Abstract
Peripheral artery disease (PAD) is a common disorder and a major cause of morbidity and mortality worldwide. Therapy is directed at reducing the risk of major adverse cardiovascular events, and at ameliorating symptoms. Medical therapy is effective at reducing the incidence of myocardial infarction and stroke to which these patients are prone, but is inadequate in relieving limb-related symptoms, such as intermittent claudication, rest pain and ischemic ulceration. Limb related morbidity is best addressed with surgical and endovascular interventions that restore perfusion. Current medical therapies have only modest effects on limb blood flow. Accordingly, there is an opportunity to develop medical approaches to restore limb perfusion. Vascular regeneration to enhance limb blood flow includes methods to enhance angiogenesis, arteriogenesis and vasculogenesis using angiogenic cytokines and cell therapies. We review the molecular mechanisms of these processes; briefly discuss what we have learned from the clinical trials of angiogenic and cell therapies; and conclude with an overview of a potential new approach based upon transdifferentiation to enhance vascular regeneration in PAD.
Peripheral Arterial Disease and Its Management
Peripheral artery disease (PAD) is the third leading cause of atherosclerotic cardiovascular morbidity, following coronary artery disease and stroke1. PAD is a common disease that is underdiagnosed2, and which has significant adverse effects on over 8 million Americans3, 4 and more than 200 million individuals worldwide1, 5. Age, tobacco use, diabetes, hypertension, hypercholesterolemia and sedentary state are the major risk factors for PAD1, 6. PAD may cause leg pain when walking (intermittent claudication, IC) which interferes with the cardiovascular benefit of regular exercise. Indeed, limitation of exercise capacity is a strong predictor of mortality in patients with PAD7. Critical limb ischemia (CLI)8 is the most advanced form of PAD, defined as chronic ischemic rest pain, ulcers, or gangrene of the lower extremity. CLI is a major cause of limb amputation9, and a harbinger of cardiovascular mortality in PAD10.
Medical therapy for PAD effectively reduces major adverse cardiovascular events (MACE) in PAD, and includes exercise, smoking cessation, anti-platelet agents, and therapies to restore normal levels of blood lipids, blood sugar and arterial pressure11. Medical therapy to relieve IC and CLI is of modest benefit. Cilostazol increases walking distance by 50%, but does not reduce MACE. Prostanoids reduce rest pain and improve ulcer healing12, and iloprost may reduce the incidence of amputation in CLI. However, vascular surgery and endovascular intervention are more effective than medical therapy at improving limb perfusion, reducing symptoms, and preventing loss of limb11, 13, 14. Clearly, there are opportunities for novel medical therapies to improve limb blood flow.
Vascular regenerative strategies include restoration of vascular function (e.g. vasodilation) and structure (e.g. plaque regression) (Figure). Restoration of vascular function, in particular endothelial function, can improve limb blood flow by enhancing vasodilation, reducing vascular inflammation, suppressing platelet aggregation and thrombosis, and promoting endogenous thrombolysis15, 16. Lipid-lowering therapy induces plaque regression, and this effect, combined with its benefit on endothelial function, may explain the increase in walking distance in PAD patients treated with statins17. In this review however, we will focus on the regenerative processes of angiogenesis, arteriogenesis and vasculogenesis; and describe a new process termed angiogenic transdifferentiation that may participate in vascular regeneration.
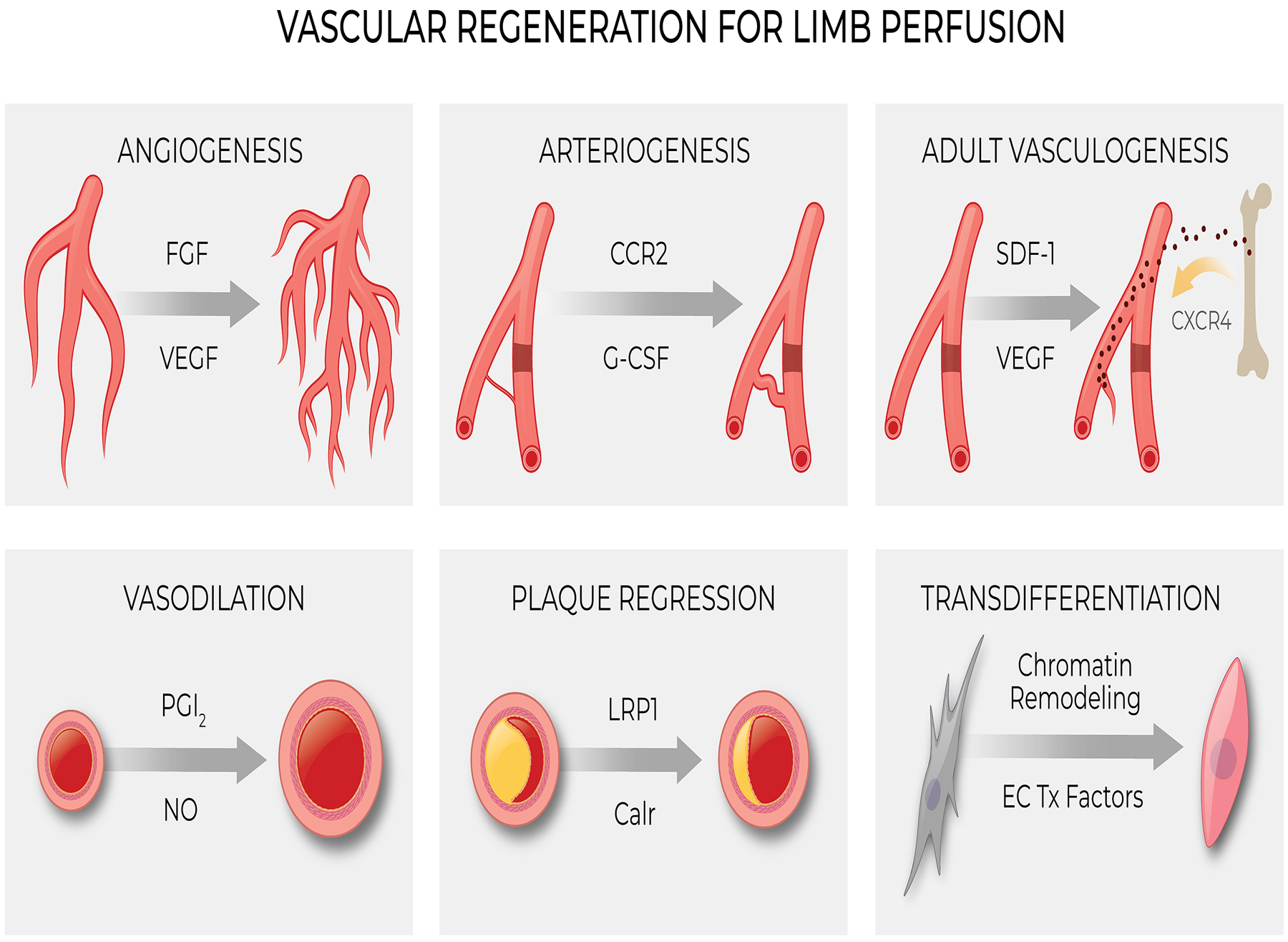
Processes involved in Vascular Regeneration. Tx = Transcriptional. See text for additional details.
