Abstract
Background
In older adults, compromised white matter tract integrity within the brain has been linked to impairments in mobility. We contend that poorer integrity disrupts mobility by altering the processing of sensorimotor and cognitive and attentional resources in neural networks. The richness of information processing in a given network can be quantified by calculating the complexity of resting-state functional MRI time series. We hypothesized that (i) older adults with lower brain complexity, specifically within sensorimotor, executive, and attention networks, would exhibit slower walking speed and greater dual-task costs (ie, dual-task cost) and (ii) such complexity would mediate the effect of white matter integrity on these metrics of mobility.
Methods
Fifty-three older adults completed a walking assessment and a neuroimaging protocol. Brain complexity was quantified by calculating the multiscale entropy of the resting-state functional MRI signal within seven previously defined functional networks. The white matter integrity across structures of the corpus callosum was quantified using fractional anisotropy.
Results
Participants with lower resting-state complexity within the sensorimotor, executive, and attention networks walked more slowly under single- and dual-task (ie, walking while performing a serial-subtraction task) conditions (β > 0.28, p ≤ .01) and had a greater dual-task cost (β < −0.28, p < .04). Complexity in these networks mediated the influence of the corpus callosum genu on both single- (indirect effects > 0.15, 95% confidence intervals = 0.02–0.32) and dual-task walking speeds (indirect effects > 0.13, 95% confidence intervals = 0.02–0.33).
Conclusion
These results suggest that the multiscale dynamics of resting-state brain activity correlate with mobility and mediate the effect of the microstructural integrity in the corpus callosum genu on walking speed in older adults.
Keywords: Resting-state BOLD complexity, Multiscale entropy, White matter integrity, Walking speed
Age-related decline in the control of walking (eg, slowed walking speed) diminishes functional independence, increases fall risk, and heightens one’s fear of falling in the future (1,2). Walking is a dynamic task regulated by a complex control system. This system includes multiple interacting brain networks related to sensory integration, motor coordination, attention, and executive function. These brain networks continuously process information from multiple physiologic systems over multiple temporal-spatial scales (3,4). Such complex control enables one to adapt their gait to ever-changing environmental constraints and interference from additional tasks; for example, walking while talking or reading a sign (ie, dual tasking) (5). Thus, although age-related declines in the control of walking have been typically attributed to multiple peripheral or skeletomuscular factors, they probably also arise from alterations in the capacity of the brain to effectively process, integrate, and communicate information within and across essential neural networks, especially those pertaining to sensorimotor, executive, and attentional control.
Even when an individual is at rest, neurons in the brain are communicating and interacting nonlinearly through multiple pathways and connections (eg, white matter tracts) over multiple scales of time (6,7), ranging from submilliseconds (eg, the transmission of neural impulses) to hours (eg, circadian rhythms). As such, the dynamics of spontaneous brain activity—detected by blood oxygen level-dependent (BOLD) signals during resting-state functional magnetic resonance imaging (rs-fMRI)—are “complex,” meaning that the active fluctuations in BOLD signals contain information-rich fractal-like patterns that are self-similar over multiple scales of time (8,9). The degree of such complexity can be captured using metrics derived from complex systems theory (10,11). Multiscale entropy (MSE) is one well-developed and widely used technique that quantifies the recurrence of patterns, or entropy, in time series data at different scales of time (12,13). The greater the likelihood of recurrent patterns (eg, as occurs in a sine wave), the less complex the signal, the poorer the system’s adaptability, and the poorer the functional outcome. For example, Yang and colleagues observed that older adults with lower rs-fMRI complexity within the default mode network had worse performance on cognitive tasks (9). In the present study, we contend that lower rs-fMRI complexity in a range of cortical networks relating to the control of sensorimotor and cognitive function would be associated with poorer walking performance in older adults.
Furthermore, because communication between functional units in the brain relies primarily on the integrity of underlying white matter, we believe white matter alterations reduce the capacity of the brain to effectively transmit information between cortical structures. For example, previous studies have shown that the neurodegeneration of the corpus callosum, as measured by lower fractional anisotropy (FA) in diffusion-weighted imaging (DWI), is predictive of impaired walking performance in older adults (14–16). We contend that lower FA within the corpus callosum is associated with impaired gait because it reflects altered information transmission across brain functional networks, as evidenced by lower resting-state complexity.
Here we hypothesize that (i) older adults who exhibit lower rs-fMRI complexity within the networks pertaining to sensorimotor, executive function, and attention will walk more slowly during both quiet and “dual-task” conditions (ie, walking while performing a cognitive task) and have a greater performance decrement between conditions (ie, dual-task cost [DTC]) and (ii) network complexity will mediate the relationship between corpus callosum integrity and these walking speeds.
