Sixty-three consecutive patients who presented to the authors' institution with cervicofacial venous malformations and underwent postcontrast MR imaging were studied. Three neuroradiologists reviewed brain MRIs for the presence of developmental venous anomalies, dural venous sinus ectasia, and cavernous malformations. The prevalence of developmental venous anomalies in this patient population was compared with an age- and sex-matched control group without venous malformations. The overall presence of developmental venous anomalies in patients with venous malformations was 36.5% (23/63) compared with 7.9% (10/126) in controls. The prevalence of dural venous sinus ectasia was 9.5% (6/63) compared with 0% for controls. The authors show a significant association between cervicofacial venous malformations and cerebral developmental venous anomalies as well as between cervicofacial venous malformations and dural venous sinus abnormalities.
Abstract
BACKGROUND AND PURPOSE:
Prior studies have suggested an association between the presence of cervicofacial venous malformations and intracranial developmental venous anomalies. We reviewed our institutional cohort of patients with cervicofacial venous malformations and examined the spectrum of intracranial venous anomalies, including developmental venous anomalies, cavernous malformations, and dural venous sinus abnormalities.
MATERIALS AND METHODS:
Consecutive patients who presented to our institution with cervicofacial venous malformations and underwent postcontrast MR imaging were studied. Three neuroradiologists reviewed brain MRIs for the presence of developmental venous anomalies, dural venous sinus ectasia, and cavernous malformations. The prevalence of developmental venous anomalies in this patient population was compared with an age- and sex-matched control group without venous malformations at a ratio of 1:2. Categoric variables were compared with χ2 tests.
RESULTS:
Sixty-three patients with venous malformations met the inclusion criteria with a mean age of 38.3 ± 24.0 years. The overall presence of developmental venous anomalies in patients with venous malformations was 36.5% (23/63) compared with 7.9% (10/126) in controls (P < .001). The prevalence of dural venous sinus ectasia was 9.5% (6/63) compared with 0% for controls (P = .002). One patient with a venous malformation had a cavernous malformation compared with 1 patient in the control group (P = .62). In 73.9% of patients (17/23), developmental venous anomalies were along the same metamere; and in 82.6% of patients, developmental venous anomalies were ipsilateral to the venous malformations.
CONCLUSIONS:
Our case-control study demonstrated a significant association between cervicofacial venous malformations and cerebral developmental venous anomalies as well as between cervicofacial venous malformations and dural venous sinus abnormalities. Our findings suggest that venous malformations may be the result of a segmental in utero insult to cells involved in cerebrofacial venous development.
Venous malformations (VMs) are slow-flow vascular malformations characterized by soft-tissue swelling and bluish skin discoloration. Pathologic studies have found that these lesions consist of dilated venous channels in the soft tissues which, in the face and neck, generally drain into the external jugular system.1 While the pathogenesis of these lesions is unclear, genetic studies suggest that most of these lesions are due to somatic mutations in either the TEK receptor tyrosine kinase (TEK) or phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) gene pathways.2,3
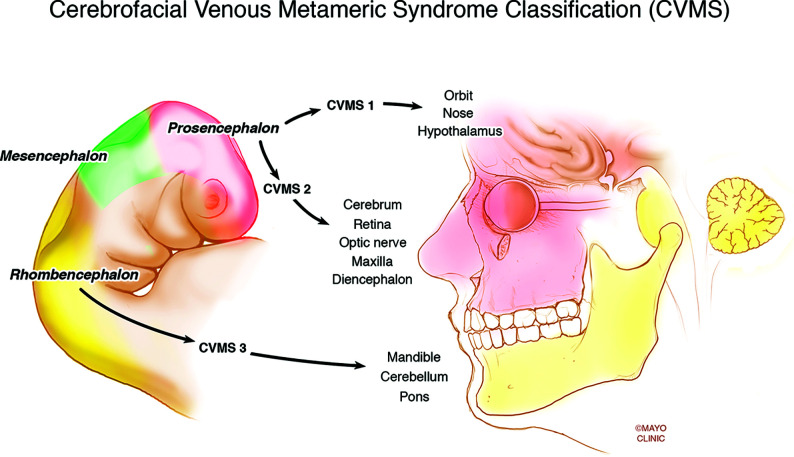
Several recent studies have demonstrated an association between VMs and intracranial venous anomalies, specifically developmental venous anomalies (DVAs). The Toronto Western Hospital group found that patients with VMs were 3 times more likely to have DVAs compared with age-matched controls.1 This association makes sense from a pathophysiologic point of view because somatic mutations affecting venous endothelial progenitor cells could presumably affect the cerebral venous system if they occur before cell migration (Fig 1).4 This mechanism is proposed for metameric disorders such as cerebrofacial arteriovenous metameric syndrome, cerebrofacial venous metameric syndrome (CVMS), and spinal arteriovenous metameric syndrome.5 Recently, our group observed that a subset of patients with VMs also have abnormalities affecting the dural venous sinuses, including the presence of a persistent falcine sinus and persistent ballooning of the torcula.
