Abstract
In this study, we investigated age and sex differences in acute and chronic pain in rats. Groups of young (3–6 months) and aged (20–24 months) male and female Fischer 344 rats were used to assess basal thermal and mechanical thresholds, capsaicin-induced acute nocifensive responses and c-Fos expression in the spinal cord, and monoiodoacetate (MIA)-induced knee osteoarthritis (OA)-like pain responses. There was a significant sex, but not age, effect on thermal threshold on the hindpaw and mechanical threshold on the knee joint. No significant age and sex differences in capsaicin-induced nocifensive and c-Fos responses were observed. MIA induced a greater peak reduction of weight-bearing responses in aged males than young rats. Aged females developed the most profound weight-bearing deficit. With knee joint sensitivity as a primary outcome measure, MIA induced more pronounced and longer-lasting hyperalgesia in older rats, with aged female rats showing the worst effect. These data suggest that age may not have significant effect on acute nociceptive processing, but it significantly impacts OA-like pain, making aged rats, especially females, more vulnerable to chronic pain conditions. These preclinical models should provide important tools to investigate basic mechanisms underlying the impact of age and sex in chronic pain conditions.
Keywords: Gender differences, Osteoarthritis, Animal model
According to a recent report by Center for Disease Control and Prevention, the prevalence of high impact chronic pain in the United States is highest among women and older adults (1). Given that the elderly comprise the fastest-growing segment of the population (2), age-related chronic pain conditions are also anticipated to increase. Therefore, effective pain management in the elderly population is an important clinical concern. However, pain management in the elderly is complicated by factors such as multi-morbidity, poly-pharmacy, and cognitive impairment (3). The development of more effective treatments for use in the elderly is further hampered by our limited understanding of the basic mechanisms of how pain and analgesia are processed in this population.
Preclinical studies on the effects of age on nociceptive sensitivity have been conducted over the past few decades. Although the results from these studies are equivocal, sufficient information is available to evaluate the impact of various experimental conditions and age-related biological changes on basal nociceptive sensitivity (4–6), and increasingly more studies report potential causal relationships between age and nociceptive systems (7–9). Age-related changes in pain processing under inflammatory and neuropathic pain conditions have also been demonstrated (10,11), but specific animal models that address the effects of aging on clinically relevant chronic pain are relatively scarce. Consequentially, our knowledge on neurobiological mechanisms that underlie chronic pain conditions that are prevalent in the elderly is limited. For example, to the best of our knowledge, the overwhelming majority of preclinical models of osteoarthritis (OA)-related pain utilize young male animals, even though age is one of the strongest risk factors in this chronic pain condition (12). There have been no preclinical reports on how OA-induced pain responses change with advancing age.
Both clinical and preclinical studies have amply demonstrated that sex is another important biological variable that affects pain and analgesia (13,14). The overwhelming majority of preclinical studies on sex differences in pain were conducted in young animals. Thus, it is assumed that any mechanistic clues learned from these studies apply uniformly across different age groups. Chronic pain conditions that show an increase in prevalence with age, such as fibromyalgia and OA, also show prominent sex differences (15,16). Early epidemiological studies show that advanced age exacerbates or even causes the development of sex differences in the prevalence of joint pain, chronic widespread pain, and fibromyalgia, with all conditions showing higher prevalence in women than in men (17–19). This pattern of sex differences varies from other types chronic pain conditions such as temporomandibular disorders and irritable bowel syndrome, which are more prevalent in women during reproductive age (20,21). These studies suggest that age and sex might lead to more complicated biological changes depending on the types of pain conditions. However, there is limited information on how sex and age intersect at various levels of the nociceptive circuitries and how such interactions lead to more profound chronic pain conditions in females in different stages of life.
Therefore, in this study, we sought to develop animal models that assess the impact of both sex and age under naïve, acute, and chronic inflammatory conditions. Under naïve conditions, we assessed sex and age differences in cutaneous thermal and mechanical sensitivities and in joint mechanical sensitivity. Capsaicin was used as an acute inflammatory agent to compare nociceptive responses as well as the spinal cord nociceptive processing between different sex and age groups. We adapted the monoiodoacetate (MIA)-induced OA model to examine the effects of sex and age under chronic joint pain conditions.
Materials and Methods
Animals
Male and female Fischer 344 rats of young (3–6 months old) and old (20–24 months old) age were obtained from the National Institute on Aging. All animals were housed in a temperature-controlled room under a 12:12 hour light–dark cycle with access to food and water ad libitum. All rats were fed Teklad Global 18% Protein Rodent Diet 2018 (Envigo) (5% fat, 5% fiber). All behavioral assays were conducted in the morning (between 8 and 10 am) with the exception of capsaicin assays, which were conducted in the afternoon (between 2 and 4 PM). Thus, we acknowledge the possibility of circadian rhythm affecting pain responses in this pain assay. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and under a University of Maryland–approved Institutional Animal Care and Use Committee protocol.
Drug Preparation and Administration
Capsaicin (Millipore Sigma, St. Louis, MO) was dissolved in ethanol (20%), Tween 80 (7%) and PBS (70%). Capsaicin (0.3% in 100 μl) was administered once intradermally in the left hindpaw using a 27-gauge needle. The same volume of PBS was injected in the same manner in control animals. MIA (Sigma–Aldrich, St. Louis, MO) was dissolved in sterile saline. For the weight-bearing behavioral analysis, a single intraarticular injection of MIA (3 mg/50 µL saline) was made through the infra-Patella ligament of the left knee using a 30-gauge needle. For the assessment of primary mechanical hyperalgesia at the knee joint, the same dose of MIA was administered for the duration of 10 minutes with an infusion pump (Harvard Apparatus, Pump 11) via a 30-gauge needle connected to PE10 tubing. Control animals were given the same volume of sterile saline in the same manner. Rats were anesthetized with isoflurane (1.5%–2%) for all injection procedures.
Thermal Sensitivity Assay
The thermal sensitivity assay was adapted from a widely established protocol (22). Rats were allowed to habituate to the experimental room for 30 minutes for three consecutive days. For each day, rats were placed on an elevated glass surface and allowed to acclimate for 10–20 minutes. A radiant heat source was directed to the plantar surface of the hindpaw from underneath the glass floor. A motion detector halted both lamp and timer when the paw was withdrawn. The voltage of the bulb was adjusted to result in an average paw withdrawal latency of 10–12 seconds in naïve animals. A 20 seconds cutoff was used to prevent tissue damage. Three trials (with an inter-trial interval of at least 5 minutes) were determined for each hindpaw, and the average of the trials was used as the mean thermal paw withdrawal latency.
