2018). The genes which were differentially expressed (−1.5 < Log FC < 1.5) were selected and the gene ontology analysis were performed using DAVID tool (Huang et al., 2007). Bubble plots, circle plot, chord plots were generated from the gene ontology and pathway enrichment results generated by DAVID tool, using the R package GOplot (Walter et al., 2015).
Data availability: The NGS (RNA-Sequencing) data for expression profiling reported in this paper have been deposited in the GenBank database (accession no. GSE147227).
2.12. Statistical analysis
All experiments were carried out along with the appropriate controls, indicated as untreated/uninfected cells (Control) or treated/infected with the appropriate reagent/virus infection alone (Mock). Experiments were performed in duplicates or triplicates for at least two or three times independently. GraphPad Prism 8.0 (GraphPad Software, La Jolla, CA, USA) was used for statistical analysis. The differences between two groups were compared by using an unpaired two-tailed Student’s t-test. While the differences between three groups or more were compared by using analysis of variance (ANOVA) with Tukey test. Differences were considered to be statistically significant when p < 0.05. Statistical significance in the figures is indicated as follows: ∗∗∗ p < 0.001, ∗∗ p < 0.01, ∗ p < 0.05; ns, not significant.
3. Results
3.1. Exposure of PM10 reduces innate immunity upon RNA virus infection
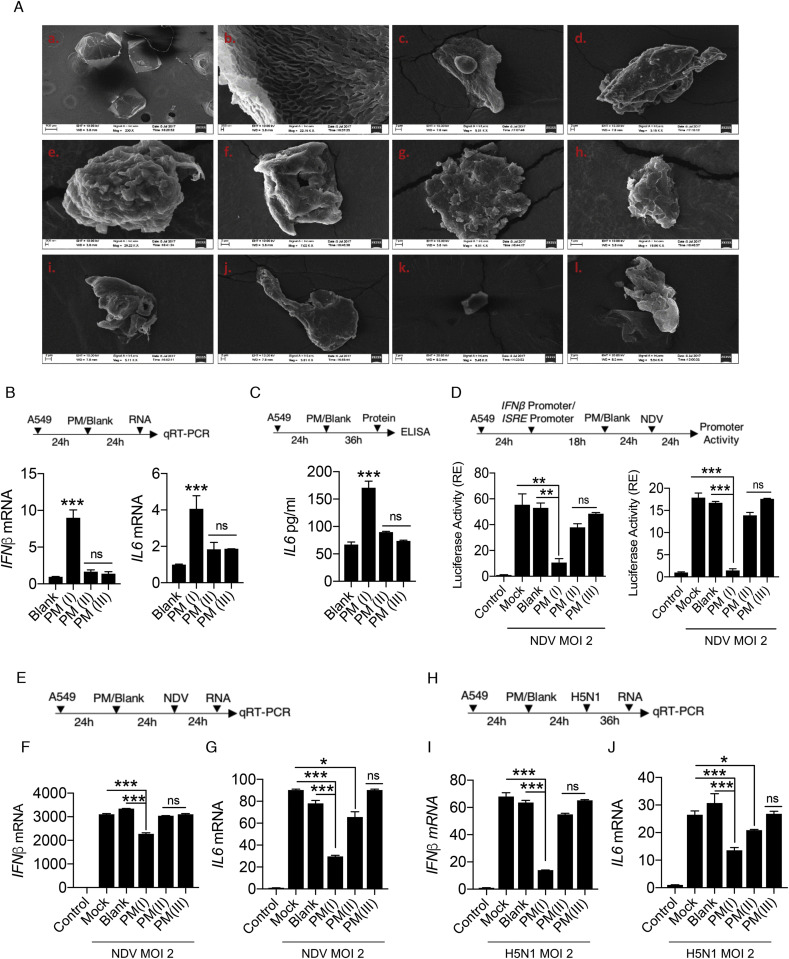
As reported previously, PM or similar substances like smog, diesel exhaust,cigarette smoke extract causes activation of the inflammatory responses when comes in contact with host’s airways and lungs (Oeder et al., 2012; Proud et al., 2012). To understand the effect of PM10 on host cells, we initially characterized the coarse size particulate matter (PM10), that were collected and used in the study. We performed SEM-EDS (scanning electron microscopy – energy dispersive X-ray spectrometer) analysis of PM10 collected from Bengaluru city, India and concluded that various shapes were embedded in the PM10. We found different characteristic morphological features within the PM10 (Fig. 1 A), consists of biologically active shapes like air ash, spherical, irregular, well-defined, aggregates and rounded. We additionally, performed energy dispersive spectroscopy (EDS) analysis and got different peaks in the spectrum obtained upon analysing the sample at different points with the pulse of electrons (Fig. S1A). The peaks in the spectra correspond to the presence of different elements including both metals and non-metals (% by weight) in the PM10 (Fig. S1B) which are hazardous and have potency to cause lung damage. Therefore, characterization of PM10 prompted us to examine whether PM10 can induce any innate immune responses in human lung epithelial carcinoma cells, A549. Interestingly, we have found that when cells were exposed to PM (I), where (I) is the dose, which corresponds to equal volume of PM10 and DMEM media (50% PM10 in DMEM media) composition; the type I interferon, IFNβ (Fig. 1B) and inflammatory cytokine, interleukin 6 - IL6 (Fig. 1C) were induced moderately as compared to the other dosage forms such as PM(II) and PM(III), where (II) represents 25% of PM10 composition in DMEM media and (III) represents 10% of PM10 composition in DMEM media respectively. Furthermore, we performed IFNβ and ISRE promoter assay after infection with NDV in presence of PM10 and found that there was significant reduction in the promoter activities at the dosage of PM10 (I) compared to other dosage of PM10; PM(II) and PM(III) respectively (Fig. 1D), suggesting that though PM10 induces innate immune responses alone but in presence of virus infection that induction compromised due to combined effect of PM10 exposure and NDV infection. Additionally, we concluded that in different set of experiments dual treatment of PM10 and virus infection (NDV) to A549 cells as shown in schematic representation (Fig. 1E) reduces the mRNA transcript levels of interferon IFNβ and cytokine IL6 (Fig. 1F and G). These findings further prompted us to investigate whether the currently characterized PM10 is associated with any respiratory diseases because majority of infectious respiratory diseases are mainly caused by RNA viruses.
Fig. 1.
PM10regulates the innate immune response upon RNA virus infection – (A) Scanning electron images of coarse airborne particulate matter PM10. (a) Image of blank (control) solution with no PM10 dissolved in it. (b–l) Images of different shapes with varied structures representing the different characteristic morphological features of PM10 in the samples. Quantification of innate immune response. A549 cells were treated with PM10 (at different dosage: PM(I), PM(II), and PM(III) – details are mentioned in the methods section) and control mentioned as blank for (B) 24 h then harvested in Trizol to quantify the mRNA expression of IFNβ and IL6 by qRT-PCR. (C) 36 h then cell supernatant was collected to measure the protein level of IL6 by ELISA. (D) Schematic representation of workflow for quantification of IFNβ and ISRE promoter activities by luciferase assay as indicated in A549 cells. NDV represents New-Castle Disease Virus infection at MOI = 2. (E) Schematic work flow of PM10 exposure (at different dosage: PM(I), PM(II), and PM(III) – details are mentioned in the methods section) and NDV infection. (F–G) Quantification of IFNβ and IL6 mRNA transcripts in uninfected (control), mock infected, blank treated and PM10 exposed cells by qRT-PCR. (H) Schematic work flow of PM10 (at different dosage: PM(I), PM(II), and PM(III) – details are mentioned in the methods section) exposure and H5N1 Influenza infection. (I–J) Quantification of IFNβ and IL6 mRNA transcripts in uninfected (control), mock infected, blank treated and PM10 exposed cells by qRT-PCR. Data are mean ± SEM of triplicate samples from single experiment and are representative of three independent experiments. ∗∗∗p < 0.001, ∗∗p < 0.01 and ∗p < 0.05 by one-way ANOVA Tukey test and unpaired t-test.
Previously, it has been shown that cigarette smoke extract (CSE) affects various regulatory pathways during Rhinovirus (RV) infection using human bronchial cell lines by microarray analysis (Proud et al., 2012). We re-analyse the microarray-based gene expression omnibus (GEO) dataset: GSE27973 in context to our prospective and found that there are several important cellular machineries associated genes (Fig. S2A) were modulated due to CSE exposure and RV infection. We next analysed the regulation of important genes involved in diseases particularly Influenza (flu) virus infection and key immune signalling pathways (Figs. S2B–D). Gene profile analysis concluded that various antiviral genes were prominently downregulated upon CSE exposure and RV infection. Here, in current study we used Influenza virus infection along with PM10 treatment in the A549 cells, because Influenza virus infection is severely fatal compared to any other virus that causes respiratory damage and Influenza virus is regularly active upon the evolutionary scale and regarded as one of the hazardous threats according to WHO to humans. Therefore, to get insights about PM10 exposure and Highly Pathogenic Avian Influenza infection (HPAI), we treated the A549 cells with PM10 and infected them HPAI H5N1 (MOI 2 – multiplicity of infection 2) as shown in schematic representation (Fig. 1H). We observed that the dose PM(I) of PM10 significantly reduces the mRNA expression levels of both IFNβ and IL6 in presence of H5N1 infection (Fig. 1I and J), compared to other dosage of PM10 (PM(II) and PM(II)) and control groups, indicating that during pathogenic infection by RNA viruses, particularly Influenza virus, PM10 reduces the innate immune response in the cells.
3.2. PM10 enhances viral replication upon RNA virus infection
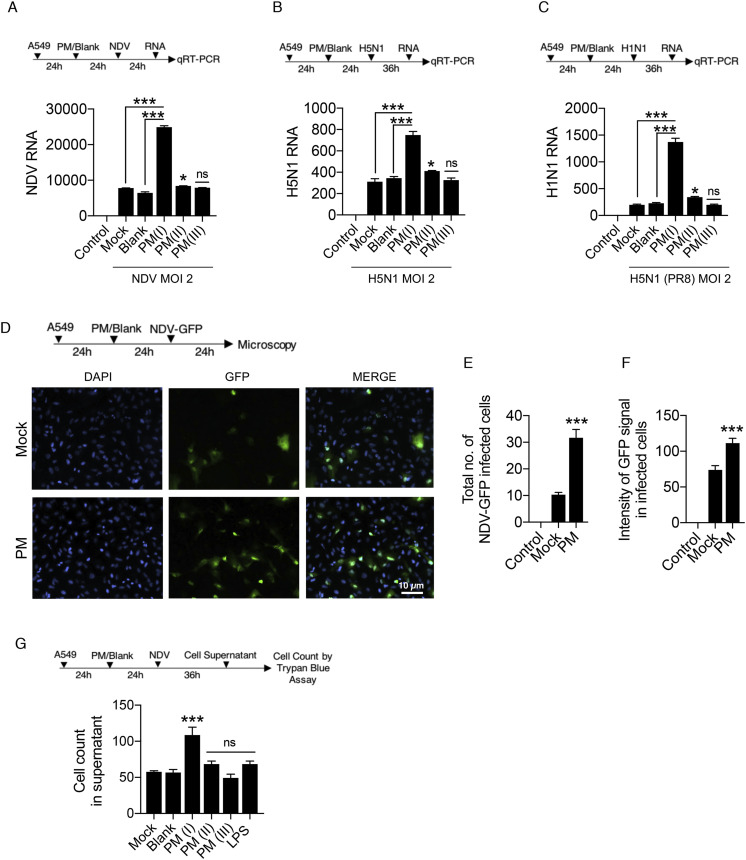
Curtailed immune responses upon PM10 treatment and virus infections: both in case of NDV and H5N1 Influenza virus infections, prompted us to measure the viral load in presence of PM10. We thus demonstrated the experiment of PM10 exposure and virus infection like NDV, H1N1 (PR8) and H5N1 in A459 cells respectively. Using virus-specific primer, it was observed that PM10 significantly enhances the viral replication of all the RNA viruses ubiquitously in its optimal concentration – PM(I) compared to other concentration (PM(II) and PM(II)) and control groups. PM10 enhances the virus replication of NDV (Fig. 2 A), H5N1 (Fig. 2B) and H1N1 (Fig. 2C). Additionally, microscopy analysis demonstrates similar results in which GFP (green fluorescent protein) tagged NDV was used to infect the PM10 pre-exposed cells (Fig. 2D). Increased NDV infection was quantified by measuring the intensity of GFP signal and number of GFP positive cells (Fig. 2E and F). Furthermore, presence of PM10 along with NDV infection induces the cell death as an additional detrimental effect on cells, quantified by the trypan blue assay (Fig. 2G). Altogether, our results conclude that PM10 enhances the viral replication pertaining to lower immune responses.
Fig. 2.
