Abstract
BACKGROUND AND PURPOSE:
Neurotransmitter changes in youth addicted to the Internet and smartphone were compared with normal controls and in subjects after cognitive behavioral therapy. In addition, the correlations between neurotransmitters and affective factors were investigated.
MATERIALS AND METHODS:
Nineteen young people with Internet and smartphone addiction and 19 sex- and age-matched healthy controls (male/female ratio, 9:10; mean age, 15.47 ± 3.06 years) were included. Twelve teenagers with Internet and smartphone addiction (male/female ratio, 8:4; mean age, 14.99 ± 1.95 years) participated in 9 weeks of cognitive behavioral therapy. Meshcher-Garwood point-resolved spectroscopy was used to measure γ-aminobutyric acid and Glx levels in the anterior cingulate cortex. The γ-aminobutyric acid and Glx levels in the addicted group were compared with those in controls and after cognitive behavioral therapy. The γ-aminobutyric acid and Glx levels correlated with clinical scales of Internet and smartphone addiction, impulsiveness, depression, anxiety, insomnia, and sleep quality.
RESULTS:
Brain parenchymal and gray matter volume–adjusted γ-aminobutyric acid-to-creatine ratios were higher in subjects with Internet and smartphone addiction (P = .028 and .016). After therapy, brain parenchymal- and gray matter volume–adjusted γ-aminobutyric acid-to-creatine ratios were decreased (P = .034 and .026). The Glx level was not statistically significant in subjects with Internet and smartphone addiction compared with controls and posttherapy status. Brain parenchymal- and gray matter volume–adjusted γ-aminobutyric acid-to-creatine ratios correlated with clinical scales of Internet and smartphone addictions, depression, and anxiety. Glx/Cr was negatively correlated with insomnia and sleep quality scales.
CONCLUSIONS:
The high γ-aminobutyric acid levels and disrupted balance of γ-aminobutyric acid-to-Glx including glutamate in the anterior cingulate cortex may contribute to understanding the pathophysiology and treatment of Internet and smartphone addiction and associated comorbidities.
Internet addiction is a behavioral addiction characterized by uncontrolled use of the Internet with tolerance, withdrawal symptoms, and compulsiveness. The prevalence of Internet addiction ranges from 1.5% to 8.2%1 and is much higher in adolescents and young adults in the Far East.2 Internet gaming addiction was listed as a research criterion for behavioral addiction in the fifth version of the Diagnostic and Statistical Manual of Mental Disorders.3 In recent years, a preoccupation with smartphones and their worldwide spread has resulted in smartphone addiction.
The mesocorticolimbic system is a dopaminergic projection engaged in common neurobiologic pathways of substance addiction.4 The anterior cingulate cortex (ACC) is part of the mesocorticolimbic system and is associated primarily with salient networks activated by reward-related stimuli.4,5 The ACC has been postulated to play a critical role with the insula in substance addiction.4 PET,6 SPECT,7 and electroencephalogram8 results reportedly show brain regions associated with substance addictions.9 The ACC has been one of the most frequently implicated regions in Internet addiction.10-15
Substance addiction is associated with the neurotransmitter changes in the mesocorticolimbic system. Nicotine and alcohol indirectly enhance dopamine release via modulation of γ-aminobutyric acid (GABA) and glutamatergic neurons.4,16 MR spectroscopy using an editing pulse to create J-coupling can separate GABA and glutamate signals from other stronger overlying metabolite signals17,18 and has been used to characterize neurotransmitter changes in dynamic and interactive psychiatric disorders. The use of MR spectroscopy to study the ACC with respect to Internet and smartphone addiction can clarify common neurobiologic mechanisms for behavioral and substance addictions and provide clinical intervention to reduce the prevalence and related functional impairments in young people.
Currently, neurotransmitter changes have not been re-searched in terms of Internet and smartphone addiction. The purpose of this study was to reveal the associations between neurotransmitter changes and Internet and smartphone addiction and compare them with those in healthy controls and subjects’ postcognitive behavioral therapy results. In addition, correlations between neurotransmitter changes and affective changes in youth diagnosed with Internet and smartphone addiction were investigated.
MATERIALS AND METHODS
Participants
The institutional review board Korea University Ansan hospital approved this prospective study, and informed consent was obtained from the adolescents and parents. We included young people between the ages of 10 and 24 years who met the following criteria: 1) >50 points on the Internet Addiction Test modified from the Young Diagnostic Questionnaire;19 2) >35 points on the Smartphone Addiction Scale-short version for adolescents;20 and 3) >75 points on the summed Internet Addiction Test and Smartphone Addiction Scale. Subsequently, the Mini-International Neuropsychiatric Interview was administered to exclude subjects who met the diagnostic criteria of psychotic disorders, such as schizophrenia spectrum and other psychotic disorders, bipolar I disorder, or substance use disorder.
We included sex- and age-matched healthy controls who met the following criteria : 1) <30 points on the Internet Addiction Test; 2) <30 points on the Smartphone Addiction Scale; and 3) <60 points on the summed Internet Addiction Test and Smartphone Addiction Scale.
Psychology Tests
The Young Internet Addiction Test measures the severity of Internet addiction and consists of 20 items scored on a 5-point Likert scale, covering the extent to which Internet use affects daily routines, social life, productivity, sleeping patterns, and feelings.19 The Smartphone Addiction Scale-short version for adolescents measures the severity of smartphone addiction. It consists of 10 items scored on a 6-point Likert scale.20
Affective and cognitive characteristics of people with addiction and controls were evaluated using the Barratt Impulsiveness Scale, Hamilton Depression Rating Scale, Spielberger State-Trait Anxiety Inventory, Pittsburgh Sleep Quality Index, Insomnia Severity Index, and Mini-International Neuropsychiatric Interview. In order to the measurement of intelligence, the Korean version of the Wechsler Adult Intelligence Scale-IV for adolescents and adults older than 16 years of age and the Korean version of the Wechsler Intelligence Scale for Children-IV for children from 6 to 16 years of age were used.
Cognitive Behavioral Therapy
Cognitive behavioral therapy was modified from the Cognitive-Behavioral Therapy For Internet Gaming Addiction,21 and the Emotional Identification and Expression Abilities Improvement Program was reinforced. The treatment program consisted of the following 7 areas: recognizing the Internet behavior, modifying the cognitive distortion, finding appropriate alternative activities, promoting self-control, recognizing self-emotions and those of others, expressing emotions, and resolving interpersonal conflicts. The cognitive behavioral therapy consisted of a weekly 75-minute program for 9 weeks. The program was administered to the young subjects with Internet and smartphone addiction who agreed to participate in the therapy. Two or more absences were defined as therapy failure.
MR Imaging Parameters
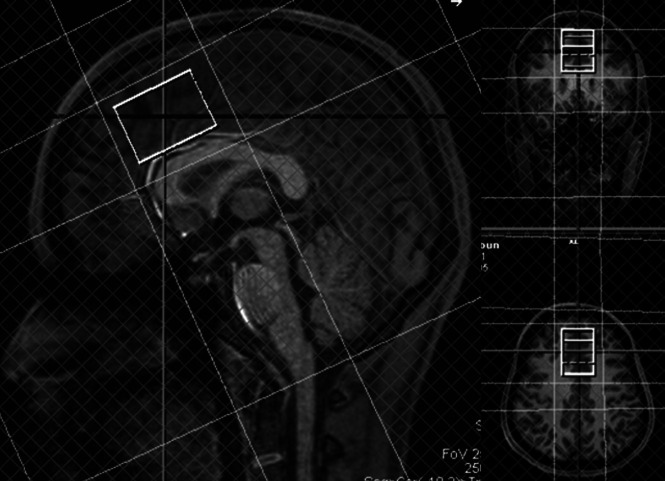
MR imaging was performed within an hour after the psychological tests. In addition, 1 or 2 days after finishing the 9-week cognitive behavioral therapy program, MR imaging rescanning was performed within an hour after the psychological retests. MR imaging data were acquired with a 3T MR imaging scanner with a 32-channel phased array head coil (MAGNETOM Skyra; Siemens Healthcare, Erlangen, Germany). Meshcher-Garwood point-resolved spectroscopy (MEGA-PRESS) was programmed by inserting a dual-band radiofrequency pulse into the manufacturer’s PRESS sequence. A dual-band radiofrequency waveform was obtained using a public domain pulse design tool, MATPULSE software (https://cind.ucsf.edu/education/software/matpulse). GABA measurement was performed using the MEGA-PRESS pulse sequence from a voxel volume of 26.25 mL (voxel dimensions, 3.5 × 3.0 × 2.5 cm) in the ACC (Fig 1), with the following acquisition parameters: TR, 2000 ms; TE, 68 ms; spectral width, 2000 Hz; number of oversampled data, 2048; number of signal averages, 256. GABA resonance at 3.01 ppm was detected by application of a refocusing pulse at 1.9 ppm during ON spectra and at 7.5 ppm during OFF spectra. Water signal suppression was achieved with the chemical shift selective imaging technique. First- and second-order shimming was performed for the voxels in the ACC, and the water line widths resulted in <16 Hz. Scan time was approximately 9 minutes.
FIG 1.
GABA, Glx, and creatine measurements in the anterior cingulate cortex. GABA and single-voxel 1H-MR spectroscopy with TE = 135 ms are obtained from a voxel volume of 26.25 mL (voxel dimensions, 3.5 × 3.0 × 2.5 cm) in the anterior cingulate cortex. The volume fractions of brain parenchyma and gray matter in the MR spectroscopy voxels are obtained from T1 MPRAGE segmentation using the SPM program. Last, GABA/Cr and Glx/Cr are adjusted by multiplying brain parenchymal and gray matter volume fractions for each individual.
Single-voxel 1H-MR spectroscopy with TE = 135 ms was obtained at the same voxels in the ACC (Fig 1), with the following parameters: TR, 2000 ms; number of samples, 64. In addition to MR spectroscopy, T1-weighted structural images were obtained using the MPRAGE sequence with the following parameters: TR, 2000 ms; TE, 3.55 ms; flip angle, 8°; section thickness, 1 mm; and acquisition matrix, 256 × 256.
MR Spectroscopy Data Processing
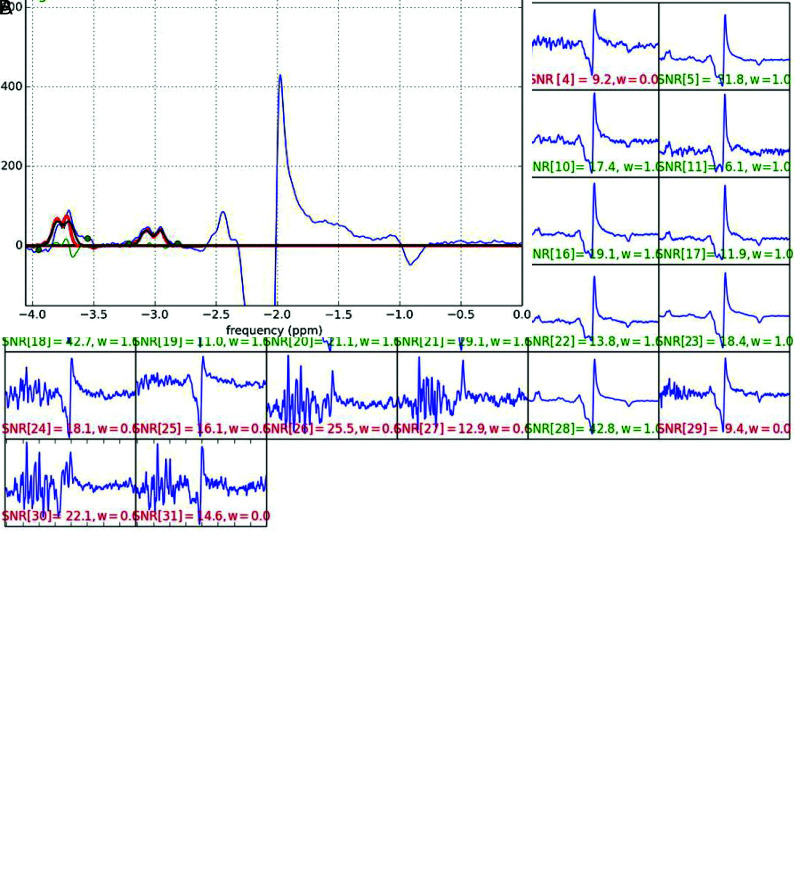
The raw GABA MR spectroscopy data were transferred to an off-line computer and processed using home-programmed MR spectroscopy analysis software, which was programmed in Python (https://www.python.org/). Raw data were averaged in the time domain and baseline-corrected, and the areas under the GABA and Glx peaks at 3.01 and 3.8 ppm were measured by fitting to double and single Gaussian functions, respectively (Fig 2). The data for 1H-MR spectroscopy at TE = 135 ms were processed using LCModel 6.3 (http://www.lcmodel.com/), and the creatine peak was measured at 3.0 ppm and used as the internal reference. GABA and Glx relative to creatine were quantified, and the GABA-to-creatine ratio (GABA/Cr) and the Glx-to-creatine ratio (Glx/Cr) were obtained. The GABA-to-Glx ratio (GABA/Glx) was also obtained.
FIG 2.
Acquisition and spectral fitting of MEGA-PRESS MR spectroscopy. A, MR imaging data are acquired with a 3T MR imaging scanner with a 32-channel phased array head coil. The signal-to-noise ratio acquired on each channel is visually checked, and raw data with a low signal-to-noise ratio (red characters) are removed from averaging. B, Selected raw data are averaged in the time domain and baseline-corrected, and the areas under the GABA and Glx peaks at 3.01 and 3.8 ppm (red lines) are measured by fitting to double and single Gaussian functions, respectively.
The volume fractions of brain parenchyma and gray matter in the MR spectroscopy voxels were obtained from T1 MPRAGE segmentation using the Statistical Parametric Mapping program (SPM; http://www.fil.ion.ucl.ac.uk/spm/software/spm12) (Fig 1). Last, GABA/Cr and Glx/Cr were adjusted by multiplying brain parenchymal and gray matter volume fractions for each individual.
Statistical Analyses
All statistical analyses were performed using SPSS Statistics 20 (IBM). Statistical significance was defined as P ≤ .05. GABA and Glx differences between the youth with Internet and smartphone addictions and controls were evaluated using the Student t test. Correlations between neurotransmitters and psychological tests were evaluated using the Pearson correlation coefficient. Differences in GABA and Glx levels between pre- and postcognitive behavioral therapy were tested using paired t tests or paired Wilcoxon signed rank tests based on whether the data met the assumption for a normal distribution.
RESULTS
The results of demographics and psychological tests are shown in Table 1. The addiction group consisted of 9 males and 10 females diagnosed with Internet and smartphone addictions. The mean age was 15.47 ± 3.06 years and ranged from 11 to 22 years. The control group consisted of 19 young healthy subjects, sex- and age-matched to the addiction group.
Table 1:
Demographics, psychological tests, and pre- and postcognitive behavioral therapy data
| Internet Addiction (Mean) |
Control (Mean) |
P Value | PreTx (Mean) |
PostTx (Mean) |
P Value | |
|---|---|---|---|---|---|---|
| (n = 19) | (n = 12) | |||||
| Sex (M/F) | 9:10 | 1.0 | 8:4 | |||
| Age (yr) | 15.47 ± 3.06 (range, 11–22) | 1.0 | 14.00 ± 1.95 (11–17) | |||
| IAT | 63.32 ± 15.15 | 27.37 ± 4.83 | < .001a | 68.42 ± 14.74 | 52.58 ± 10.24 | .001a |
| SAS | 45.53 ± 7.40 | 17.89 ± 8.12 | < .001a | 45.83 ± 6.42 | 30.67 ± 6.85 | < .001a |
| IAT+SAS | 108.84 ± 20.19 | 45.26 ± 11.72 | < .001a | 114.25 ± 19.43 | 83.25 ± 15.52 | < .001a |
| HRSD | 3.53 ± 5.92 | 0 ± 0 | .018a | 2.00 ± 3.69 | 0.25 ± 0.62 | .147 |
| State STAI | 46.11 ± 7.75 | 31.95 ± 9.99 | < .001a | 46.45 ± 8.55 | 43.82 ± 10.07 | .441 |
| Trait STAI | 50.16 ± 7.54 | 39.05 ± 4.93 | < .001a | 49.27 ± 7.84 | 45.36 ± 7.89 | .085 |
| Total STAI | 95.89 ± 14.56 | 71 ± 13.58 | < .001a | 95.06 ± 16.19 | 89.18 ± 17.55 | .253 |
| BIS | 57.53 ± 7.63 | 45.58 ± 11.72 | .001a | 54.27 ± 6.94 | 52.45 ± 6.83 | .093 |
| ISI | 6.89 ± 4.977 | 3.11 ± 2.558 | .006a | 5.17 ± 3.74 | 5.00 ± 4.43 | .891 |
| PSQI | 5.95 ± 3.24 | 3.53 ± 1.87 | .008a | 4.67 ± 2.31 | 4.83 ± 2.92 | .777 |
| IQ | 95.18 ± 2.88 | 98 ± 11.12 | .467 | |||
Note:—PreTx indicates precognitive behavioral therapy; PostTx, postcognitive behavioral therapy; IAT, Internet Addiction Test; SAS, Smartphone Addiction Scale; HRSD, Hamilton Rating Scale for Depression; STAI, State-Trait Anxiety Inventory; BIS, Barratt Impulsiveness Scale; ISI, Insomnia Severity Index; PSQI, Pittsburgh Sleep Quality Assessment; IQ, intelligence quotient.
P value ≤ .05
Internet and smartphone addiction scores were significantly higher in the addiction group compared with healthy controls (P < .001) as well as depression (P = .018), state, trait, and total anxiety scores (P < .001, P < .001, and P = .001, respectively); the impulsivity score (P = .001); insomnia severity (P = .006); and poor sleep quality (P = .008). A significant difference in the intelligence quotient was not observed between groups (P = .467).
Eight males and 4 females participated in cognitive behavioral therapy. The mean age was 14.0 ± 1.95 years and ranged from 11 to 17 years. Teenagers with Internet and smartphone addiction significantly improved after 9 weeks of cognitive behavioral therapy based on Internet and smartphone addiction scales (P = .001 and < .001, respectively), though Internet Addiction Test scores and the summed scores of the Internet Addiction Test and Smartphone Addiction Scale still met the criteria of Internet and smartphone addiction. However, psychological and sleep test scores were not significantly changed after therapy.
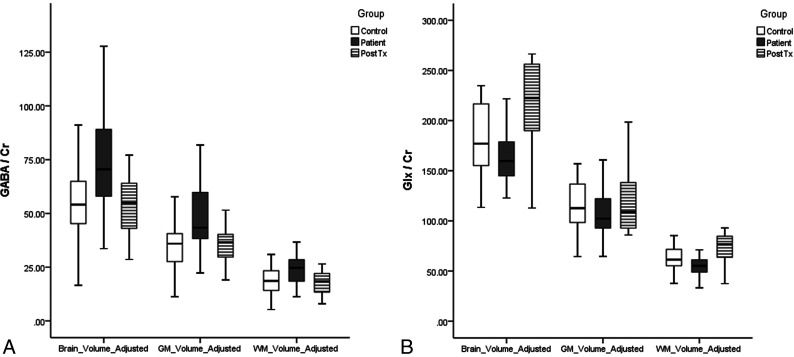
GABA levels in subjects with Internet and smartphone addictions are summarized in Table 2, and GABA levels after 9 weeks of cognitive behavioral therapy are summarized in Table 3. Brain parenchymal volume-adjusted GABA/Cr (bp-GABA/Cr) was higher in the addiction group compared with the controls (P = .028) and significantly decreased after cognitive behavioral therapy (P = .034). Gray matter volume–adjusted GABA/Cr (gm-GABA/Cr) was also higher in the addiction group (P = .016) and significantly decreased after cognitive behavioral therapy (P = .026; Fig 3A)
Table 2:
Subjects with Internet addiction versus controls
| bp-GABA/Cr | gm-GABA/Cr | wm-GABA/Cr | bp-GLX/Cr | gm-GLX/Cr | wm-GLX/Cr | |
|---|---|---|---|---|---|---|
| IA (mean) | 73.85 ± 22.74 | 49.10 ± 15.27 | 24.90 ± 8.25 | 167.74 ± 43.44 | 106.87 ± 39.90 | 56.25 ± 13.74 |
| Controls (Mean) | 56.03 ± 25.25 | 36.27 ± 16.02 | 19.77 ± 9.71 | 179.55 ± 37.30 | 117.45 ± 25.86 | 62.67 ± 13.12 |
| P value | .028a | .016a | .088 | .375 | .587 | .149 |
Note:—wm-GABA/Cr indicates white matter volume-adjusted GABA-to-creatine ratio; wm-GLX/Cr, white matter volume-adjusted glutamine and glutamate-to-creatine ratio; IA, Internet addiction.
P value ≤ 05.
Table 3:
Pre- and postcongnitive behavioral therapy data
| bp-GABA/Cr | gm-GABA/Cr | wm-GABA/Cr | bp-GLX/Cr | gm-GLX/Cr | wm-GLX/Cr | |
|---|---|---|---|---|---|---|
| PreTx (mean) | 74.20 ± 23.58 | 46.77 ± 15.95 | 24.43 ± 8.32b | 177.19 ± 47.81 | 119.07 ± 34.37 | 58.12 ± 14.81 |
| PostTx (mean) | 52.72 ± 14.52 | 34.60 ± 9.28 | 18.11 ± 5.67 | 215.65 ± 48.31 | 142.46 ± 34.54 | 73.19 ± 16.40b |
| P value | .034a | .026a | .071b | .096 | .131 | .06b |
Note:—PreTx indicates precognitive behavioral therapy; PostTx, postcognitive behavioral therapy.
P ≤ .05 based on paired t test or Wilcoxon signed rank test.
wm-GABA/Cr in precognitive behavioral therapy was not in a Gaussian distribution, and the nonparamatric Wilcoxon signed rank test was applied in statistical analysis.
FIG 3.
The boxplots of GABA/Cr (A) and Glx to Glx/Cr (B) in healthy controls and Internet and smartphone addicted subjects pre- and posttherapy. The horizontal line is the median, and the upper and lower ends of the boxes are the upper and lower quartiles, respectively. The vertical lines represent data ranges. PostTX indicates postcognitive behavioral therapy.
Glx levels in subjects with Internet and smartphone addiction are summarized in Table 2, and Glx levels after 9 weeks of cognitive behavioral therapy are summarized in Table 3. A significant difference in Glx levels was not observed in the addiction group compared with the control group. Glx/Cr adjusted brain parenchymal (bp-Glx/Cr) and gray matter volumes (gm-Glx/Cr) were lower in the addiction group but without statistical significance (P = .375 and .587, respectively; Fig 3B). The Glx level was increased after cognitive and behavioral therapy but without statistical significance (P = .096 and .131, respectively; Fig 3B).
