Abstract
BACKGROUND AND PURPOSE:
Ventricular enlargement in idiopathic normal pressure hydrocephalus is often estimated using the Evans index. However, the sensitivity of the Evans index to estimate changes in ventricular size postoperatively has been questioned. Here, we evaluated the postoperative change in ventricle size in relation to shunt response in patients with idiopathic normal pressure hydrocephalus, by comparing ventricular volume and the Evans index.
MATERIALS AND METHODS:
Fifty-seven patients with idiopathic normal pressure hydrocephalus underwent high-resolution MR imaging preoperatively and 6 months after shunt insertion. Clinical symptoms of gait, balance, cognition, and continence were assessed according to the idiopathic normal pressure hydrocephalus scale. The ventricular volume of the lateral and third ventricles and the Evans index were measured using ITK-SNAP software. Semiautomatic volumetric analysis was performed, and postoperative changes in ventricular volume and the Evans index and their relationships to postoperative clinical improvement were compared.
RESULTS:
The median postoperative ventricular volume decrease was 25 mL (P < .001). The proportional decrease in ventricular volume was greater than that in the Evans index (P < .001). The postoperative decrease in ventricular volume was associated with a postoperative increase in the idiopathic normal pressure hydrocephalus scale score (P = .004). Shunt responders (75%) demonstrated a greater ventricular volume decrease than nonresponders (P = .002).
CONCLUSIONS:
Clinical improvement after shunt surgery in idiopathic normal pressure hydrocephalus is associated with a reduction of ventricular size. Ventricular volume is a more sensitive estimate than the Evans index and, therefore, constitutes a more precise method to evaluate change in ventricle size after shunt treatment in idiopathic normal pressure hydrocephalus.
Idiopathic normal pressure hydrocephalus (iNPH) is characterized by progressive gait and balance disturbance, cognitive impairment, and urinary incontinence.1-3 Because iNPH is considered one of the few potentially treatable causes of dementia, its early diagnosis is imperative. Treatment primarily consists of shunt surgery, demonstrating high clinical improvement rates of 71%–84%.2-7
Ventriculomegaly on CT or MR imaging is mandatory to correctly diagnose iNPH.1,3,8 In 1942, during the era of pneumoencephalography, a method for the assessment of ventricular enlargement, in which the maximum transverse diameter of the frontal horns was divided by the greatest internal transverse diameter of the skull on coronal images, was introduced by W.A. Evans.9 Since the subsequent development of CT and MR imaging, the Evans index (EI) is calculated on an axial section as the ratio between the maximum width of the frontal horns and the maximal internal diameter of the skull.10,11
A calculation of the EI is generally used in the diagnostic work-up of iNPH, and according to international guidelines, a ratio of >0.3 constitutes a prerequisite for a diagnosis of probable iNPH.3 However, because the width of the frontal horns provides little information about the shape of the ventricular system at large, the index provides just a crude estimate of the actual size of the ventricles. Accordingly, authors have argued that the EI is not an ideal method to estimate ventricular volume (VV) in iNPH.11
Furthermore, decreases in EI have been shown to be poorly correlated with clinical improvement after shunt surgery,12,13 whereas recent studies evaluating ventricular size by means of volumetric measurements have shown mean postoperative decreases of VV of 24%–28% in patients with improved iNPH, suggesting that such measurements may constitute more clinically relevant markers.14-17
Currently, there are no large-scale studies comparing postoperative changes in VV and the EI in patients with iNPH. Here, we evaluated postoperative changes in VV and the EI and investigated their relation to clinical outcome.
MATERIALS AND METHODS
Sixty-six patients diagnosed with probable or possible iNPH3 were consecutively included at the Hydrocephalus Research Unit, Sahlgrenska University Hospital, between 2013 and 2015. All patients received a ventriculoperitoneal (n = 63) or ventriculoatrial (n = 3) shunt (PS Medical Strata Adjustable Valve; Medtronic) with the opening pressure set at a medium level (setting 1.5). The ventricular catheters were placed frontally with the tip of the ventricular catheter inside the lateral ventricle.