FIG 1.
Demonstration of the metameric distribution of somatic mutations affecting venous endothelial progenitor cells that could presumably affect the cerebral venous system if they occur before cell migration. Reprinted with permission of the Mayo Foundation for Medical Education and Research. All rights reserved.
To study the association between VMs and intracranial venous anomalies, including DVAs and dural venous sinus abnormalities, we performed a case-control study examining the prevalence of these findings in a consecutive cohort of patients with VMs and compared this cohort with a group of age- and sex-matched controls.
MATERIALS AND METHODS
Patient Population
Following institutional review board approval, we queried our data base of patients with VMs who had presented to our institution during the past 5 years and included patients who had brain or face MRIs with postcontrast T1-weighted imaging. At our institution, patients with VMs routinely undergo MRIs of the brain and face with and without intravenous contrast. VMs were confirmed by a combination of physical examination and imaging-based findings through our multidisciplinary Vascular Anomalies Clinic.6,7 Imaging findings strongly suggestive of a VM on MR imaging were the following: 1) a septate lobulated mass that was hyperintense on T2-weighted and hypointense on T1-weighted images without mass effect; 2) phleboliths, which are characteristically hypointense on T1- and T2-weighted sequences; 3) the presence of fluid-fluid levels; 4) the absence of vascular flow voids on spin-echo sequences; 5) infiltration of the lesion through tissue planes; 6) the absence of arterial or early venous enhancement; and 7) the presence of diffuse enhancement on delayed images. On clinical examination, VMs generally appeared as faint blue, soft, and easily compressible, nonpulsatile masses that enlarged with the Valsalva maneuver in dependent positions and were compressible with application of local pressure.
Both pediatric and adult patients with VMs were included in this study. We also selected a group of age- and sex-matched controls (case/control ratio of 1:2) from an institutional data base that was used to estimate the prevalence of brain DVAs in the general population. The data base consisted of consecutive patients who underwent a contrast-enhanced brain MR imaging during a 2-year period (2016–2017) with the terms “developmental venous anomaly,” “DVA,” “venous angioma,” “venous anomaly,” and “vascular anomaly” in their report, which amounted to examinations of 18,073 individuals. The findings were then confirmed by a single radiologist.
Imaging Analysis
All imaging was reviewed by 3 radiologists. Images were analyzed for the following findings: 1) the presence of a DVA, 2) deep or superficial drainage of the DVA, 3) location and side of the DVA, 4) the presence of dural venous sinus anomalies including dural ectasia and persistent falcine sinus, and 5) the presence of cavernous malformations. While vascular malformations of the CNS can consist of DVAs, cavernous malformations, or capillary telangiectasias, the latter was not included in this study.8-10 In addition to intracranial imaging, face and neck MRIs were evaluated for the location and size of the VM. For patients who had both DVAs and VMs, we also reviewed the imaging to determine whether the vascular anomalies occurred along the same metamere. The 3 metameres of the craniofacial system include the medial prosencephalic group (olfactory) with involvement of the forehead, nose, hypothalamus, corpus callosum, and hypophysis (CVMS 1); the lateral prosencephalic group (optic) with involvement of the temporo-parietal-occipital lobes, optic nerve, retina, thalamus, eye, cheek, and maxilla (CVMS 2); and the rhombencephalic/mesencephalon (otic) group with involvement of the cerebellum, brain stem, lower face, mandible, petrous bone, and maxilla (CVMS 3).11 Further analysis examined the association between age and the occurrence of malformations.