Mechanical Sensitivity Assay
Mechanical sensitivity of the hindpaw was assessed with the Randall–Selitto test, an established rodent model for testing sensitivity of the paw to a noxious mechanical stimulus (23). Rats were first allowed to habituate to the experimental room for 30 minutes for three consecutive days. The withdrawal response to noxious paw pressure was assessed using a digital paw pressure Randall–Selitto applicator for rodents (IITC Life Science, Woodland Hills, CA). Each rat was placed in a cloth holder suspended in a sling, and the probe of the pressure applicator was placed under the plantar surface of the hindpaw. The probe closes the pressure applicator and captures the pressure upon reaction. A gradually increasing pressure was applied until the rat withdrew its hindpaw. The lowest pressure necessary to elicit the withdrawal response was considered as the noxious mechanical threshold. These data were obtained as a baseline data from the animals that were used for assessing MIA-induced primary mechanical hyperalgesia.
Capsaicin-Induced Acute Nocifensive Behaviors and c-Fos Immunohistochemistry
Rats were placed individually in a 30 × 30 × 30-cm transparent plexiglass box and allowed to habituate to the experimental room and the observation box for 30 minutes for at least two consecutive days. After the adaptation period, capsaicin or vehicle was injected intradermally. Each rat was then returned immediately to the observation box and monitored with two video cameras placed on the opposite sides of the box for 10 minutes from the time of injection. The video images from both cameras were merged to create composite images using Adobe Capture, which allowed us to analyze the images from both sides simultaneously. The amount of time that animals spent licking and/or lifting the injected paw were considered as indicators of active nocifensive response (24). Rats also exhibited immobility, periods of at least 3 seconds during which no discernable movement beyond breathing, such as sniffing or whisking, was present. This was a characteristic behavior observed in capsaicin, but not vehicle, treated rats. This measure has been previously used in more prolonged inflammatory pain, such as that resulting from intraplantar CFA injection (25). Thus, we included immobility as a nocifensive response in our analysis.
Capsaicin-injected animals used for acute nocifensive behaviors received a lethal dose of sodium pentobarbital followed by transcardiac perfusion with 4% paraformaldehyde in phosphate buffer (pH 7.2) 2 hours after the capsaicin injection. The spinal cord at the level of L4 and L5 were blocked and postfixed. Blocks were serially sectioned on a cryostat (40 µm thick), and every fifth section was processed for visualization of fos-like immunoreactivity (Fos-LI). Free-floating sections were incubated successively in 5% normal donkey serum (30 minutes), affinity-purified rabbit polyclonal anti-Fos antibody (Calbiochem, Cat. No. PC38; 1:10,000 in 5% normal donkey serum KPBS overnight at room temperature), biotinylated donkey anti-rabbit antibody (Chemicon International; 1:300; 1 hour), and avidin–biotin–peroxidase complex (Vector Laboratory; 1 hour). Diaminobenzidine was used for visualization of Fos-LI. Sections were mounted on chromealum-coated slides and coverslipped without counterstaining. Fos-LI in the nucleus of positively stained nuclei appeared as a homogenous brown–black precipitate. Primary antibody was omitted from processing of selected sections to serve as a control for nonspecific staining.
The nuclei of neurons positive for Fos-LI appeared as distinctly oval-bodies that were easily distinguished from formed elements (eg, red blood corpuscles) and other non-neuronal elements. Labeled nuclei were counted at a magnification of 10×. Labeled nuclei were always less than 40 µm in diameter. Since every fifth section was collected, there was no possibility for double counting the same nucleus. We focused throughout the z-axis in each section and counted only intact oval-shaped densely stained nuclei.
MIA-Induced Weight-Bearing Responses
Weight-bearing response (WBR) was used as an index of OA-like pain responses (26,27). MIA-induced changes in body WBR were assessed using an Incapacitance Meter (Model-600, IITC Life Science). Rats were allowed to acclimate to the apparatus for 30 minutes a day for 3 consecutive days. The amount of weight supported by the hind legs was measured separately for the right and left hind legs every 3 seconds, before and 1, 2, 3, 7, 10, 14, and 21 days after the MIA injection. At each time point, five readings were taken for each rat. These were averaged for the individual and then for the group. Weight-bearing difference between the two legs was presented as the percentage of weight borne by the ipsilateral leg as determined using the formula % weight on the ipsilateral leg = weight on the ipsilateral leg/ (weight on the contralateral leg + weight on the ipsilateral leg) × 100%.
MIA-Induced Primary Mechanical Hyperalgesia
Mechanical sensitivity of the left knee joint was assessed using a digital paw pressure Randall–Selitto applicator before and 1, 4, 7, 14, 21, and 28 days after the MIA injection in the same knee joint. Rats were allowed to acclimate to the apparatus for 30 minutes a day for 3 days. A gradually increasing pressure was applied on the knee until the rat withdrew its leg. The lowest pressure necessary to elicit the withdrawal response was considered as the noxious mechanical threshold. The assessment of the knee joint’s sensitivity to pressure stimulation has been suggested as a functional index of OA-like pain responses (28), but, to the best of our knowledge, it has not been characterized in the rat model.
Statistical Analyses
A normality test was performed first and either parametric or non-parametric analysis of variance (ANOVA) on ranks were performed depending on the outcome of the normality test. Two-way ANOVA was used to analyze acute nocifensive responses with treatment and either sex or age as main effects. For MIA-induced changes in WBR and primary mechanical hyperalgesia, two-way ANOVA with repeated measures was used for each sex or age group with treatment as a between-subject variable and time as a within-subject variable. In order to assess the overall magnitude of drug-induced changes in WBR and primary mechanical hyperalgesia over time, the area under the curve (AUC) was calculated for each rat. Two-way ANOVA was used to compare the AUC between age and sex groups and Fos-LI counted in L4 and L5 from all animals. All multiple group comparisons were followed by a post hoc analysis to further delineate the differences between specific groups. Data are presented as mean ± standard error of the mean (SEM) and the significance of all analyses was set at p < .05.
Results
Baseline Nociceptive Thresholds
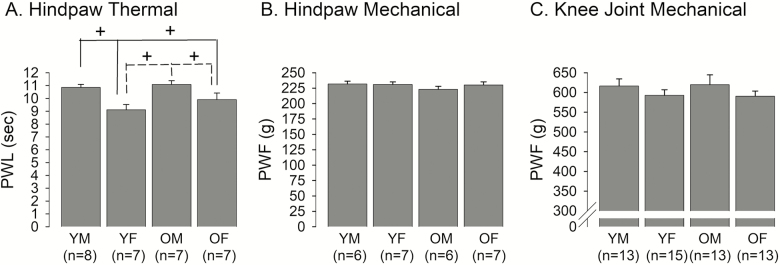
Latencies to paw withdrawal responses to noxious thermal stimulation ranged from 8.19 seconds to 12.56 seconds. Male rats exhibited significantly longer paw withdrawal latencies to noxious thermal stimulation of the hindpaw compared to those of female rats regardless of age (Figure 1A). The two-way ANOVA revealed a significant sex effect (F = 15.8, p < .001) without a significant age effect (F = 1.98, p = .17). When compared between sexes within the same age group, paw withdrawal latencies were significantly shorter in female rats compared to male rats in both age groups. When compared between the different age groups within the same sex, paw withdrawal latencies were not significantly different between young and old rats, suggesting a lower thermal threshold in females, regardless of age.
