Abstract
Objective:
Sweet syndrome (SS) is characterized by an inflammatory rash that has been associated with a number of drugs and malignant, inflammatory, and infectious conditions. Rare accounts of Hashimoto thyroiditis (HT) presenting with SS exist in the literature. HT is usually identified after the onset of skin lesions and without signs of overt thyroid dysfunction, and the stage of thyroid disease stage at presentation is variable.
Methods:
A search of the PubMed database was performed using search criteria involving combinations of “Sweet syndrome” and “Hashimoto thyroiditis,” “autoimmune thyroiditis,” or “thyroiditis,” and the search was filtered for clinical case reports. Five case reports were identified to describe the coexistence of Sweet syndrome and Hashimoto thyroiditis, and full-text versions of these reports were obtained and reviewed. Of note, cases involving subacute or other types of thyroiditis were excluded.
Results:
A 57-year-old man presented with painful eruptions on his hands; he was initially treated with antibiotics for presumed cellulitis without relief. Skin biopsy later confirmed SS and subsequent workup identified underlying HT with an elevated thyroid-stimulating hormone of 19.24 mU/L (normal, 0.30 to 4.30 mU/L) and positive thyroid peroxidase (TPO) antibody at 236.4 IU/mL.
Conclusion:
Thyroid function tests should be universally evaluated in the workup of SS, and it may be appropriate to test for TPO antibodies even in the absence of objective thyroid dysfunction. Both SS and HT show immune diathesis, so further work should be undertaken to establish whether a common immunologic trigger exists.
INTRODUCTION
Sweet syndrome (SS) is characterized by the abrupt onset of tender erythematous skin lesions and is typically associated with fever, neutrophilia, and elevated inflammatory markers (1). A number of drugs and malignant, inflammatory, and infectious conditions have been associated with SS, with inflammatory disorders such as inflammatory bowel disease being reported in up to 33% of cases (2). Rare accounts of Hashimoto thyroiditis (HT) occurring with SS exist in the literature. We report the case of a man who presented with painful eruptions on his hands who was initially treated with antibiotics for presumed cellulitis without relief. A skin biopsy later confirmed SS and a subsequent workup identified underlying HT. This case adds to a growing body of information that may lead to a better characterization and understanding of the relationship between SS and thyroiditis.
CASE REPORT
A 57-year-old man with no known past medical history was admitted to the hospital with 5 days of swelling and erythematous, tender lesions on the dorsal surfaces of both hands (Fig. 1). His recent history included a snowmobiling trip during which he wore damp leather gloves and spent time in a hot tub. He also worked as a construction worker and had recently finished tiling a bathroom. Following these exposures, he first noticed a small “pimple” on his right posterior thumb which then spread to involve multiple digits on both hands. He was seen at two outside clinics where he was treated with antibiotics for presumed cellulitis without relief. He did not have any history of weight change, heat or cold intolerance, constipation or diarrhea, fatigue, tremor, or palpitations.
Fig. 1.
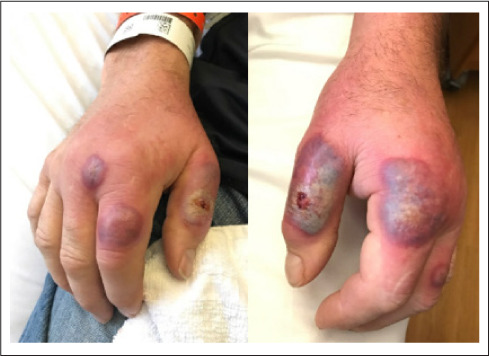
Hand exam on admission.
On presentation, he was afebrile with a physical exam notable only for the rash as shown in Fig. 1. Thyromegaly was not noted. Laboratory results showed a leukocytosis with neutrophilic predominance. He was started on broad spectrum antibiotics but showed persistent leukocytosis, worsening pain, and progressive erythema and swelling on hospital day 2. On hospital day 3 orthopedics was consulted and irrigation and debridement of suspected abscesses was performed, but no purulence was noted. Over the following days, his leukocytosis resolved but his hand lesions worsened (Fig. 2).
Fig. 2.
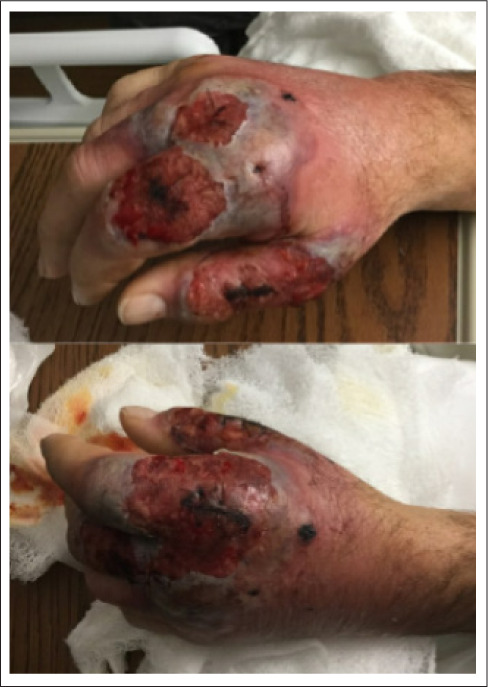
Hand exam following irrigation and debridement.