Methods
Participants
Fifty-three participants (age range: 72–96 years) were recruited from the MOBILIZE Boston Study (MBS), a population-based study aiming to identify unique risk factors surrounding falls in older adults (17). Participants of the original MBS cohort were included if they were community dwelling, aged at least 70 years, and able to walk 20 feet without personal assistance. Exclusion criteria included terminal disease, severe vision or hearing deficits, evidence of cognitive impairment (ie, Mini-Mental State Examination score < 25 within 3 years), and any condition making it difficult to perform the walking or MRI protocols. All experimental methods and protocols were approved by the Hebrew SeniorLife and VA Boston Healthcare System Institutional Review Boards (IRBs) and carried out in accordance with relevant guidelines. Written consent was required to participate in the study.
Study Protocol
Participants underwent a walking assessment within the Clinical Research Laboratory at Hebrew SeniorLife’s Marcus Institute for Aging Research and a neuroimaging protocol at the VA Boston Medical Center. Each participant completed both visits within a 2-week period.
Walking Assessment
Walking speed was measured using a 4.8-m GAITRite pressure mat (CIR Systems Inc., Havertown, PA). Two walking trials were completed during each of two conditions: normal walking (ie, single task) and walking while performing an additional cognitive task (ie, dual task). Each trial consisted of three bouts of straight walking over the mat, whereas trial order was randomized. Participants began each trial standing approximately 2 m in front of the mat and completed 180° turns 2 m after the mat, such that each pass over the mat was completed during steady-state walking. Participants performed a practice walk before starting the trials and were instructed to walk at their normal, preferred speed. In dual-task walking trials, participants were instructed to serially subtract 3 from 100. If participants could not perform this task, they were instructed to perform a task with reduced difficulty (eg, subtraction of 5 or 1). The faster walking speed of the two trials within each walking condition was used as outcome variables, because it best reflected participant walking capacity. DTCs were obtained by calculating the percent decrease in walking speed (ie, poorer performance) from the single to the dual-task condition.
MRI Assessment
To measure spontaneous brain activity over time, participants performed an eyes-open rs-fMRI scan (gradient-echo echo-planar sequence; repetition time = 3,000 ms, echo time = 26 ms, flip angle = 90°, 34 slices at 1.5 mm, in-plane resolution: 3 × 3 mm, matrix = 64 × 64, 120 volumes). A T1-weighted scan (MPRAGE; T1 = 1,000 ms, repetition time = 2.73 ms, echo time = 3.31 ms, flip angle = 7°, slice thickness = 1.3 mm, 128 slices, in-plane resolution: 1 × 1 mm, 256 × 256) was acquired for whole-brain high-resolution anatomy. A diffusion-weighted imaging scan (Acquisition Type 2D; 60 directions, b-val = 700, flip angle = 90°, repetition time = 10,000 ms, echo time = 103 ms, 2 mm iso-resolution, in-plane resolution: 2 × 2 mm, matrix = 128 × 128) was then acquired to assess white matter integrity.
These neuroimaging data were acquired across a 3T Siemens MRI scanner, before (MAGNETOM TIM Trio; 12-channel head coil) and after (Prisma Fit; 20-channel head coil) upgrade. To account for potential interscanner differences, scanner was included as a covariate in subsequent analyses.
Data Analysis
Complexity of rs-fMRI BOLD signal
Resting-state data were pre-processed using Analysis of Functional NeuroImages (18). The following steps were performed: the removal of the first three volumes, slice-timing correction, volume registration, alignment to the T1 anatomy, warp into Talairach space, 8-mm kernel smoothing, and scaling to a percentage of the mean. Data were then band-pass filtered from 0.01 to 0.08 Hz (19) and entered into a general linear model to remove the effects of 6 degrees of motion and their derivatives, nuisance cerebrospinal fluid, and white matter. The band-pass filtering and nuisance removal were completed simultaneously in Analysis of Functional NeuroImages, which avoided potential artifact re-introduced by modular preprocessing (20). We also performed these models with and without further nuisance global signal removal. The residual time series from each deconvolution reflected a “cleaned” BOLD signal for complexity calculation across the brain.
Complexity was quantified at each brain voxel by using MSE to calculate the entropies across five temporal scales (Supplementary Figure S1). Greater averaged MSE reflected greater complexity. More information on the mathematic calculation of MSE and its validation is included as Supplementary Material.