Figure 1.
Age- and sex-related differences in basal somatic sensitivity. (A) Paw withdrawal latencies (PWL) to noxious thermal stimulation on the hindpaw were compared between young male (YM), young female (YF), old male (OM), and old female (OF) rats. The solid lines indicate comparisons between YM vs. YF and OF, while the dotted lines compare OM vs. YF and OF. + denotes significant difference at p < .05. (B) Paw withdrawal force (PWF) to noxious mechanical stimulation of the hindpaw was compared between age and sex groups. (C) PWF to mechanical stimulation of the knee joint was compared between age and sex groups. The data in this figure and all subsequent figures are presented as mean ± SE.
The force required to elicit paw withdrawal response to noxious mechanical stimulation ranged from 203 g to 251 g. The paw withdrawal force was similar in all four groups (Figure 1B). There was neither a significant sex effect (F = 0.99, p = .33) nor a significant age effect (F = 0.46, p = .51) on paw withdrawal force to noxious mechanical stimulation of the hindpaw. In order to assess sex- and age-related differences in deep tissue sensitivity, we also compared the minimum force required to elicit a leg withdrawal response when applied to the knee. The threshold mechanical force ranged from 453 g to 712 g. Male rats tended to show higher pain thresholds compared to female rats, although the effect for sex did not reach statistical significance (F = 0.001, p = .98; Figure 1C). There was no significant age effect (F = 2.1, p = .15).
Capsaicin-Induced Acute Nocifensive Responses
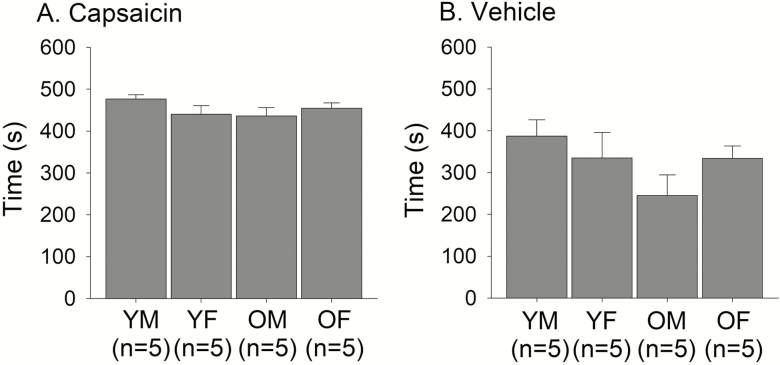
To determine sex- and age-related differences in acute inflammatory pain sensitivity, we assessed behavior immediately following intraplantar injection of capsaicin into the left hindpaw. We noted that typically recorded active nocifensive behaviors, like licking, guarding, and rapid repeated paw withdrawal, were more frequently observed in vehicle-injected animals than in capsaicin-injected ones. We suggest this is because capsaicin-treated rats, unlike vehicle-injected ones, tended towards extended periods of immobility due to intense noxious exposure. In fact, all 20 capsaicin-injected rats exhibited this behavior, with the minimum total time immobile at 243 seconds, whereas only 3 of 19 vehicle-injected rats displayed this behavior with a maximum total time of 42 seconds. Immobility, while not often recorded as an indicator of pain, is recognized as a nocifensive behavior, especially during intense pain (29,30). With this recognition and our observation of immobility as a characteristic behavior of capsaicin-induced pain, we added immobility as an additional nocifensive behavior. As the capsaicin- and vehicle-injected groups exhibited unequal variance in duration of nocifensive behaviors, the Mann–Whitney U Test was used to compare this measure. Capsaicin-induced nocifensive behavior duration (median: 454.5 seconds) was significantly longer than that of vehicle-induced duration (median: 340.0 seconds) (Mann–Whitney U = 151.0, ncap = 20, nveh = 19, p < .001; Figure 2 A and B). Two-way ANOVAs indicated there was no significant difference in duration of nocifensive behaviors by sex or age in capsaicin-injected rats (Figure 2A), nor was there a significant interaction between sex and age.
Figure 2.
Age- and sex-related differences in capsaicin-induced nocifensive responses. Total duration of nocifensive responses, including both active and immobility responses, following intradermal injection of (A) capsaicin or (B) vehicle were compared between age and sex groups.
Capsaicin-Induced c-Fos Activation in the Spinal Cord
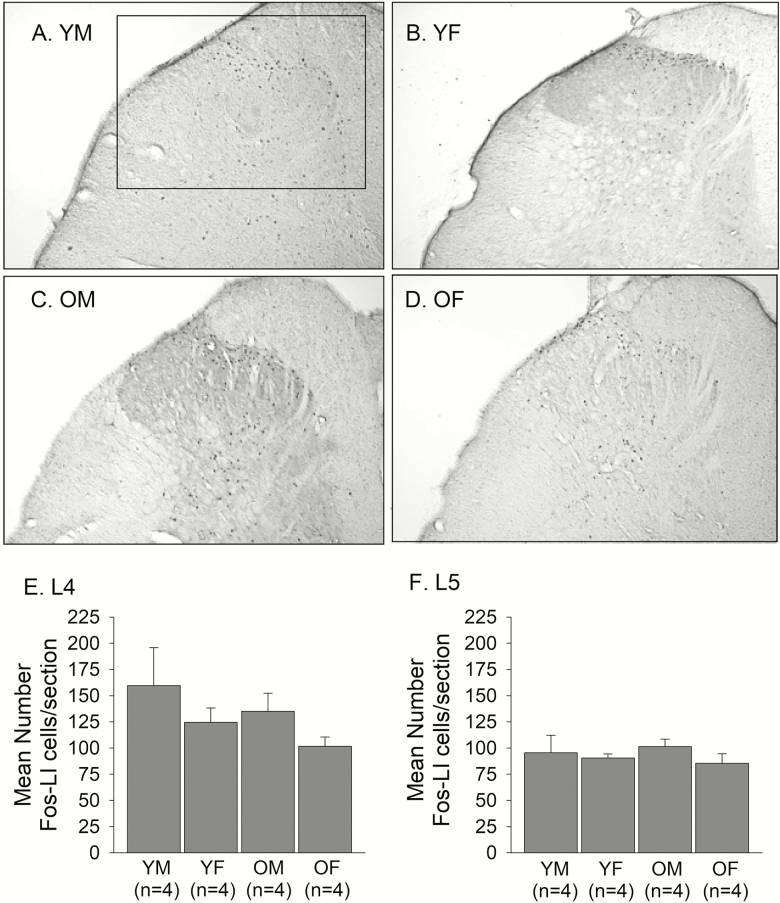
Intradermal injection of capsaicin in the hindpaw evoked intense neuronal activation in the ipsilateral spinal cord at the level of L4 and L5. The Fos‐LI was consistently seen in the laminae I, II, and III of the dorsal horn (DH) ipsilateral to the injected paw (Figure 3A–D). The neurons in deeper laminae also showed Fos‐LI, but less reliably across subjects. The Fos‐LI in the contralateral DH was minimal (data not shown). Five sections for L4 and five sections for L5 were selected and averaged for each rat. The average number of Fos-LI neurons in L4 was greater than that of L5 for all groups. The pattern of Fos-LI in the DH appeared to be similar between both age and sex groups, and the Fos activation appeared to be greater in young males and smaller in old females. Our statistical analyses confirmed that there was neither a significant sex effect nor a significant age effect on the average number of Fos-LI neurons in the L4 (Age: F = 1.21, p = .29; Sex: F = 2.5, p = .14) or the L5 (Age: F = 0.003, p = .96; Sex: F = 1.03, p = .33) (Figure 3E and F).
