Abstract
BACKGROUND AND PURPOSE:
Pathology and microbiology results for suspected spondylodiscitis on MR imaging are often negative in up to 70% of cases. We aimed to predict whether MR imaging features will add diagnostic value when combined with clinical biomarkers to predict positive findings of spondylodiscitis on pathology and/or microbiology from percutaneous biopsy.
MATERIALS AND METHODS:
In this retrospective single-center institutional review board–approved study, patients with radiologically suspected spondylodiscitis and having undergone percutaneous biopsies were assessed. Demographic characteristics, laboratory values, and tissue and blood cultures were collected. Pathology and microbiology results were used as end points. Three independent observers provided MR imaging–based scoring for typical MR imaging features for spondylodiscitis. Multivariate logistic regression and receiver operating characteristic analysis were performed to determine an optimal combination of imaging and clinical biomarkers in predicting positive findings on pathology and/or microbiology from percutaneous biopsy suggestive of spondylodiscitis.
RESULTS:
Our patient cohort consisted of 72 patients, of whom 33.3% (24/72) had spondylodiscitis. The mean age was 63 ± 16 years with a male/female ratio of 41:31. Logistic regression revealed a combination with an area under the curve of 0.72 for pathology and 0.68 for pathology and/or microbiology. Epidural enhancement on MR imaging improved predictive performance to 0.87 for pathology and 0.78 for pathology and/or microbiology.
CONCLUSIONS:
Our findings demonstrate that epidural enhancement on MR imaging added diagnostic value when combined with clinical biomarkers to help predict which patients undergoing percutaneous biopsy will have positive findings for spondylodiscitis on pathology and/or microbiology.
Diagnosis of vertebral spondylodiscitis is often difficult and determined on the basis of a combination of imaging findings, clinical context, inflammatory biomarkers, spondylodiscitis, degenerative changes, and spinal tumors, though it is not a perfect tool; more invasive sampling is frequently still required. The typical findings suggestive of spinal infection include hyperintense T2 disc signal, adjacent vertebral endplate destruction, and epidural/paraspinal enhancement.1,2 However, in the absence of these typical features, the diagnosis of spondylodiscitis can be difficult and Modic type 1 degenerative changes and inflammatory disease may often mimic spinal infections. Currently, percutaneous CT or fluoroscopy-guided biopsy is the standard of care for the diagnosis of spondylodiscitis.3
In patients with radiologically suspected spinal infection, identification of the organism is useful in directing antibiotic treatment. Per Sehn and Gilula,4 these organisms “may be identified by blood culture or biopsy and culture of site of suspected infection with reported success rates of 20–59%5,6 and 46–91%.”7 The histologic examination is useful for a correct diagnosis when the microbes responsible for spinal infection do not grow in tissue or blood culture medium or in case of anaerobic organisms.8 In clinical practice today, both microbiology and pathology are typically obtained from the tissue specimen.
Inflammatory markers such as C-reactive protein (CRP)9-11 and erythrocyte sedimentation rate (ESR)12,13 are commonly elevated in patients with spondylodiscitis. The association of spinal infection with biomarkers such as leukocytosis,10,14 fever status,15,16 alkaline phosphatase level,17,18 and hemoglobin count18,19 have been explored in the past without a clear consensus. Further studies are needed to clarify whether these clinical and laboratory biomarkers are associated with spinal infections.
MR imaging is currently the preferred technique for prediction of spondylodiscitis; however, pathology and microbiology results for radiologically suspected spondylodiscitis are only positive in up to 30% of cases.20 In this study, we aimed to evaluate whether MR imaging features add diagnostic value when combined with clinical biomarkers to help predict which patients who undergo percutaneous biopsy will have positive findings for spondylodiscitis on pathology and/or microbiology.
MATERIALS AND METHODS
Study Population
This is a single-center retrospective study, which was approved by the local institutional review board, with a waiver of informed consent. From July 2014 to August 2019, a total of 187 CT- and fluoroscopy-guided percutaneous bone biopsies were performed at our institution for suspicion of spondylodiscitis based on prior MR imaging findings. Patients were included if they had pathology reports from the biopsy, MR imaging with contrast within 3 weeks of biopsy, and laboratory markers within 2 weeks before biopsy. Patients were excluded if they did not have pathology reports from biopsy (n = 17), did not have MR imaging with contrast within 3 weeks of biopsy (n = 24), or did not have laboratory markers within 2 weeks of biopsy (n = 50). Furthermore, they were excluded if they had been on broad-spectrum IV antibiotics for >3 days before the biopsy date (n = 19). Patients were further excluded if the biopsy yielded nondiagnostic/inadequate tissue specimens (n = 5). This process yielded a final cohort of 72 patients.