Voxels were then averaged across each of seven cortical networks, as described by Yeo and colleagues (21). This widely used brain parcellation offers a reliable and unbiased localization of “functional” networks and has been utilized in our previous studies investigating functional connectivity and walking speed (22,23). The resulting network complexity estimates were used in statistical analyses.
Fractional anisotropy
FA was quantified using the following standard preprocessing diffusion imaging techniques (FSL V5.0.9 University of Oxford, UK) (24): eddy current and motion correction, brain masking, and linear fitting of diffusion tensors. Further processing included data normalization to the MNI152 template and the exclusion of FA values less than 0.2. Data were skeletonized and averaged across tracts of the corpus callosum (ie, genu, body, and splenium), as defined by the Johns Hopkins (JHU) ICBM-DTI-81 white matter atlas (25).
Statistical Analysis
Statistical analyses were performed with JMP Pro 13 (SAS Institute, Cary, NC) and SPSS 20 (IBM Corp, Armonk, NY). Since age was a potential confounder associated with decreased complexity (9), slowed walking speed, and lower white matter integrity, it was used as a covariate in all statistical models.
To test the hypothesis that older adults with lower complexity in the aforementioned frontal networks walked more slowly, we used separate multiple linear regression analyses for each walking outcome. We additionally analyzed the associations with other network complexity measures (ie, visual, limbic and default mode) using similar models.
To test the hypothesis that rs-fMRI complexity mediates the relationship between corpus callosum integrity and walking performance we utilized mediation analyses. Network complexity averages were used as mediators; age and scanner assignments were used as covariates. We calculated the total effects of the corpus callosum FA on the walking metrics (ie, total effect, path c), and the association between corpus callosum FA and the network complexity (path a). Then, we examined the association between network complexity and walking metrics (path b), which also provided the estimates for the direct effects (path c’). The percentage mediated (ie, PM) was determined by dividing the indirect effect (path a × path b) by the total effect (path c). A bootstrapping method with N = 5,000 bootstrap samples was used to calculate the 95% bias corrected and accelerated confidence intervals around the mediated and direct effects. Significance level was set to p < .05.
Results
All the participants completed the gait and MRI assessments. Nineteen completed brain imaging on the TIM Trio, whereas the other 34 participants completed imaging on the PrismaFit. Two participants were excluded from the analyses since one performed DTC lower than the mean ± three times of standard deviation (ie, mean ± 3×SD) of the group DTC and one had network complexity metrics lower than mean ± 3×SD of the group complexity metrics. Table 1 shows the demographics, most recent Mini-Mental State Exam, and walking performance of these cohorts, separately and collectively. No significant differences were observed between subcohorts (F < 0.53, p > .47).
Table 1.
Demographics, Cognitive Function, and Walking Speeds of the Entire and Subcohorts
| Complete Cohort | Subcohort 1 (n = 19) | Subcohort 2 (n = 34) | |
|---|---|---|---|
| Age (y) | 84.1 ± 4.8 | 83.7 ± 5.2 | 84.2 ± 4.6 |
| Sex | 37 females | 12 females | 25 females |
| Body mass index | 25.2 ± 4.6 | 25.2 ± 4.4 | 25.2 ± 4.7 |
| Education (y) | 16 ± 2.4 | 15.7 ± 2.7 | 16.1 ± 2.1 |
| MMSE score | 26.7 ± 1.4 | 26.5 ± 1.4 | 26.8 ± 1.4 |
| Single-task walking speed (m/s) | 1.14 ± 0.27 | 1.11 ± 0.26 | 1.15 ± 0.28 |
| Dual-task walking speed (m/s) | 0.92 ± 0.26 | 0.93 ± 0.25 | 0.92 ± 0.26 |
| Dual-task costs to walking speed (%) | −19 ± 10% | −17 ± 11% | −20 ± 9% |
Note: MMSE = Mini-Mental State Examination.
Participants walked faster during the single-task condition (1.14 ± 0.2 m/s) than the dual-task condition (0.92 ± 0.2 m/s). Walking speeds, DTC, and FA across the corpus callosum were associated with age, such that older age had slower single-task (r = −.46, p < .001) and dual-task (r = −.53, p < .001) walking speeds, greater DTC (r = .4, p = .004) and lower white matter integrity of genu, body, and splenium (r > −.32, p < .03). No significant associations were observed between age and the network complexity averages (r < −.26–.2, p > .06).