PM10elevates the RNA virus infection – (A–F) Estimation of viral replication in A549 cells exposed with PM10 for 24 h before virus infection at MOI = 2. (A) Schematic work flow of the experiment, PM10 exposure (at different dosage: PM(I), PM(II), and PM(III) – details are mentioned in the methods section; PM10 dose standardization) enhances the NDV abundance (viral transcripts) in the cells compared to the control groups (uninfected control, mock infected, and blank treated cells respectively). (B–C) Schematic work flow of the experiment, PM10 exposure (at different dosage: PM(I), PM(II), and PM(III) – details are mentioned in the methods section; PM10 dose standardization) enhances the H5N1 and H1N1 abundance (viral transcripts) in the cells compared to the control groups (uninfected control, mock infected, and blank treated cells respectively). (D) Schematic work flow for microscopy: A549 cells were exposed with PM10 (labelled as PM; corresponds to PM(I) dosage form - details are mentioned in the methods section; PM10 dose standardization) then after infected with GFP – labelled NDV for 24 h, cells on the cover slips were then fixed (as per the protocol described in methods section) and estimated for the GFP positive signals, quantified as (E) total number of NDV-GFP infected cells and (F) intensity of GFP signals in the infected cells. (G) Schematic work flow to estimate the cell death in cell supernatant after PM10 exposure (at different dosage: PM(I), PM(II), and PM(III) – details are mentioned in the methods section; PM10 dose standardization) and NDV infection in A549 cells. Cells (dead) were counted by the trypan blue counting assay. Data are mean ± SEM of triplicate samples from single experiment and are representative of three independent experiments. ∗∗∗p < 0.001 by one-way ANOVA Tukey test and unpaired t-test. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
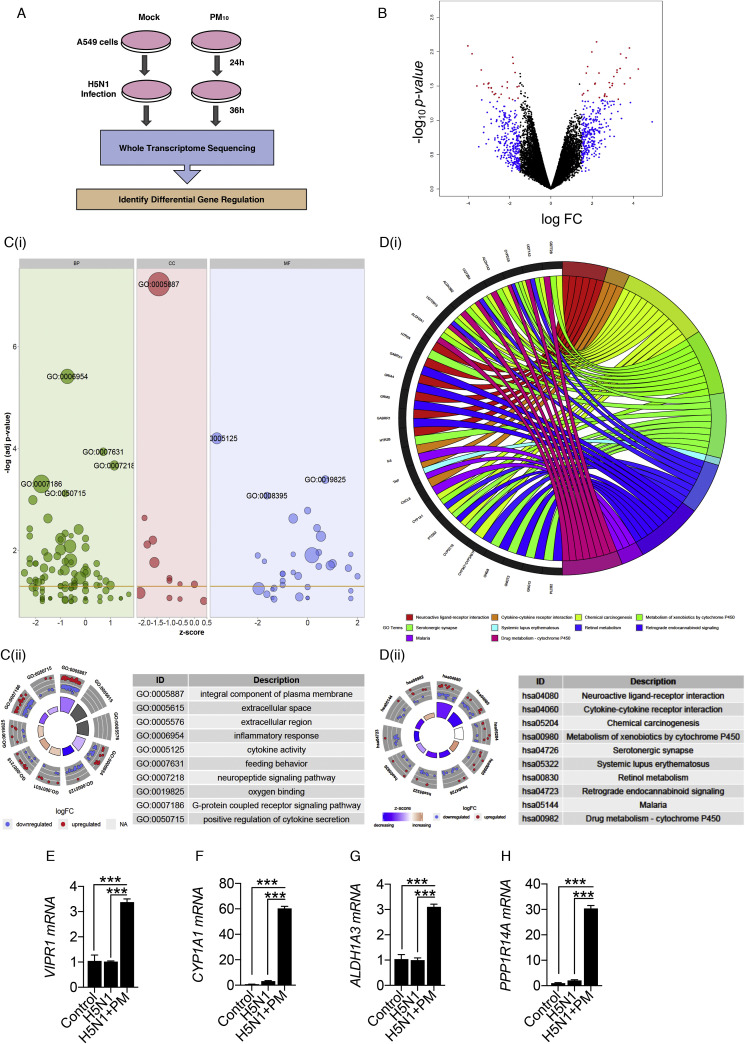
3.3. RNA-seq analysis of H5N1 infected cells in presence of PM10
PM10 enhances the viral replication and suppress the immune responses. To further understand the global outcome of immune responses within the human cell and to dissect the mechanism about the current physiological effect, we performed RNA sequencing to profile the overall changes in the host genes and cellular pathways upon PM10 treatment and HPAI H5N1 infection. Schematic workflow of the experiment and transcriptomic sequencing shown in Fig. 3 A. Differential expression of host genes analysis was performed between PM10-treated_H5N1-infected and subsequently mock-treated_H5N1-infected samples. After aligning raw RNA-sequence reads to the human transcriptome using Kallisto algorithm, the read counts of each genes were estimated and differential expression was performed using Limma package by using Biojupies tool as shown in the methods. The differential expression of genes analysis were visualised through the volcano plot (Fig. 3B), which is in general a scatter plot, plotted between the statistical significance of genes and the magnitude of change in gene expression. In the volcano plot, the genes which were upregulated are scattered towards the right of the x-axis and the genes which were downregulated are scattered towards the left of the x-axis. The genes which were statistically significant and altered more than 1.5 fold change were plotted in red colour and the genes which were altered more than 1.5 fold change between the two experimental groups (PM10-treated_H5N1-infected and mock-treated_H5N1-infected) were plotted in blue colour. And the remaining genes which were altered less than 1.5 fold were plotted in black colour. In principal, this could be noted that the enhanced viral replication, in presence of PM10 might be the result of downregulation of innate immune genes and simultaneous upregulation of other cellular factors, particularly metabolic pathways-related factors, namely, VIPR1, CYP1A1, AlDH1A3 and PPP1R14A. Moreover, gene ontology analysis performed by using the DAVID tool revealed the enriched biological processes (BP), cellular components (CC) and molecular functions (MF) for the genes which were altered more than 1.5 fold change between the experimental groups. The results were visualised through the bubble plot (Fig. 3C(i)). The Z-score obtained from the database for annotation, visualization and integrated discovery (DAVID) tool output was assigned to x-axis and the negative logarithm of the p-value was assigned at the y-axis in the bubble plot. The area of the circles in the bubble plot corresponds to the number of genes assigned to the particular ontology term and the colour corresponds to the gene ontology categories-green, pink and blue for biological process (BP), cellular components (CC) and molecular function (MF) respectively. The threshold of labelling was set to 3, based on the negative logarithm of the p-value. Inflammatory response (GO:0006954), integral component of plasma membrane (GO:0005887) and cytokine activity (GO:0005125) were the top enriched terms in the gene ontology categories; BP, CC and MF respectively. Inflammatory response and cytokine activity were known to be associated with Influenza infection as well as PM10 treatment in A549 cells. Circle plot was used to visualize the top 10 enriched ontology terms in which the outer ring shows the scatter plotting of log FC values of each genes in the ontology term, in which red circles signifies the upregulated genes and the blue circles signifies the down-regulated genes within each terms. The inner ring shows the bar plot where the size of the bar represents the significance of terms and the colour corresponds to the Z-score of the associated term (Fig. 3C(ii)). Additionally, the chord plot represents the connection of common significant differentially expressed genes with the significant enriched ontology terms (Fig. S3A). Pathway analysis was performed using the DAVID tool and top enriched pathways of differentially expressed genes with −1.5 < log FC < 1.5 were represented by the chord plot (Fig. 3D(i)) depicting the network between significant differentially expressed genes and their enriched pathways. Additionally, circle plot depicts the connection of top enriched pathways with the status of the genes contributing to the pathway represented by their log FC and Z-score (Fig. 3D(ii)).The Pathway enrichment analysis revealed neuroactive ligand-receptor interaction (hsa04080), cytokine-cytokine receptor interaction (hsa04060), chemical carcinogenesis (hsa05204) as the top enriched pathways between the experimental groups.Gene ontology analysis and pathway enrichment analysis revealed that significantly down-regulated genes during H5N1 infection in presence of PM10 were involved majorly in various immune signalling pathways and innate immune responses, in accordance with our experimentally validated results. On contrary, comprehensive analysis revealed that significantly up-regulated genes were majorly involved in various metabolic pathways. Furthermore, representative of up-regulated genes from significantly regulated metabolic pathways were validated by qRT-PCR analysis and found the enhanced mRNA expression levels of VIPR1 (Vasoactive Intestinal Polypeptide Receptor 1), CYP1A1 (Cytochrome P450 Family 1 Subfamily A Member 1), AlDH1A3 (Aldehyde Dehydrogenase Family Member A3) and PPP1R14A (Protein Phosphatase 1 Regulatory Inhibitor Subunit 14A) genes upon H5N1-infection in A549 cells in presence of PM10 (Fig. 3E–H). Similar results were obtained in NDV-infected A549 cells in presence of PM10 (Figs. S3B–E). Related results were obtained by re-analysing the GEO dataset GSE27973 of Rhinovirus infection and CSE exposure in human bronchial epithelial cell lines (Figs. S4A–B). Additionally, theses metabolic pathways-related genes were found to be associated with many pathological states (Fig. S4C). Overall our data concludes that upon PM10 treatment during RNA virus infection, particularly, Influenza virus infection, PM10 significantly enhances the virus infection by down-regulating innate immune responses and upregulating different metabolic processes, that might potentiate air pollutant to enhance virus infectivity within the cells and causes the respiratory damage.
Fig. 3.
Transcriptomic analysis shows PM10enhances abundance of metabolic pathways-related transcripts (genes) during H5N1 infection – (A) Schematic outline of PM10 exposure and H5N1 infection (MOI 2) in A549 cells at indicated time. Cells were subjected to whole transcriptome sequencing and differential gene expression analysis. (B) Volcano plot represents differential expression of genes between two groups of samples (mock H5N1 infected and PM10 exposed plus H5N1 infected) during H5N1 infection in A549 cells. For each gene: p-value is plotted against fold change (mock vs PM10). Significantly differentially expressed genes are marked in red colour while genes which are altered (>1.5-fold) are marked in blue colour. (C) Gene Ontology analysis performed as per the protocol mentioned in methods section represents the top differentially expressed genes in ontology terms: BP (biological processes), CC (cellular components) and MF (molecular functions) respectively depicted by bubble plot and circle plot generated through R package GOPlot. (D) Pathway enrichment analysis performed as per the protocol mentioned in methods section. Chord plot represents the differentially expressed genes and their connection with the top enriched pathways. Circle plot represents the top enriched pathways and status of the genes contributing to the pathways by their log FC and Z-score. (E–H) Quantification (measured by qRT-PCR) and validation of the fold changes in the abundances of significantly expressed metabolic pathways related transcripts: VIPR1, CYP1A1, ALDH1A3 and PPP1R14A in the samples of A549 cells; untreated (control), mock H5N1 infected (H5N1) and PM10 exposed (labelled as PM; corresponds to PM(I) dosage form - details are mentioned in the methods section) plus H5N1 infected (H5N1+PM), analysed by RNA- Sequencing. For figure (E–H): Data are mean ± SEM of triplicate samples from single experiment and are representative of two independent experiments. ∗∗∗p < 0.001 and ∗∗p < 0.01 by one-way ANOVA Tukey test and unpaired t-test. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.4. Knockdown of metabolism-associated genes involved in virus replication
To investigate the correlation between the upregulated metabolic pathways-related genes and their influence on virus infection upon PM10 treatment, we selected CYP1A1, VIPR1 and PPP1R14A genes because these genes were significantly upregulated in our RNA sequencing analysis and their roles are poorly understood. The CYP1A1 involved in xenobiotic metabolic pathways, which is one of the metabolic pathways in aiding virus infections, VIPR1 is associated with G-protein coupled receptor pathway and PPP1R14A involved in vascular smooth muscle contraction and oxytocin pathway which were directly or indirectly related to virus infectivity within the host cell. To this end, we performed knockdown study of CYP1A1, VIPR1 and PPP1R14A in A549 cells. We used two different short hairpin (sh)-clones for each gene to knockdown the expression of CYP1A1, VIPR1 and PPP1R14A genes respectively as shown in the schematic workflow (Fig. 4 A–C). Particularly, knock down of these genes in presence of NDV infection in A549 cells, leads to significant suppression the virus infection and upregulation of innate immune response-related or antiviral genes, IL6 (interleukin 6) and IFIT1 (interferon induced protein with tetratricopeptide repeats 1), notably, the knockdown substantially reduced the gene expression (Fig. 4A–C), suggesting that upregulated metabolic pathways-related genes in presence of ambient PM10 (Fig. 3E–H) support the virus infections and supress the innate immune responses-related genes, that further contribute to the severity of respiratory related diseases or highly pathogenic respiratory virus infections, like Influenza. Since transcriptomic analysis clearly reveals the involvement of these metabolic-pathway-related cellular genes in H5N1 infection and reduction of antiviral cytokines (Fig. 3). And these cellular factors were found to be involved in the pathogenesis of other RNA virus infection. Therefore, performing the knockdown studies of these genes in presence of an experimental RNA virus, NDV will further add to the value of these results and mechanism associated with dual effect of PM10 exposure and virus infection in enhancing the severity of respiratory illness, especially flu infection. In addition, reanalysis of GEO dataset GSE34607, reveals that the expression of these metabolic genes in BEAS-2B bronchial epithelial cells were significantly modulated in presence of PM10 (Fig. S5A) along with other cellular inflammatory genes (Fig. S5B). Moreover, VIPR1, CYP1A1 and PPP1R14A are reported to be strongly associated with the modulation of inflammatory and/or innate immune responses as their downregulation results in the elevation of immune responses (Abad et al., 2016; Blackham et al., 2010; Delgado et al., 2001; Fang et al., 2016; Paton and Renton, 1998; Yin et al., 2017; Zhang et al., 2018) during microbial infections, autoimmunity and dendritic cell differentiation respectively. Altogether, our result concludes that these metabolic pathways-related genes or cellular factors contributes in elevation of RNA virus infection and causing lung disease severity in presence of airborne ambient PM. A schematic representation of the overall study is shown in Fig. 4D.
Fig. 4.
Knockdown of validated genes reduces RNA virus infection by enhancing innate immune response – A549 cells were transiently transfected with 1.5 μg of two of the respective sh-clones of each indicated genes or scrambled control for 72 h then infected with NDV (MOI 2) for 24 h and subjected to the quantification of the respective indicated transcripts or genes; (A) CYP1A1, (B) VIPR1 and (C) PPP1R14A; NDV viral RNA transcripts and antiviral cytokines; IL6 and IFIT1 transcripts. (D) Schematic representation of the overall conclusion of the study. Data are mean ± SEM of triplicate samples from single experiment and are representative of three independent experiments. ∗∗∗p < 0.001 and ∗∗p < 0.01 by one-way ANOVA Tukey test and unpaired t-test.
4. Discussion
Airborne PM were considered as one of the abiotic hazardous determinants for the development diseases such as cancer (Quezada-Maldonado et al., 2018), respiratory (Miller and Peden, 2014), cardiovascular (Ulrich et al., 2002) and neurological disorders (Bandyopadhyay, 2016; Crinnion, 2017; Genc et al., 2012; Loane et al., 2013; Xu et al., 2016). Presently, emergence of novel infectious microbial pathogens along with air pollution is one of severe threats to the human society globally. Infection through respiratory route has been included as the top threat to global health in the 13th General Programme of work, by WHO which aims to cover more than three billion people against the health emergencies from 2019 to 2023 (WHO, 2018).
Air pollution is also one of the key risk factor for metabolism-associated diseases along with respiratory route infections, and its adverse effects including comorbidity or even death of such coinfected patients throughout the world (Landrigan, 2017). Among the different air pollutants, PM contributes to the majority of lethal effects, which differs according to the geographical area, climatic conditions and sources of occurrence or release into the environment, particularly in developing country like India, where air pollution is predominant factor in major cities like, New Delhi, Pune, Bengaluru, Kolkata, and Chennai.
