Abstract
Background
Progressive physical frailty and cognitive decline in older adults is associated with increased risk of falls, disability, institutionalization, and mortality; however, there is considerable heterogeneity in progression over time. We identified heterogeneous frailty and cognitive decline trajectory groups and examined the specific contribution of health conditions to these trajectories among older Mexican origin adults.
Methods
We use a sample from the Hispanic Established Population for the Epidemiological Study of the Elderly (HEPESE) with at least two measures of frailty criteria during 18 years follow-up: slow gait, weak handgrip strength, exhaustion, and unexplained weight loss (n = 1362, mean age 72). Cognition was measured using the Mini-Mental State Examination (MMSE).
Results
Using group-based trajectory models we identified three frailty groups—non-frail (n = 331), moderate progressive (n = 855), and progressive high (n = 149)—and three cognitive decline groups—non-cognitively impaired (476), moderate decline (677) and rapid decline (n = 209). The probability of membership in a high-frailty group given membership in a progressive cognitive decline group was 63%, while the probability of being in a non-frail group given membership in a non-cognitively impaired group was 68%. Predictors of membership into both the progressive high frailty and rapid cognitive decline groups combined were low education and diabetes. Weekly church attendance was associated with a 66% reduction in the odds of being in the combined groups.
Conclusions
Interventions to reduce frailty rates and cognitive decline might focus on the management of underlying chronic disease and on increasing participation in activities outside the home.
Keywords: Physical function, Transition, Hispanic
Progressive physical frailty in older adults is associated with increased risk of falls, disability, institutionalization, and mortality (1–4). Frailty is conceptualized as an accumulation of deficits across multiple physical systems that reduce reserve capacity and render older adults vulnerable to acute events and adverse outcomes (1,2,4). Due to the complex interaction of systems involved, frailty states vary over time.
Strong associations between increased physical frailty and cognitive impairment have also been reported (5–7). In a study of adults 74 years and older, Kumala and colleagues found that cognitive impairment and dementia were both eight times more likely among frail individuals compared to non-frail individuals (5). Similarly, Gray and colleagues found in a study of adults 65 and older that frailty was associated with a more than twofold increase in the hazard of non-Alzheimer’s dementia (7). In addition, in their examination of older Mexican Americans, Samper-Ternent and colleagues found that frail individuals had a significantly greater decline (.67 points) in cognitive function as measured by the Mini-Mental State Examination (MMSE) than the non-frail over 10 years of follow-up (8). These combined associations of frailty and cognition have resulted in the conceptualization of cognitive frailty (9), which defined the condition as possessing both physical frailty and cognitive impairment with the exclusion of Alzheimer’s disease and other dementias.
Most frailty research has focused on non-Hispanic white populations. Research on frailty among Hispanics is limited. However, similar to existing reports on non-Hispanic whites, research has shown a strong relationship between age and frailty among Hispanics (10,11). Other factors associated with increased frailty among Hispanics include adverse life events, low social support, acculturation, and neighborhoods with low proportions of Hispanics (12–14). It should be noted that findings regarding neighborhood composition have been mixed as other research has found the opposite effect (15). Importantly, frailty is associated with decreased quality of life and increased risk of mortality, disability, and cognitive decline among Hispanics (6,11,16–18).
Considerable heterogeneity has been observed in health, function, and mobility at older ages (19–21). Additionally, substantial heterogeneity in change in disability and cognitive function over time has been observed among Hispanics (22,23). Given the variation in health and function and the nature of frailty transitions over time, we investigate the heterogeneity of both frailty and cognitive function over time in a sample of older Mexican origin adults from the Hispanic Established Population for the Epidemiological Study of the Elderly (HEPESE). We hypothesized that sub-groups within the sample will exhibit different rates of change in frailty and cognitive decline over time and that differences in health conditions will explain much of this variation. Additionally, we hypothesized that there would be overlap in membership between frailty and cognitive decline groups.
Materials and Methods
Sample
We used data from the HEPESE, an on-going population-based study of 3050 non-institutionalized Mexican Americans aged 65 and older at baseline (1993–1994) from five Southwestern U.S. states (Texas, California, New Mexico, Colorado, and Arizona). Nine waves of data have been collected (1993–1994 n = 3050; 1995–1996 n = 2438; 1998–1999 n = 1981; 2000–2001 n = 1682; 2004–2005 n = 1167; 2006–2007 n = 921; 2010–2011 n = 659; 2012–2013 n = 444; 2016 n = 283). Respondents were interviewed in Spanish or English based on their preference. Details regarding the methods have been described elsewhere (24). Our study sample includes respondents beginning in Wave 2 because this was the first wave where an important component of our frailty measure (weight loss) could be assessed. Subjects were then followed over the six subsequent waves of data collection resulting in nearly 18 years of follow-up time. Respondents were included if they were able to complete the frailty assessment and had complete data for included variables at baseline and had at least one follow-up frailty measurement (N = 1362). All research protocols and informed consents were approved by the University’s Institutional Review Board.