Relationships Between Network Complexity and Walking Speed
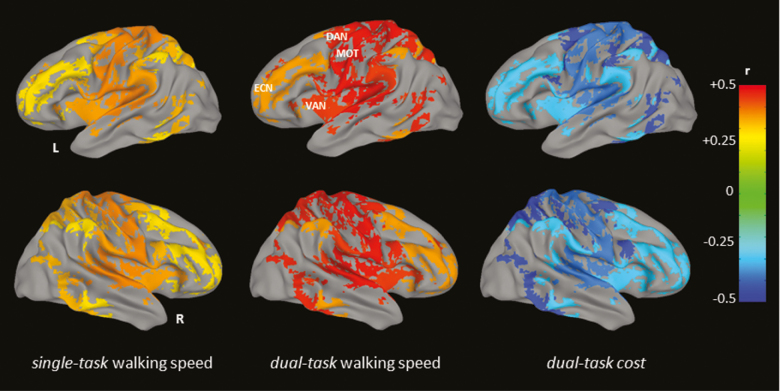
Linear regression analyses demonstrated that lower complexity within the sensorimotor, dorsal attention, and ventral attention were each associated with slower single-task (β = 0.33–0.37, p = .008–.01) and dual-task (β = 0.44–0.48, p = .0005–0.002) walking speeds (Table 2 and Figure 1). Lower complexity of executive network was significantly associated with slower dual-task walking speed (r = .35, p = .01), whereas a marginal significant association was observed between lower complexity of executive network and slower single-task walking speed (β = 0.28, p = .05; Table 2 and Figure 1). Moreover, lower complexity of these networks was associated with greater DTC (β = −0.42 to −0.28, p = .002–.04). All the associations were independent of scanner assignment and age. No significant associations were observed between walking speed and the complexity of the visual, limbic, or default mode networks (p < .23, p > .11). Similar results were observed when global regression was not performed as a preprocessing step (see Supplementary Table 1).
Table 2.
The Associations Between Walking Speeds During the Single- and Dual-Task Conditions, Dual-Task Costs to Walking Speed, and the Complexity of Brain Networks
| Single Task | Dual Task | Dual-Task Cost | ||||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| Sensorimotor | .37 | .008* | .48 | .0005* | −.37 | .008* |
| Dorsal attention | .33 | .01* | .46 | .0008* | −.42 | .002* |
| Ventral attention | .35 | .01* | .44 | .002* | −.29 | .03* |
| Executive | .28 | .05 | .35 | .01* | −.28 | .04* |
| Visual | .13 | .36 | .23 | .11 | −.19 | .17 |
| Limbic | .06 | .68 | .06 | .66 | −.02 | .87 |
| Default mode | .15 | .30 | .22 | .13 | −.21 | .13 |
Note: *p < .05.
Figure 1.
The networks in which complexity was associated with single- and dual-task walking speeds and dual-task costs to walking speed. Multiple linear regression analyses demonstrated that the complexity of sensorimotor, attention, and executive networks was associated with single- (β > 0.28, p ≤ .05) and dual-task (β > 0.35, p < .01) walking speeds and the dual-task costs (r < −.28, p < .04). Different colors in the figure represent the different values of the standardized beta coefficients. Brighter colors reflect greater coefficients. No associations were observed between visual, limbic, and default mode networks and walking speed (p < .23, p > .11).
Mediation Effects of Network Complexity on the Relationship Between Corpus Callosum FA and Walking Speed
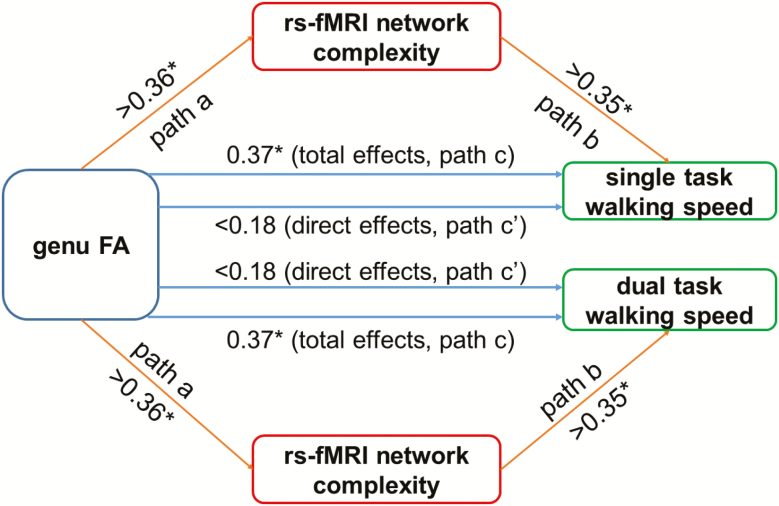
Only the genu of the corpus callosum was associated with both brain network complexity and walking speeds, such that older adults with lower FA had lower complexity in sensorimotor, ventral attention, dorsal attention, and executive networks (r > .36, p < .01), as well as slower walking speeds in both single- and dual-task conditions (r > .37, p < .01). No walking speed associations were observed with the corpus callosum body or splenium (r < .25, p > .08) or with DTC as the primary outcome (r < .13, p > .33). As a result, we focused on the associations between the genu of the corpus callosum and walking speeds in the mediation analyses.