PM exists in different sizes and directly interacts with various cells particularly epithelial cells of respiratory tract and lungs through complex physicochemical and biochemical interactions. They are classified into three sizes; PM0.1, PM2.5 and PM10. PM2.5 were considered to be the most dangerous and hazardous airborne particles as they have the tendency to penetrate deeper into the lung tissues and cause permanent damage (de Barros Mendes Lopes et al., 2018; Horne et al., 2018). However in recent past, severe lung damage and occupational respiratory tract-related disorders has been reported to be caused by the PM10, the coarse size PM, mainly found in road dust, agricultural dust, construction and demolition work site, mining operations, desert dust and dust storm apart from industrial and vehicle exhaust (Celis et al., 2004; Chirino et al., 2015; Falcon-Rodriguez et al., 2016; Goudarzi et al., 2019; Hutchison et al., 2005; Kliengchuay et al., 2018; Naimabadi et al., 2016; Song et al., 2016; Van Den Heuvel et al., 2016). PM10 have been extensively reported to cause toxic effects due to its diverse shape, size and composition in different parts of the world and a study additionally suggests that PM10 risk coefficient is several times higher than the usual risk caused by PM2.5 (Gerlofs-Nijland et al., 2007; Janssen et al., 2013; Mallone et al., 2011; Perez et al., 2008; Pope et al., 1992; Sandstrom et al., 2005).
To date, there were few studies which links PM with respiratory viral infections (Khilnani and Tiwari, 2018; MacNee and Donaldson, 2003). Though these pollutants modulate the host defense and enhance the susceptibility and severity during virus infection, the underline molecular mechanisms are poorly understood (Becker and Soukup, 1999). Although, top pandemics and global threats were majorly caused by viral outbreaks, particularly RNA viruses, PM could be one of the key determinants in the spread of these viruses. Viruses are broadly, categorized as DNA and RNA based on the type of genetic material. It is evident that RNA viruses are critically harmful to the mankind and cause epidemics or pandemic for example, Influenza (flu virus), Severe Acute Respiratory Syndrome (SARS) CoV, SARS CoV-2, Middle East Respiratory Syndrome (MERS) CoV (coronaviruses), Dengue virus and HIV (human immunodeficiency virus). Recently, before coronavirus outbreak, Influenza is also included among the topmost threats by WHO and suggested to have pandemic potential. As among all the RNA viruses, its genetic complexities allow to evolve at the rapid rate and infect the host in its completely new format with high rate of fatality. Influenza infection peaks during the winter season and cause frequent seasonal epidemics, as well as sudden unforeseen pandemics. It spreads readily, and there is no proper vaccination available, although drug (Tamiflu) for Influenza is available, drug-resistant Influenza viruses are also emerged and are severe threat to the mankind. Therefore, it has been a major health as well as an economic burden throughout the world. Among various controlling factors, environmental factors, especially air pollution play an essential role in emergence of Influenza virus with pandemic potential. Combined effect of both air pollutant and virus infection is the current interest of research community to underpin the mechanism that contributes towards the severity of the respiratory damage and spread of lung infection. Few studies explained the direct causative effects of ambient pollutants and other similar causative agents like cigarette smoke extracts, diesel exhaust on various viral lung infections especially on the severity of common cold caused by Rhinovirus (MacNee and Donaldson, 2003; Paulin and Hansel, 2016; Proud et al., 2012). Different studies provide the range of impacts by PM to the lung infections from different geographical origins (Feng et al., 2016; Huang et al., 2016; Liu et al., 2019b). In a developing country like India, the levels of airborne ambient PM, especially PM10, increased in the past decade due to heavy industrialization. PM10 isolation from Indian subcontinent and its deleterious effects on human health in terms of innate immunity during RNA virus infections are not being reported yet.
In this study, we sought to understand whether PM10 exposure leads to significant modification of innate immune responses and viral infectivity in human lung epithelial cell lines, A549. Additionally, we focused to explore the overall molecular changes occurred when cells were exposed to PM10 and virus together. We also aimed to underpin the mechanism behind the enhanced viral replication of Influenza (H5N1) virus and other RNA virus infections like NDV in presence of airborne (PM10). In continuation, the cellular transcriptomic changes in presence of PM10 during viral infection were analysed by RNA sequencing data analysis. We used PM10 in our study obtained from the Bengaluru city. Bengaluru is one of the heavily industrialized, populated and urbanized cities in India. Therefore, studying about the ambient PM10 and its impact on respiratory health holds great importance. Initially, we characterized the PM10 by performing SEM-EDS analysis, and reported the topological features and chemical composition of the PM10 as revealed by imaging analysis (Fig. 1A and S1). Characterization of PM10 is of significant importance as it classifies the air pollutant into active and hazardous as well as non-active and unharmful particles. Many studies support the notion of PM characterization through SEM-EDS analysis and has reported different morphological shapes and chemical composition of PM, that cause severe lung diseases and illness (Anake et al., 2016; Bahadar Zeb et al., 2018; Galvez et al., 2013; Genga et al., 2013; Grassi et al., 2004; Labrada-Delgado et al., 2012; Ličbinský et al., 2010; Ramirez-Leal et al., 2009; Ramirez-Leal et al., 2014; Tuvjargal et al., 2019). Then, we demonstrated the effectiveness of PM10 exposure on A549 cells, by testing its inflammatory property within the cells, as it was previously reported that PM causes dysregulated inflammation in lungs to cause severe damage and toxicity (Cevallos et al., 2017; de Barros Mendes Lopes et al., 2018; Horne et al., 2018).
PM10 and its impact on lung environment and airway was investigated by exposing the lung epithelial cells with PM10 and infecting them with different RNA viruses like NDV and H5N1 flu virus. Our results demonstrate the consequences of both air pollutant and virus infection. Interestingly, we observed that PM10 suppress innate immunity and significantly elevated the viral replication. We have performed several experiments with different dosage of PM10 to demonstrate the phenomena of reduced immune responses (Fig. 1B–J) and enhanced viral load (Fig. 2). We also demonstrate by using luciferase assay, that PM10 exposed cells in presence of NDV infection reduced the promoter activity of the key antiviral genes of innate immunity, IFNβ and ISRE (Fig. 1D). The transcripts of antiviral innate immune genes were quantified to be suppressed during NDV and H5N1 infection estimated by qRT-PCR (Fig. 1E–J). Similarly, by quantifying the viral transcripts and viral GFP signals through qRT-PCR and microscopy respectively, it was concluded that PM10 at its optimal dosage significantly enhance the virus replication (Fig. 2A–F) and contributes to the severity of infection. Additionally, optimal dose of PM10 exposer along with NDV infection to the cells showed highest cell death, as estimated by the trypan blue assay (Fig. 3F). This demonstrates ex-vivo toxicity and severity of air pollutant and virus infection on cells. Previously, it has been shown that antiviral response was supressed upon CSE exposure during Rhinovirus infection in human bronchial epithelial cell lines (Proud et al., 2012). Reports also revealed that geogenic PM10 modulates Influenza infection (Clifford et al., 2015). This prompted us to test the effect of PM10 on the enhanced infectivity of highly pathogenic avian H5N1 Influenza virus infection and decipher the molecular mechanism. Next, to support the notion in connection to these studies related to the respiratory disease severity and its dual effect of PM exposure and virus infection, we extensively reviewed previous studies along with recent published work and preprints research related to unprecedented COVID-19, caused by SARS CoV-2 through respiratory route leading to pandemic and its correlation with disease severity under air pollution, particularly in presence of PM.
PM concentration has been shown to correlate with increased mortality and morbidities in many viral diseases. PM2.5 and PM10 showed a positive correlation with RSV (respiratory syncytial virus) infection rates (Ye et al., 2016). Increased spread of measles virus is strongly correlated with the PM2.5 concentration in China (Chen et al., 2017a; Peng et al., 2020). A global mortality impact modelling study showed PM10 concentration among one of the factors, associated with increased mortality during Influenza (H1N1) 2009 pandemic (Morales et al., 2017). An ecological study showed that exposure to air pollutants such as PM10 has been associated with increased mortality during the previous SARS-CoV outbreak in China in 2003. The study showed that the regions having moderated Air Pollution Index (API) has increased risk of death than those areas having less API. It is important to note that PM10 was the major pollutant among the five air pollution indices considered in this study (Cui et al., 2003). Similar to previous coronavirus outbreak (SARS-CoV), PM is also shown to be strongly associated with increased death during the ongoing coronavirus pandemic, COVID-19 (Conticini et al., 2020; Dutheil et al., 2020b; Peng et al., 2020; Sciomer et al., 2020; Setti et al., 2020a; Zhu et al., 2020). According to a United States nationwide cross-sectional study, 1 μg/m3 increase in the PM2.5 concentration has been linked with 15% increase in death rate due to COVID-19 (Wu et al., 2020). Additionally, in a recent study it is shown that the regions with higher Air Quality Index (AQI) also reported higher mortality rate due to COVID-19 (Conticini et al., 2020; Fattorini and Regoli, 2020; Piazzalunga-Expert, 2020). As discussed earlier, PM strongly results in high mortality when associated with any respiratory diseases. In this connection, another study revealed strong correlation of air quality data in 71 provinces of Italy with the number of deaths occurred in those provinces due to COVID-19 (Fattorini and Regoli, 2020). Air pollutants including PM, thus, promotes the viral spread. Moreover, a recent study showed the first evidence of the presence of SARS-CoV2 RNA from the PM10 collected from Bergamo region of Italy (Dutheil et al., 2020a; Setti et al., 2020b). These studies strongly support the positive association of PM including PM10, with the increased mortality during virus outbreaks. Although, there may be certain uncontrollable confound factors which might be biased towards the observations, future studies which includes more variables must be considered along with the PM to find the important co-factors and/or host’s cellular factors, cellular machineries and signalling pathways as suggested by the Italian aerosol study (Italian-Aerosol-Society, 2020). As one such report suggests the role nitrogen dioxide (NO2), another class of air pollutant, might severely contributes towards the COVID-19 fatality (Ogen, 2020).
Although, few studies reported the global transcriptomic changes, in presence PM10 by microarray analysis. Our study for the first time, used high throughput RNA sequencing to study the overall changes in the gene expression upon PM10 exposure during the viral infection of highly pathogenic avian Influenza (HPAI) H5N1 virus in the lung epithelial carcinoma cells, A549. RNA sequencing analysis identified that majority of genes are significantly downregulated were involved in immune-related pathways, cytokine signalling, and few other inflammatory pathways (Fig. 3). In addition to this, we observed a significant increase in the expression of genes involved in various metabolic pathways, which were previously remain uncategorized, particularly in case of airborne ambient PM exposure during flu infection. We validated RNA sequencing results for four of the top upregulated genes, namely VIPR1 (vasoactive intestinal peptide 1), CYP1A1 (cytochrome P450, family 1, subfamily A memeber1 also known as aryl hydrocarbon hydroxylase), ALDH1A3 (aldehyde dehydrogenase 1, family member 3A) and PPP1R14A (protein phosphatase 1 regulatory inhibitor subunit 14A) using quantitative qRT-PCR analysis. These selected genes - VIPR1, mainly located on plasma membrane and PPP1R14A majorly located on nucleus and cytoskeleton were moderately found to be involved in virus infections like HIV-1 and Influenza as reported by an in-vitro study and an in-silico phosphoproteomics study in human macrophages respectively (Bokaei et al., 2006; Soderholm et al., 2016; Temerozo et al., 2018). CYP1A1 was recently reported to be involved in many virus infections especially hepatitis B and hepatitis C virus (Fattahi et al., 2018; Ohashi et al., 2018; Stavropoulou et al., 2018). One such report superficially uncovers the induction of CYP1A1 in presences of PM10 (Kim et al., 2018). Additionally, induction of CYP1A1 in presence of diesel exhaust particles were extensively reported in human bronchial cells (Totlandsdal et al., 2010). ALDH1A3, apart from being involved in studies related to different types of cancers (Croker et al., 2017; Flahaut et al., 2016), was also previously reported in connection with virus infections like human papilloma virus and respiratory syncytial virus (Diamond et al., 2012; Puttini et al., 2018; Tulake et al., 2018). Altogether, differentially expressed genes identified in our study related to different metabolic modifications were reasonably linked to virus infections. Therefore, we selected these genes to validate further, in context to RNA virus infectivity. We demonstrated by short hairpin (sh)-RNA mediated transient silencing, that these genes significantly reduced the viral replication and moderately elevated the expression of antiviral genes (Fig. 4), suggesting the importance of these metabolic pathway-related genes in regulation of pathogenic burden during viral infection.
Overall, this study highlights the effect of PM10 exposure upon virus (flu) infection that affects the lung airways to cause severe respiratory damage. The high throughput RNA sequencing was performed in our study for the first time, in context to Indian subcontinent distribution of PM10. PM10 which is collected and isolated in this study demonstrate the transcriptomic changes upon its exposure during Influenza virus infection in A549 cell lines. There were very few studies that reported the link between PM10 exposure and enhanced viral infections (Hirota et al., 2015; Wang et al., 2015). However, the implication attached to our study model is that, we have identified and validated the host’s cellular metabolic-pathway related genes and these genes contribute in enhancing the severity of infection (flu infection) in presence of ambient air pollutant, PM10. Since we have incorporated both air pollutant and H5N1 strain of virus from the Indian origin and obtained the conclusions related to the mechanism by which PM10 enhances the virus replication within the lung epithelial cells, therefore, it could be proposed that possibly adoption of our study model applies to other strains of Influenza virus and additionally to the other class of RNA viruses, for example coronavirus in connection with different types and standards of PM. One such strong hypothesis could be built through the transcriptomic analysis performed in the study, and opens up the interesting question to test in future to decipher the mechanism how air pollutant could affect the virus infection or its severity. ACE2 is the receptor for SARS-CoV-2 (Hoffmann et al., 2020) and there are reports that ACE2 expression increases upon exposure to PM2.5 (Lin et al., 2018) and PM10 (Miyashita et al., 2020). This frames an important question whether the prolonged exposure to PM increases the susceptibility to infection by SARS-CoV-2 due to the enhanced expression of ACE2. Also, increased ACE2 is crucial for the regulation of lung tissue homeostasis and prevention of lung injury due to excessive production of pro-inflammatory cytokines (Imai et al., 2005). ACE2 expression is downregulated not only after the SARS-CoV (Glowacka et al., 2010) and SARS-CoV-2 infection (Verdecchia et al., 2020), but also after the H5N1 (Zou et al., 2014), H1N1 (Liu et al., 2014) infection. From the extended RNA-seq results in our study (Fig. S4), ACE2 expression was downregulated in PM10 treated and subsequently H5N1 infected samples, compared to the mock treated H5N1infected samples. Downregulation of ACE2 has been associated with severe lung injury and increased lethality of H5N1 virus (Zou et al., 2014) as well as increased viral replication of human coronavirus (NL-63) (Dijkman et al., 2012). Meanwhile, Ace2-knockout mice develop severe lung injury after the exposure to PM2.5 (Lin et al., 2018). We can speculate that PM exposure leads to the upregulation of ACE2, which increases the susceptibility to SARS-CoV-2 and virus mediated downregulation of ACE2 post infection, which in turn contributes to the severe lung injury and increased lethality. This observation supports the “double-hit” hypothesis, in a recent study (Frontera et al., 2020), which explores the involvement of PM2.5 in SARS-CoV-2 leading to severe complications that might be applicable in case of PM10 association with RNA viruses, particularly H5N1 and/or SARS-CoV2.