Dermatology was consulted and a skin biopsy revealed neutrophilic dermatosis consistent with SS. Further evaluation for conditions associated with SS showed an elevated erythrocyte sedimentation rate and C-reactive protein, an elevated thyroid-stimulating hormone of 19.24 mU/L (normal, 0.30 to 4.30 mU/L), and a low free thyroxine of 0.6 ng/dL (normal, 0.8 to 1.6 ng/dL). A chest X-ray (to evaluate for sarcoidosis) incidentally suggested an enlarged thyroid, and a thyroid ultrasound (US) later revealed thyroiditis and thyromegaly. Subsequent thyroid peroxidase (TPO) antibody was positive at 236.4 IU/mL, confirming the diagnosis of HT.
Following diagnosis, the patient was started on oral and topical steroids with rapid resolution of the skin lesions. He was also started on levothyroxine and discharged with a close follow-up in place. At his 1-week dermatology follow-up, his hands continued to demonstrate further improvement (Fig. 3).
Fig. 3.
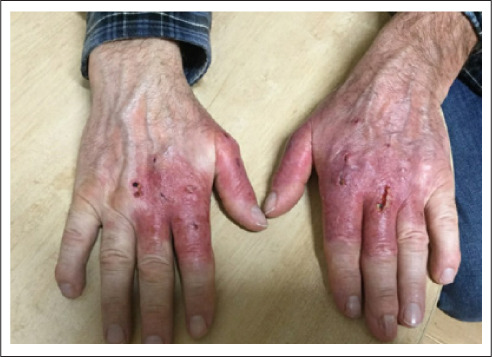
One-week dermatology follow-up after continued steroid therapy.
DISCUSSION
SS is a collection of findings characterized by an inflammatory rash and has been associated with a wide variety of conditions (1). HT is an autoimmune condition that has now been described on multiple occasions to accompany a diagnosis of SS. The exact relationship between these 2 conditions has yet to be objectively characterized, but previous reports in addition to the case described here seem to suggest that the dermatologic onset of SS precedes clinical signs of HT. It is unclear whether the conditions share a pathologic trigger or if perhaps the onset of SS provides the immunologic stimulus for HT.
SS has a multifactorial pathogenesis impacted by elements of hypersensitivity, pathergy, cytokine dysregulation, and a potential underlying genetic susceptibility (2). Immunohistochemical studies of SS have suggested that T helper type 1 (Th1) cells play a more pivotal role than T helper type 2 cells in disease causation. Similarly, T-helper cells have long been thought to be implicated in the pathogenesis of HT, and a recent study proposed a correlation between the predominant type of T-helper cell and the stage of HT (3). This study found that proportions of Th1 cells were significantly higher with overt hypothyroidism compared to subclinical hypothyroidism, suggesting that a predominance of Th1 cells in SS may provide an impetus to push a susceptible individual into clinical thyroid disease.
Per our literature review, 5 coexisting cases of HT and SS have been previously reported (4–8). While the clinical presentations of these cases varied (Table 1), they all occurred in women presenting with the characteristic skin lesions and all but 1 case had either subjective or objective fever. Furthermore, the corresponding thyroid dysfunction was most often discovered incidentally during thorough workup after the onset of skin lesions and was not suggested by clinical symptoms. One case of a patient hospitalized with SS was reported to have normal thyroid function laboratory results on the second day of hospitalization, but subsequently had a thyroid function panel diagnostic of HT, suggesting the possibility of a temporal relationship between the onset of SS and development of HT (8). The cases also presented a variability of thyroid disease stage at presentation, ranging from subclinical disease to overt hypothyroidism or hyperthyroidism. Four of the 5 cases were noted to have a goiter identifiable by physical exam or US. Of note, at least 1 case reported resolution of fever and improvement of skin lesions within 5 days, and normalization of thyroid labs and a decrease in anti-TPO after 1 month of corticosteroid treatment (8).
Table 1.