Procedure
All biopsies were performed with the patient under CT or fluoroscopic guidance. A 40-section CT scanner (Somatom Definition AS; Siemens) was used. Drill systems varied depending on operator comfort. All biopsies were by a transpedicular approach by 1 of 3 procedural neuroradiologists at our institution.
Data Collection
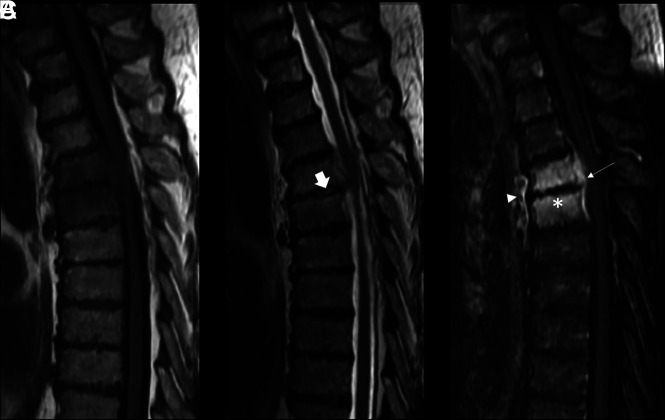
Demographic characteristics, comorbidities, fever status, laboratory values, tissue and blood culture results, and antibiotic exposure were collected from our institutional electronic medical record system. Three independent observers, blinded to clinical data and diagnosis, provided MR imaging–based scoring for the presence of hyperintense T2 disc signal, adjacent vertebral endplate erosion, epidural enhancement, and paraspinal enhancement (Fig 1). Each score was obtained in a binary fashion for the presence or absence of these features. Observers were neuroradiologists with 2, 6, and 10 years of attending experience. The presence of inflammatory histology was obtained from the surgical pathology report, and microbiology culture growth was obtained from the microbiology report of the biopsied tissue specimen.
FIG 1.
Imaging panel in a patient with osteomyelitis with hyperintense T2 disc signal, adjacent vertebral endplate erosions, and paraspinal/epidural enhancement. Thoracic spine MR imaging of a 72-year-old man with radiologically suspected infection at T4–T5. A, Disc-centered and bone marrow hypointensity on a T1-weighted image. B, Mild hyperintensity of the disc and adjacent bone marrow on T2-weighted image. The arrow represents hyperintense T2 disc signal. C, Contrast-enhanced T1-weighted image demonstrates epidural (arrow) and paraspinal enhancement (arrowhead). The asterisk represents adjacent vertebral endplate erosion. Pathology demonstrated that inflammatory histology and microbiology had no growth. ESR was 123 mm/h, and CRP was 176 mg/L. The patient was febrile on presentation with leukocytosis.
Statistical Analysis
The SPSS statistical package for Macintosh, Version 25 (IBM) was used for statistical computations. The Spearman rank correlation coefficient was used for categoric/rank variables, and the Pearson correlation coefficient was used for continuous variables. Positive predictive value (PPV), negative predictive value (NPV), sensitivity, and specificity were calculated from cross-tabulation entry. The Cohen κ analysis was used to assess paired interobserver agreement among the 3 independent observers scoring the presence of MR imaging features. Additionally, majority consensus analysis for the 3 observers was performed for each MR imaging feature for each case. Univariate analysis for each clinical and imaging biomarker was used to assess the association with positive pathology and/or microbiology indicating spondylodiscitis. Subsequently, multivariate backward stepwise logistic regression and receiver operating characteristic analysis using clinical and imaging biomarkers were used to find the optimal combination of biomarkers for predicting spondylodiscitis. This analysis was performed for 2 end points: 1) spondylodiscitis as proved on pathology only, and 2) spondylodiscitis as proved on pathology and/or microbiology because clinicians use different criteria to diagnose spondylodiscitis.
RESULTS
Clinical Characteristics of the Patient Population
Our cohort consisted of 72 patients, of whom 50 had CT-guided and 22 had fluoroscopy-guided imaging (Table 1). The mean age was 63 ± 16 years with a median of 62 years and a male/female ratio of 41:31. Forty-six percent of our patient cohort was immunosuppressed. All of our patients presented with back pain, and approximately one-third of the patients presented with fever. Additional neurologic symptoms on presentation included radiculopathy, paresthesia, and incontinence. There were 24 patients found to have positive pathology and 12 patients found to have positive microbiology growth in support of spondylodiscitis. A total of 29 patients had either positive pathology or microbiology results (7 patients had both positive pathology and microbiology, and 5 patients only had positive microbiology with negative pathology results for spondylodiscitis). Of the bacterial isolates from tissue culture, Staphylococci and Streptococci were most commonly observed.