The mediation procedures revealed that the association between genu FA and single-task and dual-task walking speeds was mediated by network complexity (Figure 2). Specifically, the white matter integrity of the genu was associated with complexity in sensorimotor, ventral attention, dorsal attention, and executive networks (paths a; r > .36, p < .01). Though the total effects (ie, path c) of genu integrity on single- and dual-task walking speed were statistically significant (r > .37, p < .01), its direct effects (path c’) on walking speed were no longer significant after introducing network complexity as mediator (single task: r < .18, p > .18; dual task: r < .15, p > .22). The sensorimotor, dorsal and ventral attention, and executive network complexity measures accounted for greater than 43% of the total effects on both single-task (indirect effects (ie, path a × path b) > 0.15, PM > 43%, 95% bias corrected and accelerated confidence intervals = 0.02–0.32) and dual-task walking speed (indirect effects > 0.13, PM > 48%, 95% bias corrected and accelerated confidence intervals = 0.02–0.33).
Figure 2.
The rs-MRI complexity of brain BOLD signal mediated the relationship between genu FA and walking speed. White matter integrity of the corpus callosum (genu FA) was associated with the complexity in sensorimotor, dorsal attention, ventral attention, and executive networks (ie, path a: r > .36, p < .01). The complexity in these networks was associated with single-task (path b, top right pathway: r > .35, p < .01) and dual-task (path b, bottom right pathway: r > .35, p < .01) walking speeds. Significant total effects (path c) of genu FA on single- (top, r = .37, p = .009) and dual-task (bottom, r = .37, p = .01) walking speed were observed, whereas the direct effects (path c’) of genu FA on walking speed were not significant after using the complexity as the mediator (single task: r < .18, p > .18; dual task: r < .15, p > .22). *p < .05.
Discussion
The present study is the first to demonstrate that (i) lower physiologic complexity of spontaneous (ie, resting state) BOLD activity in sensorimotor, ventral attention, dorsal attention, and executive networks of the brain is associated with slower walking speeds and greater dual-task costs and (ii) such complexity mediates the influence of microstructural white matter integrity in the corpus callosum on walking speed. Thus, spontaneous brain activity over multiple temporal scales, as quantified by “complexity,” provides unique insight into the neural regulation of walking and may motivate future rehabilitative strategies for balance and mobility.
The degree of complexity observed within the spontaneous patterns of a given physiologic system’s behavior (or output) during rest or a “free-running” state is associated with the capacity of that system to adapt to stress and thus its functional performance (26). In the “complexity theory of aging,” aging and age-related diseases often alter the quantity and/or quality of the regulatory elements of a physiologic system as well as their structural and functional connections within the system (27). The age- and disease-related loss or degradation of these regulatory elements manifests as a loss of the physiologic complexity in the dynamics of the system’s output in its “free-running” state (28). Previous studies have linked lower resting-state complexity of the BOLD signal to aging, impaired cognitive performance (9) and diseases such as schizophrenia (19). In a recent study, Wang and colleagues demonstrated that greater complexity of the brain’s regional rs-MRI signals, as quantified using MSE, was correlated with greater functional connectivity within the same regions (29). This suggests that the complexity metric captures the brain’s capacity for information processing, such that greater complexity represents greater interactions between brain regions. Here, we have linked the complexity of frontal brain networks to the walking performance in older adults; those with lower resting-state complexity walked slower in both single- and dual-task conditions and had greater DTC. Future work should explore longitudinal relationships between changes in resting-state brain complexity and walking performance in older adults.