Literature Review of Previously Reported Cases of Sweet Syndrome and Hashimoto Thyroiditis
| Article (Ref #) | Clinical Course | Therapy & Resolution | Country | |
|---|---|---|---|---|
| 1 | Nakayama et al (4) | 39-year-old female. Subclinical phase. |
Prednisolone 50 mg daily, rapid resolution, duration unavailable. | Japan |
| 2 | Medeiros et al (5) | 65-year-old female. SS onset with HT discovered on further workup. Subclinical phase. |
Prednisolone 30 mg daily, rapid resolution, duration unavailable. Levothyroxine continued. No recurrence at 24 months. |
Portugal |
| 3 | Francisco et al (6) | 48-year-old female. Goiter 30 years prior to SS onset. Hypothyroid phase. |
Skin lesions resolved with colchicine 1.5 μg daily within 1 week. Recurrent lesions occurred. No recurrence after definitive total thyroidectomy. Levothyroxine continued. |
Philippines |
| 4 | Saeed et al (7) | 48-year-old female. Hypothyroid phase. |
Prednisone 40 mg and levothyroxine continued. |
United States |
| 5 | Rattan R et al (8) | 52-year-old female. Initial TFTs normal, hyperthyroid on re-check. Hyperthyroid phase. |
Symptom resolution in 5 days on oral steroids. Thyroid function normal at 1 month follow-up with steroids alone. |
India |
Abbreviations: HT = Hashimoto thyroiditis; SS = sweet syndrome; TFT = thyroid function test.
Compared to the cases referenced above, the patient described here is the first male patient with SS and HT and also differed from most prior cases by the absence of fever. Thyroid dysfunction was similarly found incidentally on workup following the diagnosis of SS, and a thyroid US revealed a goiter, although a thyroid exam was noted to be unremarkable throughout the hospitalization.
The clinical presentation of this case was consistent with the previously described cases, exhibiting initial dermatologic findings and subsequent identification of HT without overt symptoms of thyroid dysfunction. We propose that thyroid function tests should be universally evaluated in the workup of SS to allow for a timely diagnosis and management of thyroid dysfunction. Because of the varying stages of HT evolution that have been identified with SS, it may be appropriate to test for TPO antibodies even in the absence of objective thyroid dysfunction. Given this variation in presentation, it is unclear from our analysis whether one condition precedes the other or if they occur concomitantly from a shared stimulus. Hyperthyroidism has been observed on at least one occasion to normalize with steroid treatment (8), supporting the possibility of a shared pathologic process that may have a common treatment strategy if initiated in the early stages of HT. Since our patient presented with hypothyroidism and was started on levothyroxine prior to discharge, it is unclear whether this same effect would have been observed in him since hypothyroidism reflects a later stage of HT. Our hope is that this case encourages further reporting of other similar cases so that more generalizations can be made about a possible association between SS and HT and the outcomes of adopting a common treatment strategy.
CONCLUSION
The coexistence of Sweet syndrome and Hashimoto thyroiditis appears to be a rare but clearly demonstrated occurrence in the medical literature. Further work should be undertaken to better characterize this correlation and establish whether a common immunologic trigger exists since both conditions exhibit immune diathesis. Finally, thyroid function tests should be universally evaluated in the workup of SS, and it may be appropriate to test for TPO antibodies even in the absence of objective thyroid dysfunction.
Abbreviations
- HT
Hashimoto thyroiditis
- SS
Sweet syndrome
- Th1
T helper type 1
- TPO
thyroid peroxidase
- US
ultrasound
Footnotes
DISCLOSURE
The authors have no multiplicity of interest to disclose.
REFERENCES
- 1.Cohen PR. Sweet's syndrome-a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34. doi: 10.1186/1750-1172-2-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nelson CA, Stephen S, Ashchyan HJ, James WD, Micheletti RG, Rosenbach M. Neutrophilic dermatoses: pathogenesis, sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018;79:987–1006. doi: 10.1016/j.jaad.2017.11.064. [DOI] [PubMed] [Google Scholar]
- 3.Guo Y, Zynat J, Xing S et al. Immunological changes of T helper cells in flow cytometer-sorted CD4+ T cells from patients with Hashimoto's thyroiditis. Exp Ther Med. 2018;15:3596–3602. doi: 10.3892/etm.2018.5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakayama H, Shimao S, Hamamoto T, Munemura C, Nakai A. Neutrophilic dermatosis of the face associated with aortitis syndrome and Hashimoto's thyroiditis. Acta Derm Venereol. 1993;73:380–381. doi: 10.2340/0001555573380381. [DOI] [PubMed] [Google Scholar]
- 5.Medeiros S, Santos R, Carneiro V, Estrela F. Sweet syndrome associated with Hashimoto thyroiditis. Dermatol Online J. 2008;14:10. [PubMed] [Google Scholar]
- 6.Francisco CRI, Patal PC, Cubillan EA, Isip-Tan IT. Sweet's syndrome associated with Hashimoto's thyroiditis. BMJ Case Rep. 2010;2011:bcr0220113921. doi: 10.1136/bcr.02.2011.3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saeed M, Brown GE, Agarwal A et al. Autoimmune clustering: sweet syndrome, Hashimoto thyroiditis, and psoriasis. J Clin Rheumatol. 2011;17:76–78. doi: 10.1097/RHU.0b013e31820e624f. [DOI] [PubMed] [Google Scholar]
- 8.Rattan R, Sharma A, Shanker V, Tegta GR, Verma GK. Transient autoimmune thyroiditis associated with Sweet's syndrome. Indian J Dermatol Venereol Leprol. 2016;82:112. doi: 10.4103/0378-6323.162323. [DOI] [PubMed] [Google Scholar]