Clinical assessments and MR imaging examinations were performed preoperatively and after 6 months in all patients. Two patients demonstrated shunt obstructions before the postoperative examinations. These 2 patients underwent shunt revision and were included in the study, with clinical assessment and MR imaging performed 6 months after the shunt revision. One patient was excluded due to a delay in follow-up after undergoing shunt revision. One patient presented with a subdural hematoma on postoperative MR imaging and was excluded. Seven patients demonstrated motion artifacts on preoperative (n = 4) or postoperative (n = 3) MR imaging and were excluded from the study. Characteristics of the remaining 57 patients are given in Table 1.
Table 1:
Demographic data of the patients in the study (n = 57)
| Demographics | |
|---|---|
| Age (mean) (range) (yr) | 74 ± 7 (49–91) |
| Sex (male/female) | 42:15 |
| Preoperative iNPH score (mean) (SD) | 54 (20) |
| Postoperative iNPH score (mean) (SD) | 66 (22) |
| Months from surgery to postoperative follow-up (mean) (range) | 6 ± 1.6 (3–9) |
Clinical Assessment
The patients were clinically evaluated before and 6 months after shunt insertion according to the iNPH scale,18 comprising 4 symptom domains (gait, balance, neuropsychology, and continence) and yielding a total score (iNPH scale score) ranging between 0 and 100, with 100 representing normal performance among healthy individuals in an iNPH typical age range of 70–74 years. In cases without clear postoperative improvement, shunt dysfunction was ruled out using a head CT and radionuclide shuntography19 or a lumbar infusion test.20 Responders were defined as patients demonstrating a postoperative increase in the iNPH scale score of ≥5 points.18
MR Imaging Volumetry and the Evans Index
Identical MR imaging scans with T1-weighted volume sequences with 1-mm scan resolution, from a 1.5T Intera (Philips Healthcare) or a 1.5T Achieva dStream (Philips Healthcare) scanner, were obtained at baseline and at the 6-month postoperative follow-up. Scan parameters were as follows: FOV = 260 × 260 × 190 mm3, TR = 25 ms, TE = 4.6 ms, and flip angle = 30°. The scan was reconstructed to a 0.5-mm image resolution. The MR imaging datasets of all images were transmitted in DICOM format from the MR imaging storage unit to a personal computer. All image analyses were performed by J.N. and D.Z., who were blinded to clinical data.
The pre- and postoperative volumes of the third and the lateral ventricles were semiautomatically measured using the ITK-SNAP software (Version 3.6.0; www.itksnap.org).21 Comparable images with clearly visualized ventricles and a histogram function of the image contrast were acquired using the Image Layer Inspector, Contrast Adjustment.21 Mean image intensity was 1368.9 ± 239.4 (arbitrary units). The volumetric measurement was performed automatically and was modified manually. The Thresholding Segmentation mode21 was used for automatic segmentation, whereas the Paintbrush Mode and Polygon Mode21 were used for manual modifications. The segmented volume was presented in voxels and in cubic millimeters. The voxel size was 0.5 × 0.5 × 0.5 mm in all examinations. The ventricular volume was the product of the number of voxels in each segmentation and the voxel volume (0.125 mm3).
The EI was measured using the Image Annotation Mode in ITK-SNAP21 on axial MR imaging slices (aligned to the anterior and posterior commissures) and defined as the maximum width of the frontal horns anterior to the foramina of Monro divided by the maximum width of the inner skull, both measured on the same section.
Statistics
Responders and nonresponders were compared with regard to postoperative decreases in ventricular volume and changes in the EI by means of the Mann-Whitney U test. Furthermore, the decreases in ventricular volume and the EI within the groups of responders and nonresponders, respectively, were tested using the Wilcoxon signed rank test. Correlations between preoperative and postoperative VVs and changes in the iNPH scale score were analyzed using the Spearman rank correlation test.
We evaluated the correlation between postoperative change in the iNPH scale score and postoperative decreases in ventricular volume and the EI, respectively, using regression models, assuming approximate normal distributions and adjusting for heteroscedasticity. We examined nonlinear effects for linear, piecewise linear, quadratic, and cubic functions of the explanatory variables. The best correlation was selected on the basis of the highest adjusted R2. The effects per a 1-SD decrease were also calculated to compare the effects of the 2 explanatory variables. The 2 measurements were adjusted for each other in a multivariable model.
