Rapid scale-up of preexposure prophylaxis resulted in a redistribution of human immunodeficiency virus and sexually transmitted infection testing across services in Victoria, Australia and did not negatively impact testing among gay and bisexual and other men who have sex with men not using preexposure prophylaxis.
Objective
Scale-up of human immunodeficiency virus (HIV) preexposure prophylaxis (PrEP) has raised concerns regarding its impact on clinic capacity and access to HIV testing. We describe enrolment in PrEPX, a large PrEP implementation study in Victoria, Australia, and the impact of PrEP uptake and maintenance on existing health services.
Methods
We describe enrolment between July 26, 2016, and March 31, 2018, and trends in HIV testing among PrEPX participating and nonparticipating gay and bisexual and other men who have sex with men (GBM) at 5 study clinics participating in a sentinel surveillance system (ACCESS). We evaluated HIV and STI testing trends using segmented linear regression across the prestudy (January 2015 to June 2016) and PrEPX study (July 2016 to March 2018) periods.
Findings
There were 2,049 individuals who registered interest in study participation: 72% enrolled into the study. Study clinics enrolled participants rapidly; of 4265 people enrolled in PrEPX (98% GBM), 1000 enrolled by week 3, 88% (n = 876) of whom enrolled at ACCESS sites.
Prestudy period HIV testing rates were increasing at all ACCESS sites. In the month PrEPX commenced, there was an additional 247 HIV tests among PrEPX participants (P < 0.01) and no significant change among non-PrEPX GBM (P = 0.72). Across the study period, HIV testing increased by 7.2 (P < 0.01) and 8.9 (P < 0.01) tests/month among PrEPX participants and non-PrEPX GBM, respectively. The HIV testing increased among non-PrEPX GBM at sexual health clinics (18.8 tests/month, P < 0.01) and primary care clinics (7.9 tests/month, P < 0.01). Similar trends were observed across testing for all measured STIs.
Conclusions
Rapid PrEP scale-up is possible without a reduction in HIV testing among GBM not using PrEP.
Daily use of tenofovir and emtricitabine for human immunodeficiency virus (HIV) preexposure prophylaxis (PrEP) is shown to be highly efficacious at preventing transmission among gay, bisexual and other men who have sex with men (GBM),1,2 heterosexuals,3 transgender women,4 and people who inject drugs5 in the setting of high medication adherence. The World Health Organization recommends that daily PrEP be offered as a prevention option for people at substantial risk of HIV infection as part of a combination prevention approach and includes PrEP on their list of essential medicines.6
Despite these recommendations, the scale-up of PrEP has been suboptimal in many countries.7 In Organization for Economic Co-operation and Development countries, the pace of PrEP scale-up has been slow. The overall number of people on PrEP is highest in the United States; however, coverage varies considerably by jurisdiction, race, and gender.8 In the United Kingdom, the Netherlands, and many other European countries, there are no subsidies for PrEP, and debate in these jurisdictions has focused on health care costs of PrEP and concerns about the ability of sexual health services to meet service demands for requisite quarterly clinic visits for HIV and STI testing.9,10 In Eastern Europe and Central Asia, where HIV incidence has risen by 30% since 2010, PrEP was available through the public health system only in the Republic of Moldova, Georgia, and the Ukraine.11
In Australia, HIV is highly concentrated among GBM with over 70% of annual notifications occurring within this group.12 Preexposure prophylaxis was approved for use in HIV prevention by the Therapeutic Goods Administration in 2017, national PrEP prescribing guidelines were published in 2017,13 and PrEP was listed on the Australia's list of subsidized medicines (Pharmaceutical Benefits Scheme [PBS]) in April 2018.14 Before PBS subsidization of PrEP most Australian jurisdictions commenced large PrEP demonstration projects during 2016 and 2017. Personal importation of PrEP via online pharmacies and through private unsubsidized prescriptions also occurred at modest levels.15
There are limited data regarding the impact of PrEP scale-up on access to services among priority populations not engaged in PrEP. In this article, we describe the implementation of the PrEPX study (a large implementation study in Victoria, Australia), the rate of PrEP uptake, and the impact on HIV testing rates for nonstudy participating GBM attending study clinics.
METHODS
Study Design
The PrEPX was a prospective, population-level PrEP implementation study that commenced on July 26, 2016, and ran for 21 months. The study protocol has been described in detail elsewhere.16 The PrEPX was designed on the basis of an a priori estimate that maintaining 2600 individuals at risk of HIV infection on PrEP would reduce state-wide HIV incidence by 25% overall and by 30% among GBM within 36 months of study commencement. In the 7 months before study commencement (January 26, 2016 to July 25, 2016), individuals could register interest in the study by completing an online form. After the study commenced on July 26, 2016, waitlisted participants were contacted and offered an enrolment appointment at their clinic of choice or at an alternative clinic if their preferred study site did not have capacity. Due to participant demand, an additional 600 study places were funded on January 19, 2017, with a further 600 study places funded on March 28, 2017, bringing the total number of available places to 3800. However, due to the staggered enrolment and study withdrawals, when study enrolment closed on March 31, 2018, because PrEP became publicly subsidized through the PBS, 4265 people had been prescribed PrEP through the study.
Each study clinic had the latitude to implement PrEPX in a manner that suited their service. The primary care clinics obtain funding for each consultation through a government rebate and may charge an additional private consultation fee. Primary care clinics incorporated PrEPX patients into their existing available appointment schedule and were paid by the study for each participant they enrolled. The 2 sexual health services receive block funding for services. The sexual health clinic developed a new system to participate in PrEPX whereupon they obtained a government rebate for each PrEP consultation, separate to their existing funding. This allowed them to establish separate clinics to enroll PrEPX participants. One sexual health clinic received funding for a nurse to support their clinic, the other received payment for each participant enrolled.