Table 1:
Demographic characteristics of patient cohort
| Variable | No (%) |
|---|---|
| Age (mean) (yr) | 63 ± 16 |
| Sex (M/F) | 41:31 |
| Immunosuppression | 33 (46) |
| Cancer | 8 (11) |
| COPD | 6 (8) |
| Cirrhosis | 3 (4) |
| Diabetes | 15 (21) |
| HIV | 4 (6) |
| Steroid use | 12 (17) |
| IV drug abuse | 9 (13) |
| Postoperative status (within 1 wk) | |
| Symptoms relevant to discitis | 0 (0) |
| Back pain | 72 (100) |
| Febrile | 25 (35) |
| Radiation | 12 (17) |
| Numbness/weakness | 10 (14) |
| Bowel or bladder incontinence | 3 (4) |
| Time to diagnosis (days)a | 55 (1–270) |
| Site of involvement | |
| Cervical | 3 (4) |
| Thoracic | 20 (28) |
| Lumbar | 49 (68) |
| Biopsy technique | |
| CT | 50 (69) |
| Fluoroscopy | 22 (31) |
| Either surgical pathology (+)/microbiology (+) | 29 (40) |
| Surgical pathology (+) | 24 (33) |
| Microbiology growth from tissue (+) | 12 (17) |
| Both | 7 (10) |
| Bacterial isolates from tissue culture | 12 |
| Staphylococci | 5 (42) |
| Streptococci | 3 (25) |
| Pseudomonas | 2 (17) |
| Klebsiella | 1 (8) |
| Mycobacteria | 1 (8) |
| Blood culture growth (+) | 2 (3) |
| Laboratory leukocyte count (cells/mm3)a | 8.0 (4.1–17.5) |
| CRP (mg/L)a | 46.4 (1–303) |
| ESR (mm/h)a | 59.3 (6–156) |
Note:—COPD indicates chronic obstructive pulmonary disease.
Mean followed by range in parenthesis.
CRP values for patients with spondylodiscitis averaged 93.4 mg/L with a range of 7–303 mg/L, while they averaged 40.2 mg/L with range of 1–156 mg/L for patients without spondylodiscitis (Table 2). ESR values averaged 65.1 mm/h in patients with spondylodiscitis with a range of 12–150 mm/h, while they averaged 51.0 mm/h and ranged from 6–106 mm/h in patients without spondylodiscitis.
Table 2:
Inflammatory biomarker characteristics associated with pathology and/or microbiology for spondylodiscitis
| Pathology |
Pathology and/or Microbiologyy |
|||
|---|---|---|---|---|
| Positive | Negative | Positive | Negative | |
| CRP (mean) (mg/L) | 93.4 | 40.2 | 86.4 | 38.3 |
| Median | 50 | 31.5 | 60.5 | 22.0 |
| Range | 7–303 | 1–156 | 2–303 | 1–156 |
| SD | 90.3 | 38.7 | 84.9 | 42.4 |
| ESR (mean) (mm/h) | 65.1 | 51.0 | 66.8 | 51.6 |
| Median | 59.0 | 46 | 61.5 | 38.5 |
| Range | 12–150 | 6–106 | 22–156 | 6–109 |
| SD | 36.5 | 27.5 | 34.9 | 33.3 |
Clinical Biomarkers and Imaging Features Associating with Spondylodiscitis
Univariate analysis showed that fever status (r = 0.26, P = .03), CRP (r = 0.29, P = .02), hyperintense T2 disc signal (r = 0.29, P = .03), adjacent vertebral endplate erosion (r = 0.31, P = .02), epidural enhancement (r = 0.41, P = .001), and paraspinal enhancement (r = 0.33, P = .01) were associated with positive pathology and/or positive microbiology for spondylodiscitis (Tables 3 and 4). Blood culture growth, leukocytosis, hemoglobin count, platelet count, alkaline phosphatase level, and ESR did not have statistically significant associations (P > .05). In the setting of epidural enhancement, the PPV and NPV ranged from 40.7% to 61.3% and 75.6% to 100% for positive pathology and either positive pathology or positive microbiology, respectively. For paraspinal enhancement, PPV and NPV ranged from 36.9% to 53.8% and 75.8% to 100%, respectively. Hyperintense T2 disc signal and adjacent endplate erosion had similar PPV and NPV ranges with relatively low PPV (ranging from 31.2% to 55.5%) and high NPV (ranging from 79.2% to 100%).