Angiogenesis and PAD
Angiogenesis is the formation of new blood vessels from pre-existing vessels18–20 (Figure). The process begins with endothelial cell (EC) sprouting from existing capillaries, followed by EC migration, proliferation and lumen formation. In addition, intussusception of existing capillaries18, 21 also contributes to expansion of the microvasculature. In ischemic tissues, hypoxia activates the transcription factor hypoxia-inducible factor 1 (HIF-1), a basic-helix-loop-helix-pas heterodimeric protein that is responsive to oxygen tension22. This transcription factor contains two subunits, HIF-1α and HIF-1β. HIF-1α has an oxidation dependent degradation domain. Under normoxic conditions, two prolyl residues in this domain are hydroxylated by prolyl hydroxylase, which leads to the ubiquitination and destruction of HIF-1α. In the setting of hypoxia, this degradation process is inactive, HIF-1α becomes stable, and accumulates in the nucleus. There HIF-1α dimerizes with HIF-1β to activate target genes including angiogenic cytokines such as vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), and angiopoietin, and matrix metalloproteinases MMP2 and MMP923.
In PAD animal models, VEGF24, FGF25, hepatocyte growth factor (HGF)26, platelet-derived growth factor (PDGF)27 and angiopoietins28, prokineticin 2 (PROK2)29, and other angiogenic cytokines have been shown to enhance angiogenesis and limb blood flow. VEGF is the most widely studied angiogenic cytokine and is essential for endothelial proliferation, migration and lumen formation mediated by its receptors VEGFR-1 and VEGFR-230.
Angiogenic cytokines have also been implicated in the effect of adult stem cell therapy to improve perfusion in preclinical models of PAD. Mesenchymal stem cells derived from bone marrow or adipose tissue, mononuclear cells isolated from the bone marrow or peripheral blood, and endothelial cells derived from embryonic stem cells or induced pluripotent stem cells, have each been shown to generate angiogenic cytokines and home to sites of ischemia, where they increase microvascular density, and improve perfusion in the ischemic limb in murine models31–33.
Arteriogenesis
Arteriogenesis is the positive remodeling of preexisting collateral arterioles to generate larger conductance vessels that compensate for occluded arteries34, 35 (Figure). These preexisting collateral arterioles are narrow and high resistance channels that generally conduct little or no blood flow in healthy tissue36. However, severe arterial obstruction or occlusion creates a pressure gradient that favors blood flow through the collateral channels. The increase in blood flow, and shear stress, in the collateral channels induces a positive remodeling. This vascular remodeling process increases both diameter and wall thickness, and is accompanied by alterations in cellular proliferation and extracellular matrix degradation and deposition37, 38.
Early in the process, monocytes and macrophages are observed adhering to the endothelium and infiltrating into the subintimal space of collateral arterioles39–41. These cells play an essential role in the remodeling process by the secretion of growth factors, chemokines and metalloproteinases42. This pericollateral macrophage recruitment is mediated in part by an ICAM-1 dependent mechanism43. In addition, the CC-chemokine receptor-2 (CCR2) plays a critical role in monocyte/macrophage recruitment to the perivascular space of collateral vessels and is required for the increase in vessel diameter44. Monocyte differentiation and maturation into macrophages is required for arteriogenesis, and is controlled by Notch ligand Delta-like 1 (Dll1) expressed on vascular ECs and macrophage Notch effector Rbpj45. Granulocyte-colony stimulating factor (G-CSF)46, 47 and granulocyte macrophage-colony stimulating factor (GM-CSF)48, 49 also promote arteriogenesis. Other immune cells such as T cells50 and mast cells51 have also been reported to play a role in arteriogenesis.
During arteriogenesis, smooth muscle cells in the media of the collateral vessel transform from a contractile to a proliferative phenotype, and form a neo-intima41. The P2Y2 nucleotide receptor, which mediates vascular cell proliferation and migration, also participates in this positive remodeling50. Importantly, the smooth muscle cells return to a contractile phenotype in the end stage of this remodeling process37. It is well known that there are species-specific variation in collateral arterioles35, 51. Furthermore, in patients with PAD, there is substantial heterogeneity in the generation of collateral channels in the limb, which may contribute to individual differences in the severity of limb symptoms.
Adult Vasculogenesis
Vasculogenesis refers to the establishment of primary vasculature from mesodermal progenitors during early development. The incorporation of circulating progenitor and stem cells into regenerating blood vessels after development is termed adult vasculogenesis (Figure). Asahara’s discovery of “endothelial progenitor cells” (EPCs) in 199752 galvanized interest in adult stem cells for vascular regeneration. These cells originate in the bone marrow and can be isolated from the blood using cell surface markers such as CD34, CD133 and VEGFR252. Ischemia and hypoxia triggers EPC mobilization from the bone marrow, mediated by VEGF53, stromal-derived factor 1 (SDF-1)54, 55, FGF and angiopoietin-1. G-CSF and GM-CSF also stimulate mobilization of hematopoietic and progenitor cells from the bone marrow56. In the murine hind limb ischemia model, GM-CSF administered by injection or by plasmid transfer augments circulating levels of EPCs and increases capillary density57.
After mobilization, EPCs home to the ischemic tissue under the influence of VEGF and SDF-1, the latter binding to EPC chemokine receptor CXCR-455. Recently, it has been shown that GDF11 improves angiogenic function of EPCs in diabetic limb ischemia58. Inhibition of macrophage inflammatory protein-1β (MIP-1β) improves EPC homing and angiogenesis in diabetic models59.