Study Visits
The PrEPX study visits were scheduled to occur every 2 months. At each study visit, participants underwent a clinical review, laboratory testing for HIV and STIs (syphilis, gonorrhea, and chlamydia), and received a 3-month PrEP prescription. Drug was dispensed from participating community and hospital study pharmacies.17 In addition to scheduled PrEPX study visits, participants could attend their PrEPX participating site for other health care, including assessment for symptomatic STI.
Data Collection
Registration of Interest
Individuals expressed their interest in participating in the PrEPX study through an online waiting list form. Data were collected and managed using REDCap online survey manager electronic data capture tools.18 Demographic characteristics, history of prior PrEP use, and preferred PrEPX site for study enrolment were asked as part of waiting list registration.
Enrolment and Withdrawal
At the baseline enrolment visit, the clinician completed an enrolment eligibility survey with the participant, using REDCap.18 This questionnaire collected data on participant demographic characteristics, reasons for enrolment, previous PrEP use, HIV/STI testing history, and sexual and injecting behaviors.
Participant withdrawal forms were also completed by the clinician using REDCap,18 and included free text information on reasons for withdrawal, which were categorized into 9 domains for this analysis.
HIV and STI Testing
Prestudy and study period HIV and STI testing was available at the 5 PrEPX study sites (3 metropolitan primary care clinics and 2 metropolitan sexual health services) that also participated in the Australian Collaboration for Coordinated Enhanced Sentinel Surveillance of Blood Borne Viruses and Sexually Transmitted Infections system (ACCESS). These ACCESS study sites enrolled 3317 (78%) of 4265 PrEPX participants. ACCESS automatically extracts deidentified HIV and STI test information from clinic patient management systems through the GRHANITE data extraction software. ACCESS collected continuous HIV and STI test data from 2013 across the 5 sites and links individuals tests within a single clinic and across clinics in the ACCESS network.19–21
Data Analysis
We report the monthly number of people registering their interest in PrEPX through the online waitlist between January 30, 2016, and March 31, 2018, and the monthly PrEPX participant enrolments based on enrolment survey completions between July 26, 2016, and March 31, 2018. Characteristics of all participants enrolling in PrEPX are described. Variables examined included age, gender, date of enrolment, PrEPX study site, PrEP eligibility criteria, and any previous PrEP or postexposure prophylaxis (PEP) use.
We estimated shifting demand for HIV testing services before enrolment date among PrEPX participants who enrolled at sites within the ACCESS network. We report the clinic type where they enrolled in PrEPX (sexual health service or primary care service) and the clinic type they attended for HIV testing between January 1, 2015, and their enrolment date to describe movement of study participants after their enrolment into the PrEPX study.
We assessed the impact of the PrEPX study on HIV testing by comparing the aggregated number of monthly HIV tests conducted at the 5 PrEPX study sites in the 18 months before PrEPX (prestudy; January 2015 to June 2016) and the 21 months of the PrEPX study (study; July 2016 to March 2018) using segmented linear regression. This analysis focused on 5 PrEPX clinics (2 sexual health services and 3 primary care settings) where historical HIV testing data were available through the ACCESS network. Specific regression analyses were conducted examining HIV testing at the 2 sexual health clinics and the 3 primary care clinics to determine if there was a difference in trends between the 2 clinic types. All regression analyses were disaggregated by HIV testing among PrEPX participants and non-PrEPX participant GBM. The PrEPX participants were defined as those enrolling in the first 12 months of the study, and GBM not participating in PrEPX were identified using previously defined methods.20,22
We repeated the analyses to assess the impact of the PrEPX study on bacterial STI testing. Each individual could contribute a maximum of 1 test event per day in each bacterial STI analysis. In Victoria, the overwhelming majority of Chlamydia trachomatis (CT) and Neisseria gonorrhea (NG) testing are conducted using duplex testing, we therefore collapsed testing for these bacteria at any anatomical site to assess CT/NG testing. We separately assessed syphilis testing. For 1 sexual health clinic which enrolled 329 study participants, CT/NG and syphilis testing was not available before the introduction of STI testing at this clinic in February 2016.
All segmented linear regression models used Newey-West standard errors and were adjusted for a maximum of 1 lag. We report the y-intercept (β0), the prestudy trend (β1, January 2015–June 2016), the immediate change in testing at the time of study commencement (β2, PrEPX commences July 2016), and the change in trend (β3) from prestudy to study periods (β1–β3, July 2016–March 2018). All statistical analyses were performed using Stata version 14 (StataCorp LP, College Station, TX) with a significance cutoff of P less than 0.05.
Human Research Ethics
Written informed consent was obtained from all study participants at study enrolment. The study was approved by the Alfred Health Human Research and Ethics Committee (HREC100/16) and registered on the Australian New Zealand Clinical Trials Registry 2 September 2016 (ACTRN12616001215415). The ACCESS study was approved by Alfred Health Human Research and Ethics Committee (HREC248/17) and this ethics committee waived the need for the individuals' consent.20
Role of the Funding Source
Individuals from the Victorian Department of Health and Human Services and the Thorne Harbor Health (previously Victorian AIDS Council) were PrEPX study coinvestigators and are coauthors on this article. They had a role in the design, review, and approval of the article and in the decision to submit the article for publication.
RESULTS
Study Enrolment
At study commencement on July 26, 2016, 2049 individuals had registered their interest in participating in PrEPX, of whom 1471 (72%) enrolled in PrEPX within the first 12 months. An average of 183 new people registered their interest each month throughout the 21 months of the study (Fig. 1).
Figure 1.