Table 3:
Correlation between individual biomarkers and pathology-proved spondylodiscitisa
| Positive Pathologyy |
||||||
|---|---|---|---|---|---|---|
| r | P | PPV | NPV | Sensitivity | Specificity | |
| Epidural enhancement | ||||||
| Observer 1 | 0.52 | <.001c | 61.3 | 87.8 | 79.2 | 75.0 |
| Observer 2 | 0.36 | .002c | 42.1 | 100 | 100 | 31.3 |
| Observer 3 | 0.33 | .004c | 40.7 | 100 | 100 | 27.1 |
| Majority consensus | 0.41 | .001c | 44.4 | 100 | 100 | 37.5 |
| Paraspinal enhancement | ||||||
| Observer 1 | 0.27 | .02c | 40.7 | 88.9 | 91.7 | 33.3 |
| Observer 2 | 0.23 | .05c | 36.9 | 100 | 100 | 14.6 |
| Observer 3 | 0.24 | .04c | 43.6 | 78.8 | 70.8 | 54.2 |
| Majority consensus | 0.33 | .01c | 40.7 | 100 | 100 | 27.1 |
| Hyperintense T2 disc signal | ||||||
| Observer 1 | 0.37 | .02c | 55.5 | 80.1 | 86.5 | 45.0 |
| Observer 2 | 0.19 | .03c | 33.0 | 100 | 100 | 20.2 |
| Observer 3 | 0.21 | .03c | 41.2 | 79.2 | 73.1 | 51.0 |
| Majority consensus | 0.29 | .03c | 51.0 | 100 | 100 | 33.2 |
| Vertebral endplate erosion | ||||||
| Observer 1 | 0.333 | .01c | 46.0 | 83.4 | 88.8 | 30.2 |
| Observer 2 | 0.22 | .05c | 35.6 | 95.5 | 93.2 | 25.7 |
| Observer 3 | 0.30 | .0c | 39.4 | 80.6 | 75.6 | 56.7 |
| Majority consensus | 0.31 | .02c | 42.1 | 92.3 | 92.0 | 35.6 |
| Blood culture growth | 0.06 | .63 | 50 | 66.7 | 4.2 | 97.9 |
| Fever status | 0.26 | .03c | 48.3 | 76.7 | 58.3 | 68.8 |
| Leukocytosis | 0.032 | .80 | 40 | 64.3 | 16.7 | 85.7 |
| Hemoglobin countb | –0.05 | .67 | NA | NA | NA | NA |
| Platelet countb | 0.20 | .11 | NA | NA | NA | NA |
| ALP levelb | –0.03 | .84 | NA | NA | NA | NA |
| ESRb | –0.09 | .49 | NA | NA | NA | NA |
| CRPb | 0.29 | .02c | NA | NA | NA | NA |
Note:—NA indicates not applicable; ALP, alkaline phosphatase.
The Spearman correlation was used for rank/categoric variables. PPV, NPV, sensitivity, and specificity numbers are represented in percentages. Continuous variables will not have PPV, NPV, sensitivity, or specificity values without established thresholds.
Continuous variables in which the Pearson correlation was used.
Statistically significant P values (<.05).
Table 4:
Correlation between individual biomarkers and spondylodiscitis as proved on pathology and/or microbiologya
| Positive Pathology and/or Microbiologyy |
||||||
|---|---|---|---|---|---|---|
| r | P | PPV | NPV | Sensitivity | Specificity | |
| Epidural enhancement | ||||||
| Observer 1 | 0.37 | .00b | 61.3 | 75.6 | 65.5 | 72.1 |
| Observer 2 | 0.42 | .001b | 50.9 | 100 | 100 | 34.9 |
| Observer 3 | 0.39 | .001b | 49.2 | 100 | 100 | 30.2 |
| Majority consensus | 0.47 | .001b | 53.7 | 100 | 100 | 41.9 |
| Paraspinal enhancement | ||||||
| Observer 1 | 0.34 | .03b | 50.0 | 88.9 | 93.1 | 37.2 |
| Observer 2 | 0.27 | .02b | 44.6 | 100 | 100 | 16.3 |
| Observer 3 | 0.30 | .01b | 53.8 | 75.8 | 72.4 | 58.1 |
| Majority consensus | 0.39 | .01b | 49.2 | 100 | 100 | 30.2 |
| Hyperintense T2 disc signal | ||||||
| Observer 1 | 0.30 | .03b | 49.2 | 79.6 | 82.5 | 42.6 |
| Observer 2 | 0.15 | .05b | 31.2 | 96.2 | 95.3 | 19.6 |
| Observer 3 | 0.25 | .02b | 45.5 | 80.2 | 74.2 | 50.4 |
| Majority consensus | 0.28 | .03b | 48.0 | 95.2 | 93.5 | 29.8 |
| Vertebral endplate erosion | ||||||
| Observer 1 | 0.29 | .02b | 48.9 | 82.4 | 89.6 | 33.6 |
| Observer 2 | 0.17 | .03b | 37.5 | 95.8 | 92.5 | 27.5 |
| Observer 3 | 0.33 | .01b | 38.1 | 82.5 | 76.3 | 59.1 |
| Majority consensus | 0.31 | .02b | 42.6 | 91.5 | 91.8 | 34.7 |
| Blood culture growth | 0.03 | .79 | 50 | 59.4 | 3.4 | 97.6 |
| Fever status | 0.25 | .03a | 55.2 | 69.8 | 55.2 | 69.8 |
| Leukocytosis | 0.039 | .77 | 40 | 55.4 | 13.8 | 83.8 |
| Hemoglobin countc | −0.02 | .86 | NA | NA | NA | NA |
| Platelet countc | 0.17 | .18 | NA | NA | NA | NA |
| ALP levelc | 0.05 | .73 | NA | NA | NA | NA |
| ESRc | −0.07 | .62 | NA | NA | NA | NA |
| CRPc | 0.26 | .04a | NA | NA | NA | NA |
Note:—NA indicates not applicable; ALP, alkaline phosphatase.