Dependent Variables
Frailty status was determined based on a modification of the frailty phenotype described by Fried and colleagues (1). The original phenotype measures included weight loss, exhaustion, slow gait, low grip strength, and low physical activity. An assessment of physical activity was not available in all waves and, thus, was excluded from our frailty measure. Weight loss was defined as a 10-pound difference in weight from prior observation. Exhaustion was assessed using responses to two items on the Center for Epidemiological Studies Depression (CES-D) scale: “I felt everything was an effort” and “I could not get going”. Assessing feelings over the past week, responses were scored as 0 for less than daily or 1–2 days and scored 1 for feelings occurring 3 or more days. Exhaustion was operationally defined as a positive response to either of the questions.
Gait speed was assessed using a timed performance of an 8-foot walk. Subjects who were unable to perform the task or were in the bottom 20% were coded as 1 (slow gait). Grip strength was assessed with a hand-held dynamometer. Subjects unable to perform the test or whose grip strength was in the bottom 20% (within sex) were coded “weak grip strength”. Each of the dichotomous indicators of the frailty phenotype were then aggregated and categorized to form a frailty rating (range 0–4). Because participants could refuse to perform assessment activities, we allowed for the range to still be reported if a single item was skipped. Thus, our aggregate may underestimate the presence of frailty measures. A list of the cut points for gait speed and grip strength are available in Supplementary Appendix Table 1.
Cognitive function was measured using the Mini-Mental State Examination (MMSE) (25) during the in-person interviews of the subjects. The MMSE employs a standard battery of items that include orientation, attention, short term recall, language and the ability to follow simple instructions. The MMSE has been shown to be valid and reliable in English (25) and Spanish (26). In our sample, the instrument was administered in both English (18% of the sample) and Spanish (82% of the sample). We use the total MMSE score as our intent was to identify heterogeneous subgroups of change over time rather than identifying cognitive impairment per se.
Covariates
Health Conditions
We included major health conditions measured at study baseline (HEPESE Wave 2) associated with functional decline (27,28). Health conditions were ascertained through self-report by asking the respondents “Since we last spoke … told by a doctor that you…” These conditions included hypertension, diabetes, arthritis, cancer, heart attack, stroke, and hip fracture. Pain was assessed with the question “In the past month, … pain or discomfort when you stood or walked?” Answers were coded as yes or no.
Other variables included age, sex (female), low education (≤5 years based on the sample mean), U.S.-born, over-weight or obese (25 ≤ BMI < 30 and BMI ≥ 30 respectively; BMI < 25 as control), current smoker, and currently married. Financial strain was assessed as “yes” if the respondent expressed either (1) difficulty paying bills or (2) not having enough money left at the end of the month.
Statistical Analysis
Demographic characteristics were examined for the selected study subjects and comparisons to non-selected survey participants were made using t-tests for continuous variables and chi-square for categorical variables. Group-based trajectory models were used to assess the trajectories of both frailty and cognitive decline within the study sample over time (29). In this approach, change over time is considered a heterogeneous mixture of groups, each with a distinct functional form (eg, linear, quadratic). The models were developed using the procedure “traj” written for Stata (30). The count of frailty measures was modeled as a Poisson distribution. MMSE was modeled as a censored normal variable using Tobit regression. Change in frailty was modeled as a function of time (months) rather than age as we were particularly interested in determining the presence of any period effects of age.
Selecting the appropriate model requires consideration of several criteria. Comparisons between models are made using Bayesian Information Criteria (BIC: lower absolute value is generally better) and minimum posterior probabilities of group assignment (0.70) (29). We examined models with 1 to 4 trajectories and tested the functional forms intercept only, linear, quadratic, and cubic. The final model for frailty which met the selection criteria contained 2 quadratic trajectories and one linear trajectory. The final model for cognitive decline included two quadratic trajectories and one linear trajectory. We then fit a joint trajectory model, which combined the frailty and cognitive decline models. This joint model afforded the opportunity to determine the probability of frailty group membership given membership in a cognitive decline trajectory.
During model specification for the joint model, a multinomial logit was used to predict group membership. The base model included covariates that have been shown to be associated with frailty and cognition in other research: age, sex (female), low education, over-weight and obese, arthritis, diabetes, and hypertension. Additional covariates were added if they satisfied two criteria: (a) there was a bivariate relationship with the outcomes in an otherwise empty model, and (b) model convergence was achieved when added to the base model. These additional covariates included U.S.-born, smoking status, financial strain, weekly church attendance, pain, hip fracture, cancer, and stroke.
Subsequent to identifying members in both the high progressive frailty and rapid decline cognition groups, we created an indicator for membership into both and performed logistic regression to assess factors associated with membership in this combined group. Because we were interested in the factors that predict membership in this rapidly declining group, our comparison was all other group combinations.
Attrition is a challenge in all analyses of longitudinal data. In our sample, intermittent missing data was treated as missing at random (MAR), which is easily handled by maximum likelihood resulting in unbiased estimates. Because of the high mean age and long follow-up period, most of the attrition in our sample was due to mortality (72% had died by the end of the study period for the entire sample). We used the approach developed by Haviland and colleagues (31) which models the joint estimation of frailty and the probability of nonrandom dropout due to death. The dropout model calculates a trajectory specific dropout probability based on prior wave observation and adjusts group-specific membership probabilities. We modeled dropout (mortality) as a function of age at prior wave. All analyses were performed using Stata 14 mp (Stata Corp., College Station, TX).