Figure 3.
Representative photomicrographs showing capsaicin-induced Fos-LI neurons in the dorsal horn of the L4 spinal cord of (A) YM, (B) YF, (C) OM, and (D) OF. The box shown in A indicates the area in the upper quadrant of the dorsal horn where all counts of Fos-LI were made. The same box was applied across all sections in all experimental groups. All counts were made from photomicrographs taken at 10 x magnification. The average number of capsaicin-induced Fos-LI neurons per section in the dorsal horn of the (E) L4 and (F) L5 spinal cord were compared between YM, YF, OM, and OF.
MIA-Induced WBR
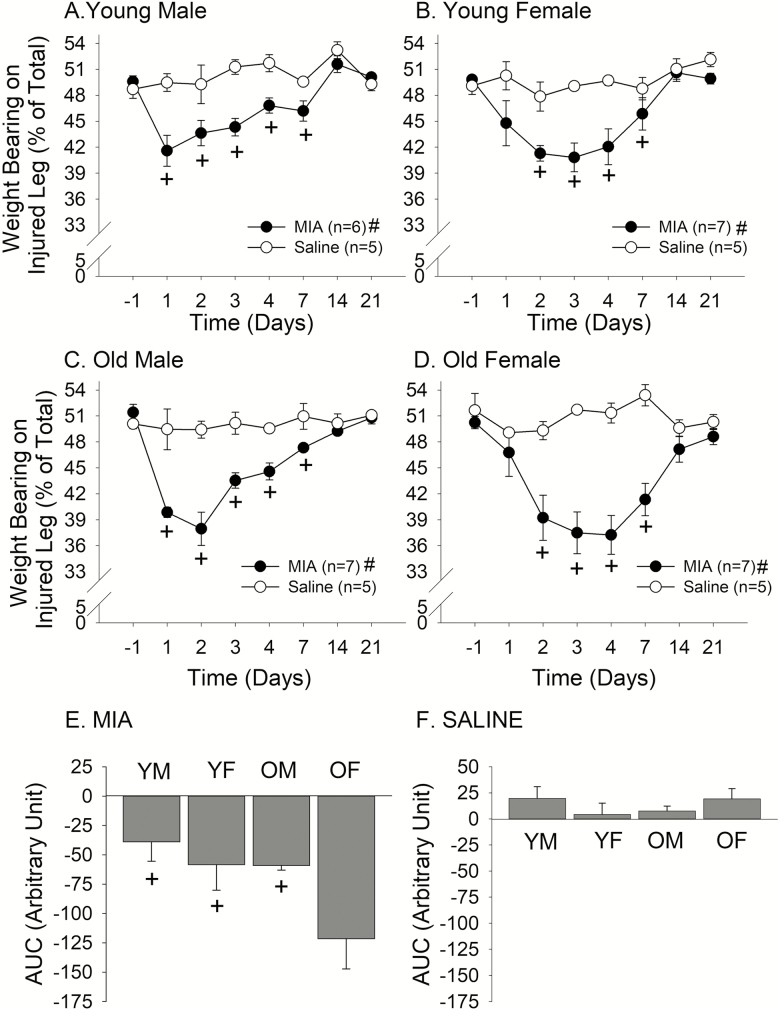
In all age and sex groups, MIA injection into the knee joint resulted in the development of significant mechanical hyperalgesia, as assessed by WBR. In young male rats, MIA-induced hyperalgesia peaked on Day 1 and slowly resolved, returning to the pre-injection level around Day 14 (Treatment: F = 11.8, p < .05, Time: F = 10.3, p < .010; Figure 4A). In young female rats, the peak hyperalgesia was observed on Day 3, but the extent and the time course of recovery seem to be similar to those of young males (Treatment: F = 12.1, p < .01, Time: F = 6.7, p < .001; Figure 4B). Aged male rats developed a greater peak reduction of weight bearing on the inflamed side compared to young rats (Treatment: F = 73.2, p < .001, Time: F = 13.5, p < .010; Figure 4C). The time course of recovery was similar to young rats. Interestingly, aged female rats developed profound hyperalgesia that persisted for a few days and did not fully recover until 3 weeks after the injection (Treatment: F = 30.9, p < .001, Time: F = 4.9, p < .010; Figure 4D). Control saline injection did not significantly alter WBR in any of the experimental groups, suggesting that the changes in weight bearing is specifically due to MIA-induced inflammation. In order to assess the extent of MIA-induced hyperalgesia in each group over the time course of 3 weeks, we compared the AUC between all experimental and control groups (Figure 4E). There was a significant age effect (F = 4.76, p < .05) as well as a significant sex effect (F = 4.61, p < .05). The overall extent of MIA-induced hyperalgesia was greatest for old female rats. Our post hoc analyses revealed that the responses from old female rats were driving both the age and sex effects. Saline treatment did not have any significant effect on AUC (Figure 4F).
Figure 4.
Effects of intraarticular MIA or saline on weight-bearing responses in (A) young male, (B) young female, (C) old male, and (D) old female rats. Line graphs show the time course of changes in weight-bearing responses. + denotes significant difference compared to the pre-injection baseline and # denotes significant treatment effect at p < .05. Bar graphs depict the overall effect of (E) MIA or (F) saline on weight-bearing responses as AUC. + denotes significant difference compared to OF at p < .05.