The limitation of our study adheres to fact that, we could have included more in-vitro lung origin cell lines/tissues (de Barros Mendes Lopes et al., 2018; Graff et al., 2012; Huang et al., 2009; O’Beirne et al., 2018) and an in-vivo mouse model in order to explore the effect of pollutant under physiological conditions after PM10 exposure which will fully elucidate the outcomes of the study. Additionally, we could have included more variety of different ambient PM related to both seasonal variations and different geographical origins (instil with different chemical composition, shape of the particle or both) of Indian subcontinent, as that would add to the strength and scientific depth of the study in providing better insights about the effects of PM10 over various lung infections including Influenza and other RNA virus infection and also add to formulate the policies related to control the air pollution. However, we understand the gravity of this research area and looking forward to answer the underexplored questions, by using in-vivo models, associated herewith in coming future studies.
5. Conclusion
In conclusion, PM10 enhances RNA virus replication by suppressing the innate immunity along with the upregulation of several metabolic pathways-related genes, as evident from the transcriptomic profiling results. Knocking down these metabolism-related upregulated genes, decreases the viral replication and subsequently increases the innate immune response. As several epidemiological studies suggest that PM10 exposure is associated with severity of lung infections, our results also support these studies. In this study, we used lung epithelial cells in which we demonstrated that Influenza (flu) virus replication were elevated in presence of PM10 through suppression of antiviral innate immune cytokines. The outcome of this study may encourage future studies to uncover the mode of action of PM10 over different RNA viruses which have the potential to cause future outbreaks and guide the policy makers to regulate the level of PM for public healthcare purposes.
Funding
This work was supported by SERB-DST grant (DST No. SB/S3/CEE/030/2014) to H.K. as principal investigator of the project and G.S. as Co-PI of the project. R.M. is supported by the IISER Bhopal institutional fellowship.
CRediT authorship contribution statement
Richa Mishra: Conceptualization, Investigation, Validation, Formal analysis, Writing - original draft, Writing - review & editing, Project administration. Pandikannan Krishnamoorthy: Data curation, Writing - review & editing. S. Gangamma: Conceptualization, Investigation, Funding acquisition, Resources. Ashwin Ashok Raut: Resources. Himanshu Kumar: Conceptualization, Formal analysis, Writing - original draft, Writing - review & editing, Project administration, Funding acquisition, Resources, Supervision.
Declaration of competing interest
The authors declare no conflict of interests.
Acknowledgments
We greatly acknowledge Director, ICAR-NIHSAD for providing BSL-3 facility to conduct H5N1 experiments. We express our humble gratitude towards Dr. Santhalembi Chingtham – for infecting the cells with Influenza (H5N1) virus in BSL-3 core facility at ICAR - NIHSAD Laboratory. We thank Dr. Harshad Ingle for proofreading and helping with illustrating the graphical abstract. We are grateful to Indian Institute of Science Education and Research (IISER) Bhopal for providing the Central Instrumentation Facility. We thank all the members of the laboratory of immunology and infectious disease biology for helpful discussions. We acknowledge the shutterstock.com for an image of endoplasmic reticulum attached to the nucleus, used in the schematic (Fig. 4D).
Footnotes
This paper has been recommended for acceptance by Da Chen.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envpol.2020.115148.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Abad C., Jayaram B., Becquet L., Wang Y., O’Dorisio M.S., Waschek J.A., Tan Y.V. VPAC1 receptor (Vipr1)-deficient mice exhibit ameliorated experimental autoimmune encephalomyelitis, with specific deficits in the effector stage. J. Neuroinflamm. 2016;13:169. doi: 10.1186/s12974-016-0626-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anake W.U., Ana G.R., Benson N.U. Study of surface morphology, elemental composition and sources of airborne fine particulate matter in Agbara industrial estate, Nigeria. Int. J. Appl. Environ. Sci. 2016;11:881–890. [Google Scholar]
- Anderson J.O., Thundiyil J.G., Stolbach A. Clearing the air: A review of the effects of particulate matter air pollution on human health. J. Med. Toxicol. 2012;8:166–175. doi: 10.1007/s13181-011-0203-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews S., Krueger F., Segonds-Pichon A., Biggins L., Krueger C., Wingett S., Montgomery J. FastQ: a Quality Control Tool for High Throughput Sequence Data. Babraham Bioinformatics: A bioinformatic Tool. 2010 https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ [Google Scholar]
- Arai T. Ministry of Heavy Industries and Public Enterprises; New Delhi: 2018. Source Apportionment of PM2.5 & PM10 of Delhi NCR for Identification of Major Sources Department of Heavy Industry. [Google Scholar]
- Bach N., Bolling A.K., Brinchmann B.C., Totlandsdal A.I., Skuland T., Holme J.A., Lag M., Schwarze P.E., Ovrevik J. Cytokine responses induced by diesel exhaust particles are suppressed by PAR-2 silencing and antioxidant treatment, and driven by polar and non-polar soluble constituents. Toxicol. Lett. 2015;238:72–82. doi: 10.1016/j.toxlet.2015.07.002. [DOI] [PubMed] [Google Scholar]
- Bahadar Zeb B., Khan Alam K., Armin Sorooshian A., Blaschke T., Ahmad I., Shahid I. On the morphology and composition of particulate matter in an urban environment. Aerosol Air Qual. Res. 2018;18:1431–1447. doi: 10.4209/aaqr.2017.09.0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay A. Neurological disorders from ambient (urban) air pollution emphasizing UFPM and PM2.5. Curr. Pollut. Rep. 2016;2:203–211. [Google Scholar]
- Bauer R.N., Diaz-Sanchez D., Jaspers I. Effects of air pollutants on innate immunity: the role of Toll-like receptors and nucleotide-binding oligomerization domain-like receptors. J. Allergy Clin. Immunol. 2012;129:14–24. doi: 10.1016/j.jaci.2011.11.004. quiz 25-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker S., Fenton M.J., Soukup J.M. Involvement of microbial components and toll-like receptors 2 and 4 in cytokine responses to air pollution particles. Am. J. Respir. Cell Mol. Biol. 2002;27:611–618. doi: 10.1165/rcmb.4868. [DOI] [PubMed] [Google Scholar]
- Becker S., Soukup J.M. Exposure to urban air particulates alters the macrophage-mediated inflammatory response to respiratory viral infection. J. Toxicol. Environ. Health. 1999;57:445–457. doi: 10.1080/009841099157539. [DOI] [PubMed] [Google Scholar]
- Bengalli R., Molteni E., Longhin E., Refsnes M., Camatini M., Gualtieri M. Release of IL-1 beta triggered by Milan summer PM10: Molecular pathways involved in the cytokine release. BioMed Res. Int. 2013;2013:158093. doi: 10.1155/2013/158093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackham S., Baillie A., Al-Hababi F., Remlinger K., You S., Hamatake R., McGarvey M.J. Gene expression profiling indicates the roles of host oxidative stress, apoptosis, lipid metabolism, and intracellular transport genes in the replication of hepatitis C virus. J. Virol. 2010;84:5404–5414. doi: 10.1128/JVI.02529-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokaei P.B., Ma X.Z., Byczynski B., Keller J., Sakac D., Fahim S., Branch D.R. Identification and characterization of five-transmembrane isoforms of human vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide receptors. Genomics. 2006;88:791–800. doi: 10.1016/j.ygeno.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Bolger A.M., Lohse M., Usadel B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray N.L., Pimentel H., Melsted P., Pachter L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 2016;34:525–527. doi: 10.1038/nbt.3519. [DOI] [PubMed] [Google Scholar]
- Carneiro M.A.F., Alves B., Gehrke F.S., Domingues J.N., Sa N., Paixao S., Figueiredo J., Ferreira A., Almeida C., Machi A., Savoia E., Nascimento V., Fonseca F. Environmental factors can influence dengue reported cases. Rev. Assoc. Med. Bras. 2017;63:957–961. doi: 10.1590/1806-9282.63.11.957. 1992. [DOI] [PubMed] [Google Scholar]
- Carugno M., Consonni D., Randi G., Catelan D., Grisotto L., Bertazzi P.A., Biggeri A., Baccini M. Air pollution exposure, cause-specific deaths and hospitalizations in a highly polluted Italian region. Environ. Res. 2016;147:415–424. doi: 10.1016/j.envres.2016.03.003. [DOI] [PubMed] [Google Scholar]
- Celis J.E., Morales J.R., Zaror C.A., Inzunza J.C. A study of the particulate matter PM10 composition in the atmosphere of Chillan, Chile. Chemosphere. 2004;54:541–550. doi: 10.1016/S0045-6535(03)00711-2. [DOI] [PubMed] [Google Scholar]
- Cevallos V.M., Diaz V., Sirois C.M. Particulate matter air pollution from the city of Quito, Ecuador, activates inflammatory signaling pathways in vitro. Innate Immun. 2017;23:392–400. doi: 10.1177/1753425917699864. [DOI] [PubMed] [Google Scholar]
- Chen G., Zhang W., Li S., Williams G., Liu C., Morgan G.G., Jaakkola J.J.K., Guo Y. Is short-term exposure to ambient fine particles associated with measles incidence in China? A multi-city study. Environ. Res. 2017;156:306–311. doi: 10.1016/j.envres.2017.03.046. [DOI] [PubMed] [Google Scholar]
- Chen R., Yin P., Meng X., Liu C., Wang L., Xu X., Ross J.A., Tse L.A., Zhao Z., Kan H., Zhou M. Fine particulate air pollution and daily mortality. A nationwide analysis in 272 Chinese cities. Am. J. Respir. Crit. Care Med. 2017;196:73–81. doi: 10.1164/rccm.201609-1862OC. [DOI] [PubMed] [Google Scholar]
- Chen X., Zhang L.W., Huang J.J., Song F.J., Zhang L.P., Qian Z.M., Trevathan E., Mao H.J., Han B., Vaughn M., Chen K.X., Liu Y.M., Chen J., Zhao B.X., Jiang G.H., Gu Q., Bai Z.P., Dong G.H., Tang N.J. Long-term exposure to urban air pollution and lung cancer mortality: A 12-year cohort study in Northern China. Sci. Total Environ. 2016;571:855–861. doi: 10.1016/j.scitotenv.2016.07.064. [DOI] [PubMed] [Google Scholar]
- Chirino Y.I., Sanchez-Perez Y., Osornio-Vargas A.R., Rosas I., Garcia-Cuellar C.M. Sampling and composition of airborne particulate matter (PM10) from two locations of Mexico City. Data Brief. 2015;4:353–356. doi: 10.1016/j.dib.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y.H., Kao S.W., Tantoh D.M., Ko P.C., Lan S.J., Liaw Y.P. Association between fine particulate matter and oral cancer among Taiwanese men. J. Investig. Med. 2019;67:34–38. doi: 10.1136/jim-2016-000263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford H.D., Perks K.L., Zosky G.R. Geogenic PM(1)(0) exposure exacerbates responses to influenza infection. Sci. Total Environ. 2015;533:275–282. doi: 10.1016/j.scitotenv.2015.07.001. [DOI] [PubMed] [Google Scholar]
