Abstract
Purpose of Review
The goal of this review is to summarize the field to date and to discuss strengths and limitations of low-level laser (light) therapy (LLLT) for the future investigation as a treatment of inflammatory disease.
Recent Findings
LLLT is a promising therapeutic, particularly for those diseases of skin and joints because they are most accessible to treatment. Indeed, the known mechanisms of LLLT support its use for anti-inflammatory purposes, as well as stimulation of tissue growth and repair. Although the standard of care for the majority of inflammatory diseases is immunosuppressive agents such as corticosteroids with undesirable toxicities, LLLT offers a unique approach by being non-invasive and incurring minimal side effects. It is also relatively inexpensive and accessible and even has the possibility to be patient directed at home.
Summary
There is evidence that LLLT is able to modulate the immune system at the skin and joint, and it has been shown to be efficacious in humans by affecting bacterial colonization as it may pertain to chronic rhinosinusitis. However, there is variability in the methods of laser application as well as a lack of evidence for laser type, dose-ranging studies, and wavelength selection that create barriers to the implementation of LLLT without further more rigorous and standardized study. The heterogeneity makes it difficult to draw strong conclusions about the efficacy of LLLT and its mechanisms.
Keywords: Low-level light/laser, Phototherapy, Photodynamic therapy, Phototherapeutics, Inflammatory disease, Autoimmunity
Introduction
Low-level laser (light) therapy (LLLT) is a continuous laser or light-emitting diode (LED) of 600 to 1000 nm wavelength applied for the purposes of analgesia, stimulating tissue repair, and/or decreasing inflammation [1••]. LLLT is also known as “cold laser therapy” because it does not cause temperature rise in treated tissue that would lead to change in the tissue architecture [2••]. This is in contrast to ablative lasers such as CO2 and Er:YAG which induce heat-related changes in the superficial tissue and are often employed in scar revision [3]. Another form of laser delivery is photodynamic therapy (PDT), involving the administration of a specific wavelength of light that activates a photosensitizer. These components are non-toxic individually, but combine to induce cellular and tissue changes in an oxygen-dependent manner. PDT has been employed for the treatment of cancer and is thought to have multiple mechanisms by which it exerts its’ effects: the production of reactive oxygen species (ROS) that is toxic to tumor cells, damage to the tumor-associated vasculature, and stimulation of an immune response against tumor cells [4]. These as well as other adaptations of laser treatment are a rapidly growing modality for targeting pain and modulation of the inflammatory response in musculoskeletal, autoimmune, and cutaneous disease as well as alternative approaches for cancer therapy.
Multiple mechanisms have been proposed to explain the observed effects of LLLT, also referred to as photobiomodulation [1••] (Fig. 1). A widely accepted hypothesis is that light therapy stimulates tissues due to the overlapping absorption spectra between oxidized cytochrome c oxidase (CCO), the terminal enzyme of the electron transport chain, and the action spectra from biological responses to light [1••, 2••]. It is thought that CCO acts as a photoacceptor from red and near-infrared regions of light via copper and iron chromophores, which consequently accelerates cellular metabolism and the production of ATP [5]. Additionally, the nitric oxide (NO) hypothesis proposes that LLLT promotes NO release as a result of photodissociation of NO from CCO which subsequently increases the rate of ATP production and promotes vasodilation [2••, 6]. NO release is believed to modulate ROS and in turn regulate transcription factors related to growth and repair [2••, 7]. A review of the molecular mechanisms of LLLT-enhanced cellular proliferation showed that LLLT reduces the concentration of signaling molecules involved in the inflammatory response such as nuclear factor kappa B (NF-κB) and can inhibit prostaglandin E2, tumor necrosis factor alpha (TNF-α), cyclooxygenase-2, and interleukin (IL)-1β [8].
Fig. 1.
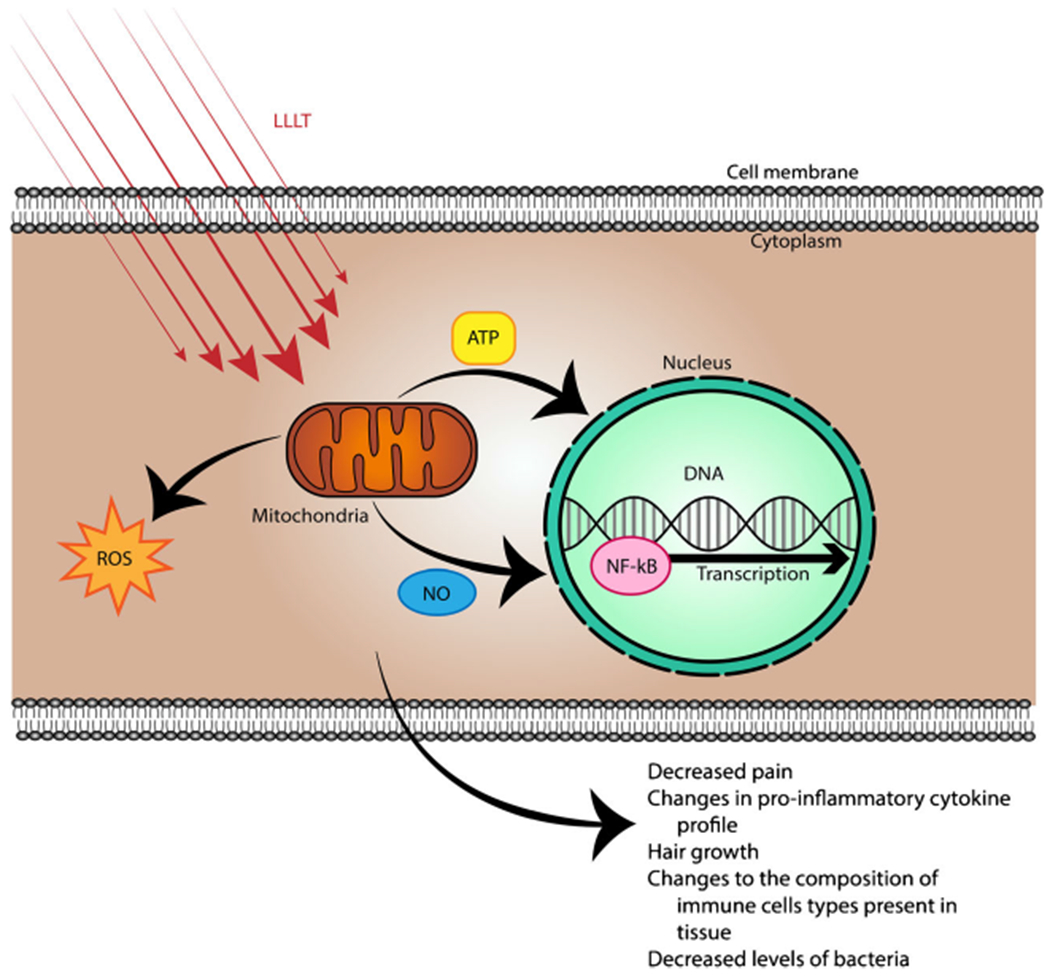
Mechanisms of low-level laser therapy (LLLT). Schematic illustrating the absorption of red/near infrared (NIR) light by mitochondrial chromophores. Subsequently, production of ATP increases, reactive oxygen species (ROS) are generated, and nitric oxide (NO) is released. These changes influence gene transcription via modulation of transcription factors such as nuclear factor kappa B (NF-κB)
These anti-inflammatory mechanisms and the non-invasive nature of LLLT suggest that it would be a desirable therapeutic option for any disease pathology defined by an inflammatory state or requiring stimulation of growth and repair. As a result, it has been welcomed as a potential treatment modality for a variety of conditions. However, studies thus far have provided conflicting results for the efficacy of LLLT, which may be a result of inconsistent treatment protocols that require further study (Table 1).
Table 1.
Summary of included experimental studies evaluating LLLT in inflammatory disease.
| Characteristics of included experimental studies | Laser information | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study | Human/animal | Model | Groups | Treatment schedule | Continuous | Location | Laser | Wavelength (nm) | Power (mW) | Density (J/cm2) |
| Ablon et al. | Human | Psoriasis | x | 10 treatments over 5 weeks OR 8 treatments over 4 weeks (varied by patient) | Y | 5 cm from skin; various locations over the body | Omnilux, Photo Therapeutics (Carlsbad, CA) | 633, 830 | NS | 126 (633 nm), 60 (830 nm) |
| Alves et al. | Male Wistar rats (12 weeks) | Collagen-induced arthritis (CIA) | Control: RA without treatment; RA treated early; RA treated late | Daily; early stage: 7 days Daily; late stage: 14 days |
Y | 1 treatment point: middle of the front side of the knee joint (spot size 0.214 cm2) | MMOptics, São Carlos, Brazil | 780 | 22 | 7.7 |
| Araki et al. | MH7A human synovial cell | Human rheumatoid arthritis | x | Once, cells harvested 3 h after irradiation | Pulsed frequency 1 Hz | Over cell plate | Linear polarized near infrared light (Super Lizer™) | 600–1600 | 250 | 3.8 |
| Female Lewis rats | Collagen-induced arthritis (CIA) | x | Once | Knee | 600–1600 | 250 | 7.64 | |||
| Goncalves et al. | Female C57BL/6 mice | Experimental autoimmune encephalitis (mouse model of multiple sclerosis) | (I) Naïve: not immunized and untreated (II) EAE: immunized and untreated |
Daily, 30 days | Y | 6 positions on the spinal cord .5 cm apart | ||||
| (III) 660 nm: immunized and treated with AlGaInP LLLT | AlGaInP | 660 | 30 | 10 | ||||||
| (IV) 904 nm: immunized and treated with GaAs LLLT | GaAS | 904 | 70 | 10 | ||||||
| Issa et al. | FVB mice | Acute arthritis: zymosan A Chronic arthritis: collagen bovine type II |
G1: acute | Once: 15 min post-injection (euthanasia 24 h) | 200 ns pulse 10,000 Hz | Knee joint | Flat top beam, Theralase | 905 | 60 | 5 |
| G2: acute | Twice: 15 min, 24 h (euthanasia 48 h) | |||||||||
| G3: chronic | 3 times/week, 4 weeks for 12 total treatments | |||||||||
| Krespi et al. | Human; positive nasal vestibular culture by swab for MSSA or MRSA |
Nasal colonization with MSSA/MRSA |
Laser only (870–930 nm, at 200 J) Laser followed by peroxide irrigation Laser and E-mycin application |
Days 1, 3, 5 | Y | Sinus | Near-infrared laser illumination | 870–930 | 3000–4000 | NS |
| Laser only (940 nm at 600 J) | 940 | 3000–4000 | ||||||||
| Krespi and Kizhner | Human; persistent chronic rhinosinusitis symptoms, with or without polyposis | Chronic rhinosinusitis | Sinus laser illumination only (GR1) | Two or three times (based on response: better responders received two treatments), 5 days between laser illuminations | Y | Sinus | NIR diode laser at 940 nm (ARC Lasers, Nurnberg, Germany) | 940 | 4000 | NS |
| Topical diluted ICG (Akorn, Lake Forest, IL) followed by sinus laser illumination (GR2) | 810-nm NIR diode laser (ARC Lasers, Nurnberg) | 810 | ||||||||
| Lazovic et al. | Human | Carpal tunnel syndrome | Experimental: active laser Control: no laser Sham: placebo, sham laser |
5 times per week for 2 weeks; 10 times every other day for 3 weeks Total: 20 treatments |
Y | 4 points perpendicular to the skin over carpal tunnel (90 s each) | GaA1As diode laser | 780 | 30 | 3.4 per point of application |
| Omi et al. | Human | Skin | 3-mm skin punch biopsies were obtained 3 h, 1 day, 3 days, 1 weeks, 2 weeks, 4 weeks, and 5 weeks after irradiation | Once | Pulsed | Skin on the fibula | Chromogenex V3 laser | 585 | NS | 3 |
| Silva et al. | Mice | Allergic asthma, mouse model | Protocol I: 4 groups: mice treated once at various times | Treated at 5 min, 1 h, 6 h or 12 h post-antigenic challenge | Y | Skin over the bronchus | CW laser diode module (MMOptics, São Paulo, Brazil) | 660 | 30 | 375 mW cm−2 |
| Protocol II: mice treated 4 consecutive times | Treated at 5 min, 1 h, 6 h and 12 h post-antigenic challenge | |||||||||
| Souza et al. | Human monocytic cell line; U937 cells | Alveolar macrophage model: U937 cells | Cells were stimulated with H2O2 and 4 h later LPS was added; all cells were irradiated once, 1 h post-LPS | Once | Y | Over cell plate | CW laser diode module (MMOptics, São Paulo, Brazil) | 660 | 30 | 4.5 |
| Stitch et al. | Dog | Canine atopic dermatitis | Prospective, randomized, double-blinded, intra-individual study; each dog served as its own placebo control | Weeks 1 and 2: 3 times/week, (no > 1 consecutive treatment). Weeks 3 and 4: 2 laser treatments/week | Y | Both the dorsal and the ventral aspects of paws | CTC Companion Compact laser (Companion Therapy Laser; LiteCure LLC, Newark, DE, USA) | 810–980 | 4000 | 4 |
| Waiz et al. | Human | Treatment-resistant alopecia areata | All patients were treated; patients with multiple patches had one patch untreated as a control | Once weekly, 4 days total | Pulsed | In contact with skin; applied to various areas of the body with patches of AA | Patented dental unit (Mario Scalvini, Italian National Research Council) | 904 | 150,000 | NS |
| Wang et al. | Wistar rats | Allergic asthma, rat model | (I) Control; (II) asthmatic; (III) LLLT; (IV) budesonide (standard of care) | Daily, 21 days total | Y | Chest | Semiconductor laser (Laser Medicine Laboratory, Biomedical Engineering Institute, Chinese Academy of Medical Science, Beijing, China | 810 | 80 | 5 |
| Yamato et al. | Rat | Glomerulonephritis—anti-GBM antibody | One experimental group | Daily, 14 days total | Y | Laser applied externally to skin overlying both kidneys | Ga-Al-As diode laser; model ZH-M143DJP, Panasonic Industrial Equipment, Tokyo, Japan | 830 | 2000 | 220 |
| Zhang et al. | Rat | Type II collagen-induced arthritis (CIA) | (I) CIA untreated (II) CIA rats treated with LLLT |
3 times per week for 2 weeks | Y | Knee joint | Ga-Al-As diode laser (model ZH-M143DJP, Panasonic Industrial Equipment, Tokyo, Japan) | 830 | 500 | 7.64 |
NS not specified
This review seeks to update the reader on recent advances using low-level laser therapy and photodynamic therapy in the treatment of inflammatory and autoimmune disease.
Musculoskeletal Disorders
Pain
LLLT or “cold laser therapy” has been widely used in pain management with proposed anti-inflammatory and analgesic effects. LLLT is a favorable option for pain as it offers a non-invasive therapeutic option that is thought to stimulate biologic activity and promote tissue healing [2••]. This is particularly relevant at a time when the incidence of chronic pain is increasing, and the medical profession is re-evaluating the use of many classic pain medications such as narcotics.
LLLT has been shown to be effective against nociceptive [9] and neuropathic pain [10], though there is no evidence yet for its use in central pain [11]. LLLT has been shown to inhibit action potentials in peripheral nerves equating to a 30% neural blockade within 10 to 20 min of application, which is reversed within 24 h [12]. It is hypothesized that this takes place as a result of decreased mitochondrial membrane potential in DRG neurons that reduces the production of ATP [13]. Studies suggest that pain reduction following LLLT occurs from a decrease in oxidative stress and edema formation [14]. LLLT is also thought to affect serotonin and endorphin release, diminishing pain signaling [15, 16]. Despite these promising results, implementing LLLT into clinical treatment has had limitations. Many of the studies that have evaluated LLLT in pain measure results over a short time course and do not provide any evidence for long-term benefit in pain treatment. As a result, the FDA has approved LLT only for temporary relief of muscle and joint pain [11]. There is also high variation in the methods of application of LLLT as well as a lack of evidence for laser type and ideal dose of laser application and wavelength [17].
A systematic literature review by Clijsen et al. assessed the effectiveness of LLLT on pain in adult patients with musculoskeletal disorders. They found a significant difference in pain between LLLT and placebo LLLT control, supporting that LLLT is an effective treatment for musculoskeletal pain (D = 0.85, 95% CI −1.22 to −0.048, p < 0.001). The authors carried out additional subgroup analyses to examine whether study design, anatomic location, and adherence to the World Association for Laser Therapy (WALT) guidelines affected outcomes. The WALT guidelines were created in an attempt to standardize laser treatment protocols based on condition and anatomical location, though many studies do not follow these recommendations. There was no difference in groups based on anatomic location that was laser treated (knee, wrist, shoulder, back) or study design (randomized control trial vs. clinical trial) [18], and there was no significant difference between studies that adhered to WALT guidelines. However, the authors suggest that WALT adherence may be clinically relevant as the mean change was greater than the minimum clinically important difference for the visual analog scale (VAS), a unidimensional measure of pain intensity used for assessment in this study as defined by multiple authors [19, 20]. This review was limited due to the multitude of treatment regimens and a high variety of anatomical locations that were treated, though the significant benefit from LLLT in this meta-analysis suggests that the benefits are independent of anatomical site. Many of the studies included in this meta-analysis employed additional treatments such as exercise or cold pack therapy, warranting further study with LLLT alone compared with the standard treatment protocol. In addition, further evaluation of appropriate laser protocol based on anatomic location should be carried out.
Carpal Tunnel Syndrome
Carpal tunnel syndrome is a mononeuropathy secondary to the entrapment of the median nerve by chronic inflammation and swelling of the transverse carpal ligament. It can cause significant functional impairment and ultimately require surgical intervention if standard treatment fails. LLLT has shown positive results in its use for injured peripheral nerves in animals, inducing Schwann cell proliferation [21]. Recently, a number of clinical studies have sought to assess the potential therapeutic benefit of LLLT in carpal tunnel syndrome.
Lazovic et al. carried out a double-blind, placebo-controlled study to assess the short-term efficacy of LLLT in patients with mild-moderate carpal tunnel syndrome lasting <1 year. They assessed active laser vs. placebo (sham) laser applied 5 times per week for 2 weeks, followed by 10 treatments every other day for 3 weeks for a total of 20 treatments. Patients were evaluated before and 3 weeks after treatment. Following LLLT treatment, there was a significant decrease in pain compared with control, based on VAS scores. There were significantly fewer cases with positive Tinel’s sign (a clinical indicator of median nerve entrapment) in the active laser group, although a decrease in the presence of Tinel’s sign was seen in both LLLT and control groups. There was also a significant decrease in the wrist to digit 2 median sensory nerve conduction velocity as compared with control [22]. This study indicates that LLLT may be beneficial for the treatment of both symptoms and nerve pathology in carpal tunnel syndrome.
A meta-analysis assessed the efficacy of LLLT in randomized control trials for carpal tunnel syndrome. Overall, LLLT was not superior to placebo in assessment of sensory nerve action potential, functional status improvement, pain reduction, or motor electrodiagnostic evaluations. However, there was a significant improvement with LLLT over placebo with regard to grip strength (MD 2.19, 95% CI 1.63–2.76) [23]. This meta-analysis was limited to a small number of trials, with a substantial amount of heterogeneity that led to the exclusion of different studies in each analysis. Further, the laser type, duration, method of application, frequency of application, and total dose varied greatly among the eight studies. Individually, many of the studies that included higher total doses of laser treatment were those that showed significant differences among LLLT and placebo groups, which may be a contributing factor. There was also inconsistent use of splinting in the studies, with some trials allowing splints to be worn, potentially undermining the efficacy of LLLT in these studies and the subsequent analysis in this review.
A Cochrane systemic review, which adheres to strict methodological and reporting standards to minimize bias and improve accuracy of results, summarized the results of randomized clinical trials assessing the use of LLLT for the treatment of carpal tunnel syndrome with similar findings. Seven clinical trials met inclusion criteria. Hand grip at 12 weeks was stronger in the LLLT group than in control (MD = 2.04, 95% CI 0.08–3.99, p = 0.04). There was also a significant improvement in the visual analog scale (VAS, as described above) at 12 weeks in the LLLT group compared with control (MD = 0.97, 95% CI 0.84–1.73, p = 0.001). There were no statistically significant differences in any other parameters measured including symptom severity scores and functional status scores, as well as nerve conduction studies [2••]. Like the aforementioned meta-analysis, this study was also limited by heterogeneity as a result of multiple laser therapy protocols involving different doses given at varying anatomical locations. Standardized laser protocols of laser beam strength and anatomic location that are compared with placebo as well as consistent outcome measurements should be studied in the future to minimize confounding variables and assess the possible benefits for carpal tunnel syndrome.
Rheumatoid Arthritis
Rheumatoid arthritis (RA) is a progressive inflammatory autoimmune disease that afflicts just under 1% of the US population [24]. RA is known to involve the influx of inflammatory cells into the synovium/joint space and the production of pro-inflammatory cytokines that result in irreversible damage and functional impairment. RA therapies are constrained by the range of moderate to severe side effects associated with long-term systemic exposure. LLLT provides a potential non-invasive, anti-inflammatory treatment with minimal side effects that could prove useful in this disease.
Alves et al. found that LLLT is able to modulate both the early and late stages of RA in a collagen-induced arthritis (CIA) rat model. This study examined the histological effects of LLLT treatment at different stages of RA progression in the knees of the CIA rat model. Groups were divided by treatment at either 12 h after arthritis induction or 7 days after arthritis induction with the same LLLT parameters (780 nm, 7.7 J/cm2, continuous). LLLT at both early and late RA progression stages significantly improved mononuclear inflammatory cells, exudate, medullary hemorrhage, hyperemia, necrosis, distribution of fibrocartilage, and number of chondroblasts and osteoblasts compared with the RA untreated control group (p < 0.05) [25].
Issa et al. sought to evaluate the clinical and histological results of LLLT in a murine model of acute (zymosan A) and chronic (collagen bovine type II) inflammation. In acute arthritis, treatment 15 min after arthritis induction was not effective in improving histologic parameters of inflammation. For animals with acute arthritis receiving two laser treatments at 15 min and 24 h, LLLT therapy at 905 nm significantly reduced resorption of the articular surface area, whereas that at 660 nm exacerbated the articular surface resorption as compared with untreated control (p = 0.044). Conversely, in the chronic arthritis group, 660 nm was the only wavelength of LLLT that showed significant reduction in articular resorption (p = 0.026). This study indicates that the different wavelengths of light may exert differential effects on acute and chronic stages of arthritis. These pre-clinical controlled studies support that optimization and study of laser wavelength, penetration, and timing are relevant to clinical endpoints being measured and may explain why different clinical trials have shown inconsistent results. A major limitation of this study is the euthanasia of animals 24 h after LLLT treatment; thus, the results are only applicable to the immediate 24 h following treatment, without evidence of how LLLT may influence inflammation over time.
It has been shown that IL-6 deficiency delays arthritis onset and reduces severity in the CIA model, suggesting a role for this inflammatory cytokine in the pathogenesis of RA [26]. Multiple studies have also suggested that IL-6 overproduction correlates with RA disease severity [27, 28]. Though the use of IL-1 and TNF-α inhibitors have changed the landscape of RA treatment, there are still a number of patients who do not respond to these treatments, suggesting that there are other potential targets that influence RA disease [29–31]. Araki and Imaoka et al. studied the role of IL-6 in the effects LLLT in rheumatoid arthritis. These authors studied linear, polarized, near-infrared light to assess the mechanism(s) of LLLT in both human cell lines isolated from the knee joint of an RA patient and those in the CIA rat model. The MH7A cell line retains the morphological and functional characteristics of synovial cells, which were incubated with or without recombinant human IL-1β for 16 h and then treated with LLLT illumination. Reverse transcription polymerase chain reaction (RT-PCR) measuring gene expression of IL-6 in these cells showed a decrease in the laser-treated group. CIA was induced in female rats followed by either laser or no treatment. Immunohistochemistry of synovial fibroblasts showed a reduction in IL-6 in the laser-treated group as compared with control [23]. Since the pro-inflammatory cytokine IL-6 is known to be involved in the pathogenesis of RA and high levels are detected in synovial fluid and serum of RA patients, LLLT-mediated reduction of this signaling molecule may be the mechanism by which LLLT exerts its therapeutic effects in RA.
RA is characterized by joint destruction as a result of leukocyte migration into the synovium in a chemokine-dependent manner [32]. Chemokines facilitate adhesion of leukocytes following migration to the site of inflammation [33]. Zhang et al. examined the immune signal changes that occur in the CIA rat model of RA and what changes occurred in these pathways following LLLT treatment. They observed that CCL2 chemokine gene expression increased in arthritis and significantly decreased with LLLT treatment. Ingenuity pathway analysis was used to reveal the role of chemokine signaling pathways in the activation of CCL2 production. This was validated with real-time PCR, RT-PCR, and immunohistochemistry [34]. This study suggests that changes to CCL2 expression may be one of the mechanisms by which LLLT reduces inflammation in RA.
LLLT is a promising treatment for RA given its non-invasive nature and anti-inflammatory effects. Current research in pre-clinical studies shows that it could play a major role in attenuating the immune response that leads to joint destruction and debilitation. LLLT as a therapeutic modality in arthritis should be studied further to determine both the mechanisms by which it may modify disease progression and its efficacy in human disease.
Dermatology
Laser therapy has long been used in the field of dermatology for cosmetic procedures such as wrinkle reduction and skin resurfacing, as well as the treatment of acne and other skin pathologies. The original laser technologies used in dermatology were based on the principle of photothermolysis or heat-induced changes to the skin [35]. Further, ultraviolet (UV) light has been used in the treatment of pathologies including acne vulgaris and psoriasis but is less favorable given its risk of skin damage and carcinogenesis. Recently, LLLT has been evaluated for the use of acne vulgaris, and its therapeutic effects are thought to be a result of ROS production that destroys bacteria [36, 37] as well as anti-inflammatory effects [38, 39]. It is hypothesized that red–near-infrared light modulates cells of the immune system to exert its effects, though this mechanism has yet to be proven in the literature. There have been many advances in laser therapy that allow illumination to be delivered to a large surface area with commercially available technology. This warrants the determination of the changes that occur at the skin level following LLLT and the mechanism of action by which it exerts anti-inflammatory effects.
Omi et al. sought to characterize the cellular and cytokine expression changes that occur in healthy human skin after LLLT. They evaluated histopathological changes in adult skin samples, as well as qualitative and quantitative changes in the inflammatory markers IL-2 and IL-4. They utilized IL-2 and IL-4 as markers of T helper 1 (TH1) and T helper 2 cell (TH2) activity, respectively. They found that laser radiation induced endothelial cell edema with hemostasis and infiltration of monocytes, neutrophils, and mast cells in the extravascular dermis at 3 h which persisted for 1 week after treatment. After 1 week of treatment, they noted the presence of both IL-2 and IL-4, with higher amounts of IL-4, in skin homing T lymphocytes. Lymphocytes and fibroblasts were still observed at 4–5 weeks after treatment [40•]. The authors propose that treatment with LLLT increases both TH1 and TH2 activity with greater activation of TH2 cells as indicated by higher IL-4 levels. Chen et al. showed that LED blue and red light illumination of healthy skin activates redox-sensitive NF-κB signaling in murine embryonic fibroblasts via generation of ROS [7]. When combined, these studies indicate that in healthy skin, laser treatment affects immune cell infiltration, which may be a potential mechanism by which LLLT could affect cellular change in immune-mediated cutaneous disease.
Psoriasis
Psoriasis is a chronic, immune-mediated disease with skin and joint manifestations that is often relapsing and remitting [41]. The pathophysiology is known to involve abnormal keratinocyte proliferation and immune cell infiltration as a result of aberrant antigen presentation [42]. This immune response is defined by the activation of NF-κB signaling and increased T helper 17 (TH17) cells that are the source of an enhanced IL-17 inflammatory response. Typical regimens for the treatment ofpsoriasis include UV light, utilizing UVB and UVA light [42]. UV phototherapy acts through multiple mechanisms including apoptosis of inflammatory cells, suppression of Th17 cells, and activation of immune-suppressing signals such as IL-10, TH2, and regulatory T (Treg) cells [43]. Phototherapy is a favorable treatment option in patients who require treatment over large areas of the body. Narrowband UVB treatment can even take place at home and has not been associated with an increased risk of skin cancer [41, 44]; however, UVB treatment has substantial insurance and co-pay costs and side effects such as photosensitization that may make it a less desirable treatment option. UVA light therapy (PUVA) is used much less frequently as it has been associated with increased risk of skin malignancy [45, 46].
Since LLLT exhibits multiple anti-inflammatory and immune-modulating effects and has shown promise in diseases defined by altered T cell signaling, psoriasis is a reasonable disease in which to pilot therapy particularly given that other forms of light therapy have been successful. In contrast to other light treatments, LLLT has stronger penetration with the potential for photobiomodulation, which may prove more promising. There have been a small number of studies utilizing LLLT for the clinical treatment of psoriasis although the literature is still in its infancy [47, 48]. Specifically, Ablon et al. treated psoriasis patients with 8–10 sessions of combined 830 and 633 nm LED therapy, with normalization of skin plaques in 60–100% of the treated surface area [47]. Future controlled studies should compare LLLT with current light-based therapies used to determine whether it is equivalent or superior.
Alopecia Areata
Alopecia areata (AA) is an autoimmune response against hair follicles, known to involve an inflammatory infiltrate primarily composed ofCD4+ T cells and Langerhans cells surrounding follicular bulbs [49]. The rate of hair loss recurrence remains relatively high despite current therapies [50–52]. The primary light therapy that has been used for the treatment of alopecia areata is the excimer laser (308 nm), a UV-based therapy [53]. The excimer laser is a well-tolerated and effective treatment, but it can be quite expensive and has been shown to only be a successful treatment of the scalp without much benefit for alopecia of other areas of the body [54]. Narrowband ultraviolet B (NB UVB) light has also been used for treatment; however, there are concerns for its carcinogenic potential [53]. Interestingly, in 1967, Mester et al. noticed that low-power laser could induce hair growth in mice [55], and paradoxical hair growth following laser hair removal treatments has been documented [56]. This suggests that in addition to the potential effects of LLLT on the immune system, it may also directly influence hair growth. Thus, LLLT has been suggested as a potential therapy for hair loss secondary to AA, especially since it has few adverse side effects while retaining the benefits of a local (as opposed to systemic) light-based therapy.
Waiz et al. carried out a clinical trial using a pulsed infrared diode laser (904 nm) on treatment-resistant AA of the scalp, beard, eyebrow, and mustache. Of the 34 patches treated, 32 showed hair regrowth, and no adverse side effects were noted. This was in contrast to consistent reporting of erythema and skin blistering with or without hyperpigmentation that was seen with excimer laser treatment as the comparator treatment [57]. A case series also showed that LLLT contributed to hair regrowth in 75% of patients with scalp AA previously resistant to other treatments even though patients were using an unspecified concomitant therapy [50]. In a prospective study, 46.7% of patients with mild patchy AA experienced hair regrowth 1.6 months earlier in treated areas compared with non-treated areas [58]. Interestingly, the three patients with comorbid atopic dermatitis were non-responders, which could indicate that the presence of other conditions, particularly those that are TH2 predominant, may affect the success of laser therapy, albeit the number of individuals treated was so low that this is difficult to conclude with certainty. Given the cost and potential adverse side effects of excimer laser, possible carcinogenicity with NB UVB, and inconsistent treatment results with current therapies based on traditional immunosuppression, LLLT has minimal toxicity and shows some promise that warrants further study in the treatment of AA, particularly in refractory cases.
Atopic Dermatitis
The use of LLLT in human studies has shown promising anti-inflammatory effects, particularly on T cell function, as discussed in previous sections. This study sought to assess LLLT as a treatment for canines with existing atopic dermatitis (AD) on either both forepaws or both hind paws (each dog served as its own control). Canine AD is a genetically predisposed, inflammatory, pruritic skin disease associated with overproduction of immunoglobulin E (IgE) antibodies in dogs, and it shares similarities with human AD, characterized by an abnormal TH2 inflammatory response [59]. A localized canine atopic dermatitis severity score (LCADSS) and owner localized pruritic visual analog score (LPVAS) were assessed as endpoints in LLLT, both of which decreased significantly from week 0 at weeks 2, 4, and 5 for both LLLT and placebo paws [60]. The authors suggest that this was likely due to a placebo effect which has been demonstrated in previous veterinary studies, among both caregivers and veterinarians [61]. However, it is also possible that the laser had systemic effects, and given that each dog served as its own control, this could have led to the placebo paw receiving treatment inadvertently from systemic effects of the laser. In the future, a non-treated or sham-treated control group should be studied as a comparator to control for potential systemic effects of LLLT. Of note, the use of LLLT in human AD has yet to be evaluated and should be studied with caution given the anecdotal experience of noted AD worsening in the AA trial described above [58].
Psoriasis and AA are skin disorders mediated by TH1 and TH17 responses in contrast to AD, which results from the aberrant TH2-mediated pathology. It is possible that the mechanism of LLLT is not advantageous for allergic skin disorders that are T cell mediated. IL-4 is a known mediator of TH2 cell recruitment, and Omi et al. showed that LLLT increased IL-4, which may affect disease outcomes in human and canine AD [40•]. Thus, the clinical efficacy and mechanistic action of LLLT must be further clarified to best direct the utility of laser therapy in immune-mediated diseases in the future.
Otolaryngology
Multiple studies have shown that nasal decolonization of Staphylococcus aureus will lead to a decreased nosocomial infection rate in hospitalized patients [62, 63]. Methicillin-sensitive S. aureus (MSSA) and methicillin-resistant S. aureus (MRSA) contribute to 25% of nosocomial infections. Antibacterial resistance of MRSA is a growing clinical problem particularly in those with nasal colonization. Near-infrared (NIR) laser, which is within the wavelength spectrum of LLLT, has been shown to successfully kill bacteria without raising tissue temperature, suggesting that it could be used in decolonization in human patients without contributing to bacterial antibiotic resistance [64].
Krespi and Kizhner demonstrated the successful use of LLLT for nasal decolonization of MSSA and MRSA in antibiotic-resistant patients. The authors found that LLLT successfully eradicated nasal decolonization with a 940-nm (600 J/cm2) laser having the greatest rate of success. They also suggest that LLLT potentiated effects of a topical antibiotic (e-mycin) to which bacteria had been previously resistant, although there was no antibiotic-only control in this study for rigorous comparison [64]. The authors propose that LLLT alters the respiratory process of the cellular plasma membrane by lowering membrane potential and then subsequently inactivates the bacterial resistance mechanism of drug efflux. This study provides promising preliminary data for the use of LLLT in nasal decolonization and should be studied further with an antibiotic-only control.
Patients with nasal colonization combined with other non-infectious factors can develop chronic rhinosinusitis [65] for which systemic antibiotics are often ineffective due to biofilm formation [66, 67]. As shown in the aforementioned study by Krespi et al., LLLT disrupts bacterial biofilms and decreases bacterial counts in patients with nasal colonization. Photoactive (PA) agents also produce cytotoxic species when excited with specific wavelengths of light causing bacterial cell death [68]. These findings lead Krespi and Kizhner to further test LLLT as a potential therapy for patients with chronic rhinosinusitis. NIR alone was compared with laser-activated indocyanine green, a PA agent which has been successful in the eradication of common bacterial pathogens in chronic rhinosinusitis (CRS) [69] as well as treatment of acne vulgaris [70]. The study evaluated 23 symptomatic, post-surgical CRS patients with nasal cultures positive for pathogenic bacteria. Group 1 was treated with 940-nm laser intra-nasally, and group 2 was treated with a combination of indocyanine green and 810-nm laser (wavelength to which indocyanine green is responsive) intra-nasally. Clinical efficacy using a validated patient-reported outcome measure (SNOT20; Sino-Nasal Outcome Test) and nasal endoscopic scoring was statistically different (p < 0.05) in both groups at the end of the study compared with readouts at the beginning of the study [71]. There was no difference between LLLT alone as compared with LLLT plus indocyanine green with regard to nasal culture growth or diversity, and the endoscopy results and SNOT20 were not different between treatment groups. This suggests that laser illumination has clinical benefit in the treatment of CRS independent of the effects of a PA agent; however, this study was not controlled, and there would be an added value from an untreated control group to compare the efficacy ofLLLT in the treatment of refractory CRS with respect to patient-reported outcome measures and bacterial diversity/amount of growth.
Nephrology
Anti-glomerular basement membrane (GBM) disease is an autoimmune disease defined by antibodies directed against the glomerular and/or pulmonary basement membrane. Human glomerulonephritis-associated renal injury is associated with the infiltration of macrophages within the glomeruli and interstitium [72]. These macrophages establish a pro-inflammatory environment via mediators including TNF-α, IL-1β, and ROS [73–75]. LLLT has shown promise as an anti-inflammatory agent that can reduce these and other components of the inflammatory response. Although LLLT has been studied as a therapeutic modality for pathologies that are either at or just below the skin surface, there has been little study of LLLT effects on internal organs given the limitations of laser depth.
Yamamoto et al. sought to assess the effect of LLLT in a rat model of crescentic glomerulonephritis. They evaluated external application of LLLT for 14 days following injection of an anti-GBM antibody into rats. RT-PCR analysis of cortical tissue isolated from the left kidney of each animal showed that following LLLT, levels of TNF-α were attenuated albeit not significantly (p = 0.07) and mRNA levels of IL-1β were significantly decreased (p < 0.05). Histologically, LLLT suppressed crescent formation as compared with control (GBM without LLLT, 82.0 ± 4.7%, GBM with LLLT, 46.8 ± 9.9%, p < 0.05). LLLT treatment also significantly decreased the number of CD8+ lymphocytes infiltrating the glomerulus compared with control (p < 0.05) and appeared to suppress the infiltration of ED1-expressing macrophages, though this difference was not significant [76]. Some important limitations of this study include a difference in laser energy delivered to the right and left kidneys (25 J/cm2 and 11 J/cm2, respectively), which is thought to be due to interference from the spleen. Further, rat kidneys are much easier to access with laser applied externally, and in order to apply this treatment modality to the human kidney, an internal source, such as an implant, would be required. Although this pre-clinical in vivo data is compelling, it highlights a major limitation of LLLT as a treatment for deeper tissues; specifically noted was a distinctly different energy dose delivered per kidney given its proximity to other organs. Even if a laser could penetrate to the appropriate depth, this study highlights that anatomical interference would still be a barrier to delivery. Nevertheless, this study is an important first step toward applying LLLT to deeper tissues and expanding beyond external application.
Neurology
Multiple sclerosis (MS) is a chronic, inflammatory disease of the CNS defined by aberrant TH1 and TH17 lymphocytes that react to myelin. Studies in experimental autoimmune encephalomyelitis (EAE) show many similarities to MS and support the hypothesis that MS originates from activation of CNS antigen-specific CD4+ T cells in the periphery [77]. Additionally, EAE is characterized by overproduction of NO by innate immune cells, such as macrophages and microglia, which is also implicated in many diseases of neurological dysfunction. LLLT has been studied in central nervous system disease as an adjunct therapy for diseases including Alzheimer’s in mouse models, as well as neurologic deficits related to stroke and traumatic brain injury in rats and rabbits [78–82]. It has also shown promising anti-inflammatory effects in immune-mediated diseases as discussed above, making LLLT a potential therapy for MS.
Goncalves et al. examined both the effect and mechanisms of LLLT in EAE [83]. In this study, the two laser wavelengths tested (660 nm, 904 nm) reduced the NO influx seen in the spinal cord with EAE induction (p < 0.01) and markedly inhibited clinical signs of disease and EAE-associated weight loss, a clinical marker for disease severity and deterioration. Although both 660-nm and 904-nm treatments significantly decreased IL-17, IFN-γ, and IL-1β levels in the spinal cord, only the 660-nm laser significantly reduced the infiltration of inflammatory cells into the CNS (p < 0.02) and attenuated demyelination. This study indicates that LLLT is effective against the neuroinflammation in this animal model of multiple sclerosis and may prove to be a successful treatment modality in the future. It also illustrates the need to consider wavelength as an important variable to study in a given disease as there were clear differences in between the two laser types tested in this study.
Pulmonology
Asthma, a chronic inflammatory disease of the airways, is characterized by an imbalance in the ratio of CD4+ T cell subtypes: TH1 and TH2. It is also known to involve various innate immune cell types (eosinophils, mast cells, neutrophils), cytokines (IL-4, IL-13, IFN-γ), and IgE [84–86]. Given that LLLT has shown promising anti-inflammatory effects in other disorders defined by an abnormal T cell response such as RA and AA as discussed above, it was evaluated as a potential treatment for allergic asthma. This is particularly relevant as most cases of allergic asthma are treated with inhaled and systemic glucocorticoid therapy, the latter ofwhich has known significant, long-term side effects [87].
Wang et al. sought to evaluate the effects of LLLT as compared with inhaled budesonide in a rat model of allergic asthma. Animals were treated once daily with LLLT or inhaled budesonide for 21 days. There was no significant difference between LLLT and budesonide groups; however, compared with the asthmatic untreated control group, LLLT decreased overall inflammatory cell numbers in bronchiolar lavage (BAL) fluid and specifically decreased eosinophils. On histopathology, LLLT reduced the infiltration of inflammatory cells similar to budesonide. LLLT also reduced IL-4 and IFN-y levels in BAL and serum and serum IgE [88].
Souza et al. carried out an in vitro study of LLLT on U937 cells that are representative of alveolar macrophages in the setting of oxidative stress and lipopolysaccharide (LPS) as a model cell system of bronchial asthma and chronic obstructive pulmonary disease (COPD). The authors specifically looked at the mechanisms of LLLT in the U937 cells subjected to oxidative stress and the ability for LLLT to restore glucocorticoid sensitivity, which is thought to be a result of histone deacetylase (HDAC) inhibition [89]. Following treatment with LLLT, an upregulation of HDAC activity was seen in vitro with inhibition of LPS/H202-induced TNF-α and IL-8 secretion. This was reliant upon cAMP-dependent protein kinase A elevation and the inhibition of PI3K signaling, mechanisms proposed by the authors by which LLLT promotes anti-inflammatory effects. Further, with restoration of HDAC activity, LLLT was able to re-sensitize the U937 cells to the action of dexamethasone, where they had previously been glucocorticoid-resistant [90].
Although these studies provide some interesting mechanistic data, the in vivo asthma study in particular shows a less robust clinical effect in a TH2-mediated disease, consistent with the AD pilot data. Given the small number of pre-clinical and in vivo studies in humans and animals, the varying applications and wavelength of lasers, and the timing/duration of LLLT application during disease, it is difficult to conclude whether or not LLLT mechanistically is more efficacious in off-setting TH1/ TH17 inflammation. Repeated and more standardized clinical studies will be required to better interpret the impact of LLLT on inflammatory-mediated disease.
Phototherapeutics
As laser therapy itself has been studied for the treatment of various disease states, there have also been developments in the use of laser light to trigger delivery of drugs, referred to as phototherapeutics or photodynamic therapy (PDT). In the management of cancer, PDT has been used to deliver localized bursts of cytotoxic O2 to tissues marked for destruction [91]. It has also been studied for antimicrobial use in skin and mucosal infections with success [92, 93]. Fan et al. described its use to deliver 5-aminolevulinic acid for the treatment of rosacea, a chronic skin condition with unknown pathogenesis [94]. Light-activated pro-drugs have also been evaluated [95–97], though they have been limited by short spectral wavelengths (< 450) with little ability to penetrate tissue. The optical window of tissue defines red and NIR spectral wavelengths of 600–900-nm light that are able to penetrate tissue up to 3 cm deep and subsequently overcome limitations of light absorption and scatter [98–105]. The ability to penetrate tissue at a deeper level makes PDT a favorable option for disease pathologies that are not limited to the skin. Further, it allows for the combination of laser therapy with an additional therapeutic, potentially creating a superior treatment option that allows for selective delivery of a photoreleasable drug to a targeted area rather than systemic delivery. There have been exciting breakthroughs in the development of photoresponsive agents that can be activated by longer wavelengths that are better able to penetrate tissue [106]. Lawrence et al. conjugated anti-inflammatory drugs to the light-responsive fluorophore Cy5 anchored to vitamin B12. These photocleavable drug conjugates can be loaded into and transported by red blood cells (RBCs). In vitro studies have shown RBC-B12-colchicine successfully disrupts the microtubule network of co-incubated HeLa cells after wavelength-specific photocleavage [107]. This is a promising new technology for the treatment of inflammatory disease, particularly RA where high-dose systemic therapy with substantial side effects is often required to address joint pathology.
Conclusions
LLLT is a promising therapeutic modality in the realm of inflammatory diseases, particularly those of skin and joints that are most accessible to treatment. It offers a unique approach by being non-invasive and incurring minimal side effects. It is also relatively inexpensive and accessible and even has the possibility to be patient directed at home. For the majority of inflammatory diseases, the standard of care is immunosuppressive agents such as corticosteroids that have a multitude of undesirable systemic consequences. LLLT has the potential to be used as both an adjunct and stand-alone treatment in a variety of these conditions and could possibly minimize side effects. In particular, the known mechanisms of LLLT support its use for anti-inflammatory purposes, as well as stimulation of tissue growth and repair. There is evidence that LLLT is able to modulate the immune system at the skin [40•, 42, 52] and joint [25, 108] and has been shown to be efficacious in humans in affecting bacterial colonization as it may pertain to chronic rhinosinusitis [71, 109]. At the pre-clinical level of investigation, it has been shown to modulate immunologic processes in animal models of multiple sclerosis, [83] allergic asthma, [88, 90, 110], and rheumatoid arthritis [24, 25, 34, 108].
Importantly, there is high variability in methods of application as well as a lack of evidence for laser type, dose-ranging studies, and wavelength selection that create barriers to the implementation of LLLT without further more rigorous and standardized study. The heterogeneity among studies makes it difficult to draw strong conclusions about the efficacy of low-level laser therapy and its mechanisms. Though Clijsen et al. did not find significant differences among studies that adhered to the WALT guidelines compared with those that did not [18], standardization of laser protocols will likely be required for measuring treatment efficacy in the future. The results presented here show a foundation for the future investigation of LLLT as a treatment in inflammatory disease and a summary of the field to date.
Acknowledgments
The authors would like to acknowledge the following funding sources: Rheumatology Research Foundation (to Teresa K. Tarrant, David S. Lawrence, and Victoria A. Wickenheisser), National Institutes of Health T32 CA71341 (to Emilia M. Zywot), The Lineberger Cancer Center and The UNC Eshelman Institute for Innovation, University of North Carolina ( to David S. Lawrence), and The Eu gene A. Stead Student Research Scholarship, Duke University (to Victoria A. Wickenheisser).
Footnotes
Conflict of Interest The authors declare no conflicts of interest relevant to this manuscript.
Compliance with Ethical Standards
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.••.Chung H, Dai T, Sharma SK, Huang YY, Carroll JD, Hamblin MR. The nuts and bolts of low-level laser (light) therapy. Ann Biomed Eng. 2012;40(2):516–33 10.1007/s10439-011-0454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]; This provides foundational knowledge on the components and evolution of LLLT. This provides foundational knowledge on the components and evolution of LLLT.
- 2.••.de Freitas LF, Hamblin MR. Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J Sel Top Quantum Electron. 2016;22(3). Doi: 10.1109/JSTQE.2016.2561201. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study summarizes the current proposed mechanisms of LLLT.
- 3.Lupton JR, Alster TS. Laser scar revision. Dermatol Clin. 2002;20(1):55–65. [DOI] [PubMed] [Google Scholar]
- 4.Felsher DW. Cancer revoked: oncogenes as therapeutic targets. Nat Rev Cancer. 2003;3(5):375–80. 10.1038/nrc1070. [DOI] [PubMed] [Google Scholar]
- 5.Karu TI. Multiple roles of cytochrome c oxidase in mammalian cells under action of red and IR-A radiation. IUBMB Life. 2010;62(8):607–10. 10.1002/iub.359. [DOI] [PubMed] [Google Scholar]
- 6.Poyton RO, Ball KA. Therapeutic photobiomodulation: nitric oxide and a novel function of mitochondrial cytochrome c oxidase. Discov Med. 2011;11(57):154–9. [PubMed] [Google Scholar]
- 7.Chen AC, Arany PR, Huang YY, Tomkinson EM, Sharma SK, Kharkwal GB, et al. Low-level laser therapy activates NF-kB via generation of reactive oxygen species in mouse embryonic fibroblasts. PLoS One. 2011;6(7):e22453 10.1371/journal.pone.0022453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao X, Xing D. Molecular mechanisms of cell proliferation induced by low power laser irradiation. J Biomed Sci. 2009;16:4 10.1186/1423-0127-16-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nesioonpour S, Mokmeli S, Vojdani S, Mohtadi A, Akhondzadeh R, Behaeen K, et al. The effect of low-level laser on postoperative pain after tibial fracture surgery: a double-blind controlled randomized clinical trial. Anesth Pain Med. 2014;4(3):e17350 10.5812/aapm.17350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falaki F, Nejat AH, Dalirsani Z. The effect of low-level laser therapy on trigeminal neuralgia: a review of literature. J Dent Res Dent Clin Dent Prospects. 2014;8(1):1–5. 10.5681/joddd.2014.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cotler HB, Chow RT, Hamblin MR, Carroll J. The use of low level laser therapy (LLLT) for musculoskeletal pain. MOJ Orthop Rheumatol. 2015;2(5). 10.15406/mojor.2015.02.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bashiri H Evaluation of low level laser therapy in reducing diabetic polyneuropathy related pain and sensorimotor disorders. Acta Med Iran. 2013;51(8):543–7. [PubMed] [Google Scholar]
- 13.Chow RT, David MA, Armati PJ. 830 nm laser irradiation induces varicosity formation, reduces mitochondrial membrane potential and blocks fast axonal flow in small and medium diameter rat dorsal root ganglion neurons: implications for the analgesic effects of 830 nm laser. J Peripher Nerv Syst. 2007;12(1):28–39. 10.1111/j.1529-8027.2007.00114.x. [DOI] [PubMed] [Google Scholar]
- 14.Carati CJ, Anderson SN, Gannon BJ, Piller NB. Treatment of postmastectomy lymphedema with low-level laser therapy: a double blind, placebo-controlled trial. Cancer. 2003;98(6):1114–22. 10.1002/cncr.11641. [DOI] [PubMed] [Google Scholar]
- 15.Klein T, Magerl W, Hopf HC, Sandkuhler J, Treede RD. Perceptual correlates of nociceptive long-term potentiation and long-term depression in humans. J Neurosci. 2004;24(4):964–71. 10.1523/JNEUROSCI.1222-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hagiwara S, Iwasaka H, Okuda K, Noguchi T. GaAlAs (830 nm) low-level laser enhances peripheral endogenous opioid analgesia in rats. Lasers Surg Med. 2007;39(10):797–802. 10.1002/lsm.20583. [DOI] [PubMed] [Google Scholar]
- 17.Dima R, Tieppo Francio V, Towery C, Davani S. Review of literature on low-level laser therapy benefits for nonpharmacological pain control in chronic pain and osteoarthritis. Altern Ther Health Med. 2017. [PubMed] [Google Scholar]
- 18.Clijsen R, Brunner A, Barbero M, Clarys P, Taeymans J. Effects of low-level laser therapy on pain in patients with musculoskeletal disorders: a systematic review and meta-analysis. Eur J Phys Rehabil Med. 2017;53(4):603–10. 10.23736/S1973-9087.17.04432-X. [DOI] [PubMed] [Google Scholar]
- 19.Todd KH, Funk JP The minimum clinically important difference in physician-assigned visual analog pain scores. Acad Emerg Med. 1996;3(2):142–6. [DOI] [PubMed] [Google Scholar]
- 20.Gallagher EJ, Bijur PE, Latimer C, Silver W. Reliability and validity of a visual analog scale for acute abdominal pain in the ED. Am J Emerg Med. 2002;20(4):287–90. [DOI] [PubMed] [Google Scholar]
- 21.Rochkind S, Drory V, Alon M, Nissan M, Ouaknine GE. Laser phototherapy (780 nm), a new modality in treatment of long-term incomplete peripheral nerve injury: a randomized double-blind placebo-controlled study. Photomed Laser Surg. 2007;25(5):436–42. 10.1089/pho.2007.2093. [DOI] [PubMed] [Google Scholar]
- 22.Lazovic M, Ilic-Stojanovic O, Kocic M, Zivkovic V, Hrkovic M, Radosavljevic N. Placebo-controlled investigation of low-level laser therapy to treat carpal tunnel syndrome. Photomed Laser Surg. 2014;32(6):336–44. 10.1089/pho.2013.3563. [DOI] [PubMed] [Google Scholar]
- 23.Bekhet AH, Ragab B, Abushouk AI, Elgebaly A, Ali OI. Efficacy of low-level laser therapy in carpal tunnel syndrome management: a systematic review and meta-analysis. Lasers Med Sci. 2017;32(6):1439–48. 10.1007/s10103-017-2234-6. [DOI] [PubMed] [Google Scholar]
- 24.Majithia V, Geraci SA. Rheumatoid arthritis: diagnosis and management. Am J Med. 2007;120(11):936–9. 10.1016/j.amjmed.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Alves AC, de Carvalho PT, Parente M, Xavier M, Frigo L, Aimbire F, et al. Low-level laser therapy in different stages of rheumatoid arthritis: a histological study. Lasers Med Sci. 2013;28(2):529–36. 10.1007/s10103-012-1102-7. [DOI] [PubMed] [Google Scholar]
- 26.Sasai M, Saeki Y, Ohshima S, Nishioka K, Mima T, Tanaka T, et al. Delayed onset and reduced severity of collagen-induced arthritis in interleukin-6-deficient mice. Arthritis Rheum. 1999;42(8):1635–43. . [DOI] [PubMed] [Google Scholar]
- 27.Sack U, Kinne RW, Marx T, Heppt P, Bender S, Emmrich F. Interleukin-6 in synovial fluid is closely associated with chronic synovitis in rheumatoid arthritis. Rheumatol Int. 1993;13(2):45–51. [DOI] [PubMed] [Google Scholar]
- 28.Madhok R, Crilly A, Watson J, Capell HA. Serum interleukin 6 levels in rheumatoid arthritis: correlations with clinical and laboratory indices of disease activity. Ann Rheum Dis. 1993;52(3):232–4. 10.1136/ard.52.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gomez-Reino JJ, Carmona L, Group B. Switching TNF antagonists in patients with chronic arthritis: an observational study of 488 patients over a four-year period. Arthritis Res Ther. 2006;8(1):R29 10.1186/ar1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hyrich KL, Lunt M, Watson KD, Symmons DP, Silman AJ, British Society for Rheumatology Biologics R. Outcomes after switching from one anti-tumor necrosis factor alpha agent to a second anti-tumor necrosis factor alpha agent in patients with rheumatoid arthritis: results from a large UK national cohort study. Arthritis Rheum 2007;56(1):13–20. doi: 10.1002/art.22331. [DOI] [PubMed] [Google Scholar]
- 31.Nuki G, Bresnihan B, Bear MB, McCabe D, European Group Of Clinical I. Long-term safety and maintenance of clinical improvement following treatment with anakinra (recombinant human interleukin-1 receptor antagonist) in patients with rheumatoid arthritis: extension phase of a randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2002;46(11):2838–2846. doi: 10.1002/art.10578. [DOI] [PubMed] [Google Scholar]
- 32.Serafin DS, Allyn B, Sassano MF, Timoshchenko RG, Mattox D, Brozowski JM, et al. Chemerin-activated functions of CMKLR1 are regulated by G protein-coupled receptor kinase 6 (GRK6) and beta-arrestin 2 in inflammatory macrophages. Mol Immunol. 2019;106:12–21. 10.1016/j.molimm.2018.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tarrant TK, Patel DD. Chemokines and leukocyte trafficking in rheumatoid arthritis. Pathophysiology. 2006;13(1):1–14. 10.1016/j.pathophys.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 34.Zhang L, Zhao J, Kuboyama N, Abiko Y. Low-level laser irradiation treatment reduces CCL2 expression in rat rheumatoid synovia via a chemokine signaling pathway. Lasers Med Sci. 2011;26(5):707–17. 10.1007/s10103-011-0917-y. [DOI] [PubMed] [Google Scholar]
- 35.Avci P, Gupta A, Sadasivam M, Vecchio D, Pam Z, Pam N, et al. Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Semin Cutan Med Surg. 2013;32(1):41–52. [PMC free article] [PubMed] [Google Scholar]
- 36.Lee SY, You CE, Park MY. Blue and red light combination LED phototherapy for acne vulgaris in patients with skin phototype IV. Lasers Surg Med. 2007;39(2):180–8. 10.1002/lsm.20412. [DOI] [PubMed] [Google Scholar]
- 37.Ross EV Optical treatments for acne. Dermatol Ther. 2005;18(3):253–66. 10.1111/j.1529-8019.2005.05024.x. [DOI] [PubMed] [Google Scholar]
- 38.Rotunda AM, Bhupathy AR, Rohrer TE. The new age of acne therapy: light, lasers, and radiofrequency. J Cosmet Laser Ther. 2004;6(4):191–200. 10.1080/14764170410008124. [DOI] [PubMed] [Google Scholar]
- 39.Sadick NS. Handheld LED array device in the treatment of acne vulgaris. J Drugs Dermatol. 2008;7(4):347–50. [PubMed] [Google Scholar]
- 40.•.Omi T, Kawana S, Sato S, Takezaki S, Honda M, Igarashi T, et al. Cutaneous immunological activation elicited by a low-fluence pulsed dye laser. Br J Dermatol. 2005;153(Suppl 2):57–62. 10.1111/j.1365-2133.2005.06971.x [DOI] [PubMed] [Google Scholar]; This study describes the changes induced by LLLT in healthy human skin and supports its immunomodulatory effects.
- 41.Greb JE, Goldminz AM, Elder JT, Lebwohl MG, Gladman DD, Wu JJ, et al. Psoriasis. Nat Rev Dis Primers. 2016;2:16082 10.1038/nrdp.2016.82. [DOI] [PubMed] [Google Scholar]
- 42.Zhang P, Wu MX. A clinical review of phototherapy for psoriasis. Lasers Med Sci. 2018;33(1):173–80. 10.1007/s10103-017-2360-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tartar D, Bhutani T, Huynh M, Berger T, Koo J. Update on the immunological mechanism of action behind phototherapy. J Drugs Dermatol. 2014;13(5):564–8. [PubMed] [Google Scholar]
- 44.Pittelkow MR, Perry HO, Muller SA, Maughan WZ, O’Brien PC. Skin cancer in patients with psoriasis treated with coal tar. A 25-year follow-up study. Arch Dermatol. 1981;117(8):465–8. [PubMed] [Google Scholar]
- 45.Stern RS, Study PF-U. The risk of squamous cell and basal cell cancer associated with psoralen and ultraviolet A therapy: a 30-year prospective study. J Am Acad Dermatol. 2012;66(4):553–62. 10.1016/j.jaad.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 46.Stem RS, Study PF. The risk of melanoma in association with long-term exposure to PUVA. J Am Acad Dermatol. 2001;44(5):755–61. 10.1067/mjd.2001.114576. [DOI] [PubMed] [Google Scholar]
- 47.Combination Ablon G. 830-nm and 633-nm light-emitting diode phototherapy shows promise in the treatment of recalcitrant psoriasis: preliminary findings. Photomed Laser Surg. 2010;28(1):141–6. 10.1089/pho.2009.2484. [DOI] [PubMed] [Google Scholar]
- 48.Weinstabl A, Hoff-Lesch S, Merk HF, von Felbert V. Prospective randomized study on the efficacy of blue light in the treatment of psoriasis vulgaris. Dermatology. 2011;223(3):251–9. 10.1159/000333364. [DOI] [PubMed] [Google Scholar]
- 49.Bernardez C, Molina-Ruiz AM, Requena L. Histologic features of alopecias-part I: nonscarring alopecias. Actas Dermosifiliogr. 2015;106(3):158–67. 10.1016/j.ad.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 50.Tzung TY, Chen CY, Tzung TY, Kao FJ, Chen WC. Infrared irradiation as an adjuvant therapy in recalcitrant alopecia areata. Dermatol Surg. 2009;35(4):721–3. 10.1111/j.1524-4725.2009.01120.x. [DOI] [PubMed] [Google Scholar]
- 51.Yoo KH, Kim MN, Kim BJ, Kim CW. Treatment of alopecia areata with fractional photothermolysis laser. Int J Dermatol. 2010;49(7):845–7. 10.1111/j.1365-4632.2009.04230.x. [DOI] [PubMed] [Google Scholar]
- 52.Lanzafame RJ, Blanche RR, Chiacchierini RP, Kazmirek ER, Sklar JA. The growth of human scalp hair in females using visible red light laser and LED sources. Lasers Surg Med. 2014;46(8):601–7. 10.1002/lsm.22277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mlacker S, Aldahan AS, Simmons BJ, Shah V, McNamara CA, Samarkandy S, et al. A review on laser and light-based therapies for alopecia areata. J Cosmet Laser Ther. 2017;19(2):93–9. 10.1080/14764172.2016.1248440. [DOI] [PubMed] [Google Scholar]
- 54.Darwin E, Arora H, Hirt PA, Wikramanayake TC, Jimenez JJ. A review of monochromatic light devices for the treatment of alopecia areata. Lasers Med Sci. 2018;33(2):435–44. 10.1007/s10103-017-2412-6. [DOI] [PubMed] [Google Scholar]
- 55.Mester E, Szende B, Gartner P. The effect of laser beams on the growth of hair in mice. Radiobiol Radiother (Berl). 1968;9(5):621–6. [PubMed] [Google Scholar]
- 56.Lolis MS, Marmur ES. Paradoxical effects of hair removal systems: a review. J Cosmet Dermatol. 2006;5(4):274–6. 10.1111/j.1473-2165.2006.00269.x. [DOI] [PubMed] [Google Scholar]
- 57.Waiz M, Saleh AZ, Hayani R, Jubory SO. Use of the pulsed infrared diode laser (904 nm) in the treatment of alopecia areata. J Cosmet Laser Ther. 2006;8(1):27–30. 10.1080/14764170600607368. [DOI] [PubMed] [Google Scholar]
- 58.Yamazaki M, Miura Y, Tsuboi R, Ogawa H. Linear polarized infrared irradiation using Super Lizer is an effective treatment for multiple-type alopecia areata. Int J Dermatol. 2003;42(9):738–40. [DOI] [PubMed] [Google Scholar]
- 59.Simon D, Braathen LR, Simon HU. Eosinophils and atopic dermatitis. Allergy. 2004;59(6):561–70. 10.1111/j.1398-9995.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- 60.Stich AN, Rosenkrantz WS, Griffin CE. Clinical efficacy of low-level laser therapy on localized canine atopic dermatitis severity score and localized pruritic visual analog score in pedal pruritus due to canine atopic dermatitis. Vet Dermatol. 2014;25(5):464–e74. 10.1111/vde.12144. [DOI] [PubMed] [Google Scholar]
- 61.Conzemius MG, Evans RB. Caregiver placebo effect for dogs with lameness from osteoarthritis. J Am Vet Med Assoc. 2012;241(10):1314–9. 10.2460/javma.241.10.1314. [DOI] [PubMed] [Google Scholar]
- 62.Perl TM, Cullen JJ, Wenzel RP, Zimmerman MB, Pfaller MA, Sheppard D, et al. Intranasal mupirocin to prevent postoperative Staphylococcus aureus infections. N Engl J Med. 2002;346(24):1871–7. 10.1056/NEJMoa003069. [DOI] [PubMed] [Google Scholar]
- 63.Bode LG, Kluytmans JA, Wertheim HF, Bogaers D, Vandenbroucke-Grauls CM, Roosendaal R, et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med. 2010;362(1):9–17. 10.1056/NEJMoa0808939. [DOI] [PubMed] [Google Scholar]
- 64.Krespi YP, Kizhner V. Laser-assisted nasal decolonization of Staphylococcus aureus, including methicillin-resistant Staphylococcus aureus. Am J Otolaryngol. 2012;33(5):572–5. 10.1016/j.amjoto.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 65.Benninger MS, Ferguson BJ, Hadley JA, Hamilos DL, Jacobs M, Kennedy DW, et al. Adult chronic rhinosinusitis: definitions, diagnosis, epidemiology, and pathophysiology. Otolaryngol Head Neck Surg. 2003;129(3 Suppl):S1–32. [DOI] [PubMed] [Google Scholar]
- 66.Harvey RJ, Lund VJ. Biofilms and chronic rhinosinusitis: systematic review of evidence, current concepts and directions for research. Rhinology. 2007;45(1):3–13. [PubMed] [Google Scholar]
- 67.Kariyawasam HH, Scadding GK. Chronic rhinosinusitis: therapeutic efficacy of anti-inflammatory and antibiotic approaches. Allergy Asthma Immunol Res. 2011;3(4):226–35. 10.4168/aair.2011.3.4.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nussbaum EL, Lilge L, Mazzulli T. Effects of low-level laser therapy (LLLT) of 810 nm upon in vitro growth of bacteria: relevance of irradiance and radiant exposure. J Clin Laser Med Surg. 2003;21(5):283–90. 10.1089/104454703322564497. [DOI] [PubMed] [Google Scholar]
- 69.Omar GS, Wilson M, Nair SP. Lethal photosensitization of wound-associated microbes using indocyanine green and near-infrared light. BMC Microbiol. 2008;8:111 10.1186/1471-2180-8-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tuchin VV, Genina EA, Bashkatov AN, Simonenko GV, Odoevskaya OD, Altshuler GB. A pilot study of ICG laser therapy of acne vulgaris: photodynamic and photothermolysis treatment. Lasers Surg Med. 2003;33(5):296–310. 10.1002/lsm.10211. [DOI] [PubMed] [Google Scholar]
- 71.Krespi YP, Kizhner V. Phototherapy for chronic rhinosinusitis. Lasers Surg Med. 2011;43(3):187–91. 10.1002/lsm.21042. [DOI] [PubMed] [Google Scholar]
- 72.Nikolic-Paterson DJ, Atkins RC. The role of macrophages in glomerulonephritis. Nephrol Dial Transplant. 2001;16(Suppl 5):3–7. [DOI] [PubMed] [Google Scholar]
- 73.Takemura T, Yoshioka K, Murakami K, Akano N, Okada M, Aya N, et al. Cellular localization of inflammatory cytokines in human glomerulonephritis. Virchows Arch. 1994;424(5):459–64. [DOI] [PubMed] [Google Scholar]
- 74.Bremer V, Tojo A, Kimura K, Hirata Y, Goto A, Nagamatsu T, et al. Role of nitric oxide in rat nephrotoxic nephritis: comparison between inducible and constitutive nitric oxide synthase. J Am Soc Nephrol. 1997;8(11):1712–21. [DOI] [PubMed] [Google Scholar]
- 75.Tesch GH, Yang N, Yu H, Lan HY, Foti R, Chadban SJ, et al. Intrinsic renal cells are the major source of interleukin-1 beta synthesis in normal and diseased rat kidney. Nephrol Dial Transplant. 1997;12(6):1109–15. [DOI] [PubMed] [Google Scholar]
- 76.Yamato M, Kaneda A, Kataoka Y. Low-level laser therapy improves crescentic glomerulonephritis in rats. Lasers Med Sci. 2013;28(4):1189–96. 10.1007/s10103-012-1229-6. [DOI] [PubMed] [Google Scholar]
- 77.Sospedra M, Martin R. Immunology of multiple sclerosis. Semin Neurol. 2016;36(2):115–27. 10.1055/s-0036-1579739. [DOI] [PubMed] [Google Scholar]
- 78.Oron A, Oron U, Chen J, Eilam A, Zhang C, Sadeh M, et al. Low-level laser therapy applied transcranially to rats after induction of stroke significantly reduces long-term neurological deficits. Stroke. 2006;37(10):2620–4. 10.1161/01.STR.0000242775.14642.b8. [DOI] [PubMed] [Google Scholar]
- 79.Xuan W, Vatansever F, Huang L, Wu Q, Xuan Y, Dai T, et al. Transcranial low-level laser therapy improves neurological performance in traumatic brain injury in mice: effect of treatment repetition regimen. PLoS One. 2013;8(1):e53454 10.1371/journal.pone.0053454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Oron A, Oron U, Streeter J, De Taboada L, Alexandrovich A, Trembovler V, et al. Near infrared transcranial laser therapy applied at various modes to mice following traumatic brain injury significantly reduces long-term neurological deficits. J Neurotrauma. 2012;29(2):401–7. 10.1089/neu.2011.2062. [DOI] [PubMed] [Google Scholar]
- 81.Farfara D, Tuby H, Trndler D, Doron-Mandel E, Maltz L, Vassar RJ, et al. Low-level laser therapy ameliorates disease progression in a mouse model of Alzheimer’s disease. J Mol Neurosci. 2015;55(2):430–6. 10.1007/s12031-014-0354-z. [DOI] [PubMed] [Google Scholar]
- 82.De Taboada L, Yu J, El-Amouri S, Gattoni-Celli S, Richieri S, McCarthy T, et al. Transcranial laser therapy attenuates amyloid-beta peptide neuropathology in amyloid-beta protein precursor transgenic mice. J Alzheimers Dis. 2011;23(3):521–35. 10.3233/JAD-2010-100894. [DOI] [PubMed] [Google Scholar]
- 83.Goncalves ED, Souza PS, Lieberknecht V, Fidelis GS, Barbosa RI, Silveira PC, et al. Low-level laser therapy ameliorates disease progression in a mouse model of multiple sclerosis. Autoimmunity. 2016;49(2):132–42. 10.3109/08916934.2015.1124425. [DOI] [PubMed] [Google Scholar]
- 84.Rothers J, Halonen M, Stern DA, Lohman IC, Mobley S, Spangenberg A, et al. Adaptive cytokine production in early life differentially predicts total IgE levels and asthma through age 5 years. J Allergy Clin Immunol. 2011;128(2):397–402 e2 10.1016/j.jaci.2011.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dubois A, Deruytter N, Adams B, Kanda A, Delbauve S, Fleury S, et al. Regulation of Th2 responses and allergic inflammation through bystander activation of CD8+ T lymphocytes in early life. J Immunol. 2010;185(2):884–91. 10.4049/jimmunol.0903287. [DOI] [PubMed] [Google Scholar]
- 86.Luzina IG, Lockatell V, Lavania S, Pickering EM, Kang PH, Bashkatova YN, et al. Natural production and functional effects of alternatively spliced interleukin-4 protein in asthma. Cytokine. 2012;58(1):20–6. 10.1016/j.cyto.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Basu K, Nair A, Williamson PA, Mukhopadhyay S, Lipworth BJ. Airway and systemic effects of soluble and suspension formulations of nebulized budesonide in asthmatic children. Ann Allergy Asthma Immunol. 2009;103(5):436–41. 10.1016/S1081-1206(10)60365-1. [DOI] [PubMed] [Google Scholar]
- 88.Wang XY, Ma WJ, Liu CS, Li YX. Effect of low-level laser therapy on allergic asthma in rats. Lasers Med Sci. 2014;29(3):1043–50. 10.1007/s10103-013-1456-5. [DOI] [PubMed] [Google Scholar]
- 89.Barnes PJ. Mechanisms and resistance in glucocorticoid control of inflammation. J Steroid Biochem Mol Biol. 2010;120(2–3):76–85. 10.1016/j.jsbmb.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 90.Souza NH, Marcondes PT, Albertini R, Mesquita-Ferrari RA, Fernandes KP, Aimbire F. Low-level laser therapy suppresses the oxidative stress-induced glucocorticoids resistance in U937 cells: relevance to cytokine secretion and histone deacetylase in alveolar macrophages. J Photochem Photobiol B. 2014;130:327–36. 10.1016/j.jphotobiol.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 91.Shirasu N, Nam SO, Kuroki M. Tumor-targeted photodynamic therapy. Anticancer Res. 2013;33(7):2823–31. [PubMed] [Google Scholar]
- 92.Perez-Laguna V, Gilaberte Y, Millan-Lou MI, Agut M, Nonell S, Rezusta A, et al. A combination of photodynamic therapy and antimicrobial compounds to treat skin and mucosal infections: a systematic review. Photochem Photobiol Sci. 2019;18(5):1020–9. 10.1039/c8pp00534f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huang L, Xuan W, Sarna T, Hamblin MR. Comparison of thiocyanate and selenocyanate for potentiation of antimicrobial photodynamic therapy. J Biophotonics. 2019;12(1):e201800092 10.1002/jbio.201800092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fan L, Yin R, Lan T, Hamblin MR. Photodynamic therapy for rosacea in Chinese patients. Photodiagn Photodyn Ther. 2018;24:82–7. 10.1016/j.pdpdt.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shamay Y, Adar L, Ashkenasy G, David A. Light induced drug delivery into cancer cells. Biomaterials. 2011;32(5):1377–86. 10.1016/j.biomaterials.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 96.Thompson S, Self AC, Self CH. Light-activated antibodies in the fight against primary and metastatic cancer. Drug Discov Today. 2010;15(11–12):468–73. 10.1016/j.drudis.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 97.Yavlovich A, Smith B, Gupta K, Blumenthal R, Puri A. Light-sensitive lipid-based nanoparticles for drug delivery: design principles and future considerations for biological applications. Mol Membr Biol. 2010;27(7):364–81. 10.3109/09687688.2010.507788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Strangman GE, Zhang Q, Li Z. Scalp and skull influence on near infrared photon propagation in the Colin27 brain template. Neuroimage. 2014;85(Pt 1):136–49. 10.1016/j.neuroimage.2013.04.090. [DOI] [PubMed] [Google Scholar]
- 99.Haeussinger FB, Heinzel S, Hahn T, Schecklmann M, Ehlis AC, Fallgatter AJ. Simulation of near-infrared light absorption considering individual head and prefrontal cortex anatomy: implications for optical neuroimaging. PLoS One. 2011;6(10):e26377 10.1371/journal.pone.0026377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Okada E, Delpy DT. Near-infrared light propagation in an adult head model. I. Modeling of low-level scattering in the cerebrospinal fluid layer. Appl Opt. 2003;42(16):2906–14. [DOI] [PubMed] [Google Scholar]
- 101.Jagdeo JR, Adams LE, Brody NI, Siegel DM. Transcranial red and near infrared light transmission in a cadaveric model. PLoS One. 2012;7(10):e47460 10.1371/journal.pone.0047460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tedford CE, DeLapp S, Jacques S, Anders J. Quantitative analysis of transcranial and intraparenchymal light penetration in human cadaver brain tissue. Lasers Surg Med. 2015;47(4):312–22. 10.1002/lsm.22343. [DOI] [PubMed] [Google Scholar]
- 103.Lapchak PA, Boitano PD, Butte PV, Fisher DJ, Holscher T, Ley EJ, et al. Transcranial near-infrared laser transmission (NILT) profiles (800 nm): systematic comparison in four common research species. PLoS One. 2015;10(6):e0127580 10.1371/journal.pone.0127580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pitzschke A, Lovisa B, Seydoux O, Zellweger M, Pfleiderer M, Tardy Y, et al. Red and NIR light dosimetry in the human deep brain. Phys Med Biol. 2015;60(7):2921–37. 10.1088/0031-9155/60/7/2921. [DOI] [PubMed] [Google Scholar]
- 105.Henderson TA, Morries LD. Near-infrared photonic energy penetration: can infrared phototherapy effectively reach the human brain? Neuropsychiatr Dis Treat. 2015;11:2191–208. 10.2147/NDT.S78182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shell TA, Shell JR, Rodgers ZL, Lawrence DS. Tunable visible and near-IR photoactivation of light-responsive compounds by using fluorophores as light-capturing antennas. Angew Chem Int Ed Engl. 2014;53(3):875–8. 10.1002/anie.201308816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shell TA, Lawrence DS. Vitamin B12: a tunable, long wavelength, light-responsive platform for launching therapeutic agents. Acc Chem Res. 2015;48(11):2866–74. 10.1021/acs.accounts.5b00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Issa JPM, Trawitzki BF, Ervolino E, Macedo AP, Lilge L. Low-intensity laser therapy efficacy evaluation in FVB mice subjected to acute and chronic arthritis. Lasers Med Sci. 2017;32(6):1269–77. 10.1007/s10103-017-2235-5. [DOI] [PubMed] [Google Scholar]
- 109.Naghdi S, Ansari NN, Fathali M, Bartley J, Varedi M, Honarpishe R. A pilot study into the effect of low-level laser therapy in patients with chronic rhinosinusitis. Physiother Theory Pract. 2013;29(8):596–603. 10.3109/09593985.2013.775204. [DOI] [PubMed] [Google Scholar]
- 110.Silva VR, Marcondes P, Silva M, Villaverde AB, Castro-Faria-Neto HC, Vieira RP, et al. Low-level laser therapy inhibits bronchoconstriction, Th2 inflammation and airway remodeling in allergic asthma. Respir Physiol Neurobiol. 2014;194:37–48. 10.1016/j.resp.2014.01.008. [DOI] [PubMed] [Google Scholar]