Results
The characteristics of the included and excluded (due to insufficient frailty measurement) samples are described in Table 1. The included and excluded respondents were similar in proportion U.S.-born, smoking rates, financial strain, as well as the presence of hypertension and arthritis. Those excluded from the analysis were significantly older (76 compared to 72 years, p < .001), had lower MMSE scores (mean difference −1.3, p < .001) and had higher prevalence of hip fracture and stroke. It should be noted that the excluded group had a large proportion of missing values for BMI (30%) and financial strain (23%).
Table 1.
Characteristics of Participants Included and Excluded (Based on Adequate Frailty Measurement), HEPESE Wave 2, Mean (SD) or %
| Excluded | Included | |
|---|---|---|
| N | 1493 | 1338 |
| Age | 76 (6.6) | 72 (5.2)** |
| Female | 56.8 | 60.1* |
| U.S. Born | 54.6 | 56.9 |
| Married | 47.7 | 57.5** |
| School: 5 years or less | 62.6 | 60.7 |
| Smoke | 12.4 | 10.9 |
| BMIa | 24.3 (9.2) | 25.7 (9.1)** |
| Financial strain | 19.2 | 28.5** |
| Church: weekly | 36.4 | 58.3** |
| Diabetes | 30.2 | 25.3** |
| Arthritis | 44.7 | 44.7 |
| Hip fracture | 2.9 | 0.8** |
| Hypertension | 48.1 | 43.7* |
| Heart attack | 10.2 | 7.9* |
| Stroke | 15.2 | 5.2** |
| MMSEb | 23.0 (5.0) | 25.0 (3.9)** |
| Frailtyc | 1.0 (1.0) | 0.6 (0.7)** |
Notes: Missing data apply only to the excluded group; HEPESE = Hispanic Established Populations for the Epidemiologic Study of the Elderly; MMSE = Mini-Mental State Examination.
aExcluded Group 48% missing.
bExcluded Group 45% missing.
cExcluded Group 63% missing.
*p < .05, **p < .01.
Additional covariates were tested for inclusion in the base model. Covariates excluded from the final model, which were not significantly associated with either outcome in the otherwise empty model were: U.S.-born (p-values ranged from .1 to .6), financial strain (p-values ranged from .3 to 1.0), and current smoker (p-values ranged from .3 to .9). Bivariate models with cancer, stroke, and hip fracture would not converge due to very low prevalence resulting in zero cell sizes and were excluded from the final model. Pain was significantly associated with membership in the progressive high frailty group (p < .01) but was not included in the final model due to collinearity with arthritis. Similarly, married was significantly negatively associated with membership in the progressive high frailty group (p = .03); however, inclusion in the base model resulted in non-convergence. Weekly church attendance was significantly negatively associated with membership in the moderate progressive (p < .01) rapid progressive (p = .001) groups compared to the non-cognitively impaired group and was included in the final model.
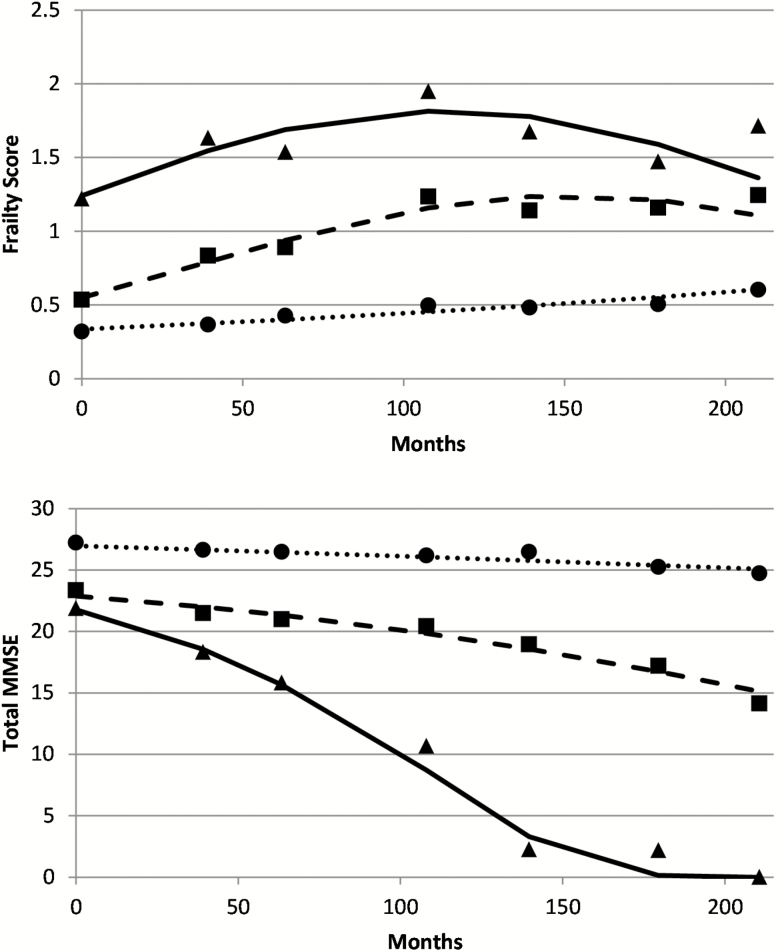
Plots of predicted counts of frailty measures for the whole sample over time are presented in Figure 1, top panel. The figure describes three trajectory groups: non-frail (29.3%), moderate progressive frailty (56.4%), and progressive high frailty (14.3%). Posterior probabilities of group membership were 0.74 (non-frail), 0.74 (moderate progressive) and 0.74 (progressive high). Wald tests comparing the groups showed a significant difference in slope between the moderate progressive and progressive high groups (Χ 2 = 53.08, p < .001) and a significant difference in intercepts between the non-frail and moderate progressive groups (Χ 2 = 38.07, p < .001) and the moderate progressive and progressive high groups (Χ 2 = 53.08, p < .001).