These complexity-walking performance relationships were also specific to certain brain functional networks. Our finding that the complexity of sensorimotor, executive, and attention networks is associated with walking speeds and DTC is consistent with the results of previous studies using traditional rs-fMRI (23,30). Yuan and colleagues, for example, reported that the resting-state functional connectivity of these networks is associated with walking speed in older adults (30). In a recent study based on the same MBS cohort, we also observed that the greater functional connectivity of sensorimotor and cognitive networks was associated with faster walking speeds and smaller DTC in older adults (23). Here, by measuring the dynamics of spontaneous BOLD fluctuations in these networks across multiple temporal scales using a complexity metric, our study provides complementary and confirmatory evidence that these networks play important roles in the control of walking.
The observed associations between the complexity of the ventral and dorsal attention networks and walking performance provide evidence that the capacity for information transfer within attentional networks is important for the regulation of walking. Specifically, these two networks subserve two attentional processes: the ventral attention network is primarily involved in task-switching and the reorientation of attention, whereas the dorsal attention network mediates goal-directed/guided attention (31,32). These resources were associated with walking speed during both conditions; however, they were more strongly associated with dual-task walking speed, probably due to the sustained attentional requirements of concurrent task performance. Future work should validate the specific roles of these two networks in the control of walking by utilizing different types of attentional cues and incorporating the cognitive-attention performance into the analyses.
In this study, we also observed that resting-state complexity of the BOLD signal was a mediator of the established association between corpus callosum structural integrity and walking performance, contributing to over 40% of this relationship. Previous studies observed that neurodegeneration within the corpus callosum, the highly dense fiber bundle responsible for interhemispheric communication, was associated with declines in balance and mobility, as well as cognitive function in older adults (14,33). Our complexity mediation findings support the following conceptual framework: lower white matter integrity in the genu of the corpus callosum may disrupt the communications and interactions between the frontal regions/networks of the brain and thus diminish the capacity of the brain to efficiently and effectively integrate and process inputs, including those pertaining to the control of walking, particularly in older adults (34).
Our observations also suggest that rehabilitative strategies for walking may be optimized by targeting the complex dynamics of spontaneous brain activity. Previous studies suggested that the loss of complexity in physiologic systems may not be an obligatory consequence of aging or age-related diseases, but instead, can be overcome with appropriate interventions (35). Several techniques, such as transcranial direct current or transcranial magnetic stimulation, can selectively and noninvasively change the neural activity of brain regions, and these interventions can be designed to facilitate the excitability of the networks we identified in this study (eg, sensorimotor, attention, and executive networks). Stimulation of these networks induces improvements in balance, mobility, and cognition (36–38). One recent pilot study, for example, showed that it is feasible to use multisession transcranial magnetic stimulation targeting both the cerebellum and sensorimotor network to alter the motor network complexity in patients with multiple systems atrophy. Such transcranial magnetic stimulation–induced change of brain complexity was associated with improvement of motor performance (38). Future studies are thus worthwhile to explore the effects of these interventions on the complexity of spontaneous brain activities and to determine whether an increase in complexity is associated with improvements in clinical and behavioral outcomes.
The present study has limitations. First, our sample size (n = 53) was still relatively small. Second, regarding our rs-fMRI paradigm, the sampling rate of the BOLD signal was relatively low (ie, 0.33 Hz) and the scan time is common in MRI studies but short compared with other techniques that record brain activity (eg, electroencephalogram). As a result, only the dynamics in relatively large scales were captured. Thus, it is thus worthwhile to implement a longer BOLD scan time in future studies, allowing the observation of multiscale dynamics on a wider range of time scales. Other rs-fMRI preprocessing techniques should also be explored to further optimize the complexity signal.
A final limitation is that we focused only on walking speed. Future studies are needed to explore the relationships between resting-state complexity and other gait metrics (eg, stride time variability or gait complexity). Nevertheless, our study is unique in demonstrating that the physiological complexity of spontaneous brain activity may serve as a novel biomarker to characterize age-related declines in walking speed.
Supplementary Material
Acknowledgments
J.Z., V.P., B.M., M.E., and L.L. designed the study; I.I. and T.W. collected the data; J.Z., V.P., and T.W. analyzed the data and performed statistical analyses; J.Z., V.P., O.L., B.M., M.E., and L.L. drafted the manuscript; and all authors contributed to and approved the final version.
Funding
This study was supported by grants from the National Institute on Aging (P01-AG004390, AG025037, AG041785, K01-AG044543, and T32-AG023480). Dr. Junhong Zhou is supported by the Hebrew SeniorLife Marcus Applebaum grant. Dr. Poole is supported by a KL2/Catalyst Medical Research Investigator Training award from the Harvard Catalyst (National Center for Advancing Translational Sciences, NIH Award KL2 TR002542 and UL 1TR002541). Dr. Lipsitz holds the Irving and Edyth S. Usen and Family Chair in Geriatric Medicine at Hebrew SeniorLife. Dr. Esterman was supported by the United States Department of Veterans Affairs Clinical Sciences R&D (CSRD) Service (Merit Review Award I01CX001653).