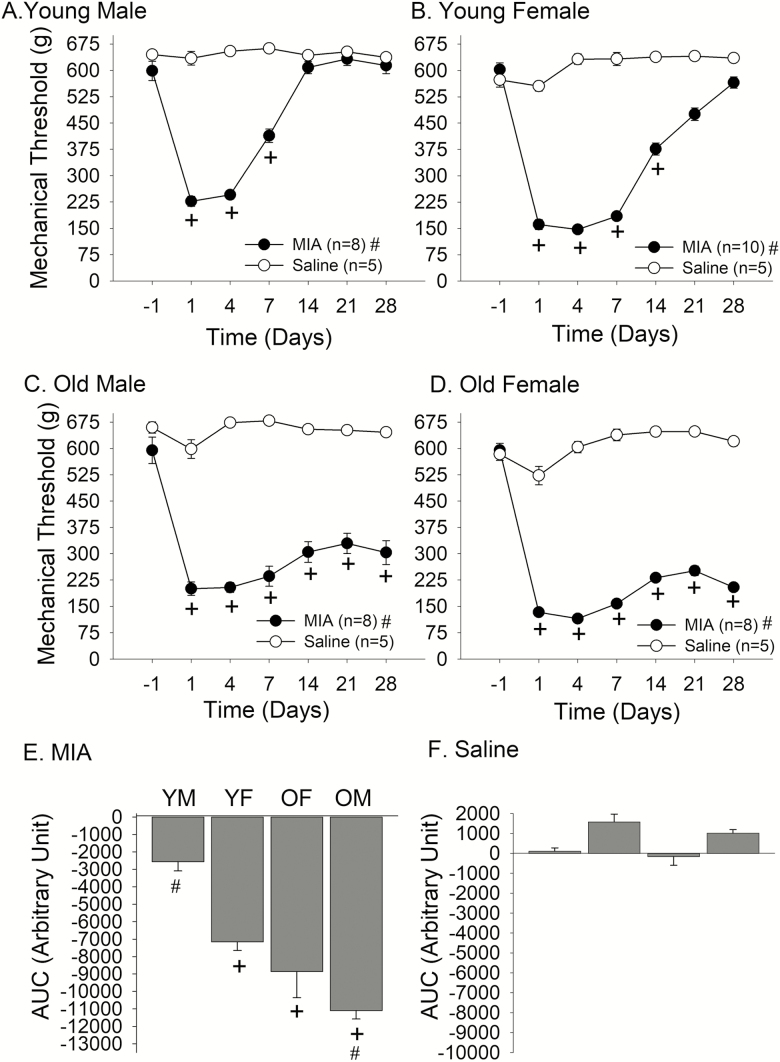
MIA-Induced Primary Mechanical Hyperalgesia
While WBR has been frequently used as an index of knee joint hyperalgesia, the data was highly variable among young female and especially in aged rats. Since aged rats were substantially larger, their mobility in the box was more limited than younger rats. Also, aged rats tended to sit rather than stand on their feet, making it more difficult to reliably assess WBR. For these reasons, we developed a second assay of mechanical hyperalgesia by directly measuring mechanical thresholds of the knee joint before and after intraarticular treatment.
As with MIA-induced WBR, MIA injection into the knee joint led to the development of significant mechanical hyperalgesia in all age and sex groups. In young male rats, there was a profound reduction of mechanical thresholds at the knee joint observed from Day 1 after MIA injection. The mechanical hyperalgesia remained significant until Day 7 before returning to the pre-injection level around Day 14 (Treatment: F = 116.3.8, p < .001, Time: F = 53.6, p < .001; Figure 5A). In young female rats, the extent of mechanical hyperalgesia was greater than that of young male rats, and the time course of recovery was also longer than that of young male rats (Treatment: F = 548.8, p < .001, Time: F = 82.4, p < .001; Figure 5B). Interestingly, aged male rats developed a peak reduction of mechanical thresholds similar to those of young male rats, but the mean threshold remained significantly lower for the entire 4 weeks of the observation period (Treatment: F = 189.6, p < .001, Time: F = 20.9, p < .001; Figure 5C). Aged female rats developed profound hyperalgesia similar in extent to that of young females. But, as with old male rats, the hyperalgesia never recovered to the pre-injection level during the 4-week observation period (Treatment: F = 981.9, p < .001, Time: F = 121.9, p < .010; Figure 5D). In order to assess the extent and duration of MIA-induced hyperalgesia over the time course of 4 weeks, we compared the AUC between all experimental and control groups. There was a significant age effect (F = 37.6, p < .001) as well as a significant sex effect (F = 16.8, p < .001) on the AUC in MIA-treated rats (Figure 5E). The post hoc analysis revealed that the AUC of young males was significantly smaller than those of young females, old males, and old females. The AUC of old female rats was significantly greater than that of young female rats. There was no significant difference between old male and old female rats. Saline treatment did not have any significant effect on AUC (Figure 5F).
Figure 5.
Effects of intraarticular MIA or saline on knee joint mechanical sensitivity in (A) young male, (B) young female, (C) old male, and (D) old female rats. Line graphs show the time course of changes in mechanical thresholds for eliciting withdrawal responses. + denotes significant difference compared to the pre-injection baseline and # denotes significant treatment effect at p < .05. Bar graphs depict the overall effect of (E) MIA or (F) saline on mechanical hyperalgesia as AUC. + denotes significant difference compared to YM and # denotes significant difference compared to YF at p < .05.
Discussion
Age and Sex Differences in Basal Nociceptive Sensitivities
This is the first study to test the effects of both sex and age on a range of pain responses. The results suggest that there are selected age and sex differences, with the older female rats showing most profound responses under chronic pain conditions. In addition, age did not significantly affect acute pain responses, and sex was a predictable variable for basal thermal sensitivity. Our results in both males and females showed no significant age-related differences in basal thermal sensitivity. The literature on age-related differences in basal thermal sensitivity shows inconsistent results. In a review of 18 studies on age differences in the basal sensitivity to noxious thermal stimuli in the rat, the majority of studies reported no age differences, a few reported an increase, and two studies reported a decrease in response latency (4). Since all but one study used only male rats, age differences based on sex could not be discerned from these studies. The inconsistent patterns of preclinical data on the effects of age on thermal sensitivity can be attributed to the types of thermal assays, operant versus reflex-based testing methods, or difference between the mouse and rat species (4–6).
Our data also showed that there were small but significant sex-related differences in both young and old rats. Among the rodent studies that show sex differences in nociceptive responses, females exhibited higher sensitivity than males (31). Again, the overwhelming majority of rodent data on sex differences in nociceptive sensitivity were generated from young animals. Therefore, our data showing that the sex differences observed at young age persist into old age is interesting. Our data suggest that age-related changes in primary afferent anatomy do not necessarily account for thermal nociception in old animals, and that sex, but not age, is a biological variable that modulates basal thermal sensitivity throughout their life span. A recent meta-analysis of human studies on the impact of age on pain thresholds concluded that pain thresholds increase with age when heat is used a stressor (32). However, the analysis revealed that data from human studies also vary depending on the types of thermal stimulus, test sites, and age ranges. Furthermore, sex was not systematically analyzed as a potential variable influencing thermal threshold in aged populations.