All tests were 2-tailed, and α was set to <.05. All analyses were performed using SAS software, Version 9.4 (SAS Institute).
Ethics Considerations
The study was approved by the local ethics committee in Gothenburg, D-number 328–14. All patient data were de-identified at the time of data analysis and presentation.
RESULTS
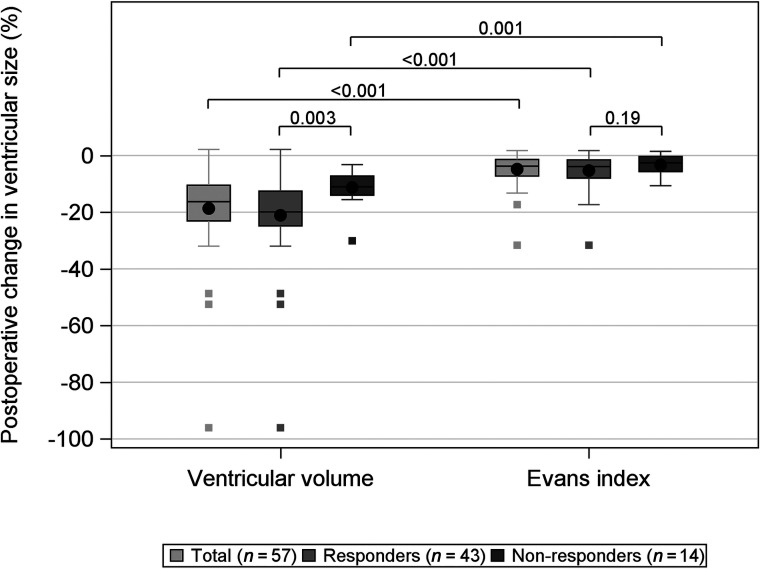
The median postoperative VV decrease was 24 mL (p25 = 16 mL, p75 = 34 mL; P < .001), equivalent to 18%, and the median postoperative decrease in the EI was 0.02 (interquartile range, 0.02; P < .001) or 5%. The proportional decrease in VV was significantly larger (P < .001) than the proportional decrease in the EI (Fig 1). Forty responders (93%) showed a >5% decrease in VV. Pre- and postoperative VVs correlated to a change in the iNPH scale score are presented in Table 2. Postoperative VV showed a weak-but-significant correlation with change in the iNPH scale score (r = –0.28, P = .036). Pre- or postoperative decreases in VVs were not significantly correlated with either pre- or postoperative iNPH scale scores or a change in the iNPH scale scores.
FIG 1.
Box-and-whisker plot showing the change in ventricular size measured by VV and the EI after shunt treatment in 57 patients with iNPH. The whiskers denote values within the 1.5 interquartile range from the first and third quartiles, and the boxes represent outliers. The P values for the difference between responders (gray) and nonresponders (dark gray) are presented as well as the total (light gray). VV decreased significantly more than the EI for all groups. Responders had a significantly larger VV decrease than nonresponders. There was no difference in the EI between responders and nonresponders.
Table 2:
Preoperative VV and postoperative absolute and relative decreases in VV among 57 patients operated on for iNPH
| Median (Minimum, p25, p75, Maximum) | VV vs iNPH Scale Scorea | |
|---|---|---|
| Preoperative VV (mL) | 141.4 (70.8, 133.4, 167.9, 317.8) | r = –0.08P = .59 |
| Postoperative VV (mL) | 120.8 (4.1, 103.7, 137.9, 309.0) | r = –0.28P = .036 |
| Postoperative absolute decrease in VV (mL) | 24.1 (−4.0, 15.9, 34.2, 100.1) | r = 0.54P < .001 |
| Postoperative relative decrease in VV (%) | 16.3 (−2.1, 10.5, 23.1, 96.1) | r = 0.56P < .001 |
Spearman rank correlation test between VV and postoperative change in the iNPH scale score.
Forty-three patients (75%) were shunt responders. A postoperative decrease in VV was significantly (P = .003) larger in shunt responders (21%, n = 43) than among nonresponders (13%, n = 14). A postoperative decrease in VV was significantly correlated with the 4 symptom domains: gait and balance disturbance (P = .002), neuropsychology (P = .010), and continence (P = .012). There was no correlation between postoperative change in the EI and each of these 3 symptom domains.