- Cohen A.J., Brauer M., Burnett R., Anderson H.R., Frostad J., Estep K., Balakrishnan K., Brunekreef B., Dandona L., Dandona R., Feigin V., Freedman G., Hubbell B., Jobling A., Kan H., Knibbs L., Liu Y., Martin R., Morawska L., Pope C.A., 3rd, Shin H., Straif K., Shaddick G., Thomas M., van Dingenen R., van Donkelaar A., Vos T., Murray C.J.L., Forouzanfar M.H. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. Lancet. 2017;389:1907–1918. doi: 10.1016/S0140-6736(17)30505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaborators G.B.D.R.F., Forouzanfar M.H., Alexander L., Anderson H.R., Bachman V.F., Biryukov S., Brauer M., Burnett R., Casey D., Coates M.M., Cohen A., Delwiche K., Estep K., Frostad J.J., Astha K.C., Kyu H.H., Moradi-Lakeh M., Ng M., Slepak E.L., Thomas B.A., Wagner J., Aasvang G.M., Abbafati C., Abbasoglu Ozgoren A., Abd-Allah F., Abera S.F., Aboyans V., Abraham B., Abraham J.P., Abubakar I., Abu-Rmeileh N.M., Aburto, TC, Achoki T., Adelekan A., Adofo K., Adou A.K., Adsuar J.C., Afshin A., Agardh E.E., Al Khabouri M.J., Al Lami F.H., Alam S.S., Alasfoor D., Albittar M.I., Alegretti M.A., Aleman A.V., Alemu Z.A., Alfonso-Cristancho R., Alhabib S., Ali R., Ali M.K., Alla F., Allebeck P., Allen P.J., Alsharif U., Alvarez E., Alvis-Guzman N., Amankwaa A.A., Amare A.T., Ameh E.A., Ameli O., Amini H., Ammar W., Anderson B.O., Antonio C.A., Anwari, P, Argeseanu Cunningham S., Arnlov J., Arsenijevic V.S., Artaman A., Asghar R.J., Assadi R., Atkins L.S., Atkinson C., Avila M.A., Awuah B., Badawi A., Bahit M.C., Bakfalouni T., Balakrishnan K., Balalla S., Balu R.K., Banerjee A., Barber R.M., Barker-Collo S.L., Barquera S., Barregard L., Barrero L.H., Barrientos-Gutierrez T., Basto-Abreu A.C., Basu A., Basu S., Basulaiman M.O., Batis Ruvalcaba C., Beardsley J., Bedi N., Bekele T., Bell M.L., Benjet C., Bennett, DA, Benzian H., Bernabe E., Beyene T.J., Bhala N., Bhalla A., Bhutta Z.A., Bikbov B., Bin Abdulhak A.A., Blore J.D., Blyth F.M., Bohensky M.A., Bora Basara B., Borges G., Bornstein N.M., Bose D., Boufous S., Bourne R.R., Brainin M., Brazinova A., Breitborde N.J., Brenner H., Briggs A.D., Broday D.M., Brooks P.M., Bruce N.G., Brugha T.S., Brunekreef B., Buchbinder R., Bui L.N., Bukhman G., Bulloch A.G., Burch M., Burney P.G., Campos-Nonato, IR, Campuzano J.C., Cantoral A.J., Caravanos J., Cardenas R., Cardis E., Carpenter D.O., Caso V., Castaneda-Orjuela C.A., Castro R.E., Catala-Lopez F., Cavalleri F., Cavlin A., Chadha V.K., Chang J.C., Charlson F.J., Chen H., Chen W., Chen Z., Chiang P.P., Chimed-Ochir O., Chowdhury R., Christophi C.A., Chuang T.W., Chugh S.S., Cirillo M., Classen T.K., Colistro V., Colomar M., Colquhoun S.M., Contreras A.G., Cooper C., Cooperrider K., Cooper L.T., Coresh J., Courville K.J., Criqui M.H., Cuevas-Nasu L., Damsere-Derry J., Danawi H., Dandona L., Dandona R., Dargan P.I., Davis A., Davitoiu D.V., Dayama A., de Castro E.F., De la Cruz-Gongora V., De Leo D., de Lima G., Degenhardt L., del Pozo-Cruz B., Dellavalle R.P., Deribe K., Derrett S., Des Jarlais D.C., Dessalegn M., deVeber G.A., Devries K.M., Dharmaratne S.D., Dherani M.K., Dicker D., Ding E.L., Dokova K., Dorsey E.R., Driscoll T.R., Duan L., Durrani A.M., Ebel B.E., Ellenbogen R.G., Elshrek Y.M., Endres M., Ermakov S.P., Erskine H.E., Eshrati B., Esteghamati A., Fahimi S., Faraon E.J., Farzadfar F., Fay D.F., Feigin V.L., Feigl A.B., Fereshtehnejad S.M., Ferrari A.J., Ferri C.P., Flaxman A.D., Fleming T.D., Foigt N., Foreman K.J., Paleo U.F., Franklin R.C., Gabbe B., Gaffikin L., Gakidou E., Gamkrelidze A., Gankpe F.G., Gansevoort R.T., Garcia-Guerra F.A., Gasana E., Geleijnse J.M., Gessner B.D., Gething P., Gibney K.B., Gillum R.F., Ginawi I.A., Giroud M., Giussani G., Goenka S., Goginashvili K., Gomez Dantes H., Gona P., Gonzalez de Cosio T., Gonzalez-Castell D., Gotay C.C., Goto A., Gouda H.N., Guerrant R.L., Gugnani H.C., Guillemin F., Gunnell D., Gupta R., Gupta R., Gutierrez R.A., Hafezi-Nejad N., Hagan H., Hagstromer M., Halasa Y.A., Hamadeh R.R., Hammami M., Hankey G.J., Hao Y., Harb H.L., Haregu T.N., Haro J.M., Havmoeller R., Hay S.I., Hedayati M.T., Heredia-Pi I.B., Hernandez L., Heuton K.R., Heydarpour P., Hijar M., Hoek H.W., Hoffman H.J., Hornberger J.C., Hosgood H.D., Hoy D.G., Hsairi M., Hu G., Hu H., Huang C., Huang J.J., Hubbell B.J., Huiart L., Husseini A., Iannarone M.L., Iburg K.M., Idrisov B.T., Ikeda N., Innos K., Inoue M., Islami F., Ismayilova S., Jacobsen K.H., Jansen H.A., Jarvis D.L., Jassal S.K., Jauregui A., Jayaraman S., Jeemon P., Jensen P.N., Jha V., Jiang F., Jiang G., Jiang Y., Jonas J.B., Juel K., Kan H., Kany Roseline S.S., Karam N.E., Karch A., Karema C.K., Karthikeyan G., Kaul A., Kawakami N., Kazi D.S., Kemp A.H., Kengne A.P., Keren A., Khader Y.S., Khalifa S.E., Khan E.A., Khang Y.H., Khatibzadeh S., Khonelidze I., Kieling C., Kim D., Kim S., Kim Y., Kimokoti R.W., Kinfu Y., Kinge J.M., Kissela B.M., Kivipelto M., Knibbs L.D., Knudsen A.K., Kokubo Y., Kose M.R., Kosen S., Kraemer A., Kravchenko M., Krishnaswami S., Kromhout H., Ku T., Kuate Defo B., Kucuk Bicer B., Kuipers E.J., Kulkarni C., Kulkarni V.S., Kumar G.A., Kwan G.F., Lai T., Lakshmana Balaji A., Lalloo R., Lallukka T., Lam H., Lan Q., Lansingh V.C., Larson H.J., Larsson A., Laryea D.O., Lavados P.M., Lawrynowicz A.E., Leasher J.L., Lee J.T., Leigh J., Leung R., Levi M., Li Y., Li Y., Liang J., Liang X., Lim S.S., Lindsay M.P., Lipshultz S.E., Liu S., Liu Y., Lloyd B.K., Logroscino G., London S.J., Lopez N., Lortet-Tieulent J., Lotufo P.A., Lozano R., Lunevicius R., Ma J., Ma S., Machado V.M., MacIntyre M.F., Magis-Rodriguez C., Mahdi A.A., Majdan M., Malekzadeh R., Mangalam S., Mapoma C.C., Marape M., Marcenes W., Margolis D.J., Margono C., Marks G.B., Martin R.V., Marzan M.B., Mashal M.T., Masiye F., Mason-Jones A.J., Matsushita K., Matzopoulos R., Mayosi B.M., Mazorodze T.T., McKay A.C., McKee M., McLain A., Meaney P.A., Medina C., Mehndiratta M.M., Mejia-Rodriguez F., Mekonnen W., Melaku Y.A., Meltzer M., Memish Z.A., Mendoza W., Mensah G.A., Meretoja A., Mhimbira F.A., Micha R., Miller T.R., Mills E.J., Misganaw, A, Mishra S., Mohamed Ibrahim N., Mohammad K.A., Mokdad A.H., Mola G.L., Monasta L., Montanez Hernandez J.C., Montico M., Moore A.R., Morawska L., Mori R., Moschandreas J., Moturi W.N., Mozaffarian D., Mueller U.O., Mukaigawara M., Mullany E.C., Murthy K.S., Naghavi M., Nahas Z., Naheed A., Naidoo K.S., Naldi L., Nand D., Nangia V., Narayan K.M., Nash D., Neal B., Nejjari C., Neupane S.P., Newton C.R., Ngalesoni F.N., Ngirabega Jde D., Nguyen G., Nguyen N.T., Nieuwenhuijsen M.J., Nisar M.I., Nogueira J.R., Nolla J.M., Nolte S., Norheim O.F., Norman R.E., Norrving B., Nyakarahuka L., Oh I.H., Ohkubo T., Olusanya B.O., Omer S.B., Opio J.N., Orozco R., Pagcatipunan R.S., Jr., Pain A.W., Pandian J.D., Panelo C.I., Papachristou C., Park E.K., Parry C.D., Paternina Caicedo A.J., Patten S.B., Paul V.K., Pavlin B.I., Pearce N., Pedraza L.S., Pedroza A., Pejin Stokic L., Pekericli A., Pereira D.M., Perez-Padilla R., Perez-Ruiz F., Perico N., Perry S.A., Pervaiz A., Pesudovs K., Peterson C.B., Petzold M., Phillips M.R., Phua H.P., Plass D., Poenaru D., Polanczyk G.V., Polinder S., Pond C.D., Pope C.A., Pope D., Popova S., Pourmalek F., Powles J., Prabhakaran D., Prasad N.M., Qato D.M., Quezada A.D., Quistberg D.A., Racape L., Rafay A., Rahimi K., Rahimi-Movaghar V., Rahman S.U., Raju, M, Rakovac I., Rana S.M., Rao M., Razavi H., Reddy K.S., Refaat A.H., Rehm J., Remuzzi G., Ribeiro A.L., Riccio P.M., Richardson L., Riederer A., Robinson M., Roca A., Rodriguez A., Rojas-Rueda D., Romieu I., Ronfani L., Room R., Roy N., Ruhago G.M., Rushton L., Sabin N., Sacco R.L., Saha S., Sahathevan R., Sahraian M.A., Salomon J.A., Salvo D., Sampson U.K., Sanabria J.R., Sanchez L.M., Sanchez-Pimienta T.G., Sanchez-Riera L., Sandar L., Santos I.S., Sapkota A., Satpathy M., Saunders J.E., Sawhney M., Saylan M.I., Scarborough P., Schmidt J.C., Schneider I.J., Schottker B., Schwebel D.C., Scott J.G., Seedat S., Sepanlou S.G., Serdar B., Servan-Mori, EE, Shaddick G., Shahraz S., Levy T.S., Shangguan S., She J., Sheikhbahaei S., Shibuya K., Shin H.H., Shinohara Y., Shiri R., Shishani K., Shiue I., Sigfusdottir I.D., Silberberg D.H., Simard E.P., Sindi S., Singh A., Singh G.M., Singh J.A., Skirbekk V., Sliwa K., Soljak M., Soneji S., Soreide K., Soshnikov S., Sposato L.A., Sreeramareddy C.T., Stapelberg N.J., Stathopoulou V., Steckling N., Stein D.J., Stein M.B., Stephens N., Stockl H., Straif K., Stroumpoulis K., Sturua L., Sunguya B.F., Swaminathan S., Swaroop M., Sykes B.L., Tabb K.M., Takahashi K., Talongwa R.T., Tandon N., Tanne D., Tanner M., Tavakkoli M., Te Ao B.J., Teixeira C.M., Tellez Rojo M.M., Terkawi A.S., Texcalac-Sangrador J.L., Thackway S.V., Thomson B., Thorne-Lyman A.L., Thrift A.G., Thurston G.D., Tillmann T., Tobollik M., Tonelli M., Topouzis F., Towbin J.A., Toyoshima H., Traebert J., Tran B.X., Trasande L., Trillini M., Trujillo U., Dimbuene Z.T., Tsilimbaris M., Tuzcu E.M., Uchendu U.S., Ukwaja K.N., Uzun S.B., van de Vijver S., Van Dingenen R., van Gool C.H., van Os J., Varakin Y.Y., Vasankari T.J., Vasconcelos A.M., Vavilala M.S., Veerman L.J., Velasquez-Melendez G., Venketasubramanian N., Vijayakumar L., Villalpando S., Violante F.S., Vlassov V.V., Vollset S.E., Wagner G.R., Waller S.G., Wallin M.T., Wan X., Wang H., Wang J., Wang L., Wang W., Wang Y., Warouw T.S., Watts C.H., Weichenthal S., Weiderpass E., Weintraub R.G., Werdecker A., Wessells K.R., Westerman R., Whiteford H.A., Wilkinson J.D., Williams H.C., Williams T.N., Woldeyohannes S.M., Wolfe C.D., Wong J.Q., Woolf A.D., Wright J.L., Wurtz B., Xu G., Yan L.L., Yang G., Yano Y., Ye P., Yenesew M., Yentur G.K., Yip P., Yonemoto N., Yoon S.J., Younis M.Z., Younoussi Z., Yu C., Zaki M.E., Zhao Y., Zheng Y., Zhou M., Zhu J., Zhu S., Zou X., Zunt J.R., Lopez A.D., Vos T., Murray C.J. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:2287–2323. doi: 10.1016/S0140-6736(15)00128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consonni D., Carugno M., De Matteis S., Nordio F., Randi G., Bazzano M., Caporaso N.E., Tucker M.A., Bertazzi P.A., Pesatori A.C., Lubin J.H., Landi M.T. Outdoor particulate matter (PM10) exposure and lung cancer risk in the EAGLE study. PLoS One. 2018;13 doi: 10.1371/journal.pone.0203539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conticini E., Frediani B., Caro D. Can atmospheric pollution be considered a co-factor in extremely high level of SARS-CoV-2 lethality in Northern Italy? Environ. Pollut. 2020;261:114465. doi: 10.1016/j.envpol.2020.114465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CPCB E.C. 2020. Air Pollution in Delhi - an Analytical Study. [Google Scholar]
- Crinnion W. Particulate matter is a surprisingly common contributor to disease. Integr. Med. 2017;16:8–12. [PMC free article] [PubMed] [Google Scholar]
- Croker A.K., Rodriguez-Torres M., Xia Y., Pardhan S., Leong H.S., Lewis J.D., Allan A.L. Differential functional roles of ALDH1A1 and ALDH1A3 in mediating metastatic bBehavior and therapy resistance of human breast cancer cells. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18102039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y., Zhang Z.F., Froines J., Zhao J., Wang H., Yu S.Z., Detels R. Air pollution and case fatality of SARS in the People’s Republic of China: An ecologic study. Environ. Health. 2003;2:15. doi: 10.1186/1476-069X-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai L., Zanobetti A., Koutrakis P., Schwartz J.D. Associations of fine particulate matter species with mortality in the United States: A multicity time-series analysis. Environ. Health Perspect. 2014;122:837–842. doi: 10.1289/ehp.1307568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daryanoosh M., Goudarzi G., Rashidi R., Keishams F., Hopke P.K., Mohammadi M.J., Nourmoradi H., Sicard P., Takdastan A., Vosoughi M., Veysi M., Kianizadeh M., Omidi Khaniabadi Y. Risk of morbidity attributed to ambient PM10 in the western cities of Iran. Toxin Rev. 2018;37:313–318. [Google Scholar]
- Dastoorpoor M., Riahi A., Yazdaninejhad H., Borsi S.H., Khanjani N., Khodadadi N., Mohammadi M.J., Aghababaeian H. Exposure to particulate matter and carbon monoxide and cause-specific cardiovascular-respiratory disease mortality in Ahvaz. Toxin Rev. 2020:1–11. [Google Scholar]
- Dastoorpoor M., Sekhavatpour Z., Masoumi K., Mohammadi M.J., Aghababaeian H., Khanjani N., Hashemzadeh B., Vahedian M. Air pollution and hospital admissions for cardiovascular diseases in Ahvaz, Iran. Sci. Total Environ. 2019;652:1318–1330. doi: 10.1016/j.scitotenv.2018.10.285. [DOI] [PubMed] [Google Scholar]
- de Barros Mendes Lopes T., Groth E.E., Veras M., Furuya T.K., de Souza Xavier Costa N., Ribeiro Junior G., Lopes F.D., de Almeida F.M., Cardoso W.V., Saldiva P.H.N., Chammas R., Mauad T. Pre- and postnatal exposure of mice to concentrated urban PM2.5 decreases the number of alveoli and leads to altered lung function at an early stage of life. Environ. Pollut. 2018;241:511–520. doi: 10.1016/j.envpol.2018.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado M., Abad C., Martinez C., Leceta J., Gomariz R.P. Vasoactive intestinal peptide prevents experimental arthritis by downregulating both autoimmune and inflammatory components of the disease. Nat. Med. 2001;7:563–568. doi: 10.1038/87887. [DOI] [PubMed] [Google Scholar]
- Deng Q., Deng L., Miao Y., Guo X., Li Y. Particle deposition in the human lung: Health implications of particulate matter from different sources. Environ. Res. 2019;169:237–245. doi: 10.1016/j.envres.2018.11.014. [DOI] [PubMed] [Google Scholar]