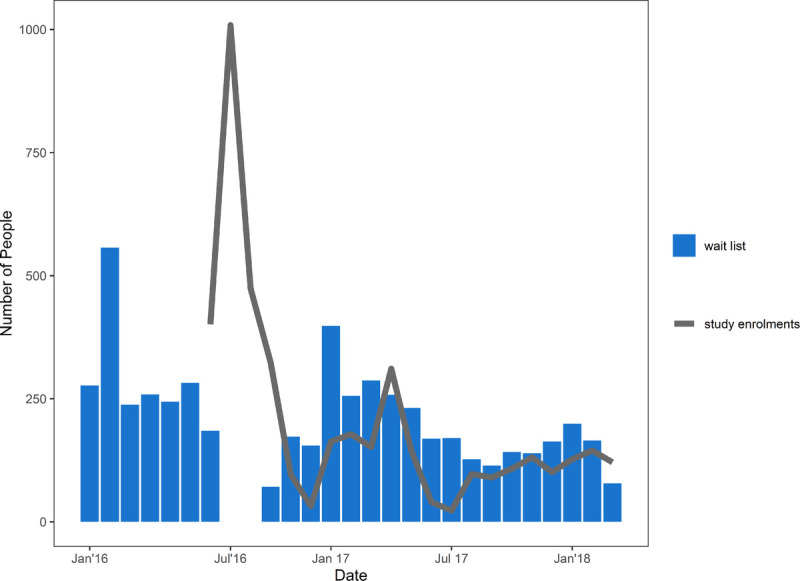
Registration (January 26, 2016 to March 31, 2018) and enrolment (July 16, 2016 to March 31, 2018) into PrEPX study.
Between July 26 2016, and March 31, 2018, 4265 people enrolled into PrEPX in Victoria. The study recorded rapid early enrolment, with 1000 people enrolled in the first 3 weeks, 87.8% (n = 876) of whom enrolled at ACCESS sites, and 80% of the original funded number of study places (2080 of 2600) filled by week 13 (Fig. 1). Two thirds of all PrEPX participants (n = 2768) enrolled at primary care clinics (Table 1).
TABLE 1.
Characteristics of PrEPX Study Participants, July 26, 2016 to March 31, 2018
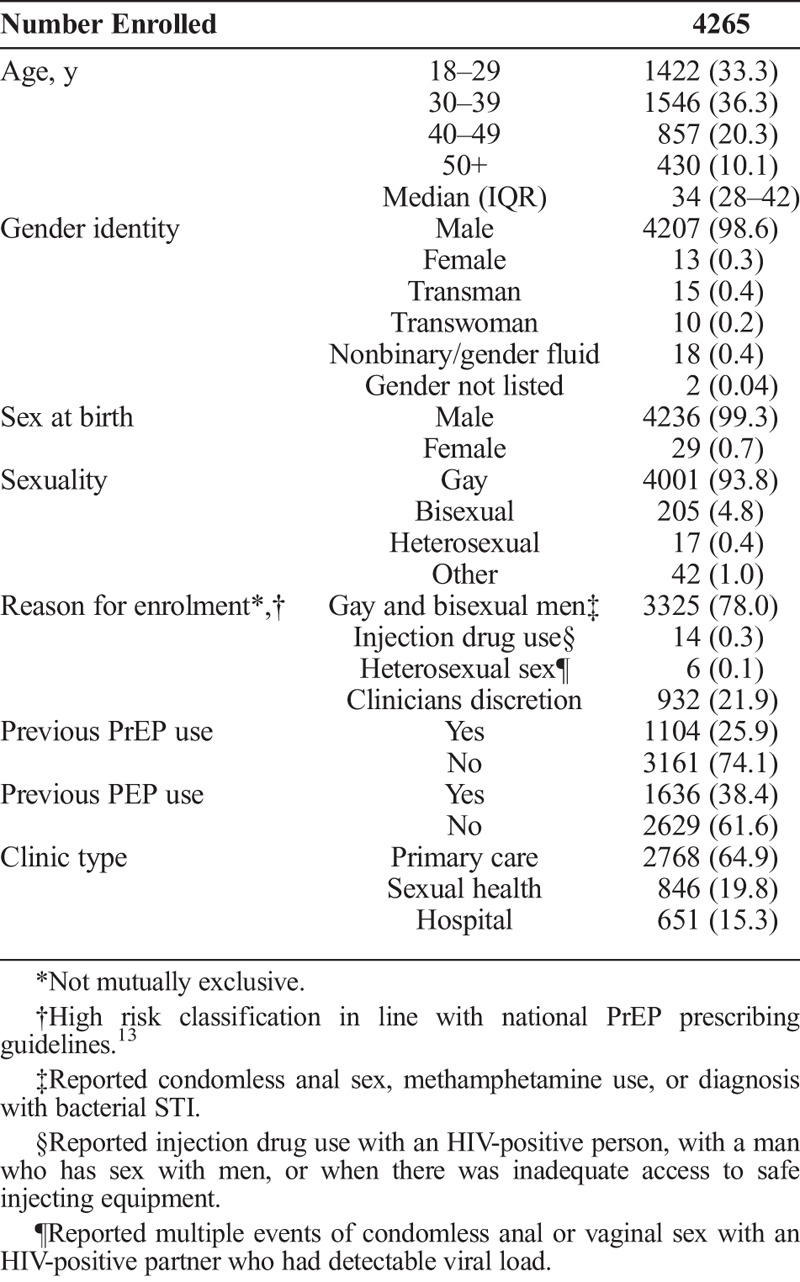
Participant Characteristics
The median age of the 4265 PrEPX participants at enrolment was 34 years, 99% were male, including 22 transmen, and 98% of participants identified as GBM. Approximately one quarter (26%) reported PrEP use before enrolment (Table 1).