Statistical Analysis
The primary outcome of this study was the prevalence of a DVA in the VM population and in controls. Prevalence rates were compared using a χ2 test. A separate χ2 analysis of DVAs in patients with and without CVMS was performed. A Student t test was used to compare continuous variables. All statistical analyses were performed using JMP 13.0 (SAS Institute).
RESULTS
Patient and Control Population
e included 63 patients with VMs and 126 controls. The mean age of the VM population was 38.3 ± 24.0 years compared with 38.3 ± 23.8 years for controls; 58.7% of patients in both the VM group (37/63) and non-VM group (74/126) were female. Of the patients in the control group, none had any documentation of a VM in their chart and there was no evidence of VMs or other cutaneous vascular malformations on review of their available brain and face MR imaging examinations. Most of the patients did not have any other CNS diagnosis (88.9%), while a small portion had a diagnosis of migraines (9.5%) and epilepsy (1.6%).
Venous Malformation Characteristics and Location
In the VM cohort, 22 patients (34.9%) had VMs isolated to the left side of the face, 27 patients (42.9%) had VMs only on the right side of the face, and 14 patients (22.2%) had bilateral VMs. When we categorized VM locations in descending frequency, the most common locations were the masticator space (44%, 28 patients), buccal space (21 patients, 33.3%), lingual space (13 patients (20.6%), orbit (8 patients, 12.7%), posterior oropharynx or hypopharynx (7 patients, 11.1%), posterior neck (7 patients, 11.1%), lip (3 patients, 4.8%), and other (3 patients, 4.8%). There was no association between age and the occurrence of malformations (P = .46).
Prevalence of DVA and DVA Characteristics
Of the 63 patients with VMs, DVAs were present in 36.5% of patients (23/63) compared with 7.9% of controls (10/126, P < .001). In 73.9% of cases (17/23), DVAs were along the same metamere, and in 82.6% of cases, DVAs were ipsilateral to the VM. In the cases in which the DVAs and VMs were along the same metamere, 8 had CVMS 3, 4 had CVMS 2, and 5 had CVMS 2 + 3. There was no significant difference in the prevalence of DVAs in patients with or without CVMS (P = .22).
In the VM + DVA cohort, 11 patients had 1 DVA and 12 patients had multiple DVAs. In 10 patients, DVAs drained to the superficial venous system; in 7 patients, DVAs drained into the deep venous system; and in 6 patients, DVAs drained into both the superficial and venous systems. DVAs were located on the right side in 10 patients and left side in 7 patients and were bilateral in 6 patients. Fourteen patients had supratentorial DVAs, 5 patients had infratentorial DVAs, and 4 patients had both supratentorial and infratentorial DVAs. One patient had a cavernous malformation associated with a DVA. There was no association between VM location and the presence of DVAs. There was no association between age (P = .63) or sex (P = .79) and DVAs. Examples of VMs associated with DVAs are provided in Figs 2–5.
FIG 2.
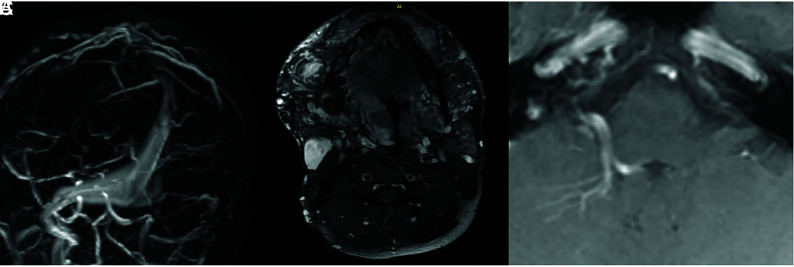
A 38-year-old man with a history of large trans-spatial cervicofacial venous malformation causing periodic airway obstruction. A, MRV shows a large and ectatic torcula and medial right transverse sinus. B, Postcontrast T1 MR imaging of the face shows an extensive venous malformation predominantly involving the right face, tongue, and hypopharynx. C, Postcontrast MR imaging shows a DVA in the right cerebellum.
FIG 5.