Relatively few preclinical studies compare age-related differences in basal mechanical thresholds (10,33). These studies reported either increased mechanical thresholds with age (33) or no age-related differences in basal mechanical thresholds (10). In our hands, thresholds for noxious mechanical pressure on neither the hindpaw nor the knee joint were significantly different between individual sex and age groups. Only when the groups were combined based on sex, regardless of age (data not shown), was there a small effect with females showing a higher sensitivity at a knee joint. Human studies also generally show minimal age-related changes in pain thresholds when mechanical stimuli are used as a stressor (32). Thus, despite age-related widespread degenerative changes in somatosensory system such as a selective loss of unmyelinated fibers (34), basal mechanical pain thresholds seem relatively uncompromised with advancing age. It seems that basal nociceptive processing is more susceptible to experimental variables than age-related anatomical changes in somatosensory system, per se.
Age and Sex Differences in Capsaicin-Induced Nocifensive Behaviors and c-Fos Activation
Capsaicin has been frequently used as an algesic substance that induces acute inflammatory pain responses in rodents (24,35). Intradermal capsaicin typically elicits active nocifensive behaviors, such as licking, lifting, and guarding. Though, to the best of our knowledge, immobility has yet to be considered as a capsaicin-induced nocifensive behavior, we take immobility to indicate a pain-induced negative affect so severe that active nocifensive behaviors are either inadequate to manage the pain or worsen it. This interpretation of immobility has similarities to the interpretation of immobility in the forced swim test, where it is used as an indicator of behavioral despair and is diminished by antidepressants (36).
Using the combined measure of active nocifensive responses and immobility, we did not find any statistical difference between male and female rats. This is in contrast to human studies demonstrating that females generally rate pain intensity following capsaicin treatment to be higher than males do (37,38). An available animal study that assessed sex differences also report greater capsaicin-induced licking/lifting responses in female rats compared to male rats (24). The discrepancy between these observations and our data can be explained by the concentration of capsaicin administered. The study by Lu et al. (24) used low concentrations of capsaicin, which led to transient responses that diminished within the first 5 minutes. The sex differences disappeared when their highest capsaicin concentration (15 µg) was used. The concentration of capsaicin we used in this study was 300 µg. Thus, it is highly likely that the lack of sex differences is due to the ceiling effect. The highly potent concentration of capsaicin may have also led to the immobility response in our study. Our data additionally showed no age-related differences in capsaicin-induced nocifensive responses. While no animal study assessing the effects of age on capsaicin responses is available, topical application of capsaicin in human subjects resulted in no age effect on the magnitude of spontaneous sensation, flare size, and area of heat hyperalgesia (39). Formalin, another acute inflammatory agent, resulted in peak nocifensive responses in the middle-aged male rats (18 months), but produces no difference between young (3 months) and old (24 months) rats (40).
There were no age- or sex-related differences in the number of Fos-LI in the spinal cord. This finding is consistent with the behavioral response data described previously. Acute inflammation induced by formalin produces an increased number of Fos-LI neurons in the spinal cord of aged rats due to the deficits in the descending 5-HT inhibitory system (41). This is at odds with our finding since formalin and capsaicin induce similar distributions of Fos-LI in the spinal cord of young male rats (42). As with the behavioral responses, it is conceivable that the high concentration of capsaicin used may have also saturated Fos-LI neurons in all groups since the effect of capsaicin on c-Fos induction in the superficial DH is concentration-dependent (42). Further studies are needed to assess whether the effect of age on capsaicin responses can be demonstrated with lower capsaicin concentrations and with middle-age groups.
MIA-Induced OA Pain Responses
There is a plethora of animal models of OA that evaluate the pathophysiological mechanisms of OA joint pathology, but relatively few models directly assess pain responses (28). Even among studies assessing pain responses as a primary end point, only young animals are used. In one study that examined the relationship between age and joint nociception in naturally occurring OA in Dunkin Hartley guinea pigs, electrophysiological responses of knee joint primary afferent neurons were assessed without evaluating OA-related pain responses (43). As far as we know, there is no preclinical data on how age impacts OA-related pain responses. This is rather surprising considering that age is one of the major risk factors for OA. In this report, we provide novel information that age and sex impact OA-related pain responses, assessed with two different outcome measures.
It has been well-documented that single intraarticular injections of MIA produce reliable and robust pain-like responses in small animals (26,27). MIA treatment in young male rodents typically leads to the development of weight-bearing deficits in the injected hindlimb that peak within the first few days and gradually returns to the pre-injection level in 2–3 weeks. The extent of initial weight-bearing deficits in young male rats in our study was consistent with published studies, but the recovery time was shorter. The differences in the pain response profiles can be attributed to different concentrations of MIA since MIA treatment shows concentration-dependent joint destruction and joint nociceptor responses (27,44). It has also been noted that the standard receptacle for incapacitance meter dissipates animals’ body weight away from the force pedals when the animals lean on the sides of the box (28). This is especially the case for old rats as they become too large to freely stand on the force pedals in a confined receptacle. Thus, WBRs can be influenced by the design of the receptacle used to restrain rodents and by how the animals are acclimated to the device during the study. In our study, the same concentration of MIA in young female rats tested in the same receptacle revealed different response profiles. The onset of peak weight-bearing deficit was slower in female rats. Interestingly, the temporal response profiles between the sexes were similar in old rats, with a significantly greater overall effect in old female rats. Though not statistically significant, the extent of MIA-induced weight-bearing deficits was also greater in old male rats compared to those in young male rats.
The effects of sex and age were more pronounced when primary mechanical hyperalgesia was used as the outcome measure of joint pain. WBR and primary mechanical hyperalgesia assays obviously measure different aspects of joint pain, but pressure application measurement of the knee joint has been suggested to provide a robust measure of knee joint pain with high inter-experimenter agreement (28). In any case, the results from both assays clearly demonstrated that old rats exhibit significantly more pain than young rats, that female rats tend to show greater pain responses, and that old female rats are the most vulnerable group. Our findings are consistent with human studies showing that women are more likely to have OA than men, that women are more likely to have more severe OA than men (45), and that the incidence and prevalence of OA increase with age (12,46).
OA in the elderly does not develop as a simple consequence of biological changes associated with age. Rather, active biological processes of joint degradation, such as joint matrix degradation stimulated by cytokine and matrix metalloproteinases and increased production of reactive oxygen species, are thought to contribute to OA development (46). Likewise, greater pain responses in elderly women stem from complex interplays between age, sex and active nociceptive processing at multiple levels of the neuroaxis. At this point, pathophysiological mechanisms underlying age-related changes in OA pain are relatively poorly understood. One can hypothesize that age- and sex-related differences peripheral nociceptive processing can contribute to more profound OA-like pain responses in old female rats. Available studies indicate that joint afferents in aged animals with naturally occurring OA show increased spontaneous activities as well as increased evoked responses to noxious joint movement (43). It is interesting to note that joint pathology, which increases with age, was not significantly correlated with nociceptor activities. Unfortunately, sex differences were not assessed in their study. The contribution of primary afferent neurons is further supported by the observation that TRPA1 channels in DRG play a critical role in the transition from acute to chronic mechanical hypersensitivity in aged animals under inflammatory conditions (47). A recent study with mice, however, showed that C fiber activities in aged animals are minimal during both acute and chronic inflammatory phases, despite strong behavioral responses (9), suggesting that central mechanisms may also play a role in aged animals. OA patients demonstrate reduced conditioned pain modulation and enhanced temporal summation of pain (48), both measures are indicative of altered central pain processing. However, neither age nor sex was investigated as a potential biological variable that affects central pain processing. Our recent fMRI study showed sex differences in functional connectivity between periaqueductal gray and anterior cingulate cortex are highly correlated with sex differences in conditioned pain modulation in rats (49). Similar studies with aged animals will reveal distinct brain circuitries involved in age-related changes in pain processing.