In studies of their angiogenic effects, most investigators have used a small set of surface markers to define EPCs60. However, the surface markers that are commonly used for identification of human EPCs include markers that are not specific for endothelial lineage, such as CD133 and VEGFR261. Only a small subset of EPCs is of true endothelial lineage in humans62, most being of hematopoietic lineage. It is unlikely that EPCs differentiate into mature endothelium in vivo60, 63. Rather, they may promote angiogenesis by secreting angiogenic cytokines and matrix metalloproteinases64, 65. Still other bone marrow derived cells can form pericytes, which may associate with and stabilize endothelial networks66. Many progenitor cell types such as EPCs52, 67, 68, endothelial colony forming cells69, 70, circulating angiogenic cells (CACs)71, 72 may promote expansion of the microvasculature in preclinical models by generating angiogenic factors. Finally, under the influence of circulating factors generated by ischemia, mature endothelial cells from other sites may be mobilized into the systemic circulation and home to the ischemic tissue73.
What have we learned from clinical trials?
Angiogenic Cytokines
Administration of angiogenic cytokines (such as VEGF, FGF and HGF) and agents that can mobilize EPCs (such as SDF-1, G-CSF and GM-CSF) have shown benefit in pre-clinical models of PAD. These data precipitated small clinical studies which were encouraging. However, larger randomized clinical trials have been largely negative for their primary endpoints. Intra-arterial or intramuscular administration of adenoviral or plasmid VEGF gene therapy failed to increase walking distance in patients with IC, or reduce amputations in patients with CLI74–76. In one phase 2b/3 study in Russia, intramuscular injections of VEGFA-165 increased the primary endpoint of pain-free walking distance at 2 years77. However, this study was not blinded, and control subjects did not receive a vehicle injection. By contrast to the Russian study, all other large randomized controlled trials (RCTs) testing angiogenic therapies, whether delivered as gene therapy or recombinant proteins, have not achieved their primary endpoints of increasing walking distance (in IC) or reducing amputations (in CLI). These include trials testing the efficacy of therapies based on FGF, HGF, HIF1α, SDF-1, G-CSF or GM-CSF78,79–80. Accordingly, there is currently no FDA-approved angiogenic therapy for relieving intermittent claudication or ischemic ulcerations.
Cell Therapies:
Since the first pilot clinical trial of cell therapy in PAD in 200281, numerous cell types have been examined in clinical trials for PAD, including bone marrow derived cells, peripheral blood derived cells, progenitor or stem cells isolated from bone marrow or blood using specific surface markers, adipose vascular stromal cell or mesenchymal stem cells. Whereas early uncontrolled series seemed promising, the initial excitement surrounding cell therapy for PAD has dimmed as larger randomized clinical trials failed to confirm the earlier results, as we and others have previously discussed63, 82, 83. However, because CLI is an unmet need, and because there have been some positive data with small trials84, clinical studies in CLI are ongoing with autologous bone marrow derived mononuclear cells (BM MNC), adipose derived stem cells, and umbilical derived mesenchymal stem cells (MSC)85,86.
Indeed, there is evidence to support further research into cell therapy for CLI82. A meta‐analysis of 10 randomized, placebo‐controlled trials (499 CLI patients) showed that cell therapy provided significant improvements in ankle-brachial index, resting pain, and pain‐free walking time, although there was no improvement in amputation rates or amputation free survival87. A more recent meta‐analysis of 19 RCT (837 CLI patients) concluded cell therapy modestly reduced the risk of amputation by 37%, improved amputation free survival by 18%, and improved wound healing by 59%88.
Lessons learned:
In brief, angiogenic therapies have failed, whereas cell therapies may yet prove useful. The failure of angiogenic therapies may be related to the fact that we have limited knowledge regarding dosing, delivery and duration of angiogenic cytokines. Angiogenesis is a complex choreographed process that cannot be mimicked by administration of a single angiogenic cytokine. Furthermore, angiogenesis increases microvascular density, which may be insufficient for PAD patients who have long segments of occluded conduit arteries that impair perfusion. Accordingly, master regulators of both angiogenesis and arteriogenesis, such as transcriptional factors that regulate a cascade of genes involved in vascular regeneration, may have greater potential for efficacy.
Furthermore, angiogenic and cell therapies have been based upon flawed animal models. A significant limitation is that much preclinical work is performed in healthy mice subjected to ligation of a femoral or iliac artery. This model induces a very different pathobiology than that in our elderly patients with multiple cardiovascular risk factors, whose vascular disease has progressed over decades. The strengths and limitations of animal models of PAD have been recently reviewed89, and must be considered in the development of new therapeutics for vascular regeneration. In this regard, autologous cell therapy in a patient with vascular disease may be comprised of fewer and/or dysfunctional stem cells. For example, in 55 patients transplanted with BM MNC, those that had a positive outcome (wound healing and limb salvage, n=33) received a significantly greater number of CD34+ cells with their transplantation compared to those individuals that required limb amputation (n=22)90.
There is much we don’t know about the dosing and delivery of cell therapies. However, there is abundant evidence that cells injected into ischemic zones do not persist. Nevertheless, in the brief time that they survive in the tissue, they may generate angiogenic cytokines that contribute to an increase in microvascular density and improved perfusion. Alternatively or in addition, they may secrete exosomes containing biological activity, e.g. as in the form of angiogenic microRNA. Indeed, therapeutic effects have been observed in preclinical PAD models of exosomes derived from a variety of progenitor cells91, 92. In this regard, HIF-1α increases MSC-exosome secretion93. Finally, the effect of injected cells may also be due to the local inflammatory signaling that they induce as they undergo cell death in the ischemic zone94. In this regard, we have uncovered a novel mechanism by which inflammation may induce adaptive changes in cell identity that could promote angiogenesis.
New Insights into Vascular Regeneration
Inflammation and Transdifferentiation:
Lineage tracing studies indicate that endothelial-to-mesenchyme transition (EndoMT) may contribute to fibrosis95. EndoMT may explain the reduced vascular density and increased interstitial fibrosis seen in many fibrotic conditions. The existence of EndoMT begs the question of whether the reverse phenomenon occurs, i.e. the transdifferentiation of mesenchymal cells to endothelial cells (Figure). To be sure, the transdifferentiation of fibroblasts to endothelial cells occurs during development96. Whether this phenomenon plays a role in the adult is controversial97, 98. We and others have shown that under specific experimental conditions, human fibroblasts can be directly reprogrammed into endothelial cells in vitro and in vivo99–102. Furthermore, our unpublished data suggests that this process may play a role in the recovery from limb ischemia. Specifically, lineage tracing studies combined with single cell RNAseq have provided preliminary support for subsets of fibroblasts which may participate in angiogenic transdifferentiation.