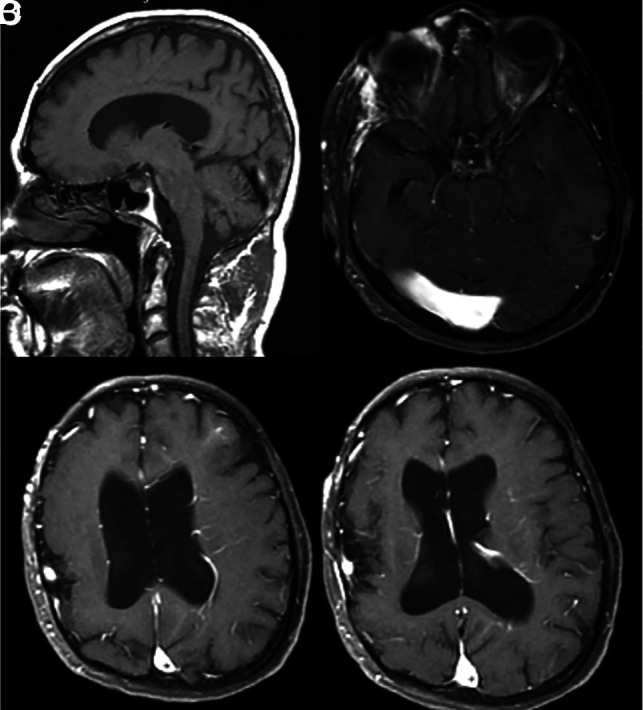
A 45-year-old man with developmental delay and bilateral facial VMs (not shown). A, Sagittal T1 MR imaging shows an ectatic torcula, which is also confirmed on axial T1 postcontrast MR imaging (B). C and D, Axial postcontrast MR imaging shows multiple extensive DVAs involving the left cerebral hemisphere. The patient also had polymicrogyria of the right hemisphere.
FIG 4.
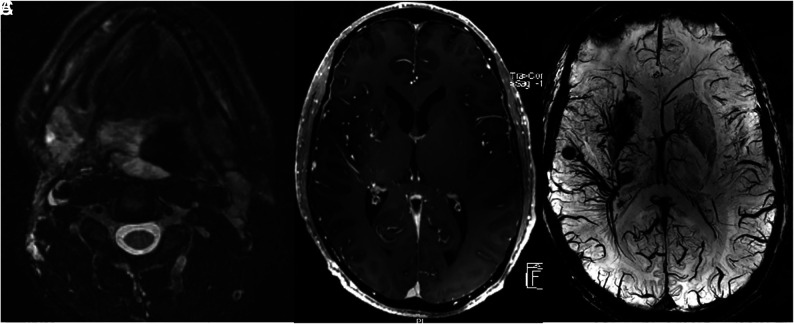
A 38-year-old man with extensive airway malformation. A, T2 FS MR imaging shows an extensive venous malformation involving the airway and tongue base. B, Axial postcontrast MR imaging shows extensive DVAs of the right frontal lobe, basal ganglia, and temporal lobe. C, 7T SWI shows the extensive DVAs and multiple cavernomas in the radicles of the DVAs.
Dural Venous Sinus Abnormalities
On review of brain MRIs, 6 patients with VMs (9.5%) had dural venous sinus abnormalities compared with 0% of controls (P = .002). All 6 patients had ectasia of the torcula. In addition, 4 patients had a persistent falcine sinus. There was no association between age and dural venous sinus abnormality (P = .46). All patients with dural venous sinus abnormalities also had intracranial DVAs, 4 of which were along the same metamere as the VM. Of the patients with CVMS, 2 had CVMS 2 and 2 had CVMS 2 + 3. Examples are provided in Figs 2, 3, and 5.
FIG 3.