The most common PrEP eligibility criterion reported at enrolment was GBM reporting specific behaviors that met the national PrEP prescribing guidelines13 (78%), whereas injecting drug use (0.3%) and heterosexual sex (0.1%) were infrequently reported. Approximately 1 in 5 participants were enrolled at the clinicians' discretion, and separate analyses found that the majority of these participants reported significant risk for HIV acquisition that did not meet the formal eligibility criteria23 (Table 1).
Impact of the PrEPX Study on HIV Testing at Study Clinics
HIV Testing Among PrEPX Participants
Of the 3317 PrEPX participants enrolled at the 5 PrEPX sites that were also ACCESS sites, 89% (n = 2794) had attended any of these 5 ACCESS sites for HIV testing between January 1, 2015, and their study enrolment date. Among participants with a testing history at ACCESS sites, 37% (n = 1044) had tested for HIV at a clinic type other than their enrolment clinic, 91% (n = 951) of whom enrolled at a primary care clinic but had previously tested for HIV at a sexual health clinic. Of the 523 PrEPX participants that did not have previous testing history at ACCESS study sites, 79% (n = 411) enrolled at primary care clinics (Fig. 2).
Figure 2.
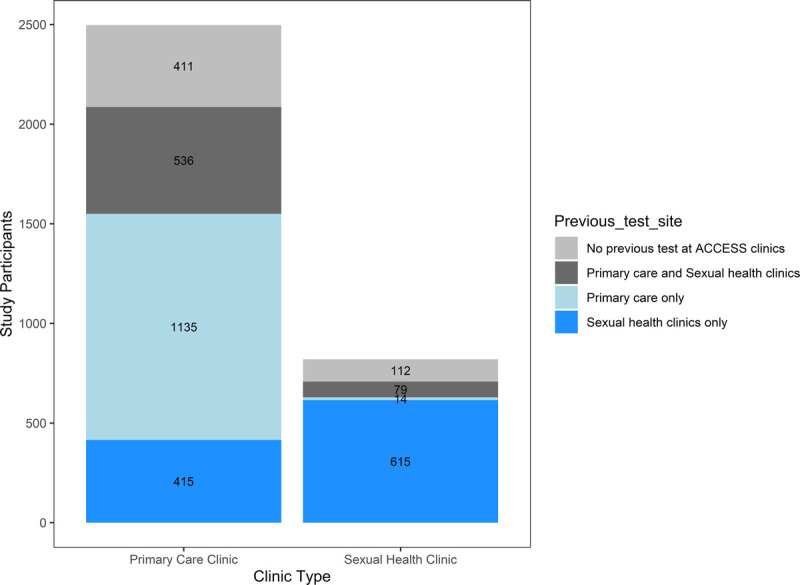
HIV testing among PrEPX participants attending ACCESS primary care and sexual health clinics, by their previous testing history from January 1, 2015 to enrolment at each clinic type.
HIV Testing Among GBM at ACCESS Study Clinics
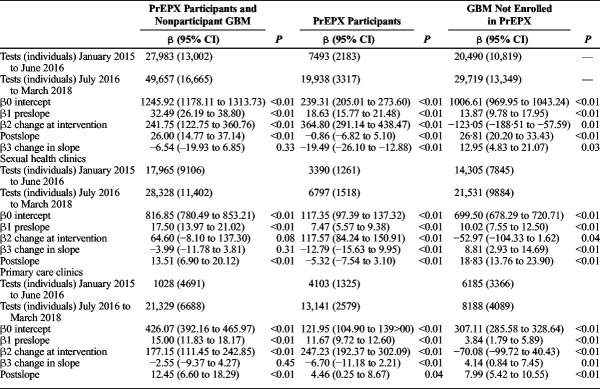
In the prestudy period, there were 27,983 HIV tests recorded among 13,002 GBM. In the study period, there were 49,657 HIV tests recorded among 16,665 GBM; 19,938 tests among 3316 PrEPX participants, and 29,719 tests among 13,349 non-PrEPX participants. In January 2015, 1246 HIV tests (β0) were conducted across the 5 ACCESS clinics, and in the prestudy period, there was a positive trend (β1, 32.49; 95% CI, 26.19–38.80) in the monthly number of HIV tests across these study sites. In the month that PrEPX commenced (July 2016), an additional 241 HIV tests (95% confidence interval [CI], 122.75–360.76) (β2) were conducted, and throughout the study period, the positive trend in the monthly number of HIV tests continued (postslope, 26.00; 95% CI, 14.77–37.14). There was no significant difference in the rate of increase in testing between the prestudy and PrEPX study periods (β3, −6.54; 95% CI, −19.93 to 6.85) (Table 2, Fig. 3).
TABLE 2.
Segmented Linear Regression of Monthly Aggregate HIV Tests Among All GBM Attending 5 PrEPX Study Sites by PrEPX Enrolment Status, January 1, 2015 to March 31, 2018
Figure 3.
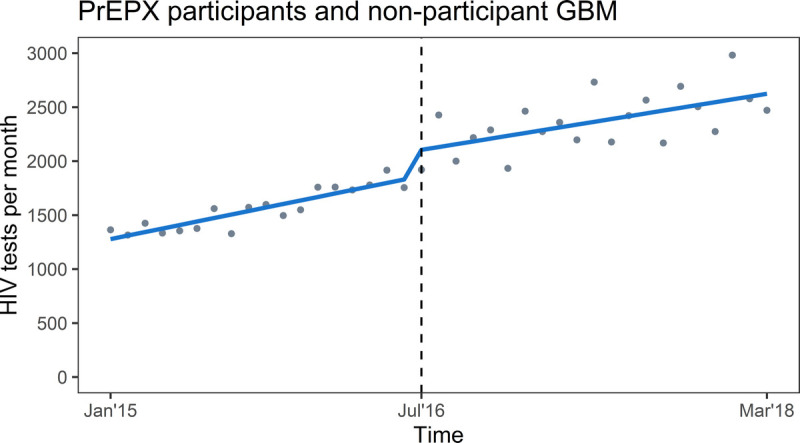
Segmented linear regression of monthly aggregate HIV tests among PrEPX participants and HIV-negative GBM not participating in PrEPX attending 2 sexual health and 3 primary care study sites, January 1, 2015 to March 31, 2018. Gray dots: estimated number of tests per month; Blue line: trend in testing; vertical dashed line: model interruption at PrEPX commencement July 2016.