The Spearman correlation was used for rank/categoric variables.
Statistically significant P values (<.05).
Continuous variables in which the Pearson correlation was used.
Combination of Imaging and Clinical Biomarkers Predicting Positive Pathology and/or Microbiology Findings Suggestive of Spondylodiscitis
Logistic regression for an optimal combination of clinical biomarkers showed that a combination of CRP, ESR, and fever status yielded the highest area under the curve (AUC) of 0.72 for positive pathology (On-line Fig 1) and 0.68 for positive pathology and/or microbiology (On-line Fig 2). When clinical biomarkers were combined with imaging features, a combination of CRP, ESR, fever status, and the presence of epidural enhancement yielded an improved AUC of 0.76–0.87 for positive pathology and 0.73–0.78 for positive pathology and/or microbiology (Table 5). The presence of hyperintense T2 disc signal, adjacent vertebral endplate erosion, and paraspinal enhancement did not improve prediction for positive pathology and/or microbiology findings suggestive of spondylodiscitis in combination with clinical biomarkers.
Table 5:
Predictive performance of clinical and image-based featuresa
| Positive Pathologyy |
Positive Pathology and/or Microbiologyy |
|||||
|---|---|---|---|---|---|---|
| AUC | Sensitivity | Specificity | AUC | Sensitivity | Specificity | |
| CRP, ESR, and fever | 0.72 | 68.2 | 67.0 | 0.68 | 60.5 | 64.5 |
| CRP, ESR, fever, and epidural enhancement (observer 1) | 0.87 | 83.1 | 79.8 | 0.76 | 68.4 | 75.6 |
| CRP, ESR, fever, and epidural enhancement (observer 2) | 0.76 | 75.0 | 66.5 | 0.73 | 59.6 | 76.2 |
| CRP, ESR, fever, and epidural enhancement (observer 3) | 0.79 | 77.2 | 70.1 | 0.78 | 69.3 | 75.8 |
| CRP, ESR, fever, and epidural enhancement (majority consensus) | 0.80 | 78.3 | 75.6 | 0.79 | 77.4 | 74.6 |
Logistic regression with backward stepwise selection was used to find the optimal combination of clinical and imaging features for 3 independent observers with majority consensus among the observers.
Interobserver Agreement among Multiple Raters and Majority Consensus
Interobserver κ agreement among 3 independent observers for hyperintense T2 disc signal, adjacent vertebral body erosions, epidural enhancement, and paraspinal enhancement was 0.58, 0.55, 0.49, and 0.33, respectively. To overcome fair κ agreement, we used a majority consensus for imaging scores. NPV was 100% for epidural and paraspinal enhancement based on majority consensus for positive pathology and positive pathology and/or microbiology (Tables 3 and 4). When epidural enhancement from majority consensus was combined with ESR, CRP, and fever status, an optimal AUC of 0.80 was obtained for positive pathology (On-line Fig 1) and 0.79 for positive pathology and/or microbiology (On-line Fig 2).
DISCUSSION
Diagnosis of spondylodiscitis can be difficult and often delayed or missed due to the insidious onset of symptoms and relative rarity of the disease in the setting of a high prevalence of patients presenting to the hospital with back pain. In our study, we assessed spondylodiscitis with 2 separate end points: as proved on pathology only and as proved on pathology and/or microbiology because clinicians use different criteria for the diagnosis of spondylodiscitis. We demonstrated that epidural enhancement on MR imaging added diagnostic value when combined with clinical biomarkers to help predict which patients undergoing percutaneous biopsy will have positive findings for spondylodiscitis on pathology and/or microbiology.