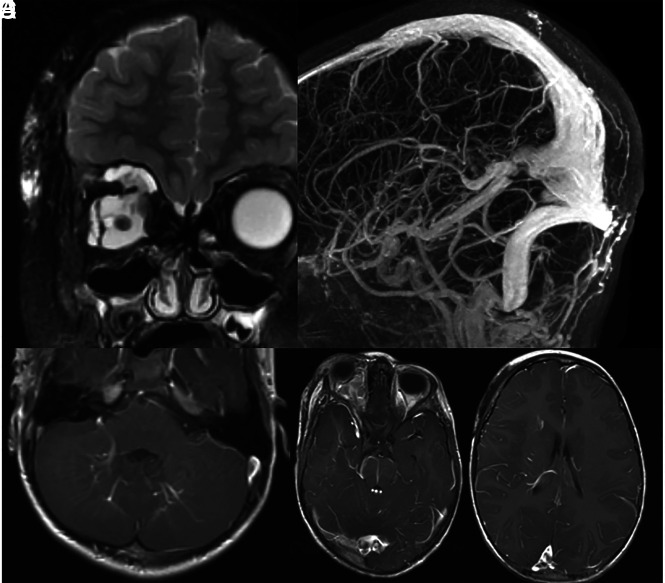
A 5-year-old child with history of right orbital venous malformation. A, T2 with fat saturation MR imaging shows a large venous malformation involving the right orbit and face. B, MRV shows a markedly ectatic torcula, which includes the entirety of the straight sinus. C–E, T1 postcontrast MRIs show multiple developmental venous anomalies, including the bilateral cerebellar hemispheres, brain stem, right temporal lobe, and right frontal lobe.
DISCUSSION
Our large series examining the prevalence of cerebral and dural venous abnormalities in patients with VMs demonstrated a number of interesting findings. First, we found that the prevalence of DVAs among patients with VMs was >4 times higher than that in our age- and sex-matched controls. As seen in 1 prior study, we found that DVAs were along the same metamere in nearly 75% of cases and were ipsilateral to the VM in nearly 80% of cases. One novel finding from our study was that nearly 10% of patients with VMs also have abnormalities associated with the dural venous sinuses, in particular ectasia of the torcula and the presence of a persistent falcine sinus. The findings from our study are important because they suggest a high likelihood of a common pathway involved in the formation of cervicofacial VMs, intracranial DVAs, and dural venous sinuses.
To date, there has been 1 case-control study examining the association between VMs and DVAs.1 The Toronto Western Hospital group recently published an article demonstrating a prevalence of DVAs in 28.6% of patients compared with just 9.5% of age- and sex-matched controls. The authors found that in 83.3% of cases, the DVAs were ipsilateral to the VM, and in 75% of cases, the DVAs were along the same metamere. In our series, we found a slightly higher rate of DVAs associated with VMs in a larger patient population, but we found a nearly identical rate of DVAs being ipsilateral or along the same metamere as the VM. The higher rate of DVA detection is likely because only patients with postcontrast MRIs were included in this study. Other series demonstrated or suggested an association between VMs and DVAs. Boukobza et al12 found that 20% of patients with facial VMs had DVAs on cerebral angiography, with most of these patients having multiple DVAs. Most of these DVAs were extensive and drained into a tortuous deep venous system, and in most cases, DVAs were ipsilateral to the VM.12 Several case reports have described patients with extensive facial venous malformations and ipsilateral DVAs.4,5,11,13-15
The pathogenesis of DVA is not clear. A number of mechanisms have been proposed for the formation of DVAs. Some authors have postulated that DVAs can form later in cerebral venous development due to functional adaptations to local venous thrombosis or due to failed development of superficial or deep veins.16 However, given the association between DVAs and VMs in our study, we believe that in at least some cases, they must be the result of errors in vasculogenesis of the venous system early in embryonic life. It would otherwise be difficult to explain how and why they are so closely linked to VMs. Unlike VMs, which have at least a few well-defined somatic mutations associated with their development, to date, there have been no genetic mutations associated with DVAs. The unilateral nature of DVAs and facial VMs can be hypothesized to be related to a prothrombotic state due to venous thrombosis and occlusion in the setting of a metameric disorder.1
Another interesting finding from our study was the high prevalence of dural venous sinus abnormalities in patients with VMs. Nearly 10% of patients had abnormal ectasia of the torcula, and approximately 6% had a persistent falcine sinus. Torcular ballooning is a well-described phenomenon during the development of the fetal dural venous sinus system. During the fourth-to-fifth fetal months, superficial veins of the expanding cerebral hemispheres increase in size and drain into the transverse sinus, resulting in a period of ballooning of both the transverse sinuses and the torcula.17,18 This in utero imaging finding was frequently misdiagnosed as a dural sinus malformation until it was recognized as a phase in the normal dural venous sinus development.