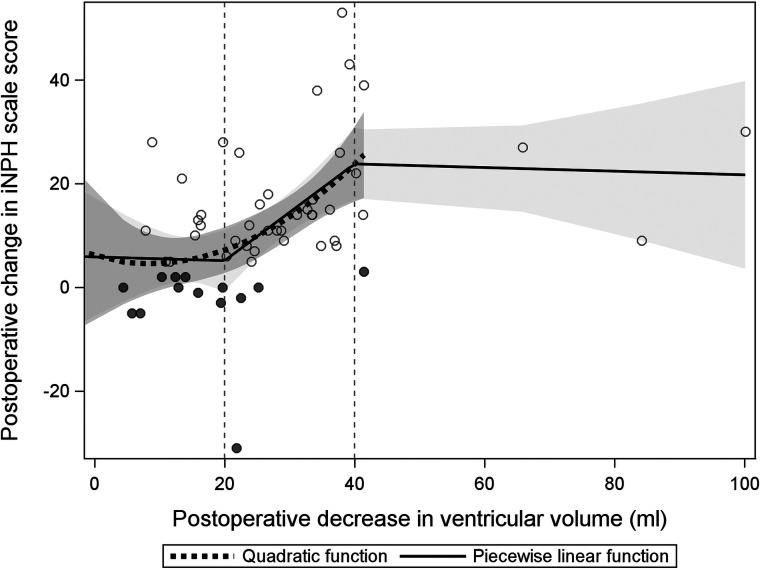
A postoperative decrease in VV and an increase in the iNPH scale score were significantly correlated; the iNPH scale increased by 16.4 (standard error, 4.6; P = .004) per 1-SD decrease in VV within the interval of 20–40 mL, compared with the relation of VV decrease of <20 and >40 mL (mean, −0.64; standard error, 5.4; and mean, −0.62; standard error, 2.9), respectively (Fig 2). The adjusted R2 was 0.22 for the amount of explained variance in the model (P < .001).
FIG 2.
Scatterplot illustrating the relation between a postoperative decrease in ventricular volume and shunt response (iNPH scale score) in the participants. Trend lines for a quadratic and piecewise linear function are shown. The shadowed gray areas represent the 95% confidence intervals for the quadratic function, and the bright gray area represents the same interval for the linear function.
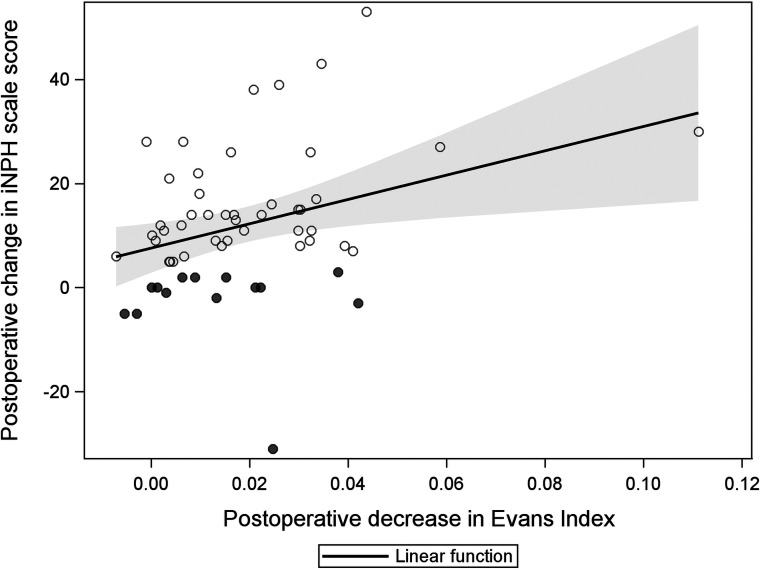
A postoperative EI decrease showed a significant linear relation to the increased iNPH scale score, with a mean increase of 7.6 (standard error, 1.7; P < .001) per 1-SD decrease in the EI (Fig 3). The adjusted R2 was 0.08 for the amount of explained variance in the model (P = .040).
FIG 3.
Scatterplot illustrating the linear relation between postoperative decrease in the EI and shunt response (iNPH scale score). The shadowed area represents the 95% confidence intervals for the linear function.