During the prestudy period, the monthly number of HIV tests increased by an average of 18 tests per month among PrEPX participants (β1, 18.63; 95% CI, 15.77–21.48) and 13 tests per month among non-PrEPX participants (β1, 13.87; 95% CI, 9.78–17.95). In the month that PrEPX commenced, an additional 364 HIV tests (95% CI, 291.14–438.47) were conducted among PrEPX participants and 123 fewer HIV tests (95% CI, −188.51 to −57.59) among non-PrEPX participants. In the PrEPX study period, the monthly number of HIV tests declined by an average of 1 test per month among PrEPX-participants (postslope, −0.86; 95% CI, −6.82 to 5.10) and increased by an average of 26 tests per month among non-PrEPX participants (postslope, 26.81; 95% CI, 20.20–33.43). Between the prestudy and PrEPX study periods, there was a negative change in rate of testing among PrEPX participants (β3, −19.49; 95% CI, −26.10 to −12.88) and a positive change in the non-PrEPX participants (β3, 12.95; 95% CI, 4.83–21.07) (Table 2, Fig. 4).
Figure 4.
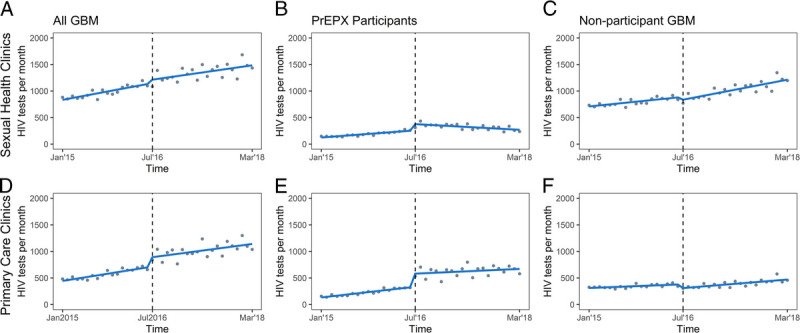
Segmented linear regression of monthly aggregate HIV tests among PrEPX participants and HIV-negative GBM not participating in PrEPX attending 2 sexual health and 3 primary care study sites, January 1, 2015 to March 31, 2018. Models represent testing estimates at sexual health clinics (A, B, C) and primary care clinics (D, E, F) among all GBM (A, D), PrEPX participants (B, E), and nonparticipant GBM (C, F). Gray dots: estimated number of tests per month; Blue line: trend in testing; vertical dashed line: model interruption at PrEPX commencement July 2016.
HIV testing at ACCESS Sexual Health Clinics
During the prestudy period, the monthly number of HIV tests at sexual health clinics increased by an average of 7 tests per month among PrEPX participants (β1, 7.47; 95% CI, 5.57–9.38) and 10 tests per month among non-PrEPX participants (β1, 10.02; 95% CI, 7.55–12.50). In the month that PrEPX commenced, an additional 118 HIV tests (95% CI, 84.24–150.91) were conducted among PrEPX participants and 53 fewer HIV tests (95% CI, −104.33 to −1.62) among non-PrEPX participants. In the PrEPX study period, the monthly number of HIV tests at sexual health clinics declined by an average of 5 tests per month among PrEPX-participants (postslope, −5.32; 95% CI, −7.54 to −3.10) and increased by 18 tests per month among non-PrEPX participants (postslope, 18.83; 95% CI, 13.76–23.90). Between the prestudy and PrEPX study periods, there was a negative change in rate of testing among PrEPX participants (β3, −12.79; 95% CI, −15.63 to −9.95) and a positive change in the non-PrEPX participants (β3, 8.81; 95% CI:, 2.93–14.69) (Table 2, Fig. 4).
HIV testing at ACCESS Primary Health Care Clinics
During the prestudy period, the monthly number of HIV tests at primary health clinics increased by an average of 11 tests per month among PrEPX participants (β1, 11.67; 95% CI, 9.72–12.60) and by 4 tests per month among non-PrEPX participants (β1:1, 3.84; 95% CI, 1.79–5.89). In the month that PrEPX commenced, an additional 247 HIV tests (95% CI, 192.37–302.09) were conducted among PrEPX participants and 70 fewer HIV tests (95% CI, −99.72 to −40.43) among non-PrEPX participants. In the PrEPX study period, the monthly number of HIV tests increased by 4 tests per month among PrEPX participants (postslope, 4.46; 95% CI, 0.25–8.67) and by 8 tests per month among non-PrEPX participants (postslope, 7.99; 95% CI, 5.42–10.55). Between the prestudy and PrEPX study periods, there was a negative change in rate of testing among PrEPX participants (β3, −6.70; 95% CI, −11.18 to −2.21) and a positive change in the non-PrEPX participants (β3, 4.14; 95% CI, 5.42–10.55) (Table 2, Fig. 4).