There have been a few case reports describing the presence of dural venous sinus abnormalities in patients with CVMS (ie, DVAs and facial VMs along the same metamere). The first reported case by Mohamed et al19 was in an infant who had multiple posterior fossa DVAs, cavernous malformations, a facial VM, and torcular ectasia from a dural sinus malformation. In this case, the dural sinus malformation had multiple points of shunting and required treatment. In a subsequent series of 30 patients with dural sinus malformations, Barbosa et al20 found that 6 patients also had vascular anomalies of the face, which were likely venous malformations. Unlike the cases referenced in the articles by Mohamed et al19 and Barbosa et al,20 in our series, no patients had any evidence of arteriovenous shunting associated with the dural venous sinus ectasia. Nevertheless, we hypothesize that the presence of VMs, DVAs, and dural venous sinus ectasia represents associations along a pathophysiologic spectrum similar to that of VM, DVA, and dural sinus malformations.
Our study may provide some insight into an interesting and not uncommon phenotype of patients with cervicofacial VMs. The pathophysiologic and genetic mechanisms of VMs associated with DVAs (with or without dural venous sinus abnormalities) almost certainly points to the presence of a somatic mutation. There have been a few somatic mutations associated with the development of venous vascular malformations, including in the PIK3CA and TEK genes.21 These genetic associations have led to promising research in the treatment of VMs. Perhaps there is a different/specific gene that results in metameric venous malformations. Further research into identifying genetic mutations in this patient population may provide insight into disease pathophysiology and targeted treatment of venous vascular malformations in this population.
Limitations
Our study has limitations. First, it was retrospective and is subject to the biases of retrospective studies, including selection bias. Only a few patients in our series underwent SWI, which would likely be significantly more sensitive for detecting tiny cavernous malformations than conventional gradient recalled-echo imaging. There was a wide spectrum of scanners and imaging protocols used in the evaluation of VMs in our study, and imaging was variably performed on 1.5T, 3T, and 7T MR imaging machines. While we did find that a substantial proportion of patients with facial venous malformations also had dural venous sinus malformations, the converse (ie, the proportion of patients with dural venous sinus malformations without facial venous malformations) is more difficult to pin down. This issue is because these malformations are often not reported and a unifying terminology for these malformations has never really been established; thus, it is difficult to identify such patients from text searches of radiology reports.
CONCLUSIONS
In the largest study to date on this topic, we found that patients with cervicofacial VMs were significantly more likely to have intracranial DVAs and dural venous sinus abnormalities compared with normal age- and sex-matched controls. In most cases, DVAs and VMs occurred along the same metamere, suggesting that the prevalence of CVMS in this patient population is underestimated. Our findings suggest that there is a developmental link in the formation of venous abnormalities affecting the brain, dura, and face in a subset of patients.
ABBREVIATIONS:
- CVMS
cerebrofacial venous metameric syndrome
- DVA
developmental venous anomaly
- VM
venous malformation
References
- 1.Brinjikji W, Hilditch CA, Tsang AC, et al. Facial venous malformations are associated with cerebral developmental venous anomalies. AJNR Am J Neuroradiol 2018;39:2103–07 10.3174/ajnr.A5811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castel P, Carmona FJ, Grego-Bessa J, et al. Somatic PIK3CA mutations as a driver of sporadic venous malformations. Sci Transl Med 2016;8:332ra 42 10.1126/scitranslmed.aaf1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ten Broek RW, Eijkelenboom A, van der Vleuten CJ, et al. Comprehensive molecular and clinicopathological analysis of vascular malformations: a study of 319 cases. Genes Chromosomes Cancer 2019;58:541–50 10.1002/gcc.22739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agid R, Terbrugge KG. Cerebrofacial venous metameric syndrome 2 plus 3: facial and cerebral manifestations. Interv Neuroradiol 2007;13:55–58 10.1177/159101990701300107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krings T, Geibprasert S, Luo CB, et al. Segmental neurovascular syndromes in children. Neuroimaging Clin N Am 2007;17:245–58 10.1016/j.nic.2007.02.006 [DOI] [PubMed] [Google Scholar]
