Schutter et al. (2019) raise concerns regarding potentially unknown risks of trigeminal nerve stimulation (TNS) in young children treated for attention-deficit/hyperactivity disorder (ADHD), written in response to our recently published double-blind sham-controlled study (McGough et al., 2019). We respect and appreciate this groups’ efforts in advancing burgeoning research on psychiatric applications of neuromodulation. Our hope is that additional clarifications will serve to alleviate their apprehensions.
A main concern suggests that observed TNS effects on ADHD might be due to direct stimulation of the frontal cortex, and not via stimulation of the trigeminal nerve. Based on computational modelling, authors posit that the electric field generated by TNS as low as 2 mA can extend to the frontal cortex and appears sufficiently high to affect neural tissue, although they admit their findings cannot indicate if generated field intensities are sufficient to have biological effects. Evidence indicates they are not.
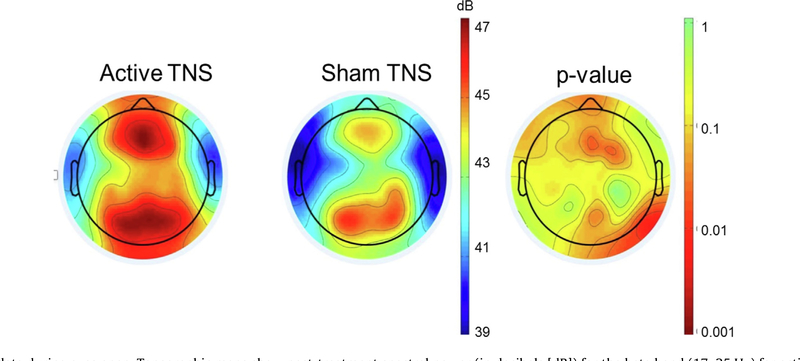
The general view is that TNS exerts its effects on cortical excitability via a “bottom-up” mechanism dependent on subcortical activation, a conclusion largely corroborated by neuroimaging studies (Shiozawa et al., 2014). This mechanism is confirmed in our own work. A positive emission tomography (PET) study of TNS in depressed adults revealed no increases in regional cerebral blood flow (rCBF) at the frontal pole where electrodes are placed, but selective activation in the anterior cingulate, right dorsolateral prefrontal cortex, and left inferior frontal gyrus (Cook et al., 2014). In our sham-controlled ADHD trial (McGough et al., 2019), resting EEG data during the eyes-open condition demonstrated primary significant spectral power differences in frontal and parietal regions lateralized to right frontal cortex (Fig. 1). A direct stimulating effect would likely affect resting EEG bilaterally in frontal regions, closer to where stimulation occurred.
Fig. 1.
Resting EEG data during eyes open. Topographic maps show post-treatment spectral power (in decibels [dB]) for the beta band (17–25 Hz) for active (left) and sham (middle) groups, as well as a p-value plot (right). A direct stimulating effect would likely affect the resting EEG profile in bilateral prefrontal regions, closer to where stimulation occurred.
An additional worry involves the paucity of current knowledge on safety and physiological effects of TNS, particularly when administered during sleep. Authors note that ADHD-affected youth often have sleep difficulties, which in themselves negatively impact ADHD symptoms. Contrary to our assertion that TNS is well tolerated with minimal risk, authors use chi-square analyses to assert that reported side effects, including fatigue, drowsiness, headache, and increased appetite occur at significantly increased rates with active TNS. Given small numbers of affected individuals, however, Fisher’s Exact Test, and not chi-square, is the appropriate statistic, and based on this test none of the differences reach significance. With particular reference to sleep, we found no group differences based on the Children’s Sleep Habits Questionnaire, a well-accepted childhood sleep measure. None of the side effects detected required clinical intervention or led to early participant withdrawal. Previous acute and long-term studies of TNS in adults similarly demonstrate treatment is well tolerated and without clinically meaningful adverse events, and that when side effects occur they are generally mild and transient (Shiozawa et al., 2014). These results supported approval as a minimal risk intervention by the U.S. Food and Drug Administration.