Bacterial STI Testing at ACCESS Clinics
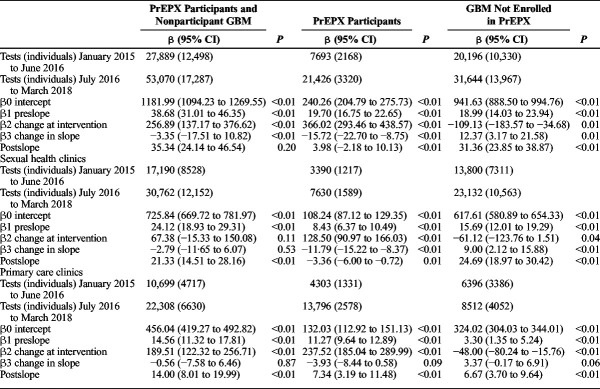
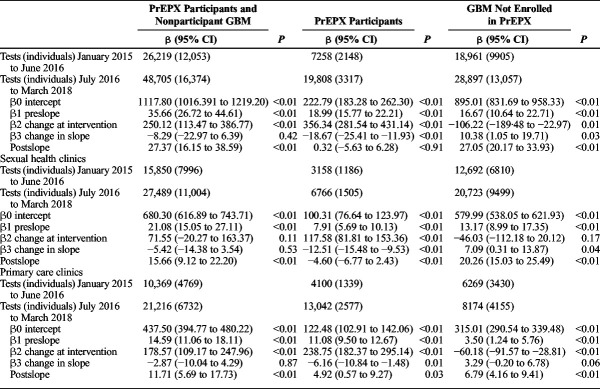
Given that STI testing among GBM is routinely provided in combination with HIV testing at study clinics, similar trends were observed in the monthly number of CT/NG and syphilis tests across PrEPX participants and nonparticipant GBM attending ACCESS participating sexual health and primary care clinics (Tables 3 and 4).
TABLE 3.
Segmented Linear Regression of Monthly Aggregate CT/NG Tests Among All GBM Attending 5 PrEPX Study Sites by PrEPX Enrolment Status, January 1, 2015 to March 31, 2018
TABLE 4.
Segmented Linear Regression of Monthly Aggregate Syphilis Tests Among All GBM Attending 5 PrEPX Study Sites by PrEPX Enrolment Status, January 1, 2015 to March 31, 2018
DISCUSSION
The PrEPX study utilized existing health services to enroll over 2000 participants in 13 weeks and achieved one of the most rapid enrolment rates into a PrEP study reported to date.24 Although there was concern that this increase in demand for health services would compromise HIV testing access for GBM who did not participate in PrEPX, we showed that PrEPX did not have a sustained negative impact on HIV testing among nonstudy GBM patients attending study clinics. Local modeling used to inform the PrEPX study suggested that rapid enrolment was needed to achieve prevention targets,25 and these findings suggest that the HIV prevention impact provided by the scale-up of PrEP was not offset by changes in HIV testing capacity. Our findings also underscore the capacity of clinics to adapt service models to meet increased demand for sexual health care after the implementation of PrEP.
The PrEPX enrolment exceeded 4000 participants, and the initial rapid enrolment reflected significant interest in PrEP among Australian GBM. This rapid enrolment may be attributed to predominance of grassroots awareness raising that was driven by Victorian PrEP activists and community organizations who were study coinvestigators,16 and establishment of an online PrEP importation assistance program.26 These activities, and findings from a number of other studies,27,28 suggest a highly engaged and health-literate GBM community in Victoria. Although these actions contributed to Victorian GBMs willingness for PrEP, a register of interest and use of existing services provided the structure necessary for rapid enrolment. The use of existing “client-centered” services for PrEP provision has been recommended recently,7 and the reliance on hospital-based prescribing in France contributing to the observed limited PrEP uptake.29 The PrEPX study used existing, highly trusted clinical services that most participants attended for HIV testing before PrEPX enrolment, and this may have further contributed to rapid enrolment and successful PrEP maintenance.
In this study, we used an existing surveillance system to assess HIV testing before and after implementation. We showed that most movement across the system was among PrEPX participants who previously tested at sexual health clinics and enrolled in primary care clinics, with the observed decline in monthly HIV tests at sexual health clinics attributable to participants moving to primary care clinics to enroll in the study. A number of factors may have contributed to primary care clinics ability to enroll greater numbers of study participants, including incorporating study participants in standard appointment slots, familiarity with federal funding for medical services, and different service priorities compared with state-funded sexual health services. The ability to accommodate a greater number of study participants contributed to movement from sexual health to primary care clinics in the PrEPX participant cohort. This movement within the testing network may have increased testing opportunities for other GBM at publically funded sexual health clinics. We also observed that before PrEPX commencement, there was an increasing trend in the monthly number of HIV tests among future PrEPX participants and non-PrEPX GBM. This increased testing may be attributed, in part, to monitoring associated with self-importation of PrEP and a positive impact of local HIV testing and prevention campaigns. These testing data show a network of sexual health and primary care services that had been able to adapt to increasing demand and shifting service needs to deliver HIV and STI testing Victorian GBM, even before PrEP was scaled up locally.
In scaling up PrEP programs, it is important to ensure that people who choose not to use PrEP are not disadvantaged in their access to sexual health care. At PrEPX implementation we observed, an immediate significant increase in the monthly number of HIV and STI tests among PrEPX participants and a decline in the number of tests among non-PrEPX participants. Importantly, the monthly number of tests among non-PrEPX participants recovered and exceeded prestudy numbers. In line with our assessment of a redistribution of HIV testing across sexual health and primary care clinics, HIV testing among non-PrEPX participants increased faster at sexual health clinics as these clinics enrolled fewer PrEPX participants and PrEPX participants moved away from sexual health clinics to enroll at primary care clinics. Recent Victorian data have shown an increasing disparity of HIV transmission, with an increased proportion of diagnoses among GBM who are not eligible for Australia's universal health care system (Medicare). These men are often diagnosed late with limited testing history, and the trend is likely driven by inequitable access to sexual health services.30 In Victoria, sexual health clinics are state-funded, and HIV testing is provided at no cost, irrespective of Medicare eligibility, whereas services at primary care clinics are rebated through Medicare, this means that Medicare ineligible may face significant out of pocket expenses when attending such services. The redistribution of testing we observed may have increased access to free testing at sexual health care services for Medicare ineligible GBM.