- Diamond D.L., Krasnoselsky A.L., Burnum K.E., Monroe M.E., Webb-Robertson B.J., McDermott J.E., Yeh M.M., Dzib J.F., Susnow N., Strom S., Proll S.C., Belisle S.E., Purdy D.E., Rasmussen A.L., Walters K.A., Jacobs J.M., Gritsenko M.A., Camp D.G., Bhattacharya R., Perkins J.D., Carithers R.L., Jr., Liou I.W., Larson A.M., Benecke A., Waters K.M., Smith R.D., Katze M.G. Proteome and computational analyses reveal new insights into the mechanisms of hepatitis C virus-mediated liver disease posttransplantation. Hepatology. 2012;56:28–38. doi: 10.1002/hep.25649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkman R., Jebbink M.F., Deijs M., Milewska A., Pyrc K., Buelow E., van der Bijl A., van der Hoek L. Replication-dependent downregulation of cellular angiotensin-converting enzyme 2 protein expression by human coronavirus NL63. J. Gen. Virol. 2012;93:1924–1929. doi: 10.1099/vir.0.043919-0. [DOI] [PubMed] [Google Scholar]
- Donaldson K., Gilmour M.I., MacNee W. Asthma and PM10. Respir. Res. 2000;1:12–15. doi: 10.1186/rr5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutheil F., Baker J.S., Navel V. COVID-19 as a factor influencing air pollution? Environ. Pollut. 2020;263:114466. doi: 10.1016/j.envpol.2020.114466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutheil F., Baker J.S., Navel V. COVID-19 as a factor influencing air pollution? Environ. Pollut. 2020;263:114466. doi: 10.1016/j.envpol.2020.114466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcon-Rodriguez C.I., Osornio-Vargas A.R., Sada-Ovalle I., Segura-Medina P. Aeroparticles, composition, and lung diseases. Front. Immunol. 2016;7:3. doi: 10.3389/fimmu.2016.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X., Zhao W., Xu J., Tu F., Wang X., Li B., Fu Y., Ren S. CYP1A1 mediates the suppression of major inflammatory cytokines in pulmonary alveolar macrophage (PAM) cell lines caused by Mycoplasma hyponeumoniae. Dev. Comp. Immunol. 2016;65:132–138. doi: 10.1016/j.dci.2016.06.023. [DOI] [PubMed] [Google Scholar]
- Fattahi S., Karimi Alivije M., Babamahmoodi F., Bayani M., Sadeghi Haddad Zavareh M., Asouri M., Lotfi M., Amirbozorgi G., Akhavan-Niaki H. Cytochrome P450 genes (CYP2E1 and CYP1A1) variants and susceptibility to chronic hepatitis B virus infection. Indian J. Clin. Biochem. 2018;33:467–472. doi: 10.1007/s12291-017-0698-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattorini D., Regoli F. Role of the chronic air pollution levels in the Covid-19 outbreak risk in Italy. Environ. Pollut. 2020;264:114732. doi: 10.1016/j.envpol.2020.114732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng C., Li J., Sun W., Zhang Y., Wang Q. Impact of ambient fine particulate matter (PM2.5) exposure on the risk of influenza-like-illness: A time-series analysis in Beijing, China. Environ. Health. 2016;15:17. doi: 10.1186/s12940-016-0115-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flahaut M., Jauquier N., Chevalier N., Nardou K., Balmas Bourloud K., Joseph J.M., Barras D., Widmann C., Gross N., Renella R., Muhlethaler-Mottet A. Aldehyde dehydrogenase activity plays a key role in the aggressive phenotype of neuroblastoma. BMC Canc. 2016;16:781. doi: 10.1186/s12885-016-2820-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frontera A., Cianfanelli L., Vlachos K., Landoni G., Cremona G. Severe air pollution links to higher mortality in COVID-19 patients: The “double-hit” hypothesis. J. Infect. 2020 doi: 10.1016/j.jinf.2020.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez M.C.D., Jayo H.C., Vallar E.A. SEM/EDX analysis of fine particulate matter from the exhaust emission of motorized pedicabs (kuligligs) IOSR J. Environ. Sci. Toxicol. Food Technol. 2013 [Google Scholar]
- Gangamma S., Patil R.S., Mukherji S. Characterization and proinflammatory response of airborne biological particles from wastewater treatment plants. Environ. Sci. Technol. 2011;45:3282–3287. doi: 10.1021/es103652z. [DOI] [PubMed] [Google Scholar]
- Genc S., Zadeoglulari Z., Fuss S.H., Genc K. The adverse effects of air pollution on the nervous system. J. Toxicol. 2012;2012:782462. doi: 10.1155/2012/782462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genga A., Baglivi F., Siciliano M., Siciliano T., Aiello D., Tortorella C. 2013. Chemical and Morphological Study of Particulate Matter Analysed by Sem-Eds, 4th Imeko TC19 Symposium on Environmental Instrumentation and Measurements Protecting Environment, Climate Changes and Pollution Control; pp. 43–46. [Google Scholar]
- Gerlofs-Nijland M.E., Dormans J.A., Bloemen H.J., Leseman D.L., John A., Boere F., Kelly F.J., Mudway I.S., Jimenez A.A., Donaldson K., Guastadisegni C., Janssen N.A., Brunekreef B., Sandstrom T., van Bree L., Cassee F.R. Toxicity of coarse and fine particulate matter from sites with contrasting traffic profiles. Inhal. Toxicol. 2007;19:1055–1069. doi: 10.1080/08958370701626261. [DOI] [PubMed] [Google Scholar]
- Glowacka I., Bertram S., Herzog P., Pfefferle S., Steffen I., Muench M.O., Simmons G., Hofmann H., Kuri T., Weber F., Eichler J., Drosten C., Pohlmann S. Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63. J. Virol. 2010;84:1198–1205. doi: 10.1128/JVI.01248-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudarzi G., Alavi N., Geravandi S., Idani E., Behrooz H.R.A., Babaei A.A., Alamdari F.A., Dobaradaran S., Farhadi M., Mohammadi M.J. Health risk assessment on human exposed to heavy metals in the ambient air PM10 in Ahvaz, southwest Iran. Int. J. Biometeorol. 2018;62:1075–1083. doi: 10.1007/s00484-018-1510-x. [DOI] [PubMed] [Google Scholar]
- Goudarzi G., Alavi N., Geravandi S., Yari A.R., Aslanpour Alamdari F., Dobaradaran S., Farhadi M., Biglari H., Dastoorpour M., Hashemzadeh B., Mohammadi M.J. Ambient particulate matter concentration levels of Ahvaz, Iran, in 2017. Environ. Geochem. Health. 2019;41:841–849. doi: 10.1007/s10653-018-0182-0. [DOI] [PubMed] [Google Scholar]
- Graff J.W., Powers L.S., Dickson A.M., Kim J., Reisetter A.C., Hassan I.H., Kremens K., Gross T.J., Wilson M.E., Monick M.M. Cigarette smoking decreases global microRNA expression in human alveolar macrophages. PLoS One. 2012;7 doi: 10.1371/journal.pone.0044066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi C., Narducci P., Tognotti L. 2004. Atmospheric Particulate Matter by SEM-EDX. 13th World Clean Air and Environmental Protection Congress, August 22nd 27th ‘04, London, UK. [Google Scholar]
- Hamra G.B., Guha N., Cohen A., Laden F., Raaschou-Nielsen O., Samet J.M., Vineis P., Forastiere F., Saldiva P., Yorifuji T., Loomis D. Outdoor particulate matter exposure and lung cancer: A systematic review and meta-analysis. Environ. Health Perspect. 2014;122:906–911. doi: 10.1289/ehp/1408092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison R.M., Yin J. Particulate matter in the atmosphere: Which particle properties are important for its effects on health? Sci. Total Environ. 2000;249:85–101. doi: 10.1016/s0048-9697(99)00513-6. [DOI] [PubMed] [Google Scholar]
- Hirota J.A., Marchant D.J., Singhera G.K., Moheimani F., Dorscheid D.R., Carlsten C., Sin D., Knight D. Urban particulate matter increases human airway epithelial cell IL-1beta secretion following scratch wounding and H1N1 influenza A exposure in vitro. Exp. Lung Res. 2015;41:353–362. doi: 10.3109/01902148.2015.1040528. [DOI] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne B.D., Joy E.A., Hofmann M.G., Gesteland P.H., Cannon J.B., Lefler J.S., Blagev D.P., Korgenski E.K., Torosyan N., Hansen G.I., Kartchner D., Pope C.A., 3rd Short-term elevation of fine particulate matter air pollution and acute lower respiratory infection. Am. J. Respir. Crit. Care Med. 2018;198:759–766. doi: 10.1164/rccm.201709-1883OC. [DOI] [PubMed] [Google Scholar]
- Huang D.W., Sherman B.T., Tan Q., Collins J.R., Alvord W.G., Roayaei J., Stephens R., Baseler M.W., Lane H.C., Lempicki R.A. The DAVID Gene Functional Classification Tool: A novel biological module-centric algorithm to functionally analyze large gene lists. Genome Biol. 2007;8:R183. doi: 10.1186/gb-2007-8-9-r183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Zhou L., Chen J., Chen K., Liu Y., Chen X., Tang F. Acute effects of air pollution on influenza-like illness in Nanjing, China: A population-based study. Chemosphere. 2016;147:180–187. doi: 10.1016/j.chemosphere.2015.12.082. [DOI] [PubMed] [Google Scholar]
- Huang Y.C., Li Z., Carter J.D., Soukup J.M., Schwartz D.A., Yang I.V. Fine ambient particles induce oxidative stress and metal binding genes in human alveolar macrophages. Am. J. Respir. Cell Mol. Biol. 2009;41:544–552. doi: 10.1165/rcmb.2008-0064OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison G.R., Brown D.M., Hibbs L.R., Heal M.R., Donaldson K., Maynard R.L., Monaghan M., Nicholl A., Stone V. The effect of refurbishing a UK steel plant on PM10 metal composition and ability to induce inflammation. Respir. Res. 2005;6:43. doi: 10.1186/1465-9921-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idani E., Geravandi S., Akhzari M., Goudarzi G., Alavi N., Yari A.R., Mehrpour M., Khavasi M., Bahmaei J., Bostan H., Dobaradaran S., Salmanzadeh S., Mohammadi M.J. Characteristics, sources, and health risks of atmospheric PM10-bound heavy metals in a populated middle eastern city. Toxin Rev. 2018:1–9. [Google Scholar]
- Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B., Yang P., Sarao R., Wada T., Leong-Poi H., Crackower M.A., Fukamizu A., Hui C.C., Hein L., Uhlig S., Slutsky A.S., Jiang C., Penninger J.M. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- India State-Level Disease Burden Initiative Air Pollution, C The impact of air pollution on deaths, disease burden, and life expectancy across the states of India: the Global Burden of Disease Study 2017. Lancet Planet Health. 2019;3:e26–e39. doi: 10.1016/S2542-5196(18)30261-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Italian-Aerosol-Society . 2020. Information on the Relationship between Air Pollution and the Spread of COVID-19. [Google Scholar]
- Jain V., Dey S., Chowdhury S. Ambient PM2.5 exposure and premature mortality burden in the holy city Varanasi, India. Environ. Pollut. 2017;226:182–189. doi: 10.1016/j.envpol.2017.04.028. [DOI] [PubMed] [Google Scholar]
- Janssen N.A., Fischer P., Marra M., Ameling C., Cassee F.R. Short-term effects of PM2.5, PM10 and PM2.5-10 on daily mortality in The Netherlands. Sci. Total Environ. 2013;463–464:20–26. doi: 10.1016/j.scitotenv.2013.05.062. [DOI] [PubMed] [Google Scholar]
- Karambelas A., Holloway T., Kinney P.L., Fiore A.M., DeFries R., Kiesewetter G., Heyes C. Urban versus rural health impacts attributable to PM 2.5 and O 3 in northern India. Environ. Res. Lett. 2018;13 [Google Scholar]
- Karr C.J., Rudra C.B., Miller K.A., Gould T.R., Larson T., Sathyanarayana S., Koenig J.Q. Infant exposure to fine particulate matter and traffic and risk of hospitalization for RSV bronchiolitis in a region with lower ambient air pollution. Environ. Res. 2009;109:321–327. doi: 10.1016/j.envres.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsouyanni K., Samet J.M., Anderson H.R., Atkinson R., Le Tertre A., Medina S., Samoli E., Touloumi G., Burnett R.T., Krewski D., Ramsay T., Dominici F., Peng R.D., Schwartz J., Zanobetti A., Committee H.E.I.H.R. Air pollution and health: A European and North American approach (APHENA) Res. Rep. Health Eff. Inst. 2009:5–90. [PubMed] [Google Scholar]
- Khaefi M., Geravandi S., Hassani G., Yari A.R., Soltani F., Dobaradaran S., Moogahi S., Mohammadi M.J., Mahboubi M., Alavi N., Farhadi M., Khaniabadi Y.O. Association of particulate matter impact on prevalence of chronic obstructive pulmonary disease in Ahvaz, southwest Iran during 2009–2013. Aerosol Air Qual. Res. 2017;17:230–237. [Google Scholar]
- Khafaie M.A., Salvi S.S., Yajnik C.S., Ojha A., Khafaie B., Gore S.D. Air pollution and respiratory health among diabetic and non-diabetic subjects in Pune, India-results from the Wellcome Trust Genetic Study. Environ. Sci. Pollut. Res. Int. 2017;24:15538–15546. doi: 10.1007/s11356-017-9148-5. [DOI] [PubMed] [Google Scholar]
- Khilnani G.C., Tiwari P. Air pollution in India and related adverse respiratory health effects: Past, present, and future directions. Curr. Opin. Pulm. Med. 2018;24:108–116. doi: 10.1097/MCP.0000000000000463. [DOI] [PubMed] [Google Scholar]
- Kim H.J., Park J.H., Seo Y.S., Holsen T.M., Hopke P.K., Sung J., Son H.Y., Yun J.M., Kwon H., Cho B., Kim J.I. CYP1A1 gene polymorphisms modify the association between PM10 exposure and lung function. Chemosphere. 2018;203:353–359. doi: 10.1016/j.chemosphere.2018.03.196. [DOI] [PubMed] [Google Scholar]
- Kliengchuay W., Cooper Meeyai A., Worakhunpiset S., Tantrakarnapa K. Relationships between meteorological parameters and particulate matter in Mae Hong Son province, Thailand. Int. J. Environ. Res. Public Health. 2018;15 doi: 10.3390/ijerph15122801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krewski D., Jerrett M., Burnett R.T., Ma R., Hughes E., Shi Y., Turner M.C., Pope C.A., 3rd, Thurston G., Calle E.E., Thun M.J., Beckerman B., DeLuca P., Finkelstein N., Ito K., Moore D.K., Newbold K.B., Ramsay T., Ross Z., Shin H., Tempalski B. Extended follow-up and spatial analysis of the American Cancer Society study linking particulate air pollution and mortality. Res. Rep. Health Eff. Inst. 2009;5–114 discussion 115-136. [PubMed] [Google Scholar]