Clinically, the initial presentation of discitis is often back pain; however, in up to 15% of patients, the initial presentation may be fever or neurologic symptoms without pain.21-24 Correct diagnosis and treatment are essential to avoid long-term sequelae involving neurologic deficits.10,15 Fever has been shown in prior studies to be associated with spondylodiscitis, specifically occurring in up to 60% of patients.15,21 We observed similar findings in our study, with roughly 58% (14/24) of patients with positive pathology found to be febrile on presentation. Prior studies have shown conflicting associations of discitis with laboratory panels such as leukocytosis,10,14 anemia,18,19 and alkaline phosphatase (ALP) levels.17,18 We did not observe a statistically significant association among these biomarkers in our study.
ESR and CRP are well-studied inflammatory biomarkers and have been shown to have high sensitivity but low specificity for spondylodiscitis in prior studies.9-13 An and Seldomridge13 showed elevation of ESR in >80% of cases, with a mean of roughly 60 mm/h. In our study, the mean value was 65 mm/h for positive pathology and 67 mm/h for either positive pathology or microbiology. Most interesting, ESR was not found to have a statistical association with spondylodiscitis as an independent biomarker; however, it was statistically significant when combined with CRP, fever status, and epidural enhancement through a multiparametric model. CRP was found to be associated with spondylodiscitis independently. In our study, CRP ranged from 1 to 156 mg/L in the negative cohort, while it ranged from 7 to 303 mg/L in the positive cohort. Thus, elevated CRP values above the range of the negative cohort (>156m g/L) may support the diagnosis of spondylodiscitis if suspected.
The presence of hyperintense T2 disc signal, adjacent vertebral body erosion, epidural enhancement, and paraspinal enhancement are well-recognized MR imaging characteristics of spondylodiscitis.1,2,25-27 In our study, we had 3 neuroradiologists independently score these characteristics blinded to the diagnosis. However, there was suboptimal interobserver agreement with κ values of 0.58, 0.55, 0.49, and 0.33 for hyperintense T2 disc signal, adjacent vertebral body erosions, and epidural and paraspinal enhancement, respectively. We attempted to overcome this discordance by applying majority consensus in our univariate analysis and prediction model.
Spira et al25 had previously demonstrated 100% sensitivity and roughly 50% specificity with paraspinal enhancement but 40% sensitivity and 80% specificity with epidural enhancement for microbiology. Our results were similar for paraspinal enhancement with a sensitivity as high as 100% and specificity ranging from 14% to 54%. However, results differed for epidural enhancement with sensitivity ranging from 79% to 100% and specificity ranging from 27% to 75%. Ledermann et al1 had previously shown high sensitivity for paraspinal/epidural enhancement (97.7% sensitivity), hyperintense T2 disc signal (93.2% sensitivity), and adjacent endplate erosion (84.1% sensitivity) in a cohort of patients with positive pathology and/or microbiology. We also found high sensitivities for these MR imaging features ranging from 70.8% to 100%, 73.1% to 100%, and 75.% to 93.2% for paraspinal/epidural enhancement, hyperintense T2 disc signal, and adjacent endplate erosion, respectively. These findings also corresponded with high NPVs in all typical MR imaging features of spondylodiscitis and, not surprisingly, indicate that the absence of these typical features can be a helpful tool in excluding pathology- and microbiology-proved spondylodiscitis.
However, the novelty of this study was in demonstrating that MR imaging features, specifically epidural enhancement, when combined with clinical biomarkers, improved predictive performance, increasing the AUC from 0.72 to 0.87. This finding can also be observed in a range of experience levels because our observers ranged from having 2–10 years of attending experience as neuroradiologists. Moreover, this finding is observed in spondylodiscitis proved by either criterion: pathology only or pathology and/or microbiology. Patients suspected of having spondylodiscitis will likely undergo MR imaging, and it is clinically important to recognize that the enhancement pattern should be considered along with clinical biomarkers in the diagnosis of spondylodiscitis.
There are several limitations to our study. First, it was retrospective. A prospective study would facilitate increasing our cohort size by obtaining MR imaging and laboratory markers at the time of tissue biopsy for more patients. There is also an inherent bias of only including patients with high radiologic suspicion of spondylodiscitis requiring biopsy. An ideal study would have included patients without radiologic suspicion for spondylodiscitis; however, this would imply performing biopsies on patients without suspicion for spinal infection. The limitation of assessing patients who have been on antibiotics for <3 days is arbitrary; however, a recent study showed that antibiotics do not affect tissue yield within 3 weeks before biopsy.28 Additionally, we acknowledge that the use of pathology and/or microbiology results from percutaneous biopsy for spondylodiscitis may have a large percentage of false-negatives, shown in up to 37% by Nam et al.29 They previously compared pathology results from open-to-percutaneous needle biopsies and found, within the same cohort, that 70.4% of patients had positive pathology results from open biopsy, whereas only 33.3% of the cohort had positive findings with percutaneous biopsies. We acknowledge that using pathology results from open biopsy may have been an ideal, or perhaps more sensitive, criterion standard; however, open biopsies are no longer routinely performed.