DISCUSSION
The median postoperative decrease in VV (25 mL) in shunted patients with iNPH was similar to that found in previous studies.14-16 The proportional postoperative decrease in ventricle size was >3 times greater when measuring VV (18%) compared with the EI (5%). Furthermore, the decrease in VV in the interval of 20–40 mL and the increase in the iNPH scale score were more strongly correlated than the decrease in the EI and the increase in the iNPH scale score; the mean increase in the iNPH scale score was 16.4 per 1-SD decrease in VV compared with 7.6 per 1-SD decrease in the EI.
The present study showed a stronger association between a decrease in VV and clinical improvement than the EI, which may indicate that increased VV may be better associated with symptoms of iNPH than the EI. Recently, Crook et al22 found that volumetric measures of ventricle size were more strongly associated with gait and cognition than the EI. Future studies on patients with iNPH correlating the different symptom domains to ventricular volume would be of great interest.
Most important, we observed that the postoperative changes in ventricle size using volumetric assessment were significantly greater in shunt responders compared with nonresponders. Using the EI for the same task did not result in a significant difference between the groups.
This finding shows that response to shunting is more closely related to changes in VV than in the EI and implies that assessment of VV could be a valuable supplementary tool in the clinical evaluation of shunt response.
We observed that a 20- to 40-mL decrease in VV was related to a significant clinical improvement, whereas neither smaller (<20 mL) nor larger VV decreases (>40 mL) were correlated with a response to shunting. That insufficient CSF drainage is associated with a lack of shunt response seems reasonable and corroborates previous studies showing that decreased shunt valve opening pressures (intended to increase the CSF drainage and potentially further decrease VV) were associated with an improved shunt response.7,23,24 On the other hand, other studies have demonstrated that low shunt valve opening pressures were not more effective than higher opening pressures.7,23 Differences in brain elasticity or CSF dynamic disturbance (eg, resistance to CSF outflow), factors not accounted for in this study, may explain differences in the postoperative reduction of VV among patients reported here.25
The improvement rate after shunt treatment of patients with iNPH in this study (75%) is consistent with that in previous studies.6,26,27
A limitation in this study is that only patients without a clear postoperative improvement underwent invasive evaluation for shunt patency, while in the remainder, significant improvement was regarded as proof of a working shunt. Because both shuntography and a lumbar infusion test are invasive procedures, we only performed these tests in cases in which shunt patency was doubted. Future studies in which shunt patency is systematically determined would be valuable in evaluating shunt patency using VV measurements.
Methodologic Aspects
Previously, studies demonstrated good interrater agreement using ITK-SNAP for volumetric measurements.21,28-30 Here, using a Thresholding Segmentation Algorithm in ITK-SNAP21 for all measurements, we standardized the data to facilitate comparison. The Thresholding Segmentation Algorithm in ITK-SNAP was able to automatically expand from a selected region to wide parts of the ventricles, but for the narrow parts of the ventricles, the CSF voxels had to be corrected manually. Similarly, manual corrections were required in areas where the borders were only thin membranes or were partly blurred due to minor head motion, because the segmentation function did not always respect the ventricular borders. These corrections have involved a degree of partial volume effect.31 We believe that partial volume effect, at ventricular borders, does not seriously affect the volumetric measurements in patients with iNPH because the ventricles occupy a relatively large proportion of the measured region. We therefore consider the volumetric measurements using ITK-SNAP accurate and reliable, albeit time-consuming because of the need for manual corrections.
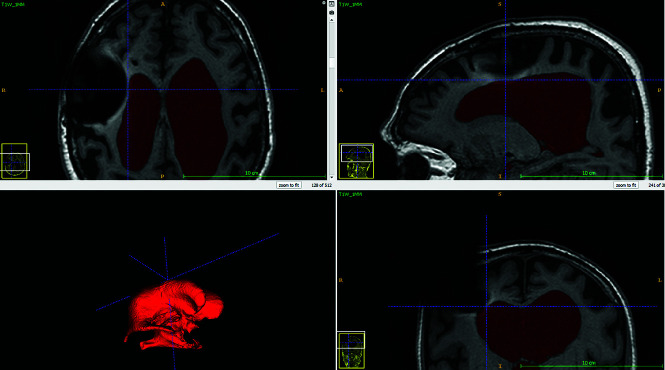
Susceptibility artifacts from metal components of the shunt valve can potentially reach into the lateral ventricle ipsilateral to the site of shunt placement and disturb the VV measurement on the postoperative MR imaging scans. We observed these susceptibility artifacts present in 3 patients, but in all of these patients, the contour of the wall of the lateral ventricle was clearly visible. Figure 4 shows postoperative MR imaging in 1 patient, in which susceptibility artifacts from the shunt valve extended into the right lateral ventricle without affecting the contour of the wall of the lateral ventricle. Therefore, we believe that the susceptibility artifacts did not affect the volumetric measurements in the present study. However, we found the presence of the susceptibility artifacts on postoperative MR imaging scans to be a limiting factor for measurement of total intracranial volumes; therefore, this measure was not assessed in this study.
FIG 4.
Ventricle volume segmentation in the ITK-SNAP software showing how susceptibility artifacts from the shunt valve appeared on the postoperative MR imaging.
There are various methods to determine volumes of brain structures. Voxel-based volume measurement uses voxel intensity to identify the desired brain structure on MR imaging. Atlas-based volumetric analysis uses a reference atlas.32 Currently, there are no volumetric reference atlases for iNPH; therefore, the use of an intensity-based method seems appropriate to evaluate VV in patients with iNPH. Previously, Ambarki et al33 used the SyntheticMR software (https://syntheticmr.com/company/) to measure intracranial volume and found it fast (<3 minutes) and reproducible. Qiu et al34 have tested ventricular volumetric measurement using different segmentation algorithms, including algorithms used in ITK-SNAP. The development of accurate, fast, and easy-to-use volumetric segmentation software is important for further studies in evaluating the standardized use of volumetry in patients with iNPH.
CONCLUSIONS
Clinical improvement after shunt surgery in iNPH is associated with a reduction in VV; shunt responders showed a greater decrease in VV than nonresponders. Furthermore, the proportional decrease in VV was significantly greater than that in the EI, showing that volumetric measurement is a more sensitive method to evaluate change in ventricular size after shunting in iNPH.
ACKNOWLEDGMENTS
We thank Aldina Pivodic at Statistiska Konsultgruppen for statistical support.
ABBREVIATIONS:
- EI
Evans index
- iNPH
idiopathic normal pressure hydrocephalus
- VV
ventricular volume
Footnotes
Disclosures: Johanna Neikter—RELATED: Grant: Göteborgs Läkaresällskap. Mats Tullberg—UNRELATED: Employment: Neuroscience and Physiology, Sahlgrenska Academy, University of Gothenburg.
References
- 1.Marmarou A, Black P, Bergsneider M, et al. ; International NPH Consultant Group. Guidelines for management of idiopathic normal pressure hydrocephalus: progress to date. Acta Neurochir Suppl 2005;95: 237–40 10.1007/3-211-32318-x_48 [DOI] [PubMed] [Google Scholar]
- 2.Ishikawa M, Hashimoto M, Kuwana N, et al. Guidelines for management of idiopathic normal pressure hydrocephalus. Neurol Med Chir (Tokyo) 2008;48(Suppl):S1–23 10.2176/nmc.48.s1 [DOI] [PubMed] [Google Scholar]
- 3.Relkin N, Marmarou A, Klinge P, et al. Diagnosing idiopathic normal-pressure hydrocephalus. Neurosurgery 2005;57(Suppl):S4–16; discussion ii–v 10.1227/01.neu.0000168185.29659.c5 [DOI] [PubMed] [Google Scholar]
- 4.Marmarou A, Bergsneider M, Klinge P, et al. The value of supplemental prognostic tests for the preoperative assessment of idiopathic normal-pressure hydrocephalus. Neurosurgery 2005;57(Supple 3):S2– 17 10.1227/01.NEU.0000168184.01002.60 [DOI] [PubMed] [Google Scholar]
- 5.Bergsneider M, Black PM, Klinge P, et al. Surgical management of idiopathic normal-pressure hydrocephalus. Neurosurgery 2005;57(Suppl):S29–39; discussion ii–v 10.1227/01.neu.0000168186.45363.4d [DOI] [PubMed] [Google Scholar]
- 6.Toma AK, Papadopoulos MC, Stapleton S, et al. Systematic review of the outcome of shunt surgery in idiopathic normal-pressure hydrocephalus. Acta Neurochir (Wien) 2013;155:1977–80 10.1007/s00701-013-1835-5 [DOI] [PubMed] [Google Scholar]
- 7.Farahmand D, Sæhle T, Eide PK, et al. A double-blind randomized trial on the clinical effect of different shunt valve settings in idiopathic normal pressure hydrocephalus. J Neurosurg 2016;124:359–67 10.3171/2015.1.JNS141301 [DOI] [PubMed] [Google Scholar]
- 8.Farahmand D, Qvarlander S, Malm J, et al. Intracranial pressure in hydrocephalus: impact of shunt adjustments and body positions. J Neurol Neurosurg Psychiatry 2015;86:222–28 10.1136/jnnp-2014-307873 [DOI] [PubMed] [Google Scholar]
- 9.Evans WA. An encephalographic ratio for estimating ventricular enlargement and cerebral atrophy. Archives of Neurology and Psychiatry 1942;47:931 10.1001/archneurpsyc.1942.02290060069004 [DOI] [Google Scholar]
- 10.Synek V, Reuben JR, Du Boulay GH. Comparing Evans’ index and computerized axial tomography in assessing relationship of ventricular size to brain size. Neurology 1976;26:231–33 10.1212/wnl.26.3.231 [DOI] [PubMed] [Google Scholar]
- 11.Toma AK, Holl E, Kitchen ND, et al. Evans’ index revisited: the need for an alternative in normal pressure hydrocephalus. Neurosurgery 2011;68:939–44 10.1227/NEU.0b013e318208f5e0 [DOI] [PubMed] [Google Scholar]
- 12.Meier U, Paris S, Gräwe A, et al. Is there a correlation between operative results and change in ventricular volume after shunt placement? A study of 60 cases of idiopathic normal-pressure hydrocephalus. Neuroradiology 2003;45:377–80 10.1007/s00234-003-0989-x [DOI] [PubMed] [Google Scholar]
- 13.Meier U, Mutze S. Correlation between decreased ventricular size and positive clinical outcome following shunt placement in patients with normal-pressure hydrocephalus. J Neurosurg 2004;100:1036–40 10.3171/jns.2004.100.6.1036 [DOI] [PubMed] [Google Scholar]
- 14.Hiraoka K, Yamasaki H, Takagi M, et al. Changes in the volumes of the brain and cerebrospinal fluid spaces after shunt surgery in idiopathic normal-pressure hydrocephalus. J Neurol Sci 2010;296:7–12 10.1016/j.jns.2010.06.021 [DOI] [PubMed] [Google Scholar]
- 15.Tsunoda A, Mitsuoka H, Bandai H, et al. Intracranial cerebrospinal fluid distribution and its postoperative changes in normal pressure hydrocephalus. Acta Neurochir (Wien) 2001;143:493–99 10.1007/s007010170079 [DOI] [PubMed] [Google Scholar]
- 16.Anderson RC, Grant JJ, de la Paz R, et al. Volumetric measurements in the detection of reduced ventricular volume in patients with normal-pressure hydrocephalus whose clinical condition improved after ventriculoperitoneal shunt placement. J Neurosurg 2002;97:73–79 10.3171/jns.2002.97.1.0073 [DOI] [PubMed] [Google Scholar]
- 17.Virhammar J, Laurell K, Cesarin KG, et al. Increase in callosal angle and decrease in ventricular volume after shunt surgery in patients with idiopathic normal pressure hydrocephalus. J Neurosurg 2018;130:130–35 10.3171/2017.8.JNS17547 [DOI] [PubMed] [Google Scholar]
- 18.Hellstrom P, Klinge P, Tans J, et al. A new scale for assessment of severity and outcome in iNPH. Acta Neurol Scand 2012;126:229–37 10.1111/j.1600-0404.2012.01677.x [DOI] [PubMed] [Google Scholar]
- 19.Wikkelso C, Andersson H, Lindberg S, et al. ‘Shuntography’: a radionuclide scanning method for evaluation of cerebrospinal fluid shunt patency. Nucl Med Commun 1983;4:88–93 [Google Scholar]
- 20.Malm J, Lundkvist B, Eklund A, et al. CSF outflow resistance as predictor of shunt function. a long-term study. Acta Neurol Scand 2004;110:154–60 10.1111/j.1600-0404.2004.00302.x [DOI] [PubMed] [Google Scholar]
- 21.Yushkevich PA, Piven J, Hazlett HC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 2006;31:1116–28 10.1016/j.neuroimage.2006.01.015 [DOI] [PubMed] [Google Scholar]
- 22.Crook JE, Gunter JL, Ball CT, et al. Linear vs volume measures of ventricle size: relation to present and future gait and cognition. Neurology 2020;94:e549–56 10.1212/WNL.0000000000008673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delwel EJ, de Jong DA, Dammers R, et al. A randomised trial of high and low pressure level settings on an adjustable ventriculoperitoneal shunt valve for idiopathic normal pressure hydrocephalus: results of the Dutch Evaluation Programme Strata Shunt (DEPSS) trial. J Neurol Neurosurg Psychiatry 2013;84:813–17 10.1136/jnnp-2012-302935 [DOI] [PubMed] [Google Scholar]
- 24.Boon AJ, Tans JT, Delwel EJ, et al. Dutch normal pressure hydrocephalus study: baseline characteristics with emphasis on clinical findings. Eur J Neurol 1997;4:39–47 10.1111/j.1468-1331.1997.tb00297.x [DOI] [PubMed] [Google Scholar]
- 25.Tans JT, Poortvliet DC. Reduction of ventricular size after shunting for normal pressure hydrocephalus related to CSF dynamics before shunting. J Neurol Neurosurg Psychiatry 1988;51:521–25 10.1136/jnnp.51.4.521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klinge P, Hellström P, Tans J, et al. ; On behalf of the European iNPH Multicentre Study Group. One-year outcome in the European multicentre study on iNPH. Acta Neurol Scand 2012;126:145–53 10.1111/j.1600-0404.2012.01676.x [DOI] [PubMed] [Google Scholar]
- 27.Kahlon B, Sjunnesson J, Rehncrona S. Long-term outcome in patients with suspected normal pressure hydrocephalus. Neurosurgery 2007;60:327–32 10.1227/01.NEU.0000249273.41569.6E [DOI] [PubMed] [Google Scholar]
- 28.Lindberg K, Kouti A, Ziegelitz D, et al. Three-dimensional volumetric segmentation of pituitary tumors: assessment of inter-rater agreement and comparison with conventional geometric equations. J Neurol Surg B Skull B 2018;79:475–81 10.1055/s-0037-1618577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.John JP, Wang L, Moffitt AJ, et al. Inter-rater reliability of manual segmentation of the superior, inferior and middle frontal gyri. Psychiatry Res 2006;148:151–63 10.1016/j.pscychresns.2006.05.006 [DOI] [PubMed] [Google Scholar]
- 30.Akudjedu TN, Nabulsi L, Makelyte M, et al. A comparative study of segmentation techniques for the quantification of brain subcortical volume. Brain Imaging Behav 2018;12:1678–95 10.1007/s11682-018-9835-y [DOI] [PubMed] [Google Scholar]
- 31.Tohka J. Partial volume effect modeling for segmentation and tissue classification of brain magnetic resonance images: a review. World J Radiol 2014;6:855–64 10.4329/wjr.v6.i11.855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Despotovic I, Goossens B, Philips W. MRI segmentation of the human brain: challenges, methods, and applications. Comput Math Methods Med 2015;2015:450341 10.1155/2015/450341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ambarki K, Lindqvist T, Wåhlin A, et al. Evaluation of automatic measurement of the intracranial volume based on quantitative MR imaging. AJNR Am J Neuroradiol 2012;33:1951–56 10.3174/ajnr.A3067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qiu W, Yuan J, Rajchl M, et al. 3D MR ventricle segmentation in pre-term infants with post-hemorrhagic ventricle dilatation (PHVD) using multi-phase geodesic level-sets. Neuroimage 2015;118:13–25 10.1016/j.neuroimage.2015.05.099 [DOI] [PubMed] [Google Scholar]