Figure 1.
Plots of predicted counts (lines) and observed values (icons) from latent class trajectory models of frailty and cognitive function across months of follow-up in the HEPESE sample (Waves 2 to 8). Three trajectory groups of frailty (top panel): non-frail (dotted line, circles, n = 331), moderate progressive (dashed line, squares, n = 855) and high progressive (solid line, triangles, n = 149); and three trajectory groups cognitive decline (bottom panel): non-cognitively impaired (dotted line, circles, n = 476), moderately decline (dashed line, squares, n = 677) and rapid decline (solid line, triangles, n = 209).
Plots of the cognitive decline trajectory groups are presented in Figure 1, bottom panel. The figure also describes three trajectory groups: a non-cognitively impaired group (37.8%), a moderately declining group (44.9%), and a rapid declining group (17.3%). Posterior probabilities for group membership were 0.87 for the non-cognitively impaired group, 0.84 for the moderate declining group and 0.91 for the rapid declining group.
Table 2 presents results of the multinomial logit the odds of membership in the trajectory groups for both frailty and cognitive decline. Covariates include those from the latent class model. In the cognitive decline portion of the model trajectory model, increased age (per year) was associated with membership in the moderate and rapid decline groups compared to the non-cognitively impaired group. Low education (less than 5 years) was also an indicator of membership in the moderate and rapid decline groups (OR 4.1, 95% CI 3.0–5.7 and OR 5.9, 95% CI 3.6–9.6, respectively). Diabetes at baseline was also associated with increased odds of membership in the rapid decline cognition group compared to the non-cognitively impaired group (OR 2.7, 95% CI 1.6–4.4).
Table 2.
Results of Latent Class Trajectory Models: Posterior Probabilities of Group Membership and Results of Multinomial Logits Predicting Odds of Membership in the Moderate Progressive and Progressive High Frailty Groups Compared to the Non-Frail Group, as well as the Moderate Decline and Rapid Decline Cognition Groups Compared to the Non-cognitively Impaired Group in the HEPESE
| Frailty | Cognitive Decline | |||||
|---|---|---|---|---|---|---|
| Non-Frail | Moderate Progressive | Progressive High | Non-Cognitively Impaired | Moderate Decline | Rapid Decline | |
| n | 331 | 855 | 149 | 476 | 677 | 209 |
| Group probability | 0.74 | 0.74 | 0.74 | 0.91 | 0.85 | 0.86 |
| <ref> | OR (95% CI) | OR (95% CI) | <ref> | OR (95% CI) | OR (95% CI) | |
| Age | 1.20 (1.09–1.32) | 1.51 (1.32–1.72) | 1.08 (1.04–1.12) | 1.26 (1.21–1.32) | ||
| Female | 1.64 (0.89–3.04) | 0.63 (0.21–1.87) | 1.00 (0.72–1.39) | 0.90 (0.57–1.42) | ||
| School: 5 years or less | 1.92 (1.06–3.48) | 3.72 (1.42–9.74) | 4.06 (2.94–5.61) | 5.65 (3.47–9.20) | ||
| Over-weight | 0.82 (0.41–1.63) | 0.82 (0.29–2.33) | 0.81 (0.56–1.19) | 0.85 (0.51–1.39) | ||
| Obese | 1.85 (0.85–4.03) | 2.43 (0.79–7.46) | 1.02 (0.69–1.52) | 0.75 (0.43–1.33) | ||
| Arthritis | 2.25 (1.10–4.60) | 9.50 (2.98–30.35) | 1.07 (0.78–1.48) | 0.88 (0.57–1.38) | ||
| Diabetes | 3.04 (1.12–8.24) | 9.55 (2.90–31.51) | 1.45 (0.99–2.12) | 2.68 (1.62–4.42) | ||
| Hypertension | 1.02 (0.53–1.97) | 2.37 (0.94–5.96) | 1.05 (0.76–1.45) | 1.20 (0.76–1.87) | ||
| Church: Weekly | 0.44 (0.23–0.86) | 0.38 (0.15–1.00) | 0.62 (0.45–0.86) | 0.44 (0.28–0.68) |
In the models for frailty, older age and low education were associated with increased odds of membership in both the moderate progressive and progressive high frail groups compared to the non-frail group. Diabetes and arthritis were both associated with increased odds of membership in the moderate progressive frailty group (OR 2.6, 95% CI 1.1–6.2 and OR 2.0, 95% CI 1.0–3.9, respectively) and progressive high frailty group (OR 9.0, 95% CI 2.8–28.3 and 9.4, 95% CI 2.7–32.7, respectively) compared to the non-frail group.