Finally, there was suboptimal interobserver agreement among the 3 observers for MR imaging features. This is likely due to varying experience levels among the observers because the highest AUC for prediction of spondylodiscitis corresponded with scores from the observer with the most experience. We acknowledge that in clinical practice, there is a broad range of experience levels, and we attempted to overcome this limitation by using a majority consensus among the 3 observers.
CONCLUSIONS
Our findings demonstrated that epidural enhancement on MR imaging added diagnostic value when combined with clinical biomarkers to help predict which patients who undergo percutaneous biopsy will have positive findings for spondylodiscitis on pathology and/or microbiology.
ABBREVIATIONS:
- AUC
area under the curve
- CRP
C-reactive protein
- ESR
erythrocyte sedimentation rate
- NPV
negative predictive value
- PPV
positive predictive value
Footnotes
Paper previously presented at: Annual Meeting of the American Society of Neuroradiology, May 30–June 4, 2020; Virtual, No. 1140.
References
- 1.Ledermann HP, Schweitzer ME, Morrison WB, et al. MR imaging findings in spinal infections: rules or myths? Radiology 2003;228:506–14 10.1148/radiol.2282020752 [DOI] [PubMed] [Google Scholar]
- 2.Hong SH, Choi JY, Lee JW, et al. MR imaging assessment of the spine: infection or an imitation? Radiographics 2009;29:599–612 10.1148/rg.292085137 [DOI] [PubMed] [Google Scholar]
- 3.Duarte RM, Vaccaro AR. Spinal infection: State of the art and management algorithm. Eur Spine J 2013;22:2787–99 10.1007/s00586-013-2850-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sehn JK, Gilula LA. Percutaneous needle biopsy in diagnosis and identification of causative organisms in cases of suspected vertebral osteomyelitis. Eur J Radiol 2012;81:940–46 10.1016/j.ejrad.2011.01.125 [DOI] [PubMed] [Google Scholar]
- 5.van Thuijl HF, Mazor T, Johnson BE, et al. Evolution of DNA repair defects during malignant progression of low-grade gliomas after temozolomide treatment. Acta Neuropathol 2015;129:597–607 10.1007/s00401-015-1403-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chew FS, Kline MJ. Diagnostic yield of CT-guided percutaneous aspiration procedures in suspected spontaneous infectious diskitis. Radiology 2001;218:211–14 10.1148/radiology.218.1.r01ja06211 [DOI] [PubMed] [Google Scholar]
- 7.Rimondi E, Staals EL, Errani C, et al. Percutaneous CT-guided biopsy of the spine: results of 430 biopsies. Eur Spine J 2008;17:975–81 10.1007/s00586-008-0678-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landi A, Grasso G, Iaiani G, et al. Spontaneous spinal discitis and spondylodiscitis: clinicotherapeutic remarks. J Neurosci Rural Pract 2017;8:42–46 10.4103/jnrp.jnrp_67_17] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dufour V, Feydy A, Rillardon L, et al. Comparative study of postoperative and spontaneous pyogenic spondylodiscitis. Semin Arthritis Rheum 2005;34:766–71 10.1016/j.semarthrit.2004.08.004 [DOI] [PubMed] [Google Scholar]
- 10.Euba G, Narváez JA, Nolla JM, et al. Long-term clinical and radiological magnetic resonance imaging outcome of abscess-associated spontaneous pyogenic vertebral osteomyelitis under conservative management. Semin Arthritis Rheum 2008;38:28–40 10.1016/j.semarthrit.2007.08.007 [DOI] [PubMed] [Google Scholar]
- 11.Gouliouris T, Aliyu SH, Brown NM. Spondylodiscitis: update on diagnosis and management. J Antimicrob Chemother 2010;65(Suppl 3):iii11–24 10.1093/jac/dkq303] [DOI] [PubMed] [Google Scholar]
- 12.Patzakis MJ, Rao S, Wilkins J, et al. Analysis of 61 cases of vertebral osteomyelitis. Clin Orthop Relat Res 1991;178–83 [PubMed] [Google Scholar]