- Kumar A., Kumar A., Ingle H., Kumar S., Mishra R., Verma M.K., Biswas D., Kumar N.S., Mishra A., Raut A.A., Takaoka A., Kumar H. MicroRNA hsa-miR-324-5p suppresses H5N1 virus replication by targeting the viral PB1 and host CUEDC2. J. Virol. 2018;92 doi: 10.1128/JVI.01057-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrada-Delgado G., Aragon-Pina A., Campos-Ramos A., Castro-Romero T., Amador-Munoz O., Villalobos-Pietrini R. Chemical and morphological characterization of PM2.5 collected during MILAGRO campaign using scanning electron microscopy. Atmos. Pollut. Res. 2012;3:289–300. [Google Scholar]
- Landrigan P.J. Air pollution and health. Lancet Public Health. 2017;2:e4–e5. doi: 10.1016/S2468-2667(16)30023-8. [DOI] [PubMed] [Google Scholar]
- Landrigan P.J., Fuller R., Acosta N.J.R., Adeyi O., Arnold R., Basu N.N., Balde A.B., Bertollini R., Bose-O’Reilly S., Boufford J.I., Breysse P.N., Chiles T., Mahidol C., Coll-Seck A.M., Cropper M.L., Fobil J., Fuster V., Greenstone M., Haines A., Hanrahan D., Hunter D., Khare M., Krupnick A., Lanphear B., Lohani B., Martin K., Mathiasen K.V., McTeer M.A., Murray C.J.L., Ndahimananjara J.D., Perera F., Potocnik J., Preker A.S., Ramesh J., Rockstrom J., Salinas C., Samson L.D., Sandilya K., Sly P.D., Smith K.R., Steiner A., Stewart R.B., Suk W.A., van Schayck O.C.P., Yadama G.N., Yumkella K., Zhong M. The Lancet Commission on pollution and health. Lancet. 2018;391:462–512. doi: 10.1016/S0140-6736(17)32345-0. [DOI] [PubMed] [Google Scholar]
- Lelieveld J., Evans J.S., Fnais M., Giannadaki D., Pozzer A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature. 2015;525:367–371. doi: 10.1038/nature15371. [DOI] [PubMed] [Google Scholar]
- Li Y.G., Gao X. Epidemiologic studies of particulate matter and lung cancer. Chin. J. Cancer. 2014;33:376–380. doi: 10.5732/cjc.014.10063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ličbinský R., Frýbort A., Huzlík J., Adamec V., Effenberger K., Mikuška P., Vojtěšek M., Křůmal K. Usage of scanning electron microscopy for particulate matter sources identification. Trans. Transp. Sci. 2010:137–144. [Google Scholar]
- Lin C.I., Tsai C.H., Sun Y.L., Hsieh W.Y., Lin Y.C., Chen C.Y., Lin C.S. Instillation of particulate matter 2.5 induced acute lung injury and attenuated the injury recovery in ACE2 knockout mice. Int. J. Biol. Sci. 2018;14:253–265. doi: 10.7150/ijbs.23489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M., Stieb D.M., Chen Y. Coarse particulate matter and hospitalization for respiratory infections in children younger than 15 years in Toronto: A case-crossover analysis. Pediatrics. 2005;116 doi: 10.1542/peds.2004-2012. e235–240. [DOI] [PubMed] [Google Scholar]
- Ling S.H., van Eeden S.F. Particulate matter air pollution exposure: Role in the development and exacerbation of chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 2009;4:233–243. doi: 10.2147/copd.s5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Chen R., Sera F., Vicedo-Cabrera A.M., Guo Y., Tong S., Coelho M., Saldiva P.H.N., Lavigne E., Matus P., Valdes Ortega N., Osorio Garcia S., Pascal M., Stafoggia M., Scortichini M., Hashizume M., Honda Y., Hurtado-Diaz M., Cruz J., Nunes B., Teixeira J.P., Kim H., Tobias A., Iniguez C., Forsberg B., Astrom C., Ragettli M.S., Guo Y.L., Chen B.Y., Bell M.L., Wright C.Y., Scovronick N., Garland R.M., Milojevic A., Kysely J., Urban A., Orru H., Indermitte E., Jaakkola J.J.K., Ryti N.R.I., Katsouyanni K., Analitis A., Zanobetti A., Schwartz J., Chen J., Wu T., Cohen A., Gasparrini A., Kan H. Ambient particulate air pollution and daily mortality in 652 cities. N. Engl. J. Med. 2019;381:705–715. doi: 10.1056/NEJMoa1817364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Yang N., Tang J., Liu S., Luo D., Duan Q., Wang X. Downregulation of angiotensin-converting enzyme 2 by the neuraminidase protein of influenza A (H1N1) virus. Virus Res. 2014;185:64–71. doi: 10.1016/j.virusres.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.X., Li Y., Qin G., Zhu Y., Li X., Zhang J., Zhao K., Hu M., Wang X.L., Zheng X. Effects of air pollutants on occurrences of influenza-like illness and laboratory-confirmed influenza in Hefei, China. Int. J. Biometeorol. 2019;63:51–60. doi: 10.1007/s00484-018-1633-0. [DOI] [PubMed] [Google Scholar]
- Loane C., Pilinis C., Lekkas T.D., Politis M. Ambient particulate matter and its potential neurological consequences. Rev. Neurosci. 2013;24:323–335. doi: 10.1515/revneuro-2013-0001. [DOI] [PubMed] [Google Scholar]
- Lu F., Xu D., Cheng Y., Dong S., Guo C., Jiang X., Zheng X. Systematic review and meta-analysis of the adverse health effects of ambient PM2.5 and PM10 pollution in the Chinese population. Environ. Res. 2015;136:196–204. doi: 10.1016/j.envres.2014.06.029. [DOI] [PubMed] [Google Scholar]
- MacNee W., Donaldson K. Mechanism of lung injury caused by PM10 and ultrafine particles with special reference to COPD. Eur. Respir. J. Suppl. 2003;40:47s–51s. doi: 10.1183/09031936.03.00403203. [DOI] [PubMed] [Google Scholar]
- Mallone S., Stafoggia M., Faustini A., Gobbi G.P., Marconi A., Forastiere F. Saharan dust and associations between particulate matter and daily mortality in Rome, Italy. Environ. Health Perspect. 2011;119:1409–1414. doi: 10.12989/ehp.1003026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manojkumar N., Srimuruganandam B. Health effects of particulate matter in major Indian cities. Int. J. Environ. Health Res. 2019:1–13. doi: 10.1080/09603123.2019.1651257. [DOI] [PubMed] [Google Scholar]
- Marzouni M.B., Moradi M., Zarasvandi A., Akbaripoor S., Hassanvand M.S., Neisi A., Goudarzi G., Mohammadi M.J., Sheikhi R., Kermani M., Shirmardi M., Naimabadi A., Gholami M., Mozhdehi S.P., Esmaeili M., Barari K. Health benefits of PM10 reduction in Iran. Int. J. Biometeorol. 2017;61:1389–1401. doi: 10.1007/s00484-017-1316-2. [DOI] [PubMed] [Google Scholar]
- Massad E., Coutinho F.A., Ma S., Burattini M.N. A hypothesis for the 2007 dengue outbreak in Singapore. Epidemiol. Infect. 2010;138:951–957. doi: 10.1017/S0950268809990501. [DOI] [PubMed] [Google Scholar]
- Miller R.L., Peden D.B. Environmental effects on immune responses in patients with atopy and asthma. J. Allergy Clin. Immunol. 2014;134:1001–1008. doi: 10.1016/j.jaci.2014.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita L., Foley G., Semple S., Grigg J. 2020. Traffic-derived Particulate Matter and Angiotensin-Converting Enzyme 2 Expression in Human Airway Epithelial Cells. 2020.2005.2015.097501. [Google Scholar]
- Momtazan M., Geravandi S., Rastegarimehr B., Valipour A., Ranjbarzadeh A., Yari A.R., Dobaradaran S., Bostan H., Farhadi M., Darabi F., Omidi Khaniabadi Y., Mohammadi M.J. An investigation of particulate matter and relevant cardiovascular risks in Abadan and Khorramshahr in 2014–2016. Toxin Rev. 2019;38:290–297. [Google Scholar]
- Morales K.F., Paget J., Spreeuwenberg P. Possible explanations for why some countries were harder hit by the pandemic influenza virus in 2009 - a global mortality impact modeling study. BMC Infect. Dis. 2017;17:642. doi: 10.1186/s12879-017-2730-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naimabadi A., Ghadiri A., Idani E., Babaei A.A., Alavi N., Shirmardi M., Khodadadi A., Marzouni M.B., Ankali K.A., Rouhizadeh A., Goudarzi G. Chemical composition of PM10 and its in vitro toxicological impacts on lung cells during the Middle Eastern Dust (MED) storms in Ahvaz, Iran. Environ. Pollut. 2016;211:316–324. doi: 10.1016/j.envpol.2016.01.006. [DOI] [PubMed] [Google Scholar]
- Ni L., Chuang C.C., Zuo L. Fine particulate matter in acute exacerbation of COPD. Front. Physiol. 2015;6:294. doi: 10.3389/fphys.2015.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Beirne S.L., Shenoy S.A., Salit J., Strulovici-Barel Y., Kaner R.J., Visvanathan S., Fine J.S., Mezey J.G., Crystal R.G. Ambient pollution-related reprogramming of the human small airway epithelial transcriptome. Am. J. Respir. Crit. Care Med. 2018;198:1413–1422. doi: 10.1164/rccm.201712-2526OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeder S., Jorres R.A., Weichenmeier I., Pusch G., Schober W., Pfab F., Behrendt H., Schierl R., Kronseder A., Nowak D., Dietrich S., Fernandez-Caldas E., Lintelmann J., Zimmermann R., Lang R., Mages J., Fromme H., Buters J.T. Airborne indoor particles from schools are more toxic than outdoor particles. Am. J. Respir. Cell Mol. Biol. 2012;47:575–582. doi: 10.1165/rcmb.2012-0139OC. [DOI] [PubMed] [Google Scholar]
- Ogen Y. Assessing nitrogen dioxide (NO2) levels as a contributing factor to coronavirus (COVID-19) fatality. Sci. Total Environ. 2020;726:138605. doi: 10.1016/j.scitotenv.2020.138605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi H., Nishioka K., Nakajima S., Kim S., Suzuki R., Aizaki H., Fukasawa M., Kamisuki S., Sugawara F., Ohtani N., Muramatsu M., Wakita T., Watashi K. The aryl hydrocarbon receptor-cytochrome P450 1A1 pathway controls lipid accumulation and enhances the permissiveness for hepatitis C virus assembly. J. Biol. Chem. 2018;293:19559–19571. doi: 10.1074/jbc.RA118.005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton T.E., Renton K.W. Cytokine-mediated down-regulation of CYP1A1 in Hepa1 cells. Biochem. Pharmacol. 1998;55:1791–1796. doi: 10.1016/s0006-2952(98)00028-8. [DOI] [PubMed] [Google Scholar]
- Paulin L., Hansel N. 2016. Particulate Air Pollution and Impaired Lung Function. F1000Res 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L., Zhao X., Tao Y., Mi S., Huang J., Zhang Q. The effects of air pollution and meteorological factors on measles cases in Lanzhou, China. Environ. Sci. Pollut. Res. Int. 2020;27:13524–13533. doi: 10.1007/s11356-020-07903-4. [DOI] [PubMed] [Google Scholar]
- Perez L., Tobias A., Querol X., Kunzli N., Pey J., Alastuey A., Viana M., Valero N., Gonzalez-Cabre M., Sunyer J. Coarse particles from Saharan dust and daily mortality. Epidemiology. 2008;19:800–807. doi: 10.1097/ede.0b013e31818131cf. [DOI] [PubMed] [Google Scholar]
- Piazzalunga-Expert A. Mimeo; 2020. Evaluation of the Potential Relationship between Particulate Matter (PM) Pollution and COVID-19 Infection Spread in Italy. [Google Scholar]
- Pope C.A., 3rd, Schwartz J., Ransom M.R. Daily mortality and PM10 pollution in Utah Valley. Arch. Environ. Health. 1992;47:211–217. doi: 10.1080/00039896.1992.9938351. [DOI] [PubMed] [Google Scholar]
- Proud D., Hudy M.H., Wiehler S., Zaheer R.S., Amin M.A., Pelikan J.B., Tacon C.E., Tonsaker T.O., Walker B.L., Kooi C., Traves S.L., Leigh R. Cigarette smoke modulates expression of human rhinovirus-induced airway epithelial host defense genes. PLoS One. 2012;7 doi: 10.1371/journal.pone.0040762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puttini S., Plaisance I., Barile L., Cervio E., Milano G., Marcato P., Pedrazzini T., Vassalli G. ALDH1A3 is the key isoform that contributes to aldehyde dehydrogenase activity and affects in vitro proliferation in cardiac atrial appendage progenitor cells. Front. Cardiovasc. Med. 2018;5:90. doi: 10.3389/fcvm.2018.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quezada-Maldonado E.M., Sanchez-Perez Y., Chirino Y.I., Vaca-Paniagua F., Garcia-Cuellar C.M. miRNAs deregulation in lung cells exposed to airborne particulate matter (PM10) is associated with pathways deregulated in lung tumors. Environ. Pollut. 2018;241:351–358. doi: 10.1016/j.envpol.2018.05.073. [DOI] [PubMed] [Google Scholar]
- Ramirez-Leal R., Esparza-Ponce H.E., Varela-Sortillón A., Astorga-Reyes A., Roman-B A. Characterization of inhalable particulate matter in ambient air by scanning electron microscopy and energy-dispersive X-ray analysis. Microsc. Microanal. 2009;15:1320–1321. [Google Scholar]
- Ramirez-Leal R., Valle-Martinez M., Cruz-Campas M. Chemical and morphological study of PM10 analysed by SEM-EDS. Open J. Air Pollut. 2014;3(4):9. [Google Scholar]
- Reyes-Zarate E., Sanchez-Perez Y., Gutierrez-Ruiz M.C., Chirino Y.I., Osornio-Vargas A.R., Morales-Barcenas R., Souza-Arroyo V., Garcia-Cuellar C.M. Atmospheric particulate matter (PM10) exposure-induced cell cycle arrest and apoptosis evasion through STAT3 activation via PKCzeta and Src kinases in lung cells. Environ. Pollut. 2016;214:646–656. doi: 10.1016/j.envpol.2016.04.072. [DOI] [PubMed] [Google Scholar]