The results of the joint trajectory model are presented in Table 3, which displays the probabilities of membership in the cognitive decline trajectory groups given membership in a frailty trajectory group. These models showed moderate to high conditional probability (68%) of membership in the non-cognitively impaired cognition group given membership in the non-frail frailty group. Membership in the progressive high frailty group was associated with a 63% probability of being in the rapidly declining cognitive trajectory, whereas members of the non-frail group had zero probability of belonging to the rapid declining cognition group.
Table 3.
Probability of Membership in a Cognitive Decline Trajectory Group Given Membership in a Frailty Trajectory Group in the HEPESE
| Frailty Group | Cognitive Decline Group | ||
|---|---|---|---|
| Non-Cognitively Impaired | Moderate Decline | Rapid Decline | |
| Non-frail | 67.8 | 32.2 | 0.0 |
| Moderate progressive | 13.5 | 65.9 | 20.7 |
| Progressive high | 14.6 | 22.2 | 63.2 |
We next ran a logistic regression to examine the predictors of membership in both the progressive high frailty group and the rapidly declining cognition group (Table 4). Low education and diabetes were both associated with more than twice the odds of being in this combined progressive high and rapid decline cognition group. Similarly, over-weight and obese, arthritis, and hypertension were also associated with increased odds of being in this combined group. In contrast, weekly church attendance was associated with a 66% reduction in odds of being in the combined progressive high frailty and rapid decline cognitive group.
Table 4.
Logistic Regression Predicting Odds of Belonging to Both the High Progressive Frailty and Rapid Decline Cognition Trajectory Groups (n = 158) Compared to all Other Combinations
| OR (95% CI) | |
|---|---|
| Age | 1.37 (1.31–1.43) |
| Female | 0.55 (0.35–0.85) |
| School: 5 years or less | 4.59 (2.75–7.65) |
| Over-weight | 1.84 (1.12–3.03) |
| Obese | 1.93 (1.10–3.39) |
| Arthritis | 2.00 (1.29–3.09) |
| Diabetes | 2.40 (1.50–3.84) |
| Hypertension | 1.96 (1.27–3.03) |
| Church: weekly | 0.62 (0.41–0.93) |
Discussion
Considerable heterogeneity exists in the rate of cognitive decline and frailty among older adults. Our group-based trajectory models examining the count of frailty measures and total MMSE scores support previous findings of heterogeneity in this sample of Mexican origin older adults. Similar to our analyses, previous work by Peek and colleagues (14) examining frailty in the same cohort also identified a three-group model; however, while their approach explicitly modeled social factors such as social support and stressors, they did not explicitly model specific health conditions. Also using the same cohort, the work by Howrey and colleagues (23) examining heterogeneity in cognitive change over time identified three cognitive trajectory groups. Our analyses of the HEPESE respondents age 90 and younger extend these prior studies with the addition of two measurement periods, approximately 6 years of additional follow-up time (a total of 18 years follow-up), model both frailty and cognitive function, and also examine the joint probability of membership in frailty and cognition trajectory groups. This joint model approach provides insight into the co-occurrence of increasing frailty and cognitive decline.
The association of diabetes and arthritis with membership in groups with increased frailty and increased cognitive decline is not surprising. Previous research using the HEPESE reported a strong association between diabetes and cognitive impairment (32). Additionally, diabetic adults age 65 and older are more likely to be frail compared to non-diabetic peers, and frail diabetics have higher rates of mortality than the non-frail (11,33,34). This association may be partially explained by glucose dysregulation and changes in micro-vascularization leading to impaired muscle function (35). The resulting weakness manifests as reduced grip strength and slower gait among older diabetics compared to non-diabetics.
Links between cognitive decline and physical frailty have been described in other research (6,8,10,36). While our findings also suggest an overlap between frailty and cognitive decline, we also found that those in the non-frail group only had a 30% probability of being in a moderately declining cognition group and zero probability of being in a rapidly declining cognitive group. Potential explanations for the associations between frailty and cognitive decline include Alzheimer’s disease-related plaque development, chronic inflammatory disease, nutritional imbalance, and cardiovascular disease. It is plausible that cognitive decline and physical frailty share a common underlying pathology (37). Our results linking trajectories of rapidly increasing frailty with rapid cognitive decline further support the supposition of a shared underlying pathology. Two common models of frailty, the phenotype proposed by Fried et al. (1) and the index proposed by Rockwood et al. (4), diverge on the inclusion of cognition. While others propose a separate measure of cognitive frailty (9). Indeed, there is considerable disagreement whether or not to include cognitive impairment in measures of frailty leading to difficulty in cross-study evaluations (38).