This study has several limitations. Assessment of the impact of PrEPX on HIV and STI testing was restricted to 5 study sites that contribute data to ACCESS, and there may be variation in the ability of smaller clinics to accommodate PrEP users which was not measured in this study This analysis assessed shifts in absolute number of PrEP-related and unrelated tests at clinics and not the frequency of testing/retesting among GBM not accessing PrEP. Separate analysis of surveillance data collected from participating clinics suggests no meaningful change in testing frequency among GBM in response PrEP scale-up.”31 The decline in the number of tests among PrEPX participants at sexual health clinics and results from our secondary analysis which showed that some participants withdrew from the study but did not inform their study clinician32 meant our analysis may have underestimated the number of study withdrawals. Our analysis may have underestimated the number of GBM within the ACCESS clinic who were using PrEP; our estimates are restricted to GBM enrolled in the PREPX study, however 25% of participants reported PrEP use before enrolment. However, if this is an underestimate of PrEP use, it would suggest that the health system could adapt to even greater need for HIV and STI testing than we have assumed in this analysis. Finally, this study was undertaken in a high-income country with universal health care, and hence, the findings may not be generalizable to other settings.
The PrEPX study design incorporated close partnerships with community, clinicians, pharmacists and researchers, and PrEPX recorded a rapid enrolment of over 2000 people in less than 3 months. Clinics independently established models of PrEP delivery to suit their service models, and this enabled clinics to accommodate PrEPX study participants with no long-term impact on sexual health care among GBM attending these clinics who were not enrolled in the PrEPX study. These findings suggest that scale up of PrEP using existing health services is highly feasible and may assist the implementation and expansion of PrEP in other settings.
Footnotes
Acknowledgments: The authors would like to acknowledge the PrEPX study participants, the researchers and participants from previous PrEP studies, and the nonhuman primates and other animals who have contributed to our current understanding of the science of PrEP. The authors would like to acknowledge the GP Clinic/sites staff and community for the work they did to ensure the study was a success. The authors would like to acknowledge, John (Tim) Lockwood, Jenny Kelsall, Robert M. Grant, and Jeff Montgomery who contributed to the study.
M.S. and E.W. are joint senior authors.
Declarations: Availability of data and material. The data sets generated and/or analyzed during the current study are not publicly available due to ongoing analysis undertaken by the research team but are available from the corresponding author on reasonable request.
Conflict of interest: M.A.S. received grants from the Commonwealth Department of Health, during the conduct of the study. J.A. received grants from the Commonwealth of Australia, Department of Health, during the conduct of the study. J.H.'s institution received reimbursement for her participation in Advisory Boards for Gilead Sciences, Merck, Sharp & Dohme and ViiV Healthcare. E.J.W. has received financial support from Gilead Sciences; Abbott Laboratories; Janssen-Cilag; Boehringer Ingelheim; ViiV Healthcare; Alfred Hospital; and Merck Sharp & Dohme. Gilead Sciences donated study drug to the VicPrEP study (precursor to the PrEPX study). All other authors declare no potential conflicts of interest.
Sources of Funding: This project was funded by the Victorian Department of Health and Human Services, the Alfred Hospital and the Victorian AIDS Council.
Authors' contributions: A.M., B.P., C.F., L.L., D.M., V.C., N.R., J. Willcox., C.C., J.Ar., B.T., M.P., G.-F.S., A.P., J.deW., J.H., and E.W. contributed to the design of the project. K.R., M.S., J.As., M.T., M.H., L.N., D.M., V.C., N.R., J. Willcox., C.C., J.Ar., B.T., G.-F.S., and E.W. contributed to data collection. K.R., A.M., M.S., C.F., J.As., V.C., J.H., and E.W. contributed to article preparation. All authors reviewed and approved the final draft of the article.
The PREPX Study team includes: Jude Armishaw, Jason Asselin, Colin Batrouney, Alison Coelho, Vincent J Cornelisse, John De Wit, Christina C Chang, Chistopher K Fairley, George Forgan-Smith, Margaret Hellard, Jennifer Hoy, Richard Keane, Luxi Lal, Anne Mak, Dean Murphy, Long Nguyen, Anna B Pierce, Brian Price, Matthew Penn, Norman Roth, Simon Ruth, Kathleen E Ryan, Garry Sattell, Mark Stoové, Ban Kiem Tee, Michael Traeger, Olga Vujovic, Michael West, Michael Whelan, Jeremy Wiggins, Jeff Willcox, Christopher Williams, Edwina Wright.