- Ritchie M.E., Phipson B., Wu D., Hu Y., Law C.W., Shi W., Smyth G.K. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas-Santiago C.E., Sarkar S., Cantarella P.t., Osornio-Vargas A., Quintana-Belmares R., Meng Q., Kirn T.J., Ohman Strickland P., Chow J.C., Watson J.G., Torres M., Schwander S. Air pollution particulate matter alters antimycobacterial respiratory epithelium innate immunity. Infect. Immun. 2015;83:2507–2517. doi: 10.1128/IAI.03018-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romieu I., Gouveia N., Cifuentes L.A., de Leon A.P., Junger W., Vera J., Strappa V., Hurtado-Diaz M., Miranda-Soberanis V., Rojas-Bracho L., Carbajal-Arroyo L., Tzintzun-Cervantes G., Committee H.E.I.H.R. Multicity study of air pollution and mortality in Latin America (the ESCALA study) Res. Rep. Health Eff. Inst. 2012:5–86. [PubMed] [Google Scholar]
- Samet J.M., Dominici F., Curriero F.C., Coursac I., Zeger S.L. Fine particulate air pollution and mortality in 20 U.S. cities, 1987-1994. N. Engl. J. Med. 2000;343:1742–1749. doi: 10.1056/NEJM200012143432401. [DOI] [PubMed] [Google Scholar]
- Sandstrom T., Cassee F.R., Salonen R., Dybing E. Recent outcomes in European multicentre projects on ambient particulate air pollution. Toxicol. Appl. Pharmacol. 2005;207:261–268. doi: 10.1016/j.taap.2005.03.031. [DOI] [PubMed] [Google Scholar]
- Sarkar S., Rivas-Santiago C.E., Ibironke O.A., Carranza C., Meng Q., Osornio-Vargas A., Zhang J., Torres M., Chow J.C., Watson J.G., Ohman-Strickland P., Schwander S. Season and size of urban particulate matter differentially affect cytotoxicity and human immune responses to Mycobacterium tuberculosis. PloS One. 2019;14 doi: 10.1371/journal.pone.0219122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saygin M., Gonca T., Ozturk O., Has M., Caliskan S., Has Z.G., Akkaya A. To investigate the effects of air pollution (PM10 and SO2) on the respiratory diseases asthma and chronic obstructive pulmonary disease. Turk. Thorac. J. 2017;18:33–39. doi: 10.5152/TurkThoracJ.2017.16016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciomer S., Moscucci F., Magri D., Badagliacca R., Piccirillo G., Agostoni P. SARS-CoV-2 spread in Northern Italy: What about the pollution role? Environ. Monit. Assess. 2020;192:325. doi: 10.1007/s10661-020-08317-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setti L., Passarini F., De Gennaro G., Barbieri P., Pallavicini A., Ruscio M., Piscitelli P., Colao A., Miani A. Searching for SARS-COV-2 on particulate matter: A possible early indicator of COVID-19 epidemic recurrence. Int. J. Environ. Res. Public Health. 2020;17 doi: 10.3390/ijerph17092986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setti L., Passarini F., De Gennaro G., Barbieri P., Perrone M.G., Borelli M., Palmisani J., Di Gilio A., Torboli V., Fontana F., Clemente L., Pallavicini A., Ruscio M., Piscitelli P., Miani A. SARS-Cov-2RNA found on particulate matter of Bergamo in northern Italy: First evidence. Environ. Res. 2020 doi: 10.1016/j.envres.2020.109754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A.K., Baliyan P., Kumar P. Air pollution and public health: The challenges for Delhi, India. Rev. Environ. Health. 2018;33:77–86. doi: 10.1515/reveh-2017-0032. [DOI] [PubMed] [Google Scholar]
- Sharma M., Kumar V.N., Katiyar S.K., Sharma R., Shukla B.P., Sengupta B. Effects of particulate air pollution on the respiratory health of subjects who live in three areas in Kanpur, India. Arch. Environ. Health. 2004;59:348–358. doi: 10.3200/AEOH.59.7.348-358. [DOI] [PubMed] [Google Scholar]
- Sharma S., Sethi G.R., Rohtagi A., Chaudhary A., Shankar R., Bapna J.S., Joshi V., Sapir D.G. Indoor air quality and acute lower respiratory infection in Indian urban slums. Environ. Health Perspect. 1998;106:291–297. doi: 10.1289/ehp.98106291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderholm S., Kainov D.E., Ohman T., Denisova O.V., Schepens B., Kulesskiy E., Imanishi S.Y., Corthals G., Hintsanen P., Aittokallio T., Saelens X., Matikainen S., Nyman T.A. Phosphoproteomics to characterize host response during influenza A virus infection of human macrophages. Mol. Cell. Proteom. 2016;15:3203–3219. doi: 10.1074/mcp.M116.057984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soneson C., Love M.I., Robinson M.D. Differential analyses for RNA-seq: transcript-level estimates improve gene-level inferences. F1000Research. 2015;4:1521. doi: 10.12688/f1000research.7563.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X., Yang S., Shao L., Fan J., Liu Y. PM10 mass concentration, chemical composition, and sources in the typical coal-dominated industrial city of Pingdingshan, China. Sci. Total Environ. 2016;571:1155–1163. doi: 10.1016/j.scitotenv.2016.07.115. [DOI] [PubMed] [Google Scholar]
- Stavropoulou E., Pircalabioru G.G., Bezirtzoglou E. The role of cytochromes P450 in infection. Front. Immunol. 2018;9:89. doi: 10.3389/fimmu.2018.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q., Huang K., Liu J., Wu S., Shen D., Dai P., Li C. Fine particulate matter from pig house induced immune response by activating TLR4/MAPK/NF-kappaB pathway and NLRP3 inflammasome in alveolar macrophages. Chemosphere. 2019;236:124373. doi: 10.1016/j.chemosphere.2019.124373. [DOI] [PubMed] [Google Scholar]
- Tecer L.H., Alagha O., Karaca F., Tuncel G., Eldes N. Particulate matter (PM(2.5), PM(10-2.5), and PM(10)) and children’s hospital admissions for asthma and respiratory diseases: A bidirectional case-crossover study. J. Toxicol. Environ. Health. 2008;71:512–520. doi: 10.1080/15287390801907459. [DOI] [PubMed] [Google Scholar]
- Temerozo J.R., de Azevedo S.S.D., Insuela D.B.R., Vieira R.C., Ferreira P.L.C., Carvalho V.F., Bello G., Bou-Habib D.C. The neuropeptides vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide control HIV-1 infection in macrophages through activation of protein kinases A and C. Front. Immunol. 2018;9:1336. doi: 10.3389/fimmu.2018.01336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre D., Lachmann A., Ma’ayan A. BioJupies: Automated generation of interactive notebooks for RNA-seq data analysis in the cloud. Cell Syst. 2018;7:556–561. doi: 10.1016/j.cels.2018.10.007. e553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totlandsdal A.I., Cassee F.R., Schwarze P., Refsnes M., Lag M. Diesel exhaust particles induce CYP1A1 and pro-inflammatory responses via differential pathways in human bronchial epithelial cells. Part. Fibre Toxicol. 2010;7:41. doi: 10.1186/1743-8977-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totlandsdal A.I., Ovrevik J., Cochran R.E., Herseth J.I., Bolling A.K., Lag M., Schwarze P., Lilleaas E., Holme J.A., Kubatova A. The occurrence of polycyclic aromatic hydrocarbons and their derivatives and the proinflammatory potential of fractionated extracts of diesel exhaust and wood smoke particles. J. Environ. Sci. Health A Tox. Hazard. Subst Environ. Eng. 2014;49:383–396. doi: 10.1080/10934529.2014.854586. [DOI] [PubMed] [Google Scholar]
- Tulake W., Yuemaier R., Sheng L., Ru M., Lidifu D., Abudula A. Upregulation of stem cell markers ALDH1A1 and OCT4 as potential biomarkers for the early detection of cervical carcinoma. Oncol. Lett. 2018;16:5525–5534. doi: 10.3892/ol.2018.9381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuvjargal N., Enkhtsetseg L., Shagjjamba D., Zuzaan P. vol. 704. 2019. Morphological study of PM2.5 by SEM-EDS. (Ulaanbaatar. IOP Conference Series: Materials Science and Engineering). [Google Scholar]
- Ulrich M.M., Alink G.M., Kumarathasan P., Vincent R., Boere A.J., Cassee F.R. Health effects and time course of particulate matter on the cardiopulmonary system in rats with lung inflammation. J. Toxicol. Environ. Health. 2002;65:1571–1595. doi: 10.1080/00984100290071676. [DOI] [PubMed] [Google Scholar]
- Valavanidis A., Fiotakis K., Vlachogianni T. Airborne particulate matter and human health: Toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2008;26:339–362. doi: 10.1080/10590500802494538. [DOI] [PubMed] [Google Scholar]
- Van Den Heuvel R., Den Hond E., Govarts E., Colles A., Koppen G., Staelens J., Mampaey M., Janssen N., Schoeters G. Identification of PM10 characteristics involved in cellular responses in human bronchial epithelial cells (Beas-2B) Environ. Res. 2016;149:48–56. doi: 10.1016/j.envres.2016.04.029. [DOI] [PubMed] [Google Scholar]
- Vandini S., Corvaglia L., Alessandroni R., Aquilano G., Marsico C., Spinelli M., Lanari M., Faldella G. Respiratory syncytial virus infection in infants and correlation with meteorological factors and air pollutants. Ital. J. Pediatr. 2013;39:1. doi: 10.1186/1824-7288-39-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdecchia P., Cavallini C., Spanevello A., Angeli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur. J. Intern. Med. 2020 doi: 10.1016/j.ejim.2020.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter W., Sanchez-Cabo F., Ricote M. GOplot: An R package for visually combining expression data with functional analysis. Bioinformatics. 2015;31:2912–2914. doi: 10.1093/bioinformatics/btv300. [DOI] [PubMed] [Google Scholar]
- Wang J., Li Q., Xie J., Xu Y. Cigarette smoke inhibits BAFF expression and mucosal immunoglobulin A responses in the lung during influenza virus infection. Respir. Res. 2015;16:37. doi: 10.1186/s12931-015-0201-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen C.P., Gao W. PM2.5: An important cause for chronic obstructive pulmonary disease? Lancet Planet Health. 2018;2:e105–e106. doi: 10.1016/S2542-5196(18)30025-1. [DOI] [PubMed] [Google Scholar]
- WHO . 2018. Thirteenth General Programme of Work2019–2023. [Google Scholar]
- Wong C.M., Vichit-Vadakan N., Kan H., Qian Z. Public Health and Air Pollution in Asia (PAPA): A multicity study of short-term effects of air pollution on mortality. Environ. Health Perspect. 2008;116:1195–1202. doi: 10.1289/ehp.11257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Nethery R.C., Sabath B.M., Braun D., Dominici F. 2020. Exposure to Air Pollution and COVID-19 Mortality in the United States: A Nationwide Cross-Sectional Study. 2020.2004.2005.20054502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Ha S.U., Basnet R. A review of epidemiological research on adverse neurological effects of exposure to ambient air pollution. Front. Public Health. 2016;4:157. doi: 10.3389/fpubh.2016.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q., Fu J.F., Mao J.H., Shang S.Q. Haze is a risk factor contributing to the rapid spread of respiratory syncytial virus in children. Environ. Sci. Pollut. Res. Int. 2016;23:20178–20185. doi: 10.1007/s11356-016-7228-6. [DOI] [PubMed] [Google Scholar]
- Yin X., Yu H., Jin X., Li J., Guo H., Shi Q., Yin Z., Xu Y., Wang X., Liu R., Wang S., Zhang L. Human blood CD1c+ dendritic cells encompass CD5high and CD5low subsets that differ significantly in phenotype, gene expression, and functions. J. Immunol. 2017;198:1553–1564. doi: 10.4049/jimmunol.1600193. [DOI] [PubMed] [Google Scholar]
- Yorifuji T., Bae S., Kashima S., Tsuda T., Doi H., Honda Y., Kim H., Hong Y.C. Health impact assessment of PM10 and PM2.5 in 27 southeast and east Asian cities. J. Occup. Environ. Med. 2015;57:751–756. doi: 10.1097/JOM.0000000000000485. [DOI] [PubMed] [Google Scholar]
- Zhang W.Y., Wang H., Qi S., Wang X., Li X., Zhou K., Zhang Y., Gao M.Q. CYP1A1 relieves lipopolysaccharide-induced inflammatory responses in bovine mammary epithelial cells. Mediat. Inflamm. 2018;2018:4093285. doi: 10.1155/2018/4093285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Richardson B., Takle E., Chai L., Schmitt D., Xin H. Airborne transmission may have played a role in the spread of 2015 highly pathogenic avian influenza outbreaks in the United States. Sci. Rep. 2019;9:11755. doi: 10.1038/s41598-019-47788-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Xie J., Huang F., Cao L. Association between short-term exposure to air pollution and COVID-19 infection: Evidence from China. Sci. Total Environ. 2020;727:138704. doi: 10.1016/j.scitotenv.2020.138704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Z., Yan Y., Shu Y., Gao R., Sun Y., Li X., Ju X., Liang Z., Liu Q., Zhao Y., Guo F., Bai T., Han Z., Zhu J., Zhou H., Huang F., Li C., Lu H., Li N., Li D., Jin N., Penninger J.M., Jiang C. Angiotensin-converting enzyme 2 protects from lethal avian influenza A H5N1 infections. Nat. Commun. 2014;5:3594. doi: 10.1038/ncomms4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.