While diabetes can independently lead to reduced physical activity and subsequently increased risk of frailty and decreased cognitive functioning, it is important to note that increases in physical exercise may diminish those effects (39,40). Such findings underscore the importance of maintaining or increasing strength and mobility in older adults as well as support for community programs aimed at increasing levels of physical exercise and social engagement. This may be of particular importance for older Mexican origin adults who report high rates of diabetes and low rates of physical activity (41).
Other research has identified links between regular religious attendance and improved cognitive performance (42), increased mobility (43), improved physical functioning following stroke (44), and reductions in hypertension (45). Similarly, our results suggest a protective effect of weekly church attendance. It is possible that religious activities offer cognitive benefits through cognitively stimulating activities such as reading and increased social engagement. Additionally, for those in the combined progressive high frailty and rapid decline cognition groups, the strong association between religious attendance and both frailty and cognition suggests opportunities for interventions aimed at helping older adults maintain community involvement and participation in social activities outside the home.
The lack of significance of several covariates warrants some discussion. We found no association between gender and frailty or cognition. While some previous studies have found significant association of gender (female) with frailty (1,6), previous work using group-based models have found little or no effect (14). The lack of evidence for an effect of nativity, marital status, or financial strain also echoes prior work (14). In regards to health conditions, it is possible that the lack of effect of hypertension in our analyses was due to early mortality before that condition could manifest as either increased frailty or declines in cognition.
Our study has several limitations. The sample was drawn from a representative sample of Mexican origin older adults from five southwestern US states. Thus, our findings cannot be compared to the broader U.S. population or Hispanic groups. Also, we used a modified measure of frailty that did not incorporate physical activity. As such, our findings are not directly comparable to those where the complete phenotype is used. We drew our sample from the second wave of the HEPESE as the measure of weight loss required a previous assessment. This may have resulted in biased estimate of frailty since only survivors at Wave 2 were included. In addition, those excluded from the study had higher rates of cancer, hip fracture, heart attack, and stroke which may have resulted in an underestimation of frailty. Finally, the time between assessments ranged from 2 to 3 years. It is possible that in the intervening time, unmeasured declines in cognition and increases in frailty occurred. Thus it is possible that our models underestimate the true rates of decline.
Conclusion
Considerable heterogeneity in frailty and cognition over time was evident in our sample of older Mexican Americans. The high conditional probability of membership in rapidly declining cognition group given membership in the rapid frailty group provides support for the arguments of a shared underlying pathology. However, it is also important to note that those in the stable frailty group had 0.0% probability of being in the rapidly declining cognition group. Interventions at reducing rapid declines in both frailty and cognition might benefit from a focus on prevention or control of underlying conditions, diabetes in particular, and also on increasing the participation of older adults in activities outside the home.
Funding
This work was supported in part by the National Institute on Aging (R01 MD01035501; R01 AG1093924; and P30 AG05930101).
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
Bret T. Howrey conceived, designed, and analyzed the data, and was responsible for interpretation of findings, primary drafting of manuscript, revisions, and final approval. Soham Al Snih, Joyce A. Middleton, and Kenneth J. Ottenbacher made substantial contributions to interpretation of data; revising the manuscript critically for important intellectual content; and had final approval of the version to be published. Sponsor’s Role: The sponsorship by the National Institute on Aging had no active role in the design, methods, subject recruitment, data collections, analysis, or preparation of the manuscript.
References
- 1. Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi:10.1093/gerona/56.3.m146 [DOI] [PubMed] [Google Scholar]
- 2. Bergman H, Ferrucci L, Guralnik J, et al. Frailty: an emerging research and clinical paradigm–issues and controversies. J Gerontol A Biol Sci Med Sci. 2007;62:731–737. doi:10.1093/gerona/62.7.731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mitnitski A, Song X, Skoog I, et al. Relative fitness and frailty of elderly men and women in developed countries and their relationship with mortality. J Am Geriatr Soc. 2005;53:2184–2189. doi:10.1111/j.1532-5415.2005.00506.x [DOI] [PubMed] [Google Scholar]