- 13.An HS, Seldomridge JA. Spinal infections: diagnostic tests and imaging studies. Clin Orthop Rel Res 2006;443:27–33 10.1097/01.blo.0000203452.36522.97 [DOI] [PubMed] [Google Scholar]
- 14.Nolla JM, Ariza J, Gómez-Vaquero C, et al. Spontaneous pyogenic vertebral osteomyelitis in nondrug users. Semin Arthritis Rheum 2002;31:271–08 10.1053/sarh.2002.29492 [DOI] [PubMed] [Google Scholar]
- 15.Mylona E, Samarkos M, Kakalou E, et al. Pyogenic vertebral osteomyelitis: a systematic review of clinical characteristics. Semin Arthritis Rheum 2009;39:10–17 10.1016/j.semarthrit.2008.03.002 [DOI] [PubMed] [Google Scholar]
- 16.Wirtz DC, Genius I, Wildberger JE, et al. Diagnostic and therapeutic management of lumbar and thoracic spondylodiscitis: an evaluation of 59 cases. Arch Orthop Trauma Surg 2000;120:245–51 10.1007/s004020050457 [DOI] [PubMed] [Google Scholar]
- 17.Colmenero JD, Jiménez-Mejías ME, Sánchez-Lora FJ, et al. Pyogenic, tuberculous, and brucellar vertebral osteomyelitis: a descriptive and comparative study of 219 cases. Ann Rheum Dis 1997;56:709–15 10.1136/ard.56.12.709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beronius M, Bergman B, Andersson R. Vertebral osteomyelitis in Göteborg, Sweden: a retrospective study of patients during 1990-95. Scand J Infect Dis 2001;33:527–32 10.1080/00365540110026566 [DOI] [PubMed] [Google Scholar]
- 19.Nather A, David V, Hee HT, et al. Pyogenic vertebral osteomyelitis: a review of 14 cases. J Orthop Surg (Hong Kong) 2005;13:240–44 10.1177/230949900501300305 [DOI] [PubMed] [Google Scholar]
- 20.Sertic M, Parkes L, Mattiassi S, et al. The Efficacy of Computed Tomography-Guided Percutaneous Spine Biopsies in Determining a Causative Organism in Cases of Suspected Infection: A Systematic Review. Can Assoc Radiol J 2019;70:96–103 10.1016/j.carj.2018.09.003 [DOI] [PubMed] [Google Scholar]
- 21.Sapico FL, Montgomerie JZ. Pyogenic vertebral osteomyelitis: report of nine cases and review of the literature. Rev Infect Dis 1979;1:754–76 10.1093/clinids/1.5.754 [DOI] [PubMed] [Google Scholar]
- 22.Torda AJ, Gottlieb T, Bradbury R. Pyogenic vertebral osteomyelitis: analysis of 20 cases and review. Clin Infect Dis 1995;20:320–28 10.1093/clinids/20.2.320 [DOI] [PubMed] [Google Scholar]
- 23.Sakkas LI, Davas EM, Kapsalaki E, et al. Hematogenous spinal infection in Central Greece. Spine (Phila Pa 1976) 2009;34:E513–18 10.1097/BRS.0b013e3181a9897e19564756 [DOI] [PubMed] [Google Scholar]
- 24.Zarrouk V, Feydy A, Sallès F, et al. Imaging does not predict the clinical outcome of bacterial vertebral osteomyelitis. Rheumatology 2006;46:292–95 10.1093/rheumatology/kel228 [DOI] [PubMed] [Google Scholar]
- 25.Spira D, Germann T, Lehner B, et al. CT-guided biopsy in suspected spondylodiscitis: the association of paravertebral inflammation with microbial pathogen detection. PLoS One 2016;11:e0146399 10.1371/journal.pone.0146399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schellinger D. Patterns of anterior spinal canal involvement by neoplasms and infections. AJNR Am J Neuroradiol 1996;17:953–59 [PMC free article] [PubMed] [Google Scholar]
- 27.Longo M, Granata F, Ricciardi GK, et al. Contrast-enhanced MR imaging with fat suppression in adult-onset septic spondylodiscitis. Eur Radiol 2003;13:626–37 10.1007/s00330-002-1411-5 [DOI] [PubMed] [Google Scholar]
- 28.Foreman SC, Schwaiger BJ, Gempt J, et al. MR and CT imaging to optimize CT-guided biopsies in suspected spondylodiscitis. World Neurosurg 2017;99:726–34.e7 10.1016/j.wneu.2016.11.017] [DOI] [PubMed] [Google Scholar]
- 29.Nam KH, Song GS, Han IH, et al. Diagnostic value of biopsy techniques in lumbar spondylodiscitis: percutaneous needle biopsy and open biopsy. Korean J Spine 2011;8:267–71 10.14245/kjs.2011.8.4.267 [DOI] [PMC free article] [PubMed] [Google Scholar]