In summary, we showed that both age and sex effects on acute and chronic pain conditions can be reliably assessed in rodent models. Using those models, we confirmed the clinical observations that old females are most vulnerable to chronic pain conditions such as OA. Our animal models that exhibit clear sex and age differences in OA pain would allow back-translation of clinical observations and provide important tools to investigate basic mechanisms underlying the impact of age and sex. Our OA model, therefore, should serve as the impetus for mechanistic research into this clinically significant and growing problem.
Funding
This work was supported by the National Institute of Aging (AG053783) and National Institute of Dental and Craniofacial Research (DE027808) at the National Institutes of Health to J.Y.R.
Conflict of Interest
None declared.
Acknowledgments
The authors thank Holly Ross for proofreading and editing of the manuscript, and Jae Il Ru, Xiecheng (Lydia) Chen, and Sean Kim for technical assistance. J.Y.R. and R.Z. designed the study, YZ, DY, and CT conducted the experiments. J.Y.R. and J.T.S. prepared the manuscript.
References
- 1. Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults - United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67:1001–1006. doi:10.15585/mmwr.mm6736a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vincent G, Velkoff V.. The Next Four Decades. The Older Population in the United States. US Department of Commerce, Economics and Statistics Administration; Suitland, MD: US Census Bureau; 2010:P25-1138. [Google Scholar]
- 3. Reid MC, Bennett DA, Chen WG, et al. Improving the pharmacologic management of pain in older adults: identifying the research gaps and methods to address them. Pain Med. 2011;12:1336–1357. doi:10.1111/j.1526-4637.2011.01211.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gagliese L, Melzack R. Age differences in nociception and pain behaviours in the rat. Neurosci Biobehav Rev. 2000;24:843–854. [DOI] [PubMed] [Google Scholar]
- 5. Gibson SJ, Farrell M. A review of age differences in the neurophysiology of nociception and the perceptual experience of pain. Clin J Pain. 2004;20:227–239. [DOI] [PubMed] [Google Scholar]
- 6. Yezierski RP. The effects of age on pain sensitivity: preclinical studies. Pain Med. 2012;13 (Suppl 2):S27–S36. doi:10.1111/j.1526-4637.2011.01311.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jimenez-Andrade JM, Mantyh WG, Bloom AP, et al. The effect of aging on the density of the sensory nerve fiber innervation of bone and acute skeletal pain. Neurobiol Aging. 2012;33:921–932. doi:10.1016/j.neurobiolaging.2010.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang S, Davis BM, Zwick M, Waxman SG, Albers KM. Reduced thermal sensitivity and Nav1.8 and TRPV1 channel expression in sensory neurons of aged mice. Neurobiol Aging. 2006;27:895–903. doi:10.1016/j.neurobiolaging.2005.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Weyer AD, Zappia KJ, Garrison SR, O’Hara CL, Dodge AK, Stucky CL. Nociceptor Sensitization Depends on Age and Pain Chronicity(1,2,3). eNeuro. 2016;3:1–26. doi:10.1523/ENEURO.0115-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chung JM, Choi Y, Yoon YW, Na HS. Effects of age on behavioral signs of neuropathic pain in an experimental rat model. Neurosci Lett. 1995;183:54–57. doi:10.1016/0304-3940(94)11113-w [DOI] [PubMed] [Google Scholar]
- 11. Zhang RX, Lao L, Qiao JT, Ruda MA. Effects of aging on hyperalgesia and spinal dynorphin expression in rats with peripheral inflammation. Brain Res. 2004;999:135–141. doi:10.1016/j.brainres.2003.11.042 [DOI] [PubMed] [Google Scholar]
- 12. Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med. 2010;26:355–369. doi:10.1016/j.cger.2010.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL 3rd. Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 2009;10:447–485. doi:10.1016/j.jpain.2008.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Greenspan JD, Craft RM, LeResche L, et al. ; Consensus Working Group of the Sex, Gender, and Pain SIG of the IASP Studying sex and gender differences in pain and analgesia: a consensus report. Pain. 2007;132 (Suppl 1):S26–S45. doi:10.1016/j.pain.2007.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Arout CA, Sofuoglu M, Bastian LA, Rosenheck RA. Gender differences in the prevalence of fibromyalgia and in concomitant medical and psychiatric disorders: A National Veterans Health Administration Study. J Womens Health (Larchmt). 2018;27:1035–1044. doi:10.1089/jwh.2017.6622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boyan BD, Tosi LL, Coutts RD, et al. Addressing the gaps: sex differences in osteoarthritis of the knee. Biol Sex Differ. 2013;4:4. doi:10.1186/2042-6410-4-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Croft P, Rigby AS, Boswell R, Schollum J, Silman A. The prevalence of chronic widespread pain in the general population. J Rheumatol. 1993;20:710–713. [PubMed] [Google Scholar]
- 18. Lawrence JS, Bremner JM, Bier F. Osteo-arthrosis. Prevalence in the population and relationship between symptoms and x-ray changes. Ann Rheum Dis. 1966;25:1–24. [PMC free article] [PubMed] [Google Scholar]
- 19. Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum. 1995;38:19–28. doi:10.1002/art.1780380104 [DOI] [PubMed] [Google Scholar]
- 20. Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol. 2014;6:71–80. doi:10.2147/CLEP.S40245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. LeResche L. Epidemiology of temporomandibular disorders: implications for the investigation of etiologic factors. Crit Rev Oral Biol Med. 1997;8:291–305. [DOI] [PubMed] [Google Scholar]
- 22. Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. [DOI] [PubMed] [Google Scholar]
- 23. Auh QS, Ro JY. Effects of peripheral κ opioid receptor activation on inflammatory mechanical hyperalgesia in male and female rats. Neurosci Lett. 2012;524:111–115. doi:10.1016/j.neulet.2012.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lu YC, Chen CW, Wang SY, Wu FS. 17Beta-estradiol mediates the sex difference in capsaicin-induced nociception in rats. J Pharmacol Exp Ther. 2009;331:1104–1110. doi:10.1124/jpet.109.158402 [DOI] [PubMed] [Google Scholar]