Conflict of interest
None reported.
References
- 1. Beauchet O, Annweiler C, Allali G, Berrut G, Herrmann FR, Dubost V. Recurrent falls and dual task-related decrease in walking speed: is there a relationship? J Am Geriatr Soc. 2008;56:1265–1269. doi:10.1111/j.1532-5415.2008.01766.x [DOI] [PubMed] [Google Scholar]
- 2. Montero-Odasso M, Verghese J, Beauchet O, Hausdorff JM. Gait and cognition: a complementary approach to understanding brain function and the risk of falling. J Am Geriatr Soc. 2012;60:2127–2136. doi:10.1111/j.1532-5415.2012.04209.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Amboni M, Barone P, Hausdorff JM. Cognitive contributions to gait and falls: evidence and implications. Mov Disord. 2013;28:1520–1533. doi:10.1002/mds.25674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hamacher D, Herold F, Wiegel P, Hamacher D, Schega L. Brain activity during walking: a systematic review. Neurosci Biobehav Rev. 2015;57:310–327. doi:10.1016/j.neubiorev.2015.08.002 [DOI] [PubMed] [Google Scholar]
- 5. Smith E, Cusack T, Blake C. The effect of a dual task on gait speed in community dwelling older adults: a systematic review and meta-analysis. Gait Posture. 2016;44:250–258. doi:10.1016/j.gaitpost.2015.12.017 [DOI] [PubMed] [Google Scholar]
- 6. Betzel RF, Bassett DS. Multi-scale brain networks. Neuroimage. 2017;160:73–83. doi:10.1016/j.neuroimage.2016.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burns SP, Santaniello S, Yaffe RB, et al. Network dynamics of the brain and influence of the epileptic seizure onset zone. Proc Natl Acad Sci USA. 2014;111:E5321–E5330. doi:10.1073/pnas.1401752111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garrett DD, Kovacevic N, McIntosh AR, Grady CL. The importance of being variable. J Neurosci. 2011;31:4496–4503. doi:10.1523/JNEUROSCI.5641-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang AC, Huang CC, Yeh HL, et al. Complexity of spontaneous BOLD activity in default mode network is correlated with cognitive function in normal male elderly: a multiscale entropy analysis. Neurobiol Aging. 2013;34:428–438. doi:10.1016/j.neurobiolaging.2012.05.004 [DOI] [PubMed] [Google Scholar]
- 10. Lipsitz LA, Goldberger AL. Loss of ‘complexity’ and aging. Potential applications of fractals and chaos theory to senescence. JAMA. 1992;267:1806–1809. [PubMed] [Google Scholar]
- 11. Lipsitz LA. Physiological complexity, aging, and the path to frailty. Sci Aging Knowledge Environ. 2009;16:pe16. doi:10.1126/sageke.2004.16.pe16 [DOI] [PubMed] [Google Scholar]
- 12. Costa M, Goldberger AL, Peng CK. Multiscale entropy analysis of complex physiologic time series. Phys Rev Lett. 2002;89:068102. doi:10.1103/PhysRevLett.89.068102 [DOI] [PubMed] [Google Scholar]
- 13. Costa M, Goldberger AL, Peng CK. Multiscale entropy analysis of biological signals. Phys Rev E Stat Nonlin Soft Matter Phys. 2005;71:021906. doi:10.1103/PhysRevE.71.021906 [DOI] [PubMed] [Google Scholar]
- 14. Bhadelia RA, Price LL, Tedesco KL, et al. Diffusion tensor imaging, white matter lesions, the corpus callosum, and gait in the elderly. Stroke. 2009;40:3816–3820. doi:10.1161/STROKEAHA.109.564765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fujiyama H, Van Soom J, Rens G, et al. Age-related changes in frontal network structural and functional connectivity in relation to bimanual movement control. J Neurosci. 2016;36:1808–1822. doi:10.1523/JNEUROSCI.3355-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gooijers J, Swinnen SP. Interactions between brain structure and behavior: the corpus callosum and bimanual coordination. Neurosci Biobehav Rev. 2014;43:1–19. doi:10.1016/j.neubiorev.2014.03.008 [DOI] [PubMed] [Google Scholar]
- 17. Leveille SG, Kiel DP, Jones RN, et al. The MOBILIZE Boston Study: design and methods of a prospective cohort study of novel risk factors for falls in an older population. BMC Geriatr. 2008;8:16. doi:10.1186/1471-2318-8-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi:10.1006/cbmr.1996.0014 [DOI] [PubMed] [Google Scholar]