Contributor Information
Collaborators: on behalf of the PrEPX Study team
REFERENCES
- 1.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363:2587–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCormack S, Dunn DT, Desai M, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): Effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet 2016; 387:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 2012; 367:423–434. [DOI] [PubMed] [Google Scholar]
- 4.Deutsch MB, Glidden DV, Sevelius J, et al. HIV pre-exposure prophylaxis in transgender women: A subgroup analysis of the iPrEx trial. Lancet HIV 2015; 2:e512–e519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir study): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2013; 381:2083–2090. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Guidelines Guideline on When to Start Antiretroviral Therapy and on Pre-Exposure Prophylaxis for HIV [Internet]. World Health Organization. Geneva, Switzerland; 2015 [cited 2018 May 1]. p. 78. Available from: http://www.who.int/hiv/pub/guidelines/earlyrelease-arv/en/. [PubMed] [Google Scholar]
- 7.Rivet Amico K, Bekker LG. Global PrEP roll-out: Recommendations for programmatic success. Lancet HIV 2019; 6:e137–e140. [DOI] [PubMed] [Google Scholar]
- 8.Huang YA, Zhu W, Smith DK, et al. HIV preexposure prophylaxis, by race and ethnicity—United States, 2014–2016. MMWR Morb Mortal Wkly Rep 2018; 67:1147–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coleman R, Prins M. Options for affordable pre-exposure prophylaxis (PrEP) in national HIV prevention programmes in Europe. Eurosurveillance 2017; 22:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCormack SM, Noseda V, Molina JM. PrEP in Europe—expectations, opportunities and barriers. J Int AIDS Soc 2016; 19(Suppl 6):6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.UNAIDS. Miles to Go: Global AIDS Update 2018. Geneva: Switzerland, 2018. [Google Scholar]
- 12.Kirby Institute. HIV in Australia: annual surveillance short report 2018 2018.
- 13.Wright E, Grulich A, Roy K, et al. Australasian society for HIV, viral hepatitis and sexual health medicine HIV pre-exposure prophylaxis: Clinical guidelines. J Virus Erad 2017; 3:168–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pharmaceutical Benefits Advisory Committee. December 2017 PBAC meeting—positive recommendations [internet]. 2017 [cited 2018 Feb 15]. p. 1–3. Available from: http://www.pbs.gov.au/industry/listing/elements/pbac-meetings/pbac-outcomes/2017-12/positive-recommendations-12-2017.pdf.
- 15.Holt M, Lea T, Mao L, et al. Adapting behavioural surveillance to antiretroviral-based HIV prevention: Reviewing and anticipating trends in the Australian Gay Community Periodic Surveys. Sex Health 2017; 14:72–79. [DOI] [PubMed] [Google Scholar]
- 16.Ryan KE, Mak A, Stoove M, et al. Protocol for an HIV pre-exposure prophylaxis (PrEP) population level intervention study in Victoria Australia: The PrEPX study. Front Public Health 2018; 29:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lal L, Ryan KE, Liu I, et al. Transformation of Australian Community Pharmacies into Good Clinical Practice Compliant Trial Pharmacies for HIV Prophylaxis. Front Pharmacol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyle D, Kong F. A systematic mechanism for the collection and interpretation of display format pathology test results from Australian primary care records. Electron J Heal Informatics 2011; 6:1–7. [Google Scholar]
- 20.Callander D, Moreira C, El-Hayek C, et al. Australian collaboration for coordinated enhanced sentinel surveillance (ACCESS): A protocol for monitoring the control of sexually transmissible infections and blood borne viruses (preprint). JMIR Res Protoc 2018; 35:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Traeger MW, Cornelisse VJ, Asselin J, et al. Association of HIV preexposure prophylaxis with incidence of sexually transmitted infections among individuals at high risk of HIV infection. JAMA 2019; 321:1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ampt FH, El Hayek C, Agius PA, et al. Anorectal swabs as a marker of male-to-male sexual exposure in STI surveillance systems. Epidemiol Infect 2017; 145:2530–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asselin J, Ryan K, Cornelisse V, et al. Understanding risk among male participants enrolled at clinicians' discretion in a PrEP demonstration trial in Victoria, Australia.In: 22nd International AIDS Conference. Amsterdam, Netherlands; 2018. [Google Scholar]
- 24.AVAC. Ongoing and Planned PrEP Open Label, Demonstration and Implementation Projects, August 2018 [Internet]. 2018 [cited 2019 Jun 3]. p. 1–5. Available from: https://www.avac.org/resource/ongoing-and-planned-prep-demonstration-and-implementation-studies.
- 25.Kirby Institute and the Centre for Social Research in Health. Discussion paper: Estimates of the number of people eligible for PrEP in Australia, and related cost-effectiveness. 2017:1–45.
- 26.prepaccessnow. prepaccessnow [Internet]. 2017 [cited 2017 Jun 15]. Available from: https://www.prepaccessnow.com.au/.
- 27.Holt M, Draper BL, Pedrana AE, et al. Comfort relying on HIV pre - exposure prophylaxis and treatment as prevention for condomless sex : Results of an online survey of Australian gay and bisexual men. Springer US 2018; 3617–3626. [DOI] [PubMed] [Google Scholar]
- 28.Wilkinson AL, Draper BL, Pedrana AE, et al. Measuring and understanding the attitudes of Australian gay and bisexual men towards biomedical HIV prevention using cross-sectional data and factor analyses. Sex Transm Infect 2018; 94:309–314. [DOI] [PubMed] [Google Scholar]
- 29.Siguier M, Mera R, Pialoux G, et al. First year of pre-exposure prophylaxis implementation in France with daily or on-demand tenofovir disoproxil fumarate/emtricitabine. J Antimicrob Chemother 2019; 74:2752–2758. [DOI] [PubMed] [Google Scholar]
- 30.Medland NA, Chow EPF, Read THR, et al. Incident HIV infection has fallen rapidly in men who have sex with men in Melbourne, Australia (2013–2017) but not in the newly-arrived Asian-born. BMC Infect Dis 2018; 18:410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burnet Institute. The Australian Collaboration for Coordinated Enhanced Sentinel Surveillance of Sexually Transmissible Infections: 2013–2018 BBV and STI Sentinel Surveillance Report for Victoria. Melbourne, 2019. [Google Scholar]
- 32.Ryan K, Asselin J, Fairley C, et al. Results from a large Australian PrEP demonstration study: Discontinuation and subsequent HIV and other sexuallytransmitted infection risk.In: 10th IAS Conference on HIV Science. Mexico City, Mexico; 2019. [Google Scholar]