- 4. Rockwood K, Hogan DB, MacKnight C. Conceptualisation and measurement of frailty in elderly people. Drugs Aging. 2000;17:295–302. doi:10.2165/00002512-200017040-00005 [DOI] [PubMed] [Google Scholar]
- 5. Kulmala J, Nykänen I, Mänty M, Hartikainen S. Association between frailty and dementia: a population-based study. Gerontology. 2014;60:16–21. doi:10.1159/000353859 [DOI] [PubMed] [Google Scholar]
- 6. Raji MA, Al Snih S, Ostir GV, Markides KS, Ottenbacher KJ. Cognitive status and future risk of frailty in older Mexican Americans. J Gerontol A Biol Sci Med Sci. 2010;65:1228–1234. doi:10.1093/gerona/glq121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gray SL, Anderson ML, Hubbard RA, et al. Frailty and incident dementia. J Gerontol A Biol Sci Med Sci. 2013;68:1083–1090. doi:10.1093/gerona/glt013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Samper‐Ternent R, Al Snih S, Raji MA, Markides KS, Ottenbacher KJ. Relationship between frailty and cognitive decline in older Mexican Americans. J Am Geriatr Soc. 2008;56:1845–1852. doi:10.1111/j.1532-5415.2008.01947.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kelaiditi E, Cesari M, Canevelli M, et al. ; IANA/IAGG. Cognitive frailty: rational and definition from an (I.A.N.A./I.A.G.G.) international consensus group. J Nutr Health Aging. 2013;17:726–734. doi:10.1007/s12603-013-0367-2 [DOI] [PubMed] [Google Scholar]
- 10. Ottenbacher KJ, Ostir GV, Peek MK, Snih SA, Raji MA, Markides KS. Frailty in older Mexican Americans. J Am Geriatr Soc. 2005;53:1524–1531. doi:10.1111/j.1532-5415.2005.53511.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ottenbacher KJ, Graham JE, Al Snih S, et al. Mexican Americans and frailty: findings from the Hispanic established populations epidemiologic studies of the elderly. Am J Public Health. 2009;99:673–679. doi:10.2105/AJPH.2008.143958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aranda MP, Ray LA, Snih SA, Ottenbacher KJ, Markides KS. The protective effect of neighborhood composition on increasing frailty among older Mexican Americans: a barrio advantage? J Aging Health. 2011;23:1189–1217. doi:10.1177/0898264311421961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Masel MC, Howrey B, Peek MK. The effect of acculturation on frailty among older Mexican Americans. J Aging Health. 2011;23:704–713. doi:10.1177/0898264310391786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peek MK, Howrey BT, Ternent RS, Ray LA, Ottenbacher KJ. Social support, stressors, and frailty among older Mexican American adults. J Gerontol B Psychol Sci Soc Sci. 2012;67:755–764. doi:10.1093/geronb/gbs081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Espinoza SE, Hazuda HP. Frailty prevalence and neighborhood residence in older Mexican Americans: the San Antonio longitudinal study of aging. J Am Geriatr Soc. 2015;63:106–111. doi:10.1111/jgs.13202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Masel MC, Ostir GV, Ottenbacher KJ. Frailty, mortality, and health-related quality of life in older Mexican Americans. J Am Geriatr Soc. 2010;58:2149–2153. doi:10.1111/j.1532-5415.2010.03146.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Graham JE, Snih SA, Berges IM, Ray LA, Markides KS, Ottenbacher KJ. Frailty and 10-year mortality in community-living Mexican American older adults. Gerontology. 2009;55:644–651. doi:10.1159/000235653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Al Snih S, Graham JE, Ray LA, Samper-Ternent R, Markides KS, Ottenbacher KJ. Frailty and incidence of activities of daily living disability among older Mexican Americans. J Rehabil Med. 2009;41:892–897. doi:10.2340/16501977-0424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Montero-Odasso M, Bergman H, Béland F, Sourial N, Fletcher JD, Dallaire L. Identifying mobility heterogeneity in very frail older adults. Are frail people all the same? Arch Gerontol Geriatr. 2009;49:272–277. doi:10.1016/j.archger.2008.09.010 [DOI] [PubMed] [Google Scholar]
- 20. Yang Y, Lee LC. Dynamics and heterogeneity in the process of human frailty and aging: evidence from the US older adult population. J Gerontol B Psychol Sci Soc Sci. 2010;65B:246–255. doi:10.1093/geronb/gbp102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lowsky DJ, Olshansky SJ, Bhattacharya J, Goldman DP. Heterogeneity in healthy aging. J Gerontol A Biol Sci Med Sci. 2014;69:640–649. doi:10.1093/gerona/glt162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Howrey BT, Al Snih S, Jana KK, Peek MK, Ottenbacher KJ. Stability and change in activities of daily living among older Mexican Americans. J Gerontol A Biol Sci Med Sci. 2016;71:780–786. doi:10.1093/gerona/glv172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Howrey BT, Raji MA, Masel MM, Peek MK. Stability in cognitive function over 18 years: prevalence and predictors among older Mexican Americans. Curr Alzheimer Res. 2015;12:614–621. doi:10.2174/1567205012666150701102947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Markides KS, Rudkin L, Angel RJ, Espino DV. Health status of hispanic elderly. In: Martin L, Soldo B, eds. Racial and Ethnic Differences in the Health of Older Americans. Washington, DC: National Academies Press; 1997:285–300. [PubMed] [Google Scholar]