- 19. Yang AC, Hong CJ, Liou YJ, et al. Decreased resting-state brain activity complexity in schizophrenia characterized by both increased regularity and randomness. Hum Brain Mapp. 2015;36:2174–2186. doi:10.1002/hbm.22763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lindquist MA, Geuter S, Wager TD, Caffo BS. Modular preprocessing pipelines can reintroduce artifacts into fMRI data. Hum Brain Mapp. 2019;40:2358–2376. doi:10.1002/hbm.24528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yeo BT, Krienen FM, Sepulcre J, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. doi:10.1152/jn.00338.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lo OY, Halko MA, Zhou J, Harrison R, Lipsitz LA, Manor B. Gait speed and gait variability are associated with different functional brain networks. Front Aging Neurosci. 2017;9:390. doi:10.3389/fnagi.2017.00390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Poole VN, Lo OY, Wooten T, Iloputaife I, Lipsitz LA, Esterman M. Motor-cognitive neural network communication underlies walking speed in community-dwelling older adults. Front Aging Neurosci. 2019;11:159. doi:10.3389/fnagi.2019.00159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi:10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- 25. Mori S, Oishi K, Jiang H, et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage. 2008;40:570–582. doi:10.1016/j.neuroimage.2007.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lipsitz LA. Dynamics of stability: the physiologic basis of functional health and frailty. J Gerontol A Biol Sci Med Sci. 2002;57:B115–B125. doi:10.1093/gerona/57.3.b115 [DOI] [PubMed] [Google Scholar]
- 27. Manor B, Lipsitz LA. Physiologic complexity and aging: implications for physical function and rehabilitation. Prog Neuropsychopharmacol Biol Psychiatry. 2013;45:287–293. doi:10.1016/j.pnpbp.2012.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Manor B, Costa MD, Hu K, et al. Physiological complexity and system adaptability: evidence from postural control dynamics of older adults. J Appl Physiol (1985). 2010;109:1786–1791. doi:10.1152/japplphysiol.00390.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang DJJ, Jann K, Fan C, et al. Correction: neurophysiological basis of multi-scale entropy of brain complexity and its relationship with functional connectivity. Front Neurosci. 2018;12:539. doi:10.3389/fnins.2018.00539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yuan J, Blumen HM, Verghese J, Holtzer R. Functional connectivity associated with gait velocity during walking and walking-while-talking in aging: a resting-state fMRI study. Hum Brain Mapp. 2015;36:1484–1493. doi:10.1002/hbm.22717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vossel S, Geng JJ, Fink GR. Dorsal and ventral attention systems: distinct neural circuits but collaborative roles. Neuroscientist. 2014;20:150–159. doi:10.1177/1073858413494269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi:10.1016/j.neuron.2008.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Charlton RA, Barrick TR, McIntyre DJ, et al. White matter damage on diffusion tensor imaging correlates with age-related cognitive decline. Neurology. 2006;66:217–222. doi:10.1212/01.wnl.0000194256.15247.83 [DOI] [PubMed] [Google Scholar]
- 34. Poole VN, Wooten T, Iloputaife I, Milberg W, Esterman M, Lipsitz LA. Compromised prefrontal structure and function are associated with slower walking in older adults. Neuroimage Clin. 2018;20:620–626. doi:10.1016/j.nicl.2018.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhou J, Lipsitz LA, Habtemariam D, Manor B. Sub-sensory vibratory noise augments the physiologic complexity of postural control in older adults. J Neuroeng Rehabil. 2016;13:44. doi:10.1186/s12984-016-0152-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Manor B, Zhou J, Jor’dan A, Zhang J, Fang J, Pascual-Leone A. Reduction of dual-task costs by noninvasive modulation of prefrontal activity in healthy elders. J Cogn Neurosci. 2016;28:275–281. doi:10.1162/jocn_a_00897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dagan M, Herman T, Harrison R, et al. Multitarget transcranial direct current stimulation for freezing of gait in Parkinson’s disease. Mov Disord. 2018;33:642–646. doi:10.1002/mds.27300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liu Z, Ma H, Poole V, et al. Effects of multi-session repetitive transcranial magnetic stimulation on motor control and spontaneous brain activity in multiple system atrophy: a Pilot Study. Front Behav Neurosci. 2018;12:90. doi:10.3389/fnbeh.2018.00090 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.