- 25. Pitzer C, Kuner R, Tappe-Theodor A. Voluntary and evoked behavioral correlates in inflammatory pain conditions under different social housing conditions. Pain Rep. 2016;1:e564. doi:10.1097/PR9.0000000000000564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fernihough J, Gentry C, Malcangio M, et al. Pain related behaviour in two models of osteoarthritis in the rat knee. Pain. 2004;112:83–93. doi:10.1016/j.pain.2004.08.004 [DOI] [PubMed] [Google Scholar]
- 27. Pomonis JD, Boulet JM, Gottshall SL, et al. Development and pharmacological characterization of a rat model of osteoarthritis pain. Pain. 2005;114:339–346. doi:10.1016/j.pain.2004.11.008 [DOI] [PubMed] [Google Scholar]
- 28. Malfait AM, Little CB, McDougall JJ. A commentary on modelling osteoarthritis pain in small animals. Osteoarthritis Cartilage. 2013;21:1316–1326. doi:10.1016/j.joca.2013.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Flecknell PA. Refinement of animal use–assessment and alleviation of pain and distress. Lab Anim. 1994;28:222–231. doi:10.1258/002367794780681660 [DOI] [PubMed] [Google Scholar]
- 30. Le Bars D, Gozariu M, Cadden SW. Animal models of nociception. Pharmacol Rev. 2001;53:597–652. [PubMed] [Google Scholar]
- 31. Mogil JS, Chesler EJ, Wilson SG, Juraska JM, Sternberg WF. Sex differences in thermal nociception and morphine antinociception in rodents depend on genotype. Neurosci Biobehav Rev. 2000;24:375–389. [DOI] [PubMed] [Google Scholar]
- 32. Lautenbacher S, Peters JH, Heesen M, Scheel J, Kunz M. Age changes in pain perception: a systematic-review and meta-analysis of age effects on pain and tolerance thresholds. Neurosci Biobehav Rev. 2017;75:104–113. doi:10.1016/j.neubiorev.2017.01.039 [DOI] [PubMed] [Google Scholar]
- 33. Akunne HC, Soliman KF. Serotonin modulation of pain responsiveness in the aged rat. Pharmacol Biochem Behav. 1994;48:411–416. doi:10.1016/0091-3057(94)90545-2 [DOI] [PubMed] [Google Scholar]
- 34. Ochoa J, Mair WG. The normal sural nerve in man. II. Changes in the axons and Schwann cells due to ageing. Acta Neuropathol. 1969;13:217–239. [DOI] [PubMed] [Google Scholar]
- 35. Sakurada T, Katsumata K, Tan-No K, Sakurada S, Kisara K. The capsaicin test in mice for evaluating tachykinin antagonists in the spinal cord. Neuropharmacology. 1992;31:1279–1285. doi:10.1016/0028-3908(92)90057-v [DOI] [PubMed] [Google Scholar]
- 36. Costa AP, Vieira C, Bohner LO, et al. A proposal for refining the forced swim test in Swiss mice. Prog Neuropsychopharmacol Biol Psychiatry. 2013;45:150–155. doi:10.1016/j.pnpbp.2013.05.002 [DOI] [PubMed] [Google Scholar]
- 37. Frot M, Feine JS, Bushnell MC. Sex differences in pain perception and anxiety. A psychophysical study with topical capsaicin. Pain. 2004;108:230–236. doi:10.1016/j.pain.2003.11.017 [DOI] [PubMed] [Google Scholar]
- 38. Gazerani P, Andersen OK, Arendt-Nielsen L. A human experimental capsaicin model for trigeminal sensitization. Gender-specific differences. Pain. 2005;118:155–163. doi:10.1016/j.pain.2005.08.009 [DOI] [PubMed] [Google Scholar]
- 39. Zheng Z, Gibson SJ, Khalil Z, Helme RD, McMeeken JM. Age-related differences in the time course of capsaicin-induced hyperalgesia. Pain. 2000;85:51–58. [DOI] [PubMed] [Google Scholar]
- 40. Gagliese L, Melzack R. Age differences in the response to the formalin test in rats. Neurobiol Aging. 1999;20:699–707. [DOI] [PubMed] [Google Scholar]
- 41. Iwata K, Kanda K, Tsuboi Y, Kitajima K, Sumino R. Fos induction in the medullary dorsal horn and C1 segment of the spinal cord by acute inflammation in aged rats. Brain Res. 1995;678:127–139. doi:10.1016/0006-8993(95)00176-q [DOI] [PubMed] [Google Scholar]
- 42. Jinks SL, Simons CT, Dessirier JM, Carstens MI, Antognini JF, Carstens E. C-fos induction in rat superficial dorsal horn following cutaneous application of noxious chemical or mechanical stimuli. Exp Brain Res. 2002;145:261–269. doi:10.1007/s00221-002-1128-3 [DOI] [PubMed] [Google Scholar]
- 43. McDougall JJ, Andruski B, Schuelert N, Hallgrímsson B, Matyas JR. Unravelling the relationship between age, nociception and joint destruction in naturally occurring osteoarthritis of Dunkin Hartley guinea pigs. Pain. 2009;141:222–232. doi:10.1016/j.pain.2008.10.013 [DOI] [PubMed] [Google Scholar]
- 44. Schuelert N, McDougall JJ. Grading of monosodium iodoacetate-induced osteoarthritis reveals a concentration-dependent sensitization of nociceptors in the knee joint of the rat. Neurosci Lett. 2009;465:184–188. doi:10.1016/j.neulet.2009.08.063 [DOI] [PubMed] [Google Scholar]
- 45. Srikanth VK, Fryer JL, Zhai G, Winzenberg TM, Hosmer D, Jones G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage. 2005;13:769–781. doi:10.1016/j.joca.2005.04.014 [DOI] [PubMed] [Google Scholar]
- 46. Shane Anderson A, Loeser RF. Why is osteoarthritis an age-related disease? Best Pract Res Clin Rheumatol. 2010;24:15–26. doi:10.1016/j.berh.2009.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Garrison SR, Stucky CL. Contribution of transient receptor potential ankyrin 1 to chronic pain in aged mice with complete Freund’s adjuvant-induced arthritis. Arthritis Rheumatol. 2014;66:2380–2390. doi:10.1002/art.38724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Edwards RR, Dolman AJ, Martel MO, et al. Variability in conditioned pain modulation predicts response to NSAID treatment in patients with knee osteoarthritis. BMC Musculoskelet Disord. 2016;17:284. doi:10.1186/s12891-016-1124-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Da Silva JT, Zhang Y, Asgar J, Ro JY, Seminowicz DA. Diffuse noxious inhibitory controls and brain networks are modulated in a testosterone-dependent manner in Sprague Dawley rats. Behav Brain Res. 2018;349:91–97. doi:10.1016/j.bbr.2018.04.055 [DOI] [PMC free article] [PubMed] [Google Scholar]