- 6.Flors L, Leiva-Salinas C, Maged IM, et al. MR imaging of soft-tissue Vascular malformations: diagnosis, classification, and therapy follow-up. Radiographics 2011;31:1321–40; discussion 1340–41 10.1148/rg.315105213 [DOI] [PubMed] [Google Scholar]
- 7.Wassef M, Blei F, Adams D, et al. ; Board and Scientific Committee. Vascular anomalies classification: recommendations from the International Society for the Study of Vascular Anomalies. Pediatrics 2015;136:e203–14 10.1542/peds.2014-3673 [DOI] [PubMed] [Google Scholar]
- 8.Abla A, Wait SD, Uschold T, et al. Developmental venous anomaly, cavernous malformation, and capillary telangiectasia: spectrum of a single disease. Acta Neurochir (Wien) 2008;150:487–89; discussion 489 10.1007/s00701-008-1570-5 [DOI] [PubMed] [Google Scholar]
- 9.McCormick PW, Spetzler RF, Johnson PC, et al. Cerebellar hemorrhage associated with capillary telangiectasia and venous angioma: a case report. Surg Neurol 1993;39:451–57 10.1016/0090-3019(93)90030-5 [DOI] [PubMed] [Google Scholar]
- 10.Rigamonti D, Johnson PC, Spetzler RF, et al. Cavernous malformations and capillary telangiectasia: a spectrum within a single pathological entity. Neurosurgery 1991;28:60–64 [PubMed] [Google Scholar]
- 11.Bhattacharya JJ, Luo CB, Suh DC, et al. Wyburn-Mason or Bonnet-Dechaume-Blanc as cerebrofacial arteriovenous metameric syndromes (CAMS): a new concept and a new classification. Interv Neuroradiol 2001;7:5–17 10.1177/159101990100700101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boukobza M, Enjolras O, Guichard JP, et al. Cerebral developmental venous anomalies associated with head and neck venous malformations. AJNR Am J Neuroradiol 1996;17:987–94 [PMC free article] [PubMed] [Google Scholar]
- 13.Goulao A, Alvarez H, Garcia Monaco R, et al. Venous anomalies and abnormalities of the posterior fossa. Neuroradiology 1990;31:476–82 10.1007/BF00340125 [DOI] [PubMed] [Google Scholar]
- 14.Portilla P, Husson B, Lasjaunias P, et al. Sturge-Weber disease with repercussion on the prenatal development of the cerebral hemisphere. AJNR Am J Neuroradiol 2002;23:490–92 [PMC free article] [PubMed] [Google Scholar]
- 15.Ramli N, Sachet M, Bao C, et al. Cerebrofacial venous metameric syndrome (CVMS) 3: Sturge-Weber syndrome with bilateral lymphatic/venous malformations of the mandible. Neuroradiology 2003;45:687–90 10.1007/s00234-003-1042-9 [DOI] [PubMed] [Google Scholar]
- 16.Pereira VM, Geibprasert S, Krings T, et al. Pathomechanisms of symptomatic developmental venous anomalies. Stroke 2008;39:3201–15 10.1161/STROKEAHA.108.521799 [DOI] [PubMed] [Google Scholar]
- 17.Robertson F. Torcular dural sinus malformation. J Neurointerv Surg 2018;10:423 10.1136/neurintsurg-2017-013654 [DOI] [PubMed] [Google Scholar]
- 18.Manjila S, Bazil T, Thomas M, et al. A review of extraaxial developmental venous anomalies of the brain involving dural venous flow or sinuses: persistent embryonic sinuses, sinus pericranii, venous varices or aneurysmal malformations, and enlarged emissary veins. Neurosurg Focus 2018;45:E9 10.3171/2018.5.FOCUS18107 [DOI] [PubMed] [Google Scholar]
- 19.Mohamed Z, Batista LL, Sachet M, et al. Growing dural sinus malformation with associated developmental venous anomaly, multiple cavernomas and facial venous malformation in an infant: an associated disease or a disease spectrum? Interv Neuroradiol 2002;8:421–30 10.1177/159101990200800412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barbosa M, Mahadevan J, Weon YC, et al. Dural sinus malformations (DSM) with giant lakes, in neonates and infants: review of 30 consecutive cases. Interv Neuroradiol 2003;9:407–24 10.1177/159101990300900413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Limaye N, Kangas J, Mendola A, et al. Somatic activating PIK3CA mutations cause venous malformation. Am J Hum Genet 2015;97:914–21 10.1016/j.ajhg.2015.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]