Abstract
Objective:
Hypophysitis is an increasingly recognized adverse effect of immune checkpoint inhibitor (ICI) therapy for malignancy. However, the mechanisms through which ICIs induce hypophysitis are largely unknown. We aim to describe 2 cases of ICI-mediated hypophysitis and perform autoantibody profiling on serial samples from these patients to determine if common autoantibodies could be identified.
Methods:
We describe 2 cases of patients with metastatic urothelial cancer who received ICI therapy and subsequently developed severe fatigue, prompting a hormonal workup consistent with hypopituitarism. Patient 1 received the ICI ipilimumab (anti-cytotoxic T-lymphocyte-associated protein 4) and patient 2 received the ICI pembrolizumab (anti-programmed cell death protein 1). Both patients had serial seromic immune biomarker profiling using high-density protein arrays before and after developing hypophysitis. Once a common autoantibody was found, zinc finger CCHC-type containing 8 (ZCCHC8), we used immunohistochemistry to assess its presence in pituitary tissue.
Results:
Of a limited number of increased autoantibodies detected, those to ZCCHC8 were the only common antibodies to increase at least 3-fold post-hypophysitis in both patients. Using immunohistochemistry staining, we show for the first time that ZCCHC8 is expressed in pituitary gland tissue.
Conclusion:
Seromic profiling identified a common autoantibody, ZCCHC8, in 2 patients who developed hypophysitis on ICI therapy, and other serial autoantibody increases in each patient. These findings warrant validation in other cohorts to determine if the response is to self or tumor antigen, and may reveal novel insights into pituitary gland physiology and the pathogenesis of ICI-mediated hypophysitis.
INTRODUCTION
Hypophysitis is a rare, but increasingly recognized immune-mediated adverse event associated with immune checkpoint inhibitor (ICI) cancer therapy. By blocking the cytotoxic T-lymphocyte antigen 4 pathway as well as the programmed cell death protein 1 and programmed cell death ligand protein 1 checkpoint pathways, ICIs augment immune responses against cancer cells (1). ICIs also can induce an immune response to host cells, leading to immune-mediated adverse events such as hypophysitis (2). The incidence of hypophysitis in clinical trials has been reported as 0.5 to 18% (3).
The role of autoantibodies in patients with ICI-mediated hypophysitis is largely unknown. Autoantibodies are required to help T cells develop and have a possible pathogenic role in the development of hypophysitis and other ICI-mediated adverse events (1,2). Proteome arrays are a tool to profile thousands of autoantibodies against many unique human antigens simultaneously. These arrays identify patterns of autoantibody response against a large number of antigens during the course of development of diseases, such as autoimmunity or malignancy (4–6). Protoarray studies have shown that certain autoantibodies may predict ICI toxicity (7,8), but only one recent study has examined autoantibody changes in hypophysitis specifically, showing increases in the autoantibodies GNAL and ITM2B in 8 patients (including melanoma, prostate cancer, and renal cell carcinoma) (9).
In this report, our objective is to describe 2 cases of ICI-mediated hypophysitis, with one patient on ipilimumab (anti-cytotoxic T-lymphocyte-associated protein 4 monoclonal antibody) and the other patient on pembrolizumab (anti-programmed cell death protein 1 monoclonal antibody) and perform autoantibody profiling on serial samples from these patients to determine if common autoantibodies could be identified. Once common autoantibodies to the zinc finger CCHC-type containing 8 (ZCCHC8) protein were identified, we also aimed to determine if ZCCHC8 is expressed in pituitary tissue.
CASE REPORT
Case 1
A 75-year-old woman with a past history of metastatic urothelial carcinoma was enrolled in a clinical trial of gemcitabine, cisplatin, and ipilimumab as first line treatment for metastatic urothelial carcinoma (Fig. 1 A). Ipilimumab treatment (10 mg/kg) was started after receiving 6 weeks of treatment with gemcitabine and cisplatin. The patient received 8 weeks of ipilimumab with a computed tomography scan showing complete radiologic response of disease after completing treatment.
Fig. 1.
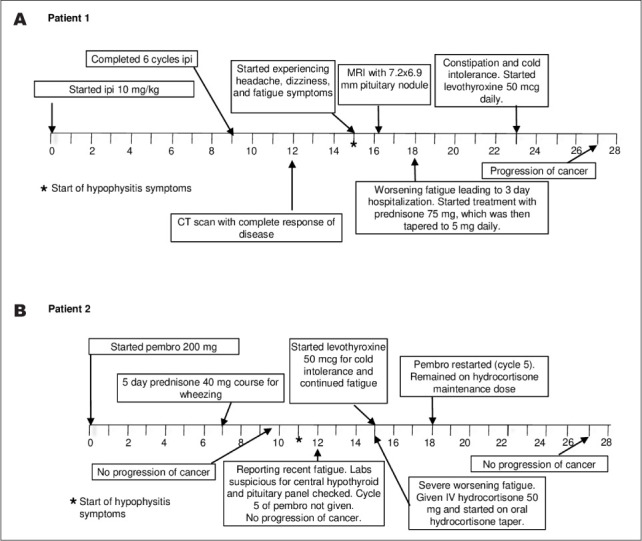
Timelines of the patients' events in weeks. (A) Timeline of patient 1's relevant clinical events after initiating ipilimimuab (ipi). (B) Timeline of patient 2's relevant clinical events after starting pembrolizumab (pembro).
One month after completing ipilimumab, the patient developed fatigue and headaches. These symptoms prompted a magnetic resonance imaging scan that showed a 7.2 × 6.9-mm enhancing pituitary nodule. Further work-up (Table 1) showed laboratory values that were consistent with hypopituitarism, including thyroid-stimulating hormone (TSH) of 0.07 IU/mL (reference range is 0.4 to 4.2 IU/mL), free thyroxine (fT4) of 1.07 ng/dL (reference range is 0.8 to 1.5 ng/dL), morning cortisol of 0.7 μg/dL (reference range is 6.7 to 22.6 μg/dL), adrenocorticotropic hormone <10 pg/mL (reference range is 0 to 46 pg/mL), follicle-stimulating hormone of 12.6 mIU/mL (reference range is 16.7 to 113.6 mIU/mL), luteinizing hormone of 5.1 mIU/mL (reference range is 10.9 to 58.6 mIU/mL), and prolactin of 22.7 ng/mL (reference range is 2.7 to 19.6 ng/mL). The patient was started on prednisone with improvement in symptoms. Approximately 5 weeks later, due to symptoms of fatigue and constipation, she was started on levothyroxine for hypothyroidism. Three months after developing hypophysitis, the patient's metastatic urothelial cancer progressed.
Table 1.
Patient 1 Pituitary Laboratory Values
| Reference range | 0 weeks | 9 weeks | 18 weeks | 23 weeks | |
|---|---|---|---|---|---|
| Thyroid-stimulating hormone (IU/mL) | 0.40–4.20 | 1.79 | 0.74 | 0.07 | 0.45 |
| Free thyroxine (ng/dL) | 0.8–1.5 | N/A | N/A | 1.00 | N/A |
| Total triiodothyronine (pg/mL) | 87–178 | N/A | N/A | 153 | N/A |
| Morning cortisol (μg/dL) | 6.7–22.6 | N/A | N/A | 0.7 | N/A |
| Adrenocorticotropic hormone (pg/mL) | 0–46 | N/A | N/A | <10 | <10 |
| Follicle-stimulating hormone (mIU/mL) | 16.7–113.6 | N/A | N/A | 12.6 | N/A |
| Luteinizing hormone (mIU/mL) | 10.9–58.6 | N/A | N/A | 5.1 | N/A |
| Prolactin (ng/mL) | 2.7–19.6 | N/A | N/A | 22.7 | N/A |
Abbreviation: N/A = not available.
Case 2
A 75-year-old man with a history of metastatic urothelial cancer progressive on chemotherapy received pembrolizumab (Fig. 1 B). After completing 4 cycles of pembrolizumab at 200 mg, the patient had no evidence of disease progression. TSH and fT4 levels at the time were suggestive of central hypothyroidism with TSH at 0.11 IU/mL and fT4 at 0.74 ng/dL. Laboratory workup at the time demonstrated hypogonadotropic hypogonadism (Table 2). Pituitary hormones were checked showing random cortisol of 21 μg/dL, ACTH at 54 pg/mL, total testosterone at 76.42 ng/dL (reference range is 300 to 180 ng/dL), follicle-stimulating hormone at 5.6 mIU/mL (reference range is 1.3 to 19.3 mIU/mL), luteinizing hormone at 2.21 mIU/mL (reference range is 1.2 to 8.6 mIU/mL), and prolactin at 2.9 ng/mL (reference range is 2.6 to 13.1 ng/mL).
Table 2.
Patient 2 Pituitary Laboratory Values
| Reference range | 0 weeks | 3 weeks | 9 weeks | 12 weeks | 12.5 weeks | 25 weeks | 27 weeks | |
|---|---|---|---|---|---|---|---|---|
| Thyroid-stimulating hormone (IU/mL) | 0.40–4.20 | 0.44 | 0.15 | 0.07 | 0.11 | N/A | 0.01 | N/A |
| Free thyroxine (ng/dL) | 0.80–1.50 | 0.92 | 0.82 | 0.69 | 0.74 | N/A | 0.92 | N/A |
| Free triiodothyronine (pg/mL) | 2.50–3.90 | 3.00 | 2.03 | 1.28 | 1.75 | N/A | 2.42 | N/A |
| Random cortisol (μg/dL) | 6.7–22.6 | N/A | N/A | N/A | N/A | 21.0 | 3.0 | N/A |
| Adrenocorticotropic hormone (pg/mL) | 0–46 | N/A | N/A | N/A | <10 | N/A | 13 | <5 |
| Follicle-stimulating hormone (mIU/mL) | 16.7–113.6 | N/A | N/A | N/A | N/A | 5.6 | N/A | N/A |
| Luteinizing hormone (mIU/mL) | 10.9–58.6 | N/A | N/A | N/A | N/A | 2.2 | N/A | N/A |
| Prolactin (ng/mL) | 2.7–19.6 | N/A | N/A | N/A | N/A | 2.9 | N/A | N/A |
| Testosterone (ng/dL) | 300–1080 | N/A | N/A | N/A | N/A | 76 | N/A | N/A |
Abbreviation: N/A = not available.
Three weeks later, the patient had severe fatigue and was found to have a random cortisol of 3 μg/dL, ACTH of 13 pg/mL, TSH of 0.008 IU/mL, and fT4 of 0.92 ng/dL. He started high-dose prednisone and levothyroxine. After fatigue symptoms resolved on treatment, he resumed pembrolizumab without further complications and without progression of urothelial cancer.
Materials and Methods
The patient samples used in this analysis were stored as part of a bladder cancer repository. Both patients provided informed consent to have their samples banked for research purposes via a protocol approved by our institutional review board (IRB # 10-1180).
Our laboratory previously validated protein array assays by comparing them to enzyme-linked immunosorbent assay antibody testing (10), as well as established normalization and calculation techniques (11). ProtoArray Human Protein Microarrays v5.1 (Thermo Fisher Scientific, Waltham, MA) were used to profile circulating antibodies against approximately 9,000 antigens (including controls) spotted in duplicate with the same methodology as done in prior studies by our laboratory (4). Median relative fluorescent unit values from the arrays were quantile normalized between the 3 time points available from each patient, as previously described with minor adjustments, namely without performing interquartile calculations (11). A fold difference >2.5 times baseline was considered significant. ZCCHC8 expression was evaluated by immunohistochemistry staining of normal pituitary glands, pituitary tumors, and testis (positive control) obtained at autopsy at our institution (IRB exempt). Antibodies used for staining were total ZCCHC8 (PA5-57969, Thermo Fisher Scientific) at 1:50 dilution. ZCCHC8 was detected using the rabbit HRP/DAB micro-polymer detection kit (Abcam, Cambridge, United Kingdom).
Results
Both patients had autoantibodies with significant fold change increases before and after hypophysitis. Patient 1 had increases in autoantibodies against 93 specific proteins between weeks 0 and 23 (Fig. 2 A and Table 3). Patient 2 had autoantibodies against 21 specific proteins between weeks 2 and 12 (Fig. 2 B and Table 4). Some autoantibodies also peaked prior to developing hypophysitis in both patients: 23 in patient 1 (Table 5) and 3 in patient 2 (Table 6). ZCCHC8 was the only common protein for which autoantibodies were found to be significantly increased after both patients developed hypophysitis. Of note, patient 1 had an increased response to control protein bovine serum albumin (BSA), though less than other reported antigens. In serum and plasma, >90% of analytes were similar and coefficients of variation for each patient were lower than the coefficients of variation observed when comparing plasma to each other or sera to each other, showing that the greater driving factor for reactivity was due to patient differences rather than specimen type (Fig. 3).
Fig. 2.
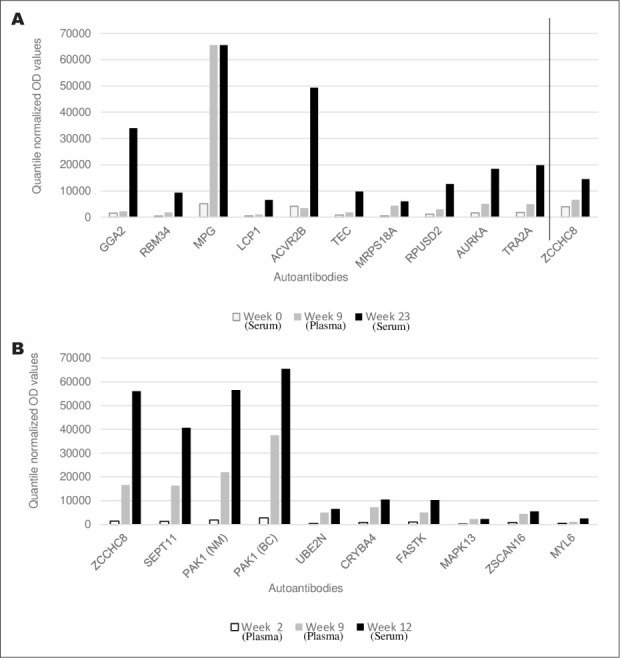
The 10 autoantibodies with the highest fold increases post-hypophysitis and ZCCHC8. (A) Quantile normalized fluorescent optical density values in patient 1 pre-hypophysitis (weeks 0 and 9) and post-hypophysitis (week 23). (B) Quantile normalized optical density values in patient 2 pre-hypophysitis (weeks 2 and 9) and post-hypophysitis (week 12).
Table 3.
Patient 1 Autoantibody Gene Loci with ≥2.5 Peak Fold Increases Post-Hypophysitis
| Quantile normalized optical density values | |||||
|---|---|---|---|---|---|
| Gene symbol | Week 0 | Week 9 | Week 23 | Ratio week 23 to week 0 | |
| BC000284.1 | GGA2 | 1571 | 2256 | 33890 | 21.6 |
| NM_015014.1 | RBM34 | 486 | 1853 | 9332 | 19.2 |
| BC014991.1 | MPG | 5159 | 65535 | 65535 | 12.7 |
| NM_002298.2 | LCP1 | 541 | 1038 | 6545 | 12.1 |
| BC096245.1 | ACVR2B | 4196 | 3428 | 49378 | 11.8 |
| NM_003215.1 | TEC | 859 | 1860 | 9777 | 11.4 |
| NM_018135.2 | MRPS18A | 548 | 4403 | 6083 | 11.1 |
| NM_152260.1 | RPUSD2 | 1139 | 3053 | 12619 | 11.1 |
| NM_003600.1 | AURKA | 1710 | 5088 | 18425 | 10.8 |
| NM_013293.1 | TRA2A | 1846 | 4948 | 19825 | 10.7 |
| BC096243.1 | ACVR2B | 1218 | 925 | 11859 | 9.7 |
| NM_003959.1 | HIP1R | 2324 | 8934 | 21092 | 9.1 |
| BC048299.1 | SPATS2 | 1471 | 6323 | 13304 | 9.0 |
| NM_001798.2 | CDK2 | 427 | 978 | 3771 | 8.8 |
| NM_016304.2 | C15orf15 | 1638 | 5552 | 14388 | 8.8 |
| BC034718.1 | EPB41L2 | 2078 | 7511 | 17692 | 8.5 |
| NM_015138.2 | RTF1 | 1267 | 973 | 10297 | 8.1 |
Table 3.
Continued
| Quantile normalized optical density values | |||||
|---|---|---|---|---|---|
| Gene symbol | Week 0 | Week 9 | Week 23 | Ratio week 23 to week 0 | |
| NM_021803.1 | IL21 | 668 | 1508 | 5100 | 7.6 |
| NM_000899.3 | KITLG | 1894 | 13873 | 14229 | 7.5 |
| BC011600.1 | - | 2193 | 14358 | 16358 | 7.5 |
| NM_002391.1 | MDK | 1076 | 2366 | 8003 | 7.4 |
| BC028059.1 | TPSAB1 | 1884 | 4789 | 13119 | 7.0 |
| NM_004216.2 | DEDD | 721 | 2318 | 4923 | 6.8 |
| NM_002904.4 | RDBP | 2951 | 14954 | 19733 | 6.7 |
| NM_198467.1 | RSBN1L | 483 | 1084 | 3189 | 6.6 |
| NM_032848.1 | C12orf52 | 2089 | 5222 | 13715 | 6.6 |
| NM_002378.2 | MATK | 872 | 3576 | 5657 | 6.5 |
| NM_012473.2 | TXN2 | 1526 | 4315 | 9799 | 6.4 |
| NM_005409.3 | CXCL11 | 1366 | 7748 | 8771 | 6.4 |
| AAH19931.1 | FCGR2A | 3060 | 7632 | 19487 | 6.4 |
| NM_032141.1 | CCDC55 | 2872 | 17943 | 17983 | 6.3 |
| NM_018553.1 | C17orf85 | 1126 | 2458 | 7045 | 6.3 |
| NM_080390.3 | TCEAL2 | 5821 | 10830 | 36228 | 6.2 |
| NM_003215.1 | TEC | 1913 | 3485 | 11148 | 5.8 |
| BC001304.1 | PCLO | 4089 | 16941 | 22119 | 5.4 |
| BC067085.1 | HIP1R | 5886 | 11949 | 31115 | 5.3 |
| BC070073.1 | ZNF365 | 847 | 3341 | 4330 | 5.1 |
| NM_033453.2 | ITPA | 988 | 4980 | 5039 | 5.1 |
| NM_001896.1 | CSNK2A2 | 1705 | 3317 | 8686 | 5.1 |
| NM_033293.1 | CASP1 | 256 | 411 | 1272 | 5.0 |
| NM_207480.1 | UNQ5830 | 1406 | 2997 | 6869 | 4.9 |
| BC040020.2 | SUHW2 | 2594 | 5657 | 12675 | 4.9 |
| BC013796.1 | AP2M1 | 540 | 1610 | 2612 | 4.8 |
| NM_012148.1 | DUX3 | 2112 | 3950 | 10126 | 4.8 |
| NP_000600.1 | CXCL12 | 2374 | 7089 | 11170 | 4.7 |
| BC059174.1 | TRAF3IP1 | 2191 | 14878 | 10280 | 4.7 |
| BC000770.1 | DIDO1 | 3298 | 5904 | 15443 | 4.7 |
| NM_022551.2 | RPS18 | 1028 | 2103 | 4733 | 4.6 |
| BC005043.1 | TNFRSF10C | 3860 | 12750 | 17339 | 4.5 |
| BC003049.1 | SERBP1 | 3382 | 11276 | 15171 | 4.5 |
| NM_014481.2 | APEX2 | 9890 | 39513 | 44263 | 4.5 |
| NM_006799.2 | PRSS21 | 592 | 1854 | 2645 | 4.5 |
| NM_003677.3 | DENR | 637 | 1149 | 2837 | 4.5 |
| NM_000975.2 | RPL11 | 589 | 1663 | 2617 | 4.4 |
| BC000903.2 | HMGB2 | 1058 | 2242 | 4638 | 4.4 |
| NM_004728.2 | DDX21 | 1617 | 24396 | 7089 | 4.4 |
| NM_002690.1 | POLB | 1080 | 2603 | 4712 | 4.4 |
| NM_207285.1 | AAA1 | 3311 | 6228 | 14439 | 4.4 |
| NM_012341.2 | GTPBP4 | 1487 | 5111 | 6472 | 4.4 |
| NM_031417.1 | MARK4 | 791 | 2927 | 3402 | 4.3 |
| BC023569.1 | UPF3A | 2724 | 5106 | 11702 | 4.3 |
Table 3.
Continued
| Quantile normalized optical density values | |||||
|---|---|---|---|---|---|
| Gene symbol | Week 0 | Week 9 | Week 23 | Ratio week 23 to week 0 | |
| BC093990.1 | SUDS3 | 3738 | 4843 | 15965 | 4.3 |
| BC060845.1 | L3MBTL3 | 566 | 603 | 2364 | 4.2 |
| BC020555.1 | SERBP1 | 2779 | 8792 | 11616 | 4.2 |
| NM_001029.2 | RPS26 | 978 | 1072 | 4075 | 4.2 |
| BC011842.2 | FLJ11184 | 1831 | 5767 | 7621 | 4.2 |
| BC014774.1 | BAG1 | 1210 | 1601 | 4926 | 4.1 |
| BC032508.1 | FLJ10781 | 1035 | 1817 | 4196 | 4.1 |
| NM_006275.2 | SFRS6 | 711 | 1153 | 2883 | 4.1 |
| BC015505.1 | DDX42 | 3751 | 6554 | 15150 | 4.0 |
| NM_080548.1 | PTPN6 | 1116 | 2235 | 4479 | 4.0 |
| BC013005.2 | IK | 482 | 1278 | 1921 | 4.0 |
| NM_133336.1 | WHSC1 | 471 | 1398 | 1874 | 4.0 |
| BC010907.1 | PAK1IP1 | 710 | 1329 | 2822 | 4.0 |
| NM_198081.1 | SCML4 | 1053 | 5168 | 4125 | 3.9 |
| BC029796.1 | LOC116349 | 5168 | 8927 | 19970 | 3.9 |
| NM_015634.2 | KIAA1279 | 920 | 2971 | 3543 | 3.9 |
| BC096708.1 | WIT1 | 4280 | 7533 | 16256 | 3.8 |
| NM_002103.3 | GYS1 | 1247 | 4040 | 4671 | 3.7 |
| NM_014303.2 | PES1 | 1448 | 4542 | 5394 | 3.7 |
| NM_017612.1 | ZCCHC8 | 3970 | 6640 | 14505 | 3.7 |
| NM_006658.1 | C7orf16 | 4176 | 10482 | 15215 | 3.6 |
| NM_199124.1 | C11orf63 | 4199 | 13185 | 15259 | 3.6 |
| NM_015891.2 | CDC40 | 3341 | 5952 | 12100 | 3.6 |
| BC099907.1 | GTF2I | 8025 | 37447 | 28856 | 3.6 |
| NM_003583.2 | DYRK2 | 1161 | 6398 | 3912 | 3.4 |
| NM_138730.1 | HMGN3 | 1678 | 6683 | 5088 | 3.0 |
| NM_201998.1 | SF1 | 9401 | 36764 | 27587 | 2.9 |
| NM_004103.2 | PTK2B | 789 | 3607 | 2237 | 2.8 |
| BC015514.1 | TFPI | 2506 | 11012 | 6989 | 2.8 |
| BC063463.1 | COQ3 | 710 | 488 | 1973 | 2.8 |
| BC012131.1 | C14orf149 | 2554 | 9927 | 6693 | 2.6 |
| NM_005313.3 | PDIA3 | 851 | 10445 | 2179 | 2.6 |
Fig. 3.
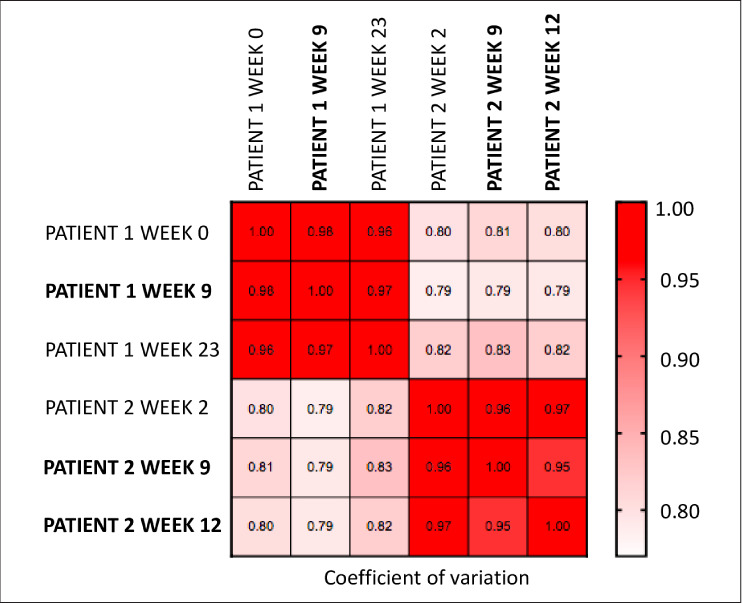
Non-parametric Spearman correlation matrix of the fluorescent optical density data after quantile normalization. Bolded text is plasma sample and normal text is serum sample.
Immunohistochemical staining demonstrated that ZCCHC8 was expressed in pituitary tissue and ACTH-secreting pituitary tumor tissue (Fig. 4 A through C). Blood vessels in the pituitary slides did not stain for ZCCHC8, serving as an internal negative control. ZCCHC8 localized to the nucleus. ZCCHC8 was also expressed in testis (Fig. 4 D), as previously reported, and was used as a positive control in this experiment (12).
Fig. 4.
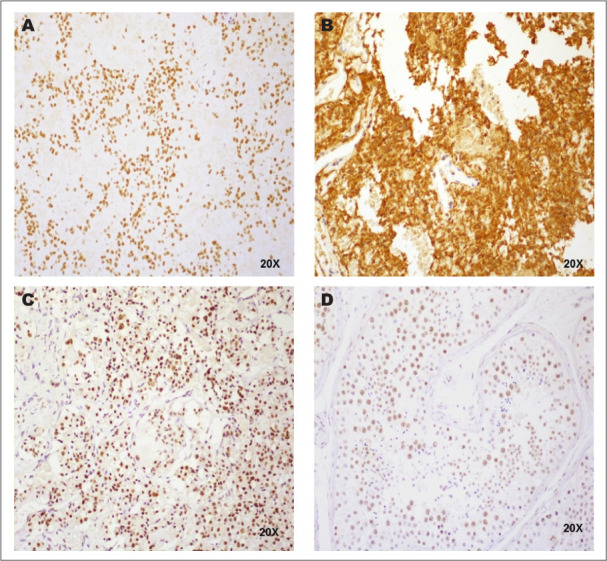
Immunohistochemical staining. (A) Adrenocorticotropic hormone-secreting pituitary adenoma tissue stained for ZCCHC8 (×20). (B) Adrenocorticotropic hormone-secreting pituitary adenoma tissue stained for adrenocorticotropic hormone (×20). (C) Normal pituitary tissue stained for ZCCHC8 (×20). (D) Testis tissue stained for ZCCHC8 (×20).
DISCUSSION
Proteome array identified a common autoantibody, ZCCHC8, in 2 patients who developed hypophysitis on ICI therapy. Immunohistochemistry staining revealed that ZCCHC8 is expressed in normal and ACTH-secreting pituitary tissue. ZCCHC8 is a zinc-knuckle protein known to be involved in cellular RNA processing (13). Gene expression studies have confirmed ZCCHC8 is present in the pituitary gland at the mRNA level (12), though its role in pituitary physiology is unknown. To our knowledge, until this study, protein expression of ZCCHC8 specifically in pituitary tissue had never been studied. Expression of ZCCHC8 has been shown in urothelial carcinoma (12).
Proteome array techniques have identified novel autoantibodies in autoimmune endocrine entities such as autoimmune polyendocrine syndrome 1 (5) and type 1 diabetes mellitus (6). In regard to ICI-mediated hypophysitis, one study showed serial increases in autoantibodies to the proteins GNAL and ITM2B in patients treated with ipilimumab. ZCCHC8 autoantibodies were not increased in this discovery cohort (9). Our study showed that patient 1 had a 2.4-fold increase in ITM2B and patient 2 had no ITM2B increases. GNAL was not part of our protoarray panel. The differences between our studies may suggest different autoantibody profiles in different malignancy types and different hypophysitis mechanisms. To our knowledge, our case series is the first to publish findings on autoantibodies in patients with ICI-mediated hypophysitis with urothelial cancer and the first to show that ZCCHC8 is expressed in pituitary tissue.
Our results must be interpreted with caution, as there are many limitations to our report. In our protoarray assays, we used both patient sera and plasma (Fig. 2). Across sera and plasma, the vast majority of analytes were similar and work pending publication in our lab has shown concordance in serum and plasma to multiple tumor antigens. Additionally, lack of a control group further impairs our autoantibody testing interpretation, as we cannot know if increased autoantibodies are to tumor or to self-antigens, or if preexisting antibodies may play a role in pathogenesis. Furthermore, patient 2 did not have a week 0 timepoint and patient 1 had more autoantibody increases than patient 2, including to the control antigen BSA. Anti-BSA antibodies may have clinical relevance, as they have been seen in patients with autoimmune conditions (14,15). Although this could lead to some false positive results due to protein preparation, the fold change in reported autoantibodies were all higher than those seen against BSA.
CONCLUSION
The finding of increased autoantibodies in patients with ICI-mediated hypophysitis warrants validation in other cohorts to determine if the response is to self or tumor antigen and may reveal insights into pituitary physiology and predictors of ICI-mediated hypophysitis. Future studies should include proteome assay autoantibody testing in a control population and a larger cohort of patients who developed hypophysitis on ICI therapy, as well as further examination of the role of ZCCHC8 in pituitary physiology.
ACKNOWLEDGMENT
This project was presented, in part, at the Endocrine Society Conference in Chicago, Illinois in March of 2018. All procedures involving human participants were in accordance with the ethical standards of our institutional review board (IRB # 10-1180) and with the 1964 Helsinki declaration. Informed consent was obtained from all included individual participants. The Tisch Cancer Institute is supported by NCI Cancer Center Support Grant P30CA196521. This work was also supported by NCI/NIH U24 CA224319 (SG), P01 CA190174 (SG), NCI/NIH K08CA190770 (EG), and Alkeon Capital Management (EG).
Abbreviations
- ACTH
adrenocorticotropic hormone
- BSA
bovine serum albumin
- fT4
free thyroxine
- ICI
immune checkpoint inhibitor
- TSH
thyroid-stimulating hormone
- ZCCHC8
zinc finger CCHC-type containing 8
Footnotes
DISCLOSURE
A.L., M.F., S.K.S., and I.L. have nothing to disclose. S.G. reports consultancy/advisory roles from Merck, Neon Therapeutics, and OncoMed, and research funding from Agenus, BMS, Genentech, Immune Design, Janssen R&D, Pfizer, Regeneron, and Takeda; all unrelated to this current work. M.G. reports ownership interests in Rappta Therapeutics, research funding from AstraZeneca, Bristol-Myers Squibb, Dendreon, Genentech/Roche, Janssen Oncology, Merck, and Novartis, and consultancy/advisory roles for Aileron Therapeutics, AstraZeneca, BioMotiv, Bristol-Myers Squibb, Dendreon, EMD Serono, Estellas Pharma, Genentech, GlaxoSmithKline, Incyte, Inovio Pharmaceuticals, Janssen, Lilly, Merck, Novartis, NuMab, Pfizer, and Seattle Genetics. E.J.G. reports advisory role for Novartis, unrelated to this current work.
REFERENCES
- 1.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khoja L, Day D, Wei-Wu Chen T, Siu LL, Hansen AR. Tumourand class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol. 2017;28:2377–2385. doi: 10.1093/annonc/mdx286. [DOI] [PubMed] [Google Scholar]
- 3.Solinas C, Porcu M, De Silva P et al. Cancer immunotherapy-associated hypophysitis. Semin Oncol. 2018;45:181–186. doi: 10.1053/j.seminoncol.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Gnjatic S, Ritter E, Büchler MW et al. Seromic profiling of ovarian and pancreatic cancer. Proc Natl Acad Sci U S A. 2010;107:5088–5093. doi: 10.1073/pnas.0914213107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Landegren N, Sharon D, Freyhult E et al. Proteome-wide survey of the autoimmune target repertoire in autoimmune polyendocrine syndrome type 1. Sci Rep. 2016;6:20104. doi: 10.1038/srep20104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koo BK, Chae S, Kim KM et al. Identification of novel autoantibodies in type 1 diabetic patients using a high-density protein microarray. Diabetes. 2014;63:3022–3032. doi: 10.2337/db13-1566. [DOI] [PubMed] [Google Scholar]
- 7.Da Gama Duarte J, Parakh S, Andrews MC et al. Autoantibodies may predict immune-related toxicity: results from a phase I study of intralesional bacillus Calmette-Guérin followed by ipilimumab in patients with advanced metastatic melanoma. Front Immunol. 2018;9:411. doi: 10.3389/fimmu.2018.00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gowen MF, Giles KM, Simpson D et al. Baseline antibody profiles predict toxicity in melanoma patients treated with immune checkpoint inhibitors. J Transl Med. 2018;16:82. doi: 10.1186/s12967-018-1452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tahir SA, Gao J, Miura Y et al. Autoimmune antibodies correlate with immune checkpoint therapy-induced toxicities. Proc Natl Acad Sci U S A. 2019;116:22246–22251. doi: 10.1073/pnas.1908079116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gnjatic S, Old LJ, Chen YT. Autoantibodies against cancer antigens. Methods Mol Biol. 2009;520:11–19. doi: 10.1007/978-1-60327-811-9_2. [DOI] [PubMed] [Google Scholar]
- 11.Gnjatic S, Wheeler C, Ebner M et al. Seromic analysis of antibody responses in non-small cell lung cancer patients and healthy donors using conformational protein arrays. J Immunol Methods. 2009;341:50–58. doi: 10.1016/j.jim.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 12.Uhlen M, Zhang C, Lee S et al. A pathology atlas of the human cancer transcriptome. Science. 2017;357:eaan2507. doi: 10.1126/science.aan2507. [DOI] [PubMed] [Google Scholar]
- 13.Falk S, Finogenova K, Melko M et al. Structure of the RBM7-ZCCHC8 core of the NEXT complex reveals connections to splicing factors. Nat Commun. 2016;7:13573. doi: 10.1038/ncomms13573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Füchtenbusch M, Karges W, Standl E, Dosch HM, Ziegler AG. Antibodies to bovine serum albumin (BSA) in type 1 diabetes and other autoimmune disorders. Exp Clin Endocrinol Diabetes. 1997;105:86–91. doi: 10.1055/s-0029-1211732. [DOI] [PubMed] [Google Scholar]
- 15.Nehring J, Schirmbeck LA, Friebus-Kardash J et al. Autoantibodies against albumin in patients with systemic lupus erythematosus. Front Immunol. 2018;9:2090. doi: 10.3389/fimmu.2018.02090. [DOI] [PMC free article] [PubMed] [Google Scholar]