The process of transdifferentiation from one somatic cell to a different lineage requires an increase in DNA accessibility so that new cell identity genes can be activated. We have shown that DNA accessibility is increased by signaling pathways known to be activated during injury and ischemia. In brief, pattern recognition receptors (PRRs; such as Toll-like receptors) can sense damage- or pathogen-associated molecular patterns (DAMPs or PAMPs respectively). Activation of PRRs induces inflammatory signaling, e.g. through NFκB, which subsequently promotes DNA accessibility through global changes in the expression and activity of epigenetic modifiers99, 100, 103–106. It is as though a cell, sensing a challenge, opens up its genetic toolbox so as to adapt and survive. Such adaptation may include a change in phenotype.
We have shown that this inflammatory signaling increases the expression of histone acetyltransferases and suppresses the expression of histone deacetylases103, 105 so as to promote epigenetic plasticity and cell fate transitions. Furthermore, inflammatory signaling causes inducible nitric oxide synthase (iNOS) to translocate to the nucleus. There iNOS S-nitrosylates epigenetic modifiers, such as the polycomb100 and NURD complexes106, to antagonize their suppressive histone markings. Intriguingly, a glycolytic switch is activated by this inflammatory signaling, and is coupled to epigenetic changes. Specifically, inflammatory signaling is associated with mitochondrial export of citrate to the nucleus, which increases nuclear acetyl-coA, the substrate for histone acetylation. These processes increase DNA accessibility as shown by micrococcal nuclease assay. In this state, the cell is rendered more tractable to reprogramming. The effect of inflammatory signaling to increase DNA accessibility and thereby facilitate changes in cell identity is called transflammation107.
Into what lineage the cell is reprogrammed is determined by the environmental milieu. For example, we observe that an inflammatory stimulus (the TLR3 agonist polyinosinic cytidilic acid) increases the epigenetic fluidity of fibroblasts so that they can transdifferentiate into endothelial cells under the influence of medium containing high levels of VEGF and other endothelial growth factors99, 100. Thus it seems possible that in the setting of ischemic injury, the local injury and inflammatory signaling, together with the release of angiogenic cytokines, might induce transdifferentiation of fibroblasts to endothelial cells, thereby enhancing angiogenesis. There is abundant evidence that an inflammatory response is necessary for tissue regeneration108. Future elucidation of this process may provide a novel therapeutic avenue for ischemic syndromes.
Summary
Since Judah Folkman’s early work on angiogenesis in the 1970s, much has been learned about angiogenesis and vascular regeneration. Although angiogenic therapy in preclinical models appeared to be very promising, the clinical trials of angiogenic factors have disappointed, failing to show a consistent effect on claudication distance, ischemic pain relief or ulcer healing78, 109. Cellular therapies to improve perfusion also showed promise in preclinical studies as well as small trials110, 111 but larger randomized clinical trials have been in general negative. The lack of benefit may be due to incomplete understanding regarding the appropriate dose, duration, delivery method and/or mechanisms of angiogenic or cell therapies. Angiogenic expansion of the microvasculature is probably insufficient to improve perfusion in human PAD, which is typically characterized by long segments of obstructed vessels. Combined with arteriogenesis, angiogenic therapies might be more effective. Finally, new insights into vascular regeneration, such as transdifferentiation, may provide for effective vascular regenerative strategies in PAD.
Highlights.
Vascular regeneration comprises angiogenesis, arteriogenesis and vasculogenesis.
Clinical trials of angiogenic factors for peripheral arterial disease have failed, in part because of imperfect pre-clinical models and incomplete knowledge
Clinical trials of cell therapies for critical limb ischemia suffer from similar limitations, but some positive results provide encouragement for continued development
The role of inflammatory signaling and transdifferentiation in vascular regeneration merits further study
Sources of Funding
This work was supported by the National Institutes of Health (HL1133254 and HL148338); the George and Angelina Kostas Research Center For Cardiovascular Medicine; and the Robert J. Kleberg, Jr. and Helen C. Kleberg Foundation
Nonstandard Abbreviations and Acronyms
- PAD
peripheral artery disease
- IC
intermittent claudication
- CLI
critical limb ischemia
- MACE
major adverse cardiovascular events
- EC
endothelial cell
- EPC
endothelial progenitor cell
- CAC
circulating angiogenic cell
- RCT
randomized controlled trial
- BM MNC
bone marrow derived mononuclear cell
- MSC
mesenchymal stem cell
- EndoMT
endothelial-to-mesenchyme transition
- PRR
pattern recognition receptor
- DAMP
damage-associated molecular pattern
- PAMP
pathogen-associated molecular pattern
Footnotes
Disclosures
Stanford University is the assignee, and JPC is one of the inventors, on patents related to therapeutic modulation of innate immunity.
References
- 1.Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ, Mensah GA, Criqui MH. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: A systematic review and analysis. Lancet. 2013;382:1329–1340 [DOI] [PubMed] [Google Scholar]
- 2.Nead KT, Cooke JP, Olin JW, Leeper NJ. Alternative ankle-brachial index method identifies additional at-risk individuals. J Am Coll Cardiol. 2013;62:553–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allison MA, Ho E, Denenberg JO, Langer RD, Newman AB, Fabsitz RR, Criqui MH. Ethnic-specific prevalence of peripheral arterial disease in the united states. Am J Prev Med. 2007;32:328–333 [DOI] [PubMed] [Google Scholar]
- 4.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics−−2012 update: A report from the american heart association. Circulation. 2012;125:e2–e220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics-2018 update: A report from the american heart association. Circulation. 2018;137:e67–e492 [DOI] [PubMed] [Google Scholar]
- 6.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the united states: Results from the national health and nutrition examination survey, 1999–2000. Circulation. 2004;110:738–743 [DOI] [PubMed] [Google Scholar]
- 7.Leeper NJ, Myers J, Zhou M, Nead KT, Syed A, Kojima Y, Caceres RD, Cooke JP. Exercise capacity is the strongest predictor of mortality in patients with peripheral arterial disease. J Vasc Surg. 2013;57:728–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schanzer A, Conte MS. Critical limb ischemia. Curr Treat Options Cardiovasc Med. 2010;12:214–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fowkes FG, Aboyans V, Fowkes FJ, McDermott MM, Sampson UK, Criqui MH. Peripheral artery disease: Epidemiology and global perspectives. Nat Rev Cardiol. 2017;14:156–170 [DOI] [PubMed] [Google Scholar]
- 10.Farber A, Eberhardt RT. The current state of critical limb ischemia: A systematic review. JAMA Surg. 2016;151:1070–1077 [DOI] [PubMed] [Google Scholar]
- 11.Olin JW, White CJ, Armstrong EJ, Kadian-Dodov D, Hiatt WR. Peripheral artery disease: Evolving role of exercise, medical therapy, and endovascular options. J Am Coll Cardiol. 2016;67:1338–1357 [DOI] [PubMed] [Google Scholar]
- 12.Ruffolo AJ, Romano M, Ciapponi A. Prostanoids for critical limb ischaemia. Cochrane Database Syst Rev. 2010:CD006544 [DOI] [PubMed] [Google Scholar]
- 13.Hussain MA, Al-Omran M, Creager MA, Anand SS, Verma S, Bhatt DL. Antithrombotic therapy for peripheral artery disease: Recent advances. J Am Coll Cardiol. 2018;71:2450–2467 [DOI] [PubMed] [Google Scholar]
- 14.Morcos R, Louka B, Tseng A, Misra S, McBane R, Esser H, Shamoun F. The evolving treatment of peripheral arterial disease through guideline-directed recommendations. J Clin Med. 2018;7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maxwell AJ, Schauble E, Bernstein D, Cooke JP. Limb blood flow during exercise is dependent on nitric oxide. Circulation. 1998;98:369–374 [DOI] [PubMed] [Google Scholar]
- 16.Cooke JP. Flow, no, and atherogenesis. Proc Natl Acad Sci U S A. 2003;100:768–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Statins Markel A. and peripheral arterial disease. Int Angiol. 2015;34:416–427 [PubMed] [Google Scholar]
- 18.Ribatti D, Crivellato E. “Sprouting angiogenesis”, a reappraisal. Developmental biology. 2012;372:157–165 [DOI] [PubMed] [Google Scholar]
- 19.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nature reviews. Molecular cell biology 2007;8:464–478 [DOI] [PubMed] [Google Scholar]
- 20.Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887 [DOI] [PubMed] [Google Scholar]
- 21.Mentzer SJ, Konerding MA. Intussusceptive angiogenesis: Expansion and remodeling of microvascular networks. Angiogenesis. 2014;17:499–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-pas heterodimer regulated by cellular o2 tension. Proc Natl Acad Sci U S A. 1995;92:5510–5514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Semenza GL. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu Rev Pathol. 2014;9:47–71 [DOI] [PubMed] [Google Scholar]
- 24.Qian HS, Liu P, Huw LY, et al. Effective treatment of vascular endothelial growth factor refractory hindlimb ischemia by a mutant endothelial nitric oxide synthase gene. Gene Ther. 2006;13:1342–1350 [DOI] [PubMed] [Google Scholar]
- 25.Ferraro B, Cruz YL, Baldwin M, Coppola D, Heller R. Increased perfusion and angiogenesis in a hindlimb ischemia model with plasmid fgf-2 delivered by noninvasive electroporation. Gene Ther. 2010;17:763–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morishita R, Nakamura S, Hayashi S, Taniyama Y, Moriguchi A, Nagano T, Taiji M, Noguchi H, Takeshita S, Matsumoto K, Nakamura T, Higaki J, Ogihara T. Therapeutic angiogenesis induced by human recombinant hepatocyte growth factor in rabbit hind limb ischemia model as cytokine supplement therapy. Hypertension. 1999;33:1379–1384 [DOI] [PubMed] [Google Scholar]
- 27.Banfi A, von Degenfeld G, Gianni-Barrera R, Reginato S, Merchant MJ, McDonald DM, Blau HM. Therapeutic angiogenesis due to balanced single-vector delivery of vegf and pdgf-bb. FASEB J. 2012;26:2486–2497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lekas M, Lekas P, Mei SH, Deng Y, Dumont DJ, Stewart DJ. Tie2-dependent neovascularization of the ischemic hindlimb is mediated by angiopoietin-2. PLoS One. 2012;7:e43568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lichtenauer M, Jung C. Tbx20 and the prok2-prokr1 pathway-new kid on the block in angiogenesis research. Ann Transl Med. 2018;6:S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrara N, Gerber HP, LeCouter J. The biology of vegf and its receptors. Nat Med. 2003;9:669–676 [DOI] [PubMed] [Google Scholar]
- 31.Huang NF, Niiyama H, Peter C, De A, Natkunam Y, Fleissner F, Li Z, Rollins MD, Wu JC, Gambhir SS, Cooke JP. Embryonic stem cell-derived endothelial cells engraft into the ischemic hindlimb and restore perfusion. Arterioscler Thromb Vasc Biol. 2010;30:984–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leeper NJ, Hunter AL, Cooke JP. Stem cell therapy for vascular regeneration: Adult, embryonic, and induced pluripotent stem cells. Circulation. 2010;122:517–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frangogiannis NG. Cell therapy for peripheral artery disease. Curr Opin Pharmacol. 2018;39:27–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Royen N, Piek JJ, Buschmann I, Hoefer I, Voskuil M, Schaper W. Stimulation of arteriogenesis; a new concept for the treatment of arterial occlusive disease. Cardiovasc Res. 2001;49:543–553 [DOI] [PubMed] [Google Scholar]
- 35.Heil M, Eitenmuller I, Schmitz-Rixen T, Schaper W. Arteriogenesis versus angiogenesis: Similarities and differences. J Cell Mol Med. 2006;10:45–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buschmann I, Schaper W. The pathophysiology of the collateral circulation (arteriogenesis). J Pathol. 2000;190:338–342 [DOI] [PubMed] [Google Scholar]
- 37.Wolf C, Cai WJ, Vosschulte R, Koltai S, Mousavipour D, Scholz D, Afsah-Hedjri A, Schaper W, Schaper J. Vascular remodeling and altered protein expression during growth of coronary collateral arteries. J Mol Cell Cardiol. 1998;30:2291–2305 [DOI] [PubMed] [Google Scholar]
- 38.Cai WJ, Koltai S, Kocsis E, Scholz D, Kostin S, Luo X, Schaper W, Schaper J. Remodeling of the adventitia during coronary arteriogenesis. Am J Physiol Heart Circ Physiol. 2003;284:H31–40 [DOI] [PubMed] [Google Scholar]
- 39.Schaper J, Konig R, Franz D, Schaper W. The endothelial surface of growing coronary collateral arteries. Intimal margination and diapedesis of monocytes. A combined sem and tem study. Virchows Arch A Pathol Anat Histol. 1976;370:193–205 [DOI] [PubMed] [Google Scholar]
- 40.Arras M, Ito WD, Scholz D, Winkler B, Schaper J, Schaper W. Monocyte activation in angiogenesis and collateral growth in the rabbit hindlimb. J Clin Invest. 1998;101:40–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scholz D, Ito W, Fleming I, Deindl E, Sauer A, Wiesnet M, Busse R, Schaper J, Schaper W. Ultrastructure and molecular histology of rabbit hind-limb collateral artery growth (arteriogenesis). Virchows Arch. 2000;436:257–270 [DOI] [PubMed] [Google Scholar]
- 42.Fung E, Helisch A. Macrophages in collateral arteriogenesis. Front Physiol. 2012;3:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heuslein JL, Meisner JK, Li X, Song J, Vincentelli H, Leiphart RJ, Ames EG, Blackman BR, Blackman BR, Price RJ. Mechanisms of amplified arteriogenesis in collateral artery segments exposed to reversed flow direction. Arterioscler Thromb Vasc Biol. 2015;35:2354–2365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heil M, Ziegelhoeffer T, Wagner S, Fernandez B, Helisch A, Martin S, Tribulova S, Kuziel WA, Bachmann G, Schaper W. Collateral artery growth (arteriogenesis) after experimental arterial occlusion is impaired in mice lacking cc-chemokine receptor-2. Circ Res. 2004;94:671–677 [DOI] [PubMed] [Google Scholar]
- 45.Krishnasamy K, Limbourg A, Kapanadze T, et al. Blood vessel control of macrophage maturation promotes arteriogenesis in ischemia. Nat Commun. 2017;8:952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zbinden S, Zbinden R, Meier P, Windecker S, Seiler C. Safety and efficacy of subcutaneous-only granulocyte-macrophage colony-stimulating factor for collateral growth promotion in patients with coronary artery disease. J Am Coll Cardiol. 2005;46:1636–1642 [DOI] [PubMed] [Google Scholar]
- 47.Meier P, Gloekler S, Oezdemir B, Indermuehle A, Traupe T, Vogel R, de Marchi S, Seiler C. G-csf induced arteriogenesis in humans: Molecular insights into a randomized controlled trial. Current Vascular Pharmacology. 2013;11:38–46 [PubMed] [Google Scholar]
- 48.Grundmann S, Hoefer I, Ulusans S, Bode C, Oesterle S, Tijssen JG, Piek JJ, Buschmann I, van Royen N. Granulocyte-macrophage colony-stimulating factor stimulates arteriogenesis in a pig model of peripheral artery disease using clinically applicable infusion pumps. J Vasc Surg. 2006;43:1263–1269 [DOI] [PubMed] [Google Scholar]
- 49.Meier P, Gloekler S, de Marchi SF, Indermuehle A, Rutz T, Traupe T, Steck H, Vogel R, Seiler C. Myocardial salvage through coronary collateral growth by granulocyte colony-stimulating factor in chronic coronary artery disease: A controlled randomized trial. Circulation. 2009;120:1355–1363 [DOI] [PubMed] [Google Scholar]
- 50.McEnaney RM, Shukla A, Madigan MC, Sachdev U, Tzeng E. P2y2 nucleotide receptor mediates arteriogenesis in a murine model of hind limb ischemia. J Vasc Surg. 2016;63:216–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schaper W, Gorge G, Winkler B, Schaper J. The collateral circulation of the heart. Prog Cardiovasc Dis. 1988;31:57–77 [DOI] [PubMed] [Google Scholar]
- 52.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967 [DOI] [PubMed] [Google Scholar]
- 53.Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, Inai Y, Silver M, Isner JM. Vegf contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999;18:3964–3972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through hif-1 induction of sdf-1. Nat Med. 2004;10:858–864 [DOI] [PubMed] [Google Scholar]
- 55.Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo DW, Isner JM, Asahara T. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–1328 [DOI] [PubMed] [Google Scholar]
- 56.Kovacic JC, Muller DW, Graham RM. Actions and therapeutic potential of g-csf and gm-csf in cardiovascular disease. J Mol Cell Cardiol. 2007;42:19–33 [DOI] [PubMed] [Google Scholar]
- 57.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438 [DOI] [PubMed] [Google Scholar]
- 58.Zhang J, Li Y, Li H, Zhu B, Wang L, Guo B, Xiang L, Dong J, Liu M, Xiang G. Gdf11 improves angiogenic function of epcs in diabetic limb ischemia. Diabetes. 2018;67:2084–2095 [DOI] [PubMed] [Google Scholar]
- 59.Chang TT, Lin LY, Chen JW. Inhibition of macrophage inflammatory protein-1beta improves endothelial progenitor cell function and ischemia-induced angiogenesis in diabetes. Angiogenesis. 2019;22:53–65 [DOI] [PubMed] [Google Scholar]
- 60.Yoder MC. Defining human endothelial progenitor cells. J Thromb Haemost. 2009;7 Suppl 1:49–52 [DOI] [PubMed] [Google Scholar]
- 61.Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2008;28:1584–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–2760 [DOI] [PubMed] [Google Scholar]
- 63.Cooke JP, Losordo DW. Modulating the vascular response to limb ischemia: Angiogenic and cell therapies. Circ Res. 2015;116:1561–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Jung S, Chimenti S, Landsman L, Abramovitch R, Keshet E. Vegf-induced adult neovascularization: Recruitment, retention, and role of accessory cells. Cell. 2006;124:175–189 [DOI] [PubMed] [Google Scholar]
- 65.Yoon CH, Hur J, Park KW, Kim JH, Lee CS, Oh IY, Kim TY, Cho HJ, Kang HJ, Chae IH, Yang HK, Oh BH, Park YB, Kim HS. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: The role of angiogenic cytokines and matrix metalloproteinases. Circulation. 2005;112:1618–1627 [DOI] [PubMed] [Google Scholar]
- 66.Bababeygy SR, Cheshier SH, Hou LC, Higgins DMO, Weissman IL, Tse VCK. Hematopoietic stem cell-derived pericytic cells in brain tumor angio-architecture. Stem Cells and Development. 2008;17:11–18 [DOI] [PubMed] [Google Scholar]
- 67.Drake CJ. Embryonic and adult vasculogenesis. Birth defects research. Part C, Embryo today : reviews. 2003;69:73–82 [DOI] [PubMed] [Google Scholar]
- 68.Aicher A, Rentsch M, Sasaki K, Ellwart JW, Fandrich F, Siebert R, Cooke JP, Dimmeler S, Heeschen C. Nonbone marrow-derived circulating progenitor cells contribute to postnatal neovascularization following tissue ischemia. Circ Res. 2007;100:581–589 [DOI] [PubMed] [Google Scholar]
- 69.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600 [DOI] [PubMed] [Google Scholar]
- 70.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Bohm M, Nickenig G. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007 [DOI] [PubMed] [Google Scholar]
- 71.Rehman J, Li J, Parvathaneni L, Karlsson G, Panchal VR, Temm CJ, Mahenthiran J, March KL. Exercise acutely increases circulating endothelial progenitor cells and monocyte-/macrophage-derived angiogenic cells. J Am Coll Cardiol. 2004;43:2314–2318 [DOI] [PubMed] [Google Scholar]
- 72.Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164–1169 [DOI] [PubMed] [Google Scholar]
- 73.Heeschen C, Aicher A, Fandrich F, Cooke JP, Dimmeler S. Non-bone marrow-derived circulating progenitor cells contribute to postnatal neovascularization following tissue ischemia. Journal of Vascular Research. 2006;43:559–559 [DOI] [PubMed] [Google Scholar]
- 74.Makinen K, Manninen H, Hedman M, Matsi P, Mussalo H, Alhava E, Yla-Herttuala S. Increased vascularity detected by digital subtraction angiography after vegf gene transfer to human lower limb artery: A randomized, placebo-controlled, double-blinded phase ii study. Mol Ther. 2002;6:127–133 [DOI] [PubMed] [Google Scholar]
- 75.Rajagopalan S, Mohler ER 3rd, Lederman RJ, Mendelsohn FO, Saucedo JF, Goldman CK, Blebea J, Macko J, Kessler PD, Rasmussen HS, Annex BH. Regional angiogenesis with vascular endothelial growth factor in peripheral arterial disease: A phase ii randomized, double-blind, controlled study of adenoviral delivery of vascular endothelial growth factor 121 in patients with disabling intermittent claudication. Circulation. 2003;108:1933–1938 [DOI] [PubMed] [Google Scholar]
- 76.Kusumanto YH, van Weel V, Mulder NH, Smit AJ, van den Dungen JJ, Hooymans JM, Sluiter WJ, Tio RA, Quax PH, Gans RO, Dullaart RP, Hospers GA. Treatment with intramuscular vascular endothelial growth factor gene compared with placebo for patients with diabetes mellitus and critical limb ischemia: A double-blind randomized trial. Hum Gene Ther. 2006;17:683–691 [DOI] [PubMed] [Google Scholar]
- 77.Deev RV, Bozo IY, Mzhavanadze ND, Voronov DA, Gavrilenko AV, Chervyakov YV, Staroverov IN, Kalinin RE, Shvalb PG, Isaev AA. Pcmv-vegf165 intramuscular gene transfer is an effective method of treatment for patients with chronic lower limb ischemia. J Cardiovasc Pharmacol Ther. 2015;20:473–482 [DOI] [PubMed] [Google Scholar]
- 78.Belch J, Hiatt WR, Baumgartner I, Driver IV, Nikol S, Norgren L, Van Belle E, Committees T, Investigators. Effect of fibroblast growth factor nv1fgf on amputation and death: A randomised placebo-controlled trial of gene therapy in critical limb ischaemia. Lancet. 2011;377:1929–1937 [DOI] [PubMed] [Google Scholar]
- 79.Powell RJ, Goodney P, Mendelsohn FO, Moen EK, Annex BH, Investigators HGFT. Safety and efficacy of patient specific intramuscular injection of hgf plasmid gene therapy on limb perfusion and wound healing in patients with ischemic lower extremity ulceration: Results of the hgf-0205 trial. J Vasc Surg. 2010;52:1525–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shishehbor MH, Rundback J, Bunte M, Hammad TA, Miller L, Patel PD, Sadanandan S, Fitzgerald M, Pastore J, Kashyap V, Henry TD. Sdf-1 plasmid treatment for patients with peripheral artery disease (stop-pad): Randomized, double-blind, placebo-controlled clinical trial. Vasc Med. 2019;24:200–207 [DOI] [PubMed] [Google Scholar]
- 81.Tateishi-Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, Masaki H, Amano K, Kishimoto Y, Yoshimoto K, Akashi H, Shimada K, Iwasaka T, Imaizumi T, Therapeutic Angiogenesis using Cell Transplantation Study I. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: A pilot study and a randomised controlled trial. Lancet. 2002;360:427–435 [DOI] [PubMed] [Google Scholar]
- 82.Qadura M, Terenzi DC, Verma S, Al-Omran M, Hess DA. Concise review: Cell therapy for critical limb ischemia: An integrated review of preclinical and clinical studies. Stem Cells. 2018;36:161–171 [DOI] [PubMed] [Google Scholar]
- 83.Iyer SR, Annex BH. Therapeutic angiogenesis for peripheral artery disease: Lessons learned in translational science. JACC Basic Transl Sci. 2017;2:503–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pignon B, Sevestre MA, Kanagaratnam L, Pernod G, Stephan D, Emmerich J, Clement C, Sarlon G, Boulon C, Tournois C, Nguyen P. Autologous bone marrow mononuclear cell implantation and its impact on the outcome of patients with critical limb ischemia- results of a randomized, double-blind, placebo-controlled trial. Circ J. 2017;81:1713–1720 [DOI] [PubMed] [Google Scholar]
- 85.Bura A, Planat-Benard V, Bourin P, Silvestre JS, Gross F, Grolleau JL, Saint-Lebese B, Peyrafitte JA, Fleury S, Gadelorge M, Taurand M, Dupuis-Coronas S, Leobon B, Casteilla L. Phase i trial: The use of autologous cultured adipose-derived stroma/stem cells to treat patients with non-revascularizable critical limb ischemia. Cytotherapy. 2014;16:245–257 [DOI] [PubMed] [Google Scholar]
- 86.Yang SS, Kim NR, Park KB, Do YS, Roh K, Kang KS, Kim DI. A phase i study of human cord blood-derived mesenchymal stem cell therapy in patients with peripheral arterial occlusive disease. Int J Stem Cells. 2013;6:37–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Peeters Weem SM, Teraa M, de Borst GJ, Verhaar MC, Moll FL. Bone marrow derived cell therapy in critical limb ischemia: A meta-analysis of randomized placebo controlled trials. Eur J Vasc Endovasc Surg. 2015;50:775–783 [DOI] [PubMed] [Google Scholar]
- 88.Rigato M, Monami M, Fadini GP. Autologous cell therapy for peripheral arterial disease: Systematic review and meta-analysis of randomized, nonrandomized, and noncontrolled studies. Circ Res. 2017;120:1326–1340 [DOI] [PubMed] [Google Scholar]
- 89.Simons M, Alitalo K, Annex BH, et al. State-of-the-art methods for evaluation of angiogenesis and tissue vascularization: A scientific statement from the american heart association. Circ Res. 2015;116:e99–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Madaric J, Klepanec A, Valachovicova M, Mistrik M, Bucova M, Olejarova I, Necpal R, Madaricova T, Paulis L, Vulev I. Characteristics of responders to autologous bone marrow cell therapy for no-option critical limb ischemia. Stem Cell Res Ther. 2016;7:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mathiyalagan P, Liang Y, Kim D, Misener S, Thorne T, Kamide CE, Klyachko E, Losordo DW, Hajjar RJ, Sahoo S. Angiogenic mechanisms of human cd34(+) stem cell exosomes in the repair of ischemic hindlimb. Circ Res. 2017;120:1466–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Johnson TK, Zhao L, Zhu D, Wang Y, Xiao Y, Oguljahan B, Zhao X, Kirlin WG, Yin L, Chilian WM, Liu D. Exosomes derived from induced vascular progenitor cells promote angiogenesis in vitro and in an in vivo rat hindlimb ischemia model. Am J Physiol Heart Circ Physiol. 2019;317:H765–H776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gonzalez-King H, Garcia NA, Ontoria-Oviedo I, Ciria M, Montero JA, Sepulveda P. Hypoxia inducible factor-1alpha potentiates jagged 1-mediated angiogenesis by mesenchymal stem cell-derived exosomes. Stem Cells. 2017;35:1747–1759 [DOI] [PubMed] [Google Scholar]
- 94.Vagnozzi RJ, Maillet M, Sargent MA, Khalil H, Johansen AKZ, Schwanekamp JA, York AJ, Huang V, Nahrendorf M, Sadayappan S, Molkentin JD. An acute immune response underlies the benefit of cardiac stem cell therapy. Nature. 2020;577:405–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–961 [DOI] [PubMed] [Google Scholar]
- 96.Kovacic JC, Mercader N, Torres M, Boehm M, Fuster V. Epithelial-to-mesenchymal and endothelial-to-mesenchymal transition: From cardiovascular development to disease. Circulation. 2012;125:1795–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ubil E, Duan J, Pillai IC, Rosa-Garrido M, Wu Y, Bargiacchi F, Lu Y, Stanbouly S, Huang J, Rojas M, Vondriska TM, Stefani E, Deb A. Mesenchymal-endothelial transition contributes to cardiac neovascularization. Nature. 2014;514:585–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.He L, Huang X, Kanisicak O, et al. Preexisting endothelial cells mediate cardiac neovascularization after injury. J Clin Invest. 2017;127:2968–2981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sayed N, Wong WT, Ospino F, Meng S, Lee J, Jha A, Dexheimer P, Aronow BJ, Cooke JP. Transdifferentiation of human fibroblasts to endothelial cells: Role of innate immunity. Circulation. 2015;131:300–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Meng S, Zhou G, Gu Q, Chanda PK, Ospino F, Cooke JP. Transdifferentiation requires inos activation: Role of ring1a s-nitrosylation. Circ Res. 2016;119:e129–e138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wong WT, Cooke JP. Therapeutic transdifferentiation of human fibroblasts into endothelial cells using forced expression of lineage-specific transcription factors. J Tissue Eng. 2016;7:2041731416628329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lai L, Reineke E, Hamilton DJ, Cooke JP. Glycolytic switch is required for transdifferentiation to endothelial lineage. Circulation. 2019;139:119–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee J, Sayed N, Hunter A, Au KF, Wong WH, Mocarski ES, Pera RR, Yakubov E, Cooke JP. Activation of innate immunity is required for efficient nuclear reprogramming. Cell. 2012;151:547–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhou G, Meng S, Li Y, Ghebre YT, Cooke JP. Optimal ros signaling is critical for nuclear reprogramming. Cell Rep. 2016;15:919–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sayed N, Ospino F, Himmati F, Lee J, Chanda P, Mocarski ES, Cooke JP. Retinoic acid inducible gene 1 protein (rig1)‐like receptor pathway is required for efficient nuclear reprogramming. Stem cells. 2017;35:1197–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chanda PK, Meng S, Lee J, Leung HE, Chen K, Cooke JP. Nuclear s-nitrosylation defines an optimal zone for inducing pluripotency. Circulation. 2019;140:1081–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cooke JP. Therapeutic transdifferentiation: A novel approach for vascular disease. Circ Res. 2013;112:748–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cooke JP. Inflammation and its role in regeneration and repair. Circ Res. 2019;124:1166–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Morishita R, Makino H, Aoki M, Hashiya N, Yamasaki K, Azuma J, Taniyama Y, Sawa Y, Kaneda Y, Ogihara T. Phase i/iia clinical trial of therapeutic angiogenesis using hepatocyte growth factor gene transfer to treat critical limb ischemia. Arterioscler Thromb Vasc Biol. 2011;31:713–720 [DOI] [PubMed] [Google Scholar]
- 110.Jonsson TB, Larzon T, Arfvidsson B, Tidefelt U, Axelsson CG, Jurstrand M, Norgren L. Adverse events during treatment of critical limb ischemia with autologous peripheral blood mononuclear cell implant. Int Angiol. 2012;31:77–84 [PubMed] [Google Scholar]
- 111.Moazzami K, Majdzadeh R, Nedjat S. Local intramuscular transplantation of autologous mononuclear cells for critical lower limb ischaemia. Cochrane Database Syst Rev. 2011:CD008347 [DOI] [PubMed] [Google Scholar]