- 25. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi:10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 26. Lobo A, Escobar V, Ezquerra J, Seva Díaz A. “El Mini-Examen Cognoscitivo” (Un test sencillo, práctico, para detectar alteraciones intelectuales en pacientes psiquiátricos). [The “Mini-Examen Cognoscitiuo”: a simple and practical test to detect intellectual dysfunctions in psychiatric patients.]. Revista de Psiquiatría y Psicología Médica. 1980;14:39–57. [Google Scholar]
- 27. Raji MA, Al Snih S, Ray LA, Patel KV, Markides KS. Cognitive status and incident disability in older Mexican Americans: findings from the Hispanic established population for the epidemiological study of the elderly. Ethn Dis. 2004;14:26–31. [PubMed] [Google Scholar]
- 28. Stuck AE, Walthert JM, Nikolaus T, Büla CJ, Hohmann C, Beck JC. Risk factors for functional status decline in community-living elderly people: a systematic literature review. Soc Sci Med. 1999;48:445–469. doi:10.1016/s0277-9536(98)00370-0 [DOI] [PubMed] [Google Scholar]
- 29. Nagin DS. Analyzing developmental trajectories: a semiparametric, group-based approach. Psychol Methods. 1999;4:139–157. doi:10.1037/1082-989X.4.2.139 [DOI] [PubMed] [Google Scholar]
- 30. Jones BL, Nagin DS. A note on a stata plugin for estimating group-based trajectory models. Sociol Methods Res. 2013;42:608–613. doi:10.1177/0049124113503141 [Google Scholar]
- 31. Haviland AM, Jones BL, Nagin DS. Group-based trajectory modeling extended to account for nonrandom participant attrition. Sociol Methods Res. 2011;40:367–390. doi:10.1177/0049124111400041 [Google Scholar]
- 32. Nguyen HT, Black SA, Ray LA, Espino DV, Markides KS. Predictors of decline in MMSE scores among older Mexican Americans. J Gerontol A Biol Sci Med Sci. 2002;57:M181–M185. doi:10.1093/gerona/57.3.m181 [DOI] [PubMed] [Google Scholar]
- 33. Hubbard RE, Andrew MK, Fallah N, Rockwood K. Comparison of the prognostic importance of diagnosed diabetes, co-morbidity and frailty in older people. Diabet Med. 2010;27:603–606. doi:10.1111/j.1464-5491.2010.02977.x [DOI] [PubMed] [Google Scholar]
- 34. Cacciatore F, Testa G, Galizia G, et al. Clinical frailty and long-term mortality in elderly subjects with diabetes. Acta Diabetol. 2013;50:251–260. doi:10.1007/s00592-012-0413-2 [DOI] [PubMed] [Google Scholar]
- 35. Evans WJ, Paolisso G, Abbatecola AM, et al. Frailty and muscle metabolism dysregulation in the elderly. Biogerontology. 2010;11:527–536. doi:10.1007/s10522-010-9297-0 [DOI] [PubMed] [Google Scholar]
- 36. Mitnitski A, Fallah N, Rockwood MR, Rockwood K. Transitions in cognitive status in relation to frailty in older adults: a comparison of three frailty measures. J Nutr Health Aging. 2011;15:863–867. doi:10.1007/s12603-011-0066-9 [DOI] [PubMed] [Google Scholar]
- 37. Buchman AS, Yu L, Wilson RS, Boyle PA, Schneider JA, Bennett DA. Brain pathology contributes to simultaneous change in physical frailty and cognition in old age. J Gerontol A Biol Sci Med Sci. 2014;69:1536–1544. doi:10.1093/gerona/glu117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sternberg SA, Wershof Schwartz A, Karunananthan S, Bergman H, Mark Clarfield A. The identification of frailty: a systematic literature review. J Am Geriatr Soc. 2011;59:2129–2138. doi:10.1111/j.1532-5415.2011.03597.x [DOI] [PubMed] [Google Scholar]
- 39. Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia: a meta-analysis. Arch Phys Med Rehabil. 2004;85:1694–1704. doi:10.1016/j.apmr.2004.03.019 [DOI] [PubMed] [Google Scholar]
- 40. Peterson MJ, Giuliani C, Morey MC, et al. ; Health, Aging and Body Composition Study Research Group. Physical activity as a preventative factor for frailty: the health, aging, and body composition study. J Gerontol A Biol Sci Med Sci. 2009;64:61–68. doi:10.1093/gerona/gln001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Neighbors CJ, Marquez DX, Marcus BH. Leisure-time physical activity disparities among Hispanic subgroups in the United States. Am J Public Health. 2008;98:1460–1464. doi:10.2105/AJPH.2006.096982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hill TD, Burdette AM, Angel JL, Angel RJ. Religious attendance and cognitive functioning among older Mexican Americans. J Gerontol B Psychol Sci Soc Sci. 2006;61:P3–P9. doi:10.1093/geronb/61.1.p3 [DOI] [PubMed] [Google Scholar]
- 43. Hill TD, Burdette AM, Taylor J, Angel JL. Religious attendance and the mobility trajectories of older Mexican Americans: an application of the growth mixture model. J Health Soc Behav. 2016;57:118–134. doi:10.1177/0022146515627850 [DOI] [PubMed] [Google Scholar]
- 44. Berges IM, Kuo YF, Markides KS, Ottenbacher K. Attendance at religious services and physical functioning after stroke among older Mexican Americans. Exp Aging Res. 2007;33:1–11. doi:10.1080/03610730601005893 [DOI] [PubMed] [Google Scholar]
- 45. Bell CN, Bowie JV, Thorpe RJ Jr. The interrelationship between hypertension and blood pressure, attendance at religious services, and race/ethnicity. J Relig Health. 2012;51:310–322. doi:10.1007/s10943-010-9346-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.