In response to concerns over potentially negative effects on neuroplasticity due to TNS-induced increases in gamma-aminobutyric acid (GABA), we argue that theoretical concerns based on associations between performance on selected working memory tests and glutamate/GABA ratios are outweighed by our results which demonstrate improved executive functioning. The study cited was based on a very small sample and the negative relationship found between GABA levels and cognitive performance was in a single brain region. In addition, our preliminary secondary analyses demonstrate significant improvements in executive functioning with active TNS. These results from our sham-controlled study are being prepared for publication. Although yet to be demonstrated, we would greet the prospect of TNS effects on brain plasticity as a potential boon to ADHD therapy. Several studies suggest that stimulant treatment at key periods is associated with shifts in developmental brain trajectories in the direction of those seen in non-ADHD affected youth (Shaw et al., 2009). These findings are tentative and do not establish a causal relation between ADHD medication treatment and brain change. Nonetheless, a possible role for administering TNS during critical periods of brain development as a means to effect positive and possibly persistent changes in brain networks remains an intriguing area for future research. Any consideration of risk from TNS intervention must, of course, be weighed against known risks of medication treatment and of untreated ADHD itself.
We have one final comment in response to authors’ use of the abbreviation “TNS” to signify transcutaneous nerve stimulation, not trigeminal as used in our article and elsewhere. We do not know if this usage was in error or an implicit argument rooted in the authors’ opinion that treatment effects are direct through the skin to the frontal lobe, and not inherent to the trigeminal nerve. Ongoing research can address this question. However, consistent use of terminology within the literature is more apt to advance the debate.
Acknowledgments
This study was supported by National Institute of Mental Health grant R34 MH101282 (to Drs. McGough and Loo, Co-PIs). Study devices and some materials were provided by NeuroSigma, Inc. in response to an investigator-initiated request.
Footnotes
Disclosures
Dr. McGough has provided expert testimony on behalf of Eli Lilly Canada. Dr. Cook has been an advisor to Arctica Health, Cereve, and HeartCloud; has served as part of the management team of NeuroSigma, Inc. (on leave since 6/2016), and has been allocated stock options. His patents are assigned to the University of California. Dr. Loo reports no financial conflicts of interest.
Contributor Information
James J. McGough, Department of Psychiatry and Biobehavioral Sciences, Semel Institute for Neuroscience and Human Behavior and David Geffen School of Medicine at UCLA, Los Angeles, CA, USA.
Sandra K. Loo, Department of Psychiatry and Biobehavioral Sciences, Semel Institute for Neuroscience and Human Behavior and David Geffen School of Medicine at UCLA, Los Angeles, CA, USA
Ian A. Cook, Department of Psychiatry and Biobehavioral Sciences, Semel Institute for Neuroscience and Human Behavior and David Geffen School of Medicine at UCLA, Los Angeles, CA, USA Department of Bioengineering, Henry Samueli School of Engineering and Applied Science at UCLA, Los Angeles, CA, USA; NeuroSigma, Inc., Los Angeles, CA, USA.
References
- Cook IA, Espinoza R, Leuchter AF. Neuromodulation for depression: invasive and noninvasive (deep brain stimulation, transcranial magnetic stimulation, trigeminal nerve stimulation). Neurosurg Clin N Am 2014;25:103–16. 10.1016/j.nec.2013.10.002. [DOI] [PubMed] [Google Scholar]
- McGough JJ, Sturm A, Cowen J, et al. Double-blind, sham-controlled, pilot study of trigeminal nerve stimulation for attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2019;58:403–11. 10.1016/j.jaac.2018.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutter DJLG, Hunnius S, Rommelse N. Transcutaneous electric currents to target the peripheral and central nervous system in children with attention deficit hyperactivity disorder. Clin Neurophysiol 2019;130:2005–7. [DOI] [PubMed] [Google Scholar]
- Shaw P, Sharp WS, Morrison M, et al. Psychostimulant treatment and the developing cortex in attention deficit hyperactivity disorder. Am J Psychiatry 2009;166:143–51. 10.1176/appi.ajp.2008.08050781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozawa P, da Silva ME, de Carvalho TC, Cordeiro Q, Brunoni R, Fregni F. Transcutaneous vagus and trigeminal nerve stimulation for neuropsychiatric disorders: a systematic review. Arq Neuropsiquiatr 2014;72:542–7. 10.1590/0004-282X20140061. [DOI] [PubMed] [Google Scholar]