Abstract
Background
Walking speed during fast-paced walking task has been associated with cognitive function. It is unclear what underlying brain structures are related to fast-paced walking. We investigated the association of gray matter (GM) density with fast-paced walking speed and usual-paced walking speed.
Methods
We collected data from 284 older adults from a subset of the Health, Aging, and Body composition study (mean age = 83 [SD = 2.8], 58% women, 41% black). Voxel-wise analyses on magnetic resonance imaging data identified regions of the brain where GM density was associated with fast-paced walking speed. We then extracted GM density for all identified regions and modeled the association with fast-paced walking speed after adjusting for demographic factors, clinical factors, and cognitive function. Analyses were repeated for usual-paced walking. Regions with beta coefficients ≥0.3 m/s were considered to be meaningfully correlated.
Results
GM density of clusters from cortical regions in the right middle and superior frontal gyrus, right postcentral gyrus, and left superior temporal gyrus were positively correlated with fast-paced walking speed in adjusted models. Adjustment for cognitive function had little impact on the findings. Caudate was correlated with usual paced walking speed at coefficient ≥0.3 m/s after adjustment of demographic factors and clinical factors, but not after further adjustment of cognitive function.
Conclusions
Fast-paced walking speed was correlated with GM density of right middle and superior frontal gyrus, right postcentral gyrus, and left superior temporal gyrus, and could potentially provide evidence about subclinical structural change of brain related to aging.
Keywords: Structural magnetic resonance imaging, Mobility, Health, aging, body composition study
Slower walking speed is associated with risk of adverse outcomes, including mobility disability, cognitive decline, mortality, falls, and institutionalization, as well as worse brain integrity in older adults (1–4). To date, most studies on walking in older adults have focused on walking speed under usual conditions.
It has recently been shown that walking speed during tasks asking participants to walk as fast as possible may also be important for detecting clinical outcomes in older adults. Evidence suggests that fast-paced walking speed is associated with aging-related adverse events (5). Fast-paced walking challenges locomotor adaption and requires faster processing and integration of multiple inputs and motor response. Fast-paced walking speed also requires more active neural control than usual-paced walking (6). Previous studies suggest that fast-paced walking speed was associated with cognitive function and can predict changes in cognitive function during follow-up in older adults (7–10).
Previous evidence suggests that usual walking speed was associated with brain structure and integrity in older adults. Slower usual walking speed in older adults without any diagnosed neurological diseases was associated with lower gray matter (GM) and white matter (WM) volume, increased white matter hyperintensity burden, lower fractional anisotropy (FA), and increased mean diffusivity of both sensory- or motor-related regions and higher-order regions (see review (11)). Fewer studies have assessed the neural correlates of fast-paced walking speed. A previous study suggests that fast-paced walking speed was associated with total cerebral volume and not associated with hippocampal volume in healthy older adults (1). Fast-paced walking speed was also correlated with GM volume in right caudate nucleus, bilateral thalamus, and left putamen among older adults with memory problems (12).
In this study, we investigated the association of GM density, a measure related to both volume and cortical thickness, with fast-paced walking speed and usual-paced walking speed. We conducted voxel-wise regression to identify regions of the brain where GM density was associated with fast-paced walking speed among older adults using the Health, Aging, and Body composition (Health ABC) study (13). We hypothesized that GM density of regions associated with executive function and memory would be associated with fast-paced walking speed but not to usual-paced walking speed.
Methods
Study Population
The Health ABC study is a longitudinal cohort study that investigated physical and cognitive functional change among the elderly who were physically and cognitively healthy at baseline. Participants aged 70–79 were recruited in community-based settings from Pittsburgh, PA (n = 1,501) and Memphis, TN (n = 1,574) from 1997 to 1998 (14). Demographic information was obtained at baseline. Participants were followed annually and completed questionnaires and performance tests for the evaluation of body composition, health status, and adverse health outcomes (15).
A subset (n = 325) of the Pittsburgh site who participated in the clinical visit at Year 10 or Year 11 (2006–2008) completed magnetic resonance imaging (MRI) scanning if they met the inclusion criteria: (i) no assistive devices for walking; (ii) eligible for a 3T MRI scan; (iii) had a mobility measure at the previous visit; (iv) no medical history of neurological or psychological illnesses (16,17). Participants with valid MR measures were included in our analysis (n = 312). Participants who did not complete the fast-paced walking speed task were excluded (n = 20, 6%). Participants with missing covariates, including education, body mass index (BMI), depression severity score (Center for Epidemiologic Studies Depression Scale, CES-D), or digit symbol substitution task (DSST) score were excluded from the analyses (n = 8, 2%). Our final data set included 284 (87%) participants from the Health ABC MRI subset. Distribution of the demographics and clinical variables were similar between the original subset and our final data set (not shown).
MRI Measures
MRI scans were obtained at the MR Research Center of the University of Pittsburgh using a 3 T Siemens Tim Trio MR Scanner and a Siemens 12-channel head coil. An axial, whole-brain T1-weighted magnetization prepared rapid gradient echo (MPRAGE) was collected with repetition time (TR) = 2,300 ms, echo time (TE) = 3.43 ms, flip angle (FA) = 9°, field of view (FOV) = 224 × 256, 1 mm3 isotropic resolution, no gap, and no acceleration. An axial, whole-brain fluid attenuated inversion recovery (FLAIR) sequence to appropriately identify white/gray matter were also collected. This sequence had TR = 9160 ms, TE = 90 ms, FA = 150°, FOV = 212 × 156, 1 × 1 × 3 mm resolution, 3 mm gap, and no acceleration.
All processing steps were conducted in SPM12 (18). All image space interpolation was performed using fourth degree B-spline method and similarity metric for registrations was mutual information (for motion correction) or normalized mutual information (coregistration between different image types). FLAIR images were coregistered to the MPRAGE (12 degree of freedom transformations) then a multispectral segmentation was conducted of both the MPRAGE and coregistered FLAIR into six tissues: GM, WM, cerebrospinal fluid, skull, soft-tissue, and air. Default values were used for the segmentations in SPM. Native space segmentations of the GM were input into the diffeomorphic anatomical registration using exponentiated lie algebra (DARTEL) algorithm using the standard pipeline for generating a study-specific template and normalizing each segmentation to that template (19). This generates a study-specific GM template through iterative coregistrations and averages, then coregisters each map to the final template. This outputs a GM density map, which is highly associated with volume and cortical thickness, and is considered to reflect the amount of GM locally (20). This is because when a single participant’s GM is coregistered to a standard space (like MNI), the GM has to be compressed or expanded depending on whether the MNI space GM is smaller or larger, respectively. When it is compressed the density goes up and when it is expanded the density goes down—thus a greater volume and cortical thickness results in a greater density.
Walking Speed Measures
Walking tests were performed within 6 months of the MRI scan. Participants were asked to walk along a 20 m corridor from a standing start during both the usual-paced walking task and the fast-paced walking task. Participants were instructed to walk “as you normally would” and “as fast as you can,” respectively (14). Time to finish the tasks was recorded by stop watch and converted to speed in m/s. The fast-paced walking speed obtained was treated as the outcome.
Covariates
Demographics, clinical information, and cognitive function were obtained at baseline of the Health ABC Study or the time of MRI. Demographic characteristics included age at the time of MRI measurement, as well as sex, race, education, and body mass index (BMI) obtained at baseline. BMI (kg/m2) was calculated from self-reported height and weight values at the time of MRI. Clinical information included cardiovascular disease (CVD), stroke or TIA (transient ischemic attack), hypertension, diabetes, and depression. History of CVD and stroke or TIA was identified from the questionnaire as ever having the event at baseline and was updated by self-report annually. Participants were defined as having hypertension if they had a systolic blood pressure >140 mm Hg or diastolic blood pressure >90 mm Hg or self-reported a hypertension diagnosis or antihypertension medication use at the time of MRI measurement. Participants were defined as having diabetes if their fasting plasma glucose was >126 mg/dL or 2-hour postchallenge >200 Hg/dL, or self-reported a diabetes diagnosis or diabetes medication use (21). Depressive symptoms were described at the time of MRI based on the 20-item CES-D scale (22). The Modified Mini-Mental State Examination (3MSE) was used to assess general cognitive function at the time of MRI (23). The digit symbol substitution test (DSST) was also administered at the same time to assess processing speed (24).
Statistical Analysis
The characteristics of the study population are presented as mean (standard deviation) for continuous variables and count (percentage) for categorical variables. Unadjusted associations between the sample’s characteristics and fast-paced walking speed are reported as Spearman correlation coefficients for continuous variables and mean difference from independent t-test for categorical variables.
Voxel-wise analyses tested the associations between fast-paced walking speed and GM density from the entire brain using SnPM (25). SnPM computed nonparametric p values corrected using a cluster-wise inference method (cluster forming threshold of p < .001) that controlled the family wise error rate (FWE) at α = 0.05. This analysis was done in the whole brain and did not exclude any regions. Next, we extracted the average GM density of clusters significantly associated with fast-paced walking speed by utilizing the automated anatomical labeling atlas (26).
For each region, the association between GM density and fast-paced walking speed was modeled using nested linear models with adjustment for (i) demographics (age, sex, race, education, and BMI), (ii) plus clinical variables (cardiovascular disease, stroke or TIA, hypertension, diabetes, and depression), and (iii) plus cognitive function (3MSE and DSST scores). Demographics and clinical variables were adjusted in the models as potential confounders. 3MSE and DSST scores were included in the fully adjusted model in order to test whether this association was independent of cognitive function. Lastly, a cutoff point of 0.3 m/s was chosen for the point estimate of fast-paced walking speed change per SD change of GM density to suggest a meaningful association between the regional GM density and fast-paced walking speed after the full adjustment. This cutoff was selected to represent a medium effect, which approximates the average effect of 14-year change of age on the change of fast-paced walking speed in our study sample. Regression analyses were conducted in SAS 9.4. The same analyses were repeated for usual-paced walking speed and GM density.
Results
Participants were on average 83 years old (SD = 2.8), 58% women and 41% black. Average walking speed was 1.0 m/s (SD = 0.21) for usual-paced walking and 1.4 m/s (SD = 0.34) for fast-paced walking. Greater usual-paced walking speed was correlated with greater fast-paced walking speeds (r = .58, p < .0001). Participants who were female, black, had a lower level of education, or had a history of knee pain were more likely to have a slower fast-paced walking speed. In addition, those who were older, had greater BMI, greater depressive symptoms (CES-D), worse cognitive function (3MSE or DSST score), had hypertension, or had diabetes also were more likely to have a slower fast-paced walking speed (Table 1).
Table 1.
Characteristics of the Study Population and Association With Fast-Paced Walking Speed (n = 284): Health, Aging, and Body Composition Study, 2010–2011
| Characteristics | Mean (SD) or n (%) | Association With Fast Pace Walking Speed (m/s)* | p Value |
|---|---|---|---|
| Age (year) | 83 (2.8) | −0.15 | .01 |
| Female | 164 (58%) | −0.23 (0.04) | <.0001 |
| Black | 116 (41%) | −0.16 (0.04) | <.0001 |
| Education >high school | 146 (51%) | 0.16 (0.04) | <.0001 |
| BMI | 27 (4.4) | −0.27 | <.0001 |
| CVD | 80 (28%) | −0.02 (0.05) | .59 |
| Stroke or TIA | 21 (7.4%) | −0.09 (0.08) | .24 |
| Hypertension | 256 (90%) | −0.16 (0.05) | <.0001 |
| Diabetes | 73 (26%) | −0.09 (0.05) | .05 |
| CES-D | 6.6 (5.9) | −0.17 | .004 |
| Knee pain† | 122 (43%) | −0.19 (0.11) | <.0001 |
| 3MSE score | 93 (6.7) | 0.23 | <.0001 |
| DSST | 37 (13) | 0.25 | <.0001 |
| Usual-paced walking speed‡ (m/s) | 1.0 (0.21) | 0.58 | <.0001 |
| Fast-paced walking speed (m/s) | 1.4 (0.34) |
Note: 3MSE = Modified Mini-Mental State Examination; BMI = body mass index; CES-D = Center for Epidemiologic Studies-Depression; CVD = cardiovascular disease; DSST = digit symbol substitution test; TIA = transient ischemic attack.
*Spearman correlation coefficient for continuous variable and group mean difference (SD) from t-test for categorical variable.
†Knee pain (in the past month) was evaluated during the visit at Year 10.
‡For usual-paced walking speed, n = 269 participants.
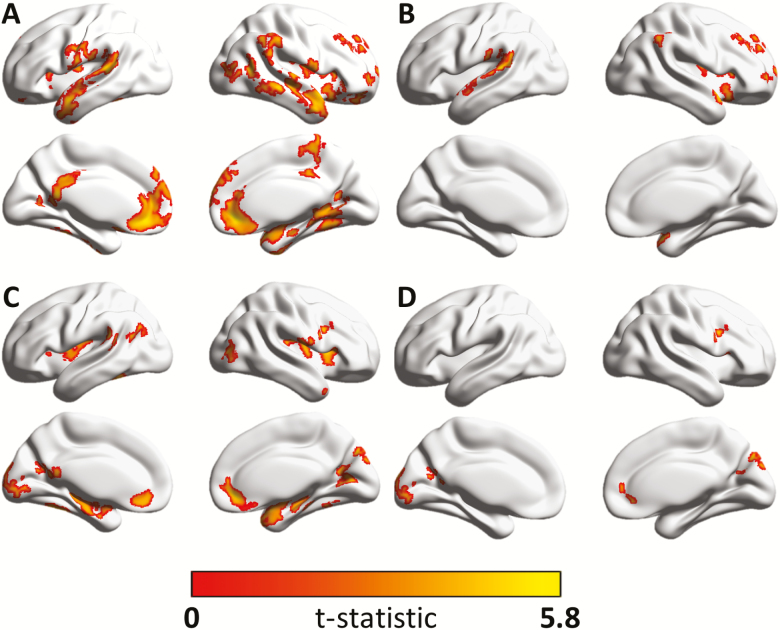
In the unadjusted voxel-wise analyses, greater fast-paced walking speed was positively correlated with greater GM density in clusters from: the frontal and temporal lobe, pre- and postcentral gyrus, inferior parietal lobule, precuneus gyrus, lingual, parahippocampal and fusiform gyrus, calcarine cortex, middle occipital gyrus, supramarginal and angular gyrus, Rolandic operculum, insular cortex, cingulum, hippocampus, amygdala, and cerebellum (Figure 1, Supplementary Table 1, and Supplementary Figure 1). Regions correlated with usual-paced walking speed are shown in Figure 1, Supplementary Table 2, and Supplementary Figure 3. Clusters from insula, cerebellum, parahippocampus, calcarine, middle and inferior frontal, temporal, middle occipital, amygdala, fusiform gyrus, lingual gyrus, and precuneus, were correlated with both fast-paced and usual-paced walking speed. Fast-paced walking speed but not usual-paced walking was correlated with GM density in clusters from precentral gyrus and inferior parietal lobule. In addition, usual-paced walking speed but not fast-paced walking speed was associated with GM density in clusters from cuneus, caudate, putamen, gyrus rectus, and superior occipital gyrus (Supplementary Figure 5).
Figure 1.
(A) Association between gray matter density and fast-paced walking speed unadjusted and (B) regions that remained significant after adjusting for demographic variables, clinical morbidities, and cognitive function—including those with smaller effect sizes, ie, <0.3 m/s. (C) Association between gray matter density and usual-paced walking speed unadjusted and (D) regions that remained significant after adjusting for demographic variables, clinical morbidities, and cognitive function—including those with smaller effect sizes, ie, <0.3 m/s. Color bar indicates value of the t-statistic testing the association between gray matter density and fast- (A and B) or usual-paced (C and D) walking speed.
Regions that were significantly associated with fast-paced walking speed after adjustment for demographic variables, clinical morbidities, and cognitive function are given in Table 2, Figure 1, and Supplementary Figure 2. After further adjusting for covariates, fast-paced walking speed was positively correlated at coefficient ≥0.3 m/s with GM density of clusters in: right middle and superior frontal gyrus, right postcentral gyrus, and left superior temporal gyrus. Adjustment for cognitive function had little impact on the findings. We found no association between fast-paced walking speed with GM density of prefrontal and hippocampal regions or with basal ganglia (Figure 1 and Table 2). Regions that are significantly associated with usual-paced walking after covariates adjustment are shown in Supplementary Table 3, Figure 1, and Supplementary Figure 4. Usual paced walking speed was positively correlated with GM density of left caudate region at coefficient ≥0.3 m/s after adjustment of demographics and morbidities, but not after further adjusting for cognition. In addition, no regions were associated with both usual and fast-paced walking speed after adjustment (Supplementary Figure 6). In general, the effect size of GM density of each region on usual-paced walking was smaller than fast-paced walking speed.
Table 2.
Association Between Fast-Paced Walking Speed and Gray Matter Density
| Model 1* | Model 2† | Model 3‡ | ||
|---|---|---|---|---|
| Region | MNI coordinates (x, y, z) | β (p value)§ | ||
| Right angular gyrus | 62, −50, 36 | 0.23 (.01) | 0.22 (.01) | 0.21 (.02) |
| Right cerebellum 4–5 | 24, −50, −20 | 0.14 (.04) | 0.13 (.06) | 0.12 (.08) |
| Right cerebellum 6 | 26, −56, −18 | 0.13 (.03) | 0.12 (.05) | 0.11 (.08) |
| Left cerebellum crus 2 | −38, −66, −38 | 0.11 (.04) | 0.10 (.06) | 0.09 (.10) |
| Right cerebellum crus 2 | 44, −68, −40 | 0.13 (.04) | 0.11 (.07) | 0.10 (.09) |
| Left middle orbital frontal gyrus | −8, 38, −12 | 0.13 (.03) | 0.11 (.07) | 0.09 (.14) |
| Right middle orbital frontal gyrus | 10, 42, −6 | 0.14 (.03) | 0.13 (.06) | 0.10 (.12) |
| Right middle frontal gyrus | 28, 54, 2 | 0.35 (.001) | 0.35 (.001) | 0.31 (.01) |
| Right superior frontal gyrus | 14, 68, 24 | 0.37 (.01) | 0.36 (.01) | 0.31 (.04) |
| Right fusiform gyrus | 26, −52, −16 | 0.17 (.04) | 0.15 (.07) | 0.13 (.14) |
| Left lingual gyrus | −16, −66, 2 | 0.11 (.05) | 0.11 (.06) | 0.10 (.08) |
| Right lingual gyrus | 16, −48, 4 | 0.20 (.03) | 0.19 (.03) | 0.18 (.04) |
| Right insula | 38, 10, 10 | 0.24 (.05) | 0.20 (.11) | 0.14 (.30) |
| Right paracentral lobule | 8, −36, 60 | 0.24 (.05) | 0.22 (.08) | 0.21 (.09) |
| Right inferior parietal gyrus | 60, −40, 46 | 0.25 (.01) | 0.23 (.01) | 0.23 (.01) |
| Right postcentral gyrus | 64, 0, 18 | 0.38 (.04) | 0.36 (.05) | 0.35 (.05) |
| Right precuneus | 14, −40, 58 | 0.21 (.03) | 0.20 (.05) | 0.19 (.05) |
| Right Rolandic operculum | 44, −14, 16 | 0.15 (.04) | 0.13 (.09) | 0.11 (.14) |
| Left supramarginal gyrus | −44, −34, 26 | 0.23 (.005) | 0.22 (.01) | 0.19 (.02) |
| Right supramarginal gyrus | 66, −36, 38 | 0.17 (.03) | 0.14 (.07) | 0.11 (.16) |
| Left middle temporal gyrus | −58, −38, 12 | 0.21 (.03) | 0.19 (.06) | 0.16 (.10) |
| Right middle temporal gyrus | 70, −18, −6 | 0.22 (.04) | 0.20 (.06) | 0.15 (.19) |
| Right superior temporal pole | 34, 10, −24 | 0.31 (.01) | 0.30 (.02) | 0.26 (.04) |
| Left superior temporal gyrus | −54, −38, 14 | 0.37 (.001) | 0.34 (.004) | 0.31 (.01) |
| Right superior temporal gyrus | 68, −18, −4 | 0.31 (.02) | 0.27 (.04) | 0.24 (.06) |
Notes: Corresponding coefficients (β) and p values are shown across three nested models. 3MSE = Modified Mini-Mental State Examination; BMI = body mass index; CES-D = Center for Epidemiologic Studies-Depression; DSST = digit symbol substitution test; TIA = transient ischemic attack.
*Model 1: general linear model adjusting for demographics (age, sex, race, education, and BMI).
†Model 2: based on Model 1, further adjusting for morbidities (cardiovascular disease, stroke or TIA, hypertension, diabetes, and CES-D score).
‡Model 3: based on Model 2, further adjusting for cognition (3MSE and DSST scores).
§The coefficient of 1 SD change of regional gray matter density on fast-paced walking speed and the corresponding p value.
Discussion
We found that fast-paced walking speed was positively associated with GM density in cortical regions including right middle and superior frontal gyrus, right postcentral gyrus, and left superior temporal gyrus. Associations were robust to adjustment for demographic factors, clinical factors, and cognitive function. Our hypothesis was accurate, as fast-paced walking speed but not usual-paced walking speed, was associated with clusters related to executive function (27). However, no memory-related clusters were associated with fast-paced or usual-paced walking speed.
In this study, we examined the whole brain to capture a wide network or regions related to fast-paced walking speed in older adults without neurological and psychological diseases. The brain regions from cortical, subcortical, and cerebellum identified from the unadjusted analyses where GM density was correlated with either fast-paced or usual-paced walking speed may reflect the functions of brain related to mobility (28). Clusters from cortical, subcortical, and cerebellum regions were correlated with both fast-paced and usual-paced walking speed. This could reflect the correlation between two walking measures, which was measured as 0.58 in our sample. On the other hand, these two measures were not perfectly correlated, and each was correlated with specific regions. Fast-paced walking speed was correlated with parietal regions, and usual-paced walking speed was correlated with regions of basal ganglia and occipital. This may indicate that different mechanisms of neuronal control were involved in fast-paced walking than usual-paced walking. It was suggested that involved a higher conscious control than usual-paced walking (10).
Previous studies have been limited. One study focused on associations of fast-paced walking speed with total brain volume and hippocampal volume (1) and only adjusted for age. Another study assessed regional GM volume associated with faster fast-paced walking speed (12), but only participants having memory complaints were recruited—limiting its generalizability to older populations without impairment of cognitive function.
After adjustment for demographic variables, clinical factors, and cognitive function, we observed that faster fast-paced walking speed was associated with GM density in right middle and superior frontal gyrus, right postcentral gyrus, and left superior temporal gyrus. These regions are specifically related to executive, somatosensory, and vestibular function (27,29,30). In a previous study of resting-state functional connectivity and walking, greater resting-state functional connectivity between the midbrain locomotor region and right superior frontal gyrus was associated with greater walking capacity, indicating that superior frontal gyrus was a relevant locomotor area (31). This is consistent with our finding, where fast-paced walking was a measure of walking capacity and was related to the structure of superior frontal gyrus. Right postcentral gyrus is a somatosensory region that facilitates the automatic process of walking (32), and has been identified to be related to walking in studies investigating brain activation (33,34). Previous studies using functional near-infrared spectroscopy observed increased activation in superior temporal gyrus during postural control and balance, indicating that this area is involved in dynamic balance (35,36). Our study suggests that superior temporal gyrus could play a role in fast-paced walking through balance control. However, future studies are needed to verify the results by analyzing functional associations with the brain.
Associations tended to be lateralized to the right hemisphere for fast-paced walking. Right hemisphere dominance for vestibular and ocular motor structures in right-handed volunteers has been previously reported in a positron emission tomography study (37). Another study using electroencephalography also observed increased right hemispheric engagement related to ventral attention (38). A previous review suggested a lateralized model for motor control: the left cortex is specialized for predictive control while the right cortex is specialized for impedance control specifying velocity and position-based impedance (39). Our results are consistent with previous evidence suggesting the increased engagement of the right hemisphere during the fast walking process that may be associated with increased engagement of vestibular function and impedance control.
Adjustment for covariates including demographics, morbidities, and cognitive function did not attenuate the associations of GM density of middle and superior frontal gyrus, right postcentral gyrus, and left superior temporal gyrus, with fast-paced walking speed. The association of usual-paced walking speed with regional GM density was not as robust to the adjustment of covariates. In addition, no regions were associated with both usual and fast-paced walking speed after adjustment. This may indicate (i) the association of fast-paced walking but not usual-paced walking was independent of age, sex, race, education, BMI, and morbidities, CVD, stroke or TIA, hypertension, diabetes, and depression. (ii) Cognitive function represented by 3MSE and DSST scores is not likely to be a mediator between GM density and fast-paced walking speed. This may not hold true for usual-paced walking speed. 3) Although there’s overlap of brain regions related to usual- and fast-paced walking speed in consistent with the correlation between the two walking measures, this overlap could be largely explained by covariates that may be common causes of the brain and mobility.
Fast-paced walking speed but not usual-paced walking speed was correlated with GM density of superior frontal gyrus. Compared with usual-paced walking, fast-paced walking was more strongly correlated with GM density of clusters in general. Our results are consistent with previous hypotheses that (i) fast-paced walking, but not usual-paced walking, was associated with cognitive function in older adults (7–10) and may be related to cognitive execution (27); (ii) complex walking like fast-paced walking provides greater levels of variability and allows difference in fitness to be identified, which would not be identified in usual-paced walking (9); and (iii) fast-paced walking necessitates a higher level of conscious control and thus is more closely correlated with cortical structure than usual-paced walking (10).
Negative findings also merit attention. We did not find a significant association of fast-paced walking speed with memory-related areas (eg, hippocampus) after adjustment with cognitive function. This is surprising given the association of fast-paced walking with dementia (28,40). Associations were also not significant with basal ganglia, which is involved in the automatic control of voluntary movements (41). Basal ganglia was observed to be associated with usual walking speed among older adults in previous studies, where a combined effect of the neurodegenerative process may be involved (12,42). Only the caudate was associated with usual-paced walking, while many studies have found the association of walking speed with GM volume in motor-related regions such as precentral gyrus, frontal, basal ganglia, hippocampal, and cerebellar regions. The negative findings could be explained by several reasons. As a more demanding task, it is possible that a different mechanism is involved in fast-paced walking that is different from usual-paced walking or complex walking tasks (10). We only studied GM density, which may not sufficiently describe the age-related changes in brain correlated with fast-paced walking. For example, there could be changes in other brain imaging parameters, including WM volume and integrity, brain activity, subclinical manifestations (eg, micro-bleeding, β-amyloid), and functional connectivity, before the changes of GM density could be detected. Previous study identified associations of slower walking speed with lower WM microstructure in thalamic radiations (43). The associations of slower walking speed with greater WM hyperintensities were observed in basal ganglia and thalamic radiation (44–47). A previous study using florbetapir PET observed significant association between β-amyloid in putamen and slow gait speed (48). It is also important to put the results of GM density in the context of other modalities because of compensatory mechanisms and functional reserve of the brain. Our voxel-wise multiple comparisons correction reflect the latest in the field (49), which may also influence the results we have observed. We used a cutoff of 0.3 m/s to select the regions in multivariate-adjusted linear regressions, thus we focused on the effect size instead of hypothesis testing results (p values). Inconsistencies may also result from different sample of participants. Specifically, if those who were unable to complete fast-paced walking had different GM density profiles than our study sample. Finally, our sample is a relatively healthy sample whose range in usual walking speed (0.47–1.64 m/s) may be lower compared with clinical samples.
There is the need to find simple markers to detect early dementia risk in older adults. Traditional methods like MRI and cognitive assessments are either invasive or require lots of time and specialist input. Gait measures are relatively simple yet important measures in the older population. Usual walking speed is a simple measure, but its association with brain integrity might not be strong enough at the individual level. More complex motor tasks, such as dual task walking, are promising because of their potential to reflect changes in the central nervous system to a greater extent compared with usual walking speed (8). However, dual task walking is complicated to administer and difficult to standardize. Fast gait may be a good compromise, as previous evidence suggest that it reveals cognitive characteristics, and it requires relatively simple and consistent protocol compared with other complex walking tasks (8–10). Our study indicates that fast gait does reflect integrity in certain brain regions, but not those that are memory-related. This may suggest that fast-paced gait speed alone is not helpful for detecting risk of memory-related dementia. However, it could be helpful to detect other general signs of advanced brain aging or problems with executive function.
There are several limitations in our study. We used a selective population: only participants who survived and completed MRI scanning in Year 10 or Year 11 and had complete data profile were included from the Health ABC study. In addition, the age range of our study sample is narrow with the average age of 83 (SD = 2.8), preventing generalization to the younger elderly. Our study is cross-sectional and so any associations are nontemporal and noncausal. Longitudinal studies may help explain the causal relationships among these variables. Last, we only investigated the association with GM density. Other brain imaging parameters should be assessed to further understand the neural correlates associated with fast-paced walking speed. As a result, it is important to interpret our results with caution, as they need to be put in the context of other neural modalities.
In this study, we conducted voxel-wise analyses between fast-paced walking speed and GM density, which allows for the comparisons across the entire brain with a high regional specificity and without prior region-specific assumptions. Our study sample is relatively large in size with sufficient sampling of male and black participants. While we did not investigate associations with WM structural integrity, we have collected diffusion weighted imaging data and plan to conduct future analyses to investigate the association between WM integrity and gait speed.
Conclusion
Our study adds to the current evidence of the neural correlates of fast-paced walking speed. We identified several clusters in the brain including right middle and superior frontal gyrus, right postcentral gyrus, and left superior temporal gyrus independent of demographic variables, clinical factors, and cognitive function among older adults. The association of frontal and temporal gyri with fast-paced but not usual-paced walking speed may explain previously observed associations between fast-paced walking speed and cognitive function (8–10).
Supplementary Material
Acknowledgment
All study participants provided written informed consent and the protocol was approved by the University of Pittsburgh institutional review board.
Funding
This work was supported by National Institute on Aging (NIA) contracts (N01-AG-6-2101, N01-AG-6-2103, N01-AG-6-2106), NIA grant (R01-AG-028050, K23-AG-028966, R01-AG-029232, P30-AG-024827-07, R01AG057671); and National Institute of Nursing Research grant (R01-NR-012459). This research was funded in part by the Intramural Research Program of the NIH, National Institute on Aging.
Conflict of Interest
None reported.
References
- 1. Camargo EC, Weinstein G, Beiser AS, et al. Association of physical function with clinical and subclinical brain disease: the Framingham Offspring Study. J Alzheimers Dis. 2016;53:1597–1608. doi: 10.3233/JAD-160229 [DOI] [PubMed] [Google Scholar]
- 2. Abellan Van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people. J Nutr Health Aging. 2009;13(10):881–889. doi: 10.1007/s12603-009-0246-z [DOI] [PubMed] [Google Scholar]
- 3. Buchman AS, Bennett DA. Loss of motor function in preclinical Alzheimer’s disease. Expert Rev Neurother. 2011;11:665–676. doi: 10.1586/ern.11.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Callisaya ML, Blizzard L, Schmidt MD, et al. Gait, gait variability and the risk of multiple incident falls in older people: a population-based study. Age Ageing. 2011;40:481–487. doi: 10.1093/ageing/afr055 [DOI] [PubMed] [Google Scholar]
- 5. Callisaya ML, Blizzard L, McGinley JL, Srikanth VK. Risk of falls in older people during fast-walking—the TASCOG study. Gait Posture. 2012;36:510–515. doi: 10.1016/j.gaitpost.2012.05.003 [DOI] [PubMed] [Google Scholar]
- 6. England SA, Granata KP. The influence of gait speed on local dynamic stability of walking. Gait Posture. 2007;25:172–178. doi: 10.1016/j.gaitpost.2006.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shumway-Cook A, Guralnik JM, Phillips CL, et al. Age-associated declines in complex walking task performance: the Walking InCHIANTI toolkit. J Am Geriatr Soc. 2007;55:58–65. doi: 10.1111/j.1532-5415.2006.00962.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rosso AL, Metti AL, Faulkner K, et al. Complex walking tasks and risk for cognitive decline in high functioning older adults. J Alzheimers Dis. 2019;71(s1):S65–S73. doi: 10.3233/JAD-181140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fitzpatrick AL, Buchanan CK, Nahin RL, et al. ; Ginkgo Evaluation of Memory (GEM) Study Investigators Associations of gait speed and other measures of physical function with cognition in a healthy cohort of elderly persons. J Gerontol A Biol Sci Med Sci. 2007;62:1244–1251. doi: 10.1093/gerona/62.11.1244 [DOI] [PubMed] [Google Scholar]
- 10. Deshpande N, Metter EJ, Bandinelli S, Guralnik J, Ferrucci L. Gait speed under varied challenges and cognitive decline in older persons: a prospective study. Age Ageing. 2009;38:509–514. doi: 10.1093/ageing/afp093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wilson J, Allcock L, Mc Ardle R, Taylor JP, Rochester L. The neural correlates of discrete gait characteristics in ageing: a structured review. Neurosci Biobehav Rev. 2019;100:344–369. doi: 10.1016/j.neubiorev.2018.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Allali G, Montembeault M, Brambati SM, et al. Brain structure covariance associated with gait control in aging. J Gerontol A Biol Sci Med Sci. 2018;74:705–713. doi: 10.1093/gerona/gly123 [DOI] [PubMed] [Google Scholar]
- 13. Newman AB, Simonsick EM, Naydeck BL, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295:2018–2026. doi: 10.1001/jama.295.17.2018 [DOI] [PubMed] [Google Scholar]
- 14. Simonsick EM, Newman AB, Nevitt MC, et al. Measuring higher level physical function in well-functioning older adults: expanding familiar approaches in the Health ABC study. J Gerontol A Biol Sci Med Sci. 2001;56A(10):M644–M649. doi: 10.1093/gerona/56.10.M644 [DOI] [PubMed] [Google Scholar]
- 15. Houston DK, Nicklas BJ, Ding JZ, et al. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the Health, Aging, and Body Composition (Health ABC) Study. Am J Clin Nutr. 2008;87:150–155. doi: 10.1093/ajcn/87.1.150 [DOI] [PubMed] [Google Scholar]
- 16. Nadkarni NK, Nunley KA, Aizenstein H, et al. Association between cerebellar gray matter volumes, gait speed, and information-processing ability in older adults enrolled in the Health ABC study. J Gerontol A Biol Sci Med Sci. 2014;69(8):996–1003. doi: 10.1093/gerona/glt151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rosso AL, Olson Hunt MJ, Yang M, et al. ; Health ABC study Higher step length variability indicates lower gray matter integrity of selected regions in older adults. Gait Posture. 2014;40:225–230. doi: 10.1016/j.gaitpost.2014.03.192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Penny WD, Friston KJ, Ashburner JT, Kiebel SJ, Nichols TE.. Statistical Parametric Mapping: The Analysis of Functional Brain Images. London, UK: Elsevier; 2011. [Google Scholar]
- 19. Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007 [DOI] [PubMed] [Google Scholar]
- 20. Gennatas ED, Avants BB, Wolf DH, et al. Age-related effects and sex differences in gray matter density, volume, mass, and cortical thickness from childhood to young adulthood. J Neurosci. 2017;37(20):5065–5073. doi: 10.1523/JNEUROSCI.3550-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yaffe K, Fiocco AJ, Lindquist K, et al. ; Health ABC Study Predictors of maintaining cognitive function in older adults: the Health ABC study. Neurology. 2009;72:2029–2035. doi: 10.1212/WNL.0b013e3181a92c36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale). Am J Prev Med. 1994;10:77–84. doi: 10.1016/S0749-3797(18)30622-6 [DOI] [PubMed] [Google Scholar]
- 23. Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 24. Wechsler D. Manual for the Wechsler Adult Intelligence Scale—Third Edition (WAIS-III). San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 25. Holmes AP, Blair RC, Watson JD, Ford I. Nonparametric analysis of statistic images from functional mapping experiments. J Cereb Blood Flow Metab. 1996;16:7–22. doi: 10.1097/00004647-199601000-00002 [DOI] [PubMed] [Google Scholar]
- 26. Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- 27. Li W, Qin W, Liu H, et al. Subregions of the human superior frontal gyrus and their connections. Neuroimage. 2013;78:46–58. doi: 10.1016/j.neuroimage.2013.04.011 [DOI] [PubMed] [Google Scholar]
- 28. Holtzer R, Epstein N, Mahoney JR, Izzetoglu M, Blumen HM. Neuroimaging of mobility in aging: a targeted review. J Gerontol A Biol Sci Med Sci. 2014;69:1375–1388. doi: 10.1093/gerona/glu052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ventre-Dominey J, Nighoghossian N, Denise P. Evidence for interacting cortical control of vestibular function and spatial representation in man. Neuropsychologia. 2003;41:1884–1898. doi: 10.1016/s0028-3932(03)00126-x [DOI] [PubMed] [Google Scholar]
- 30. Corkin S, Milner B, Rasmussen T. Somatosensory thresholds—contrasting effects of postcentral-gyrus and posterior parietal-lobe excisions. Arch Neurol. 1970;23:41–58. doi: 10.1001/archneur.1970.00480250045007 [DOI] [PubMed] [Google Scholar]
- 31. Boyne P, Maloney T, DiFrancesco M, et al. Resting-state functional connectivity of subcortical locomotor centers explains variance in walking capacity. Hum Brain Mapp. 2018;39:4831–4843. doi: 10.1002/hbm.24326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Clark DJ, Christou EA, Ring SA, Williamson JB, Doty L. Enhanced somatosensory feedback reduces prefrontal cortical activity during walking in older adults. J Gerontol A Biol Sci Med Sci. 2014;69:1422–1428. doi: 10.1093/gerona/glu125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sacco K, Cauda F, Cerliani L, Mate D, Duca S, Geminiani GC. Motor imagery of walking following training in locomotor attention. The effect of “the tango lesson”. Neuroimage. 2006;32:1441–1449. doi: 10.1016/j.neuroimage.2006.05.018 [DOI] [PubMed] [Google Scholar]
- 34. la Fougère C, Zwergal A, Rominger A, et al. Real versus imagined locomotion: a [18F]-FDG PET-fMRI comparison. Neuroimage. 2010;50:1589–1598. doi: 10.1016/j.neuroimage.2009.12.060 [DOI] [PubMed] [Google Scholar]
- 35. Rosso AL, Cenciarini M, Sparto PJ, Loughlin PJ, Furman JM, Huppert TJ. Neuroimaging of an attention demanding dual-task during dynamic postural control. Gait Posture. 2017;57:193–198. doi: 10.1016/j.gaitpost.2017.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Karim H, Schmidt B, Dart D, Beluk N, Huppert T. Functional near-infrared spectroscopy (fNIRS) of brain function during active balancing using a video game system. Gait Posture. 2012;35:367–372. doi: 10.1016/j.gaitpost.2011.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dieterich M, Bense S, Lutz S, et al. Dominance for vestibular cortical function in the non-dominant hemisphere. Cereb Cortex. 2003;13:994–1007. doi: 10.1093/cercor/13.9.994 [DOI] [PubMed] [Google Scholar]
- 38. Benwell CS, Harvey M, Thut G. On the neural origin of pseudoneglect: EEG-correlates of shifts in line bisection performance with manipulation of line length. Neuroimage. 2014;86:370–380. doi: 10.1016/j.neuroimage.2013.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mutha PK, Haaland KY, Sainburg RL. The effects of brain lateralization on motor control and adaptation. J Mot Behav. 2012;44:455–469. doi: 10.1080/00222895.2012.747482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. DiSalvio NL, Rosano C, Aizenstein HJ, et al. Gray matter regions associated with functional mobility in community-dwelling older adults. J Am Geriatr Soc. 2019. doi: 10.1111/jgs.16309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Takakusaki K, Saitoh K, Harada H, Kashiwayanagi M. Role of basal ganglia-brainstem pathways in the control of motor behaviors. Neurosci Res. 2004;50:137–151. doi: 10.1016/j.neures.2004.06.015 [DOI] [PubMed] [Google Scholar]
- 42. Dumurgier J, Crivello F, Mazoyer B, et al. MRI atrophy of the caudate nucleus and slower walking speed in the elderly. Neuroimage. 2012;60:871–878. doi: 10.1016/j.neuroimage.2012.01.102 [DOI] [PubMed] [Google Scholar]
- 43. Verlinden VJ, de Groot M, Cremers LG, et al. Tract-specific white matter microstructure and gait in humans. Neurobiol Aging. 2016;43:164–173. doi: 10.1016/j.neurobiolaging.2016.04.005 [DOI] [PubMed] [Google Scholar]
- 44. Bolandzadeh N, Liu-Ambrose T, Aizenstein H, et al. Pathways linking regional hyperintensities in the brain and slower gait. Neuroimage. 2014;99:7–13. doi: 10.1016/j.neuroimage.2014.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nadkarni NK, McIlroy WE, Mawji E, Black SE. Gait and subcortical hyperintensities in mild Alzheimer’s disease and aging. Dement Geriatr Cogn Disord. 2009;28:295–301. doi: 10.1159/000245158 [DOI] [PubMed] [Google Scholar]
- 46. Rosano C, Brach J, Longstreth WT Jr, Newman AB. Quantitative measures of gait characteristics indicate prevalence of underlying subclinical structural brain abnormalities in high-functioning older adults. Neuroepidemiology. 2006;26:52–60. doi: 10.1159/000089240 [DOI] [PubMed] [Google Scholar]
- 47. Rosario BL, Rosso AL, Aizenstein HJ, et al. ; Health ABC Study Cerebral white matter and slow gait: contribution of hyperintensities and normal-appearing parenchyma. J Gerontol A Biol Sci Med Sci. 2016;71:968–973. doi: 10.1093/gerona/glv224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Del Campo N, Payoux P, Djilali A, et al. ; MAPT/DSA Study Group Relationship of regional brain β-amyloid to gait speed. Neurology. 2016;86:36–43. doi: 10.1212/WNL.0000000000002235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Eklund A, Nichols TE, Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U S A. 2016;113:7900–7905. doi: 10.1073/pnas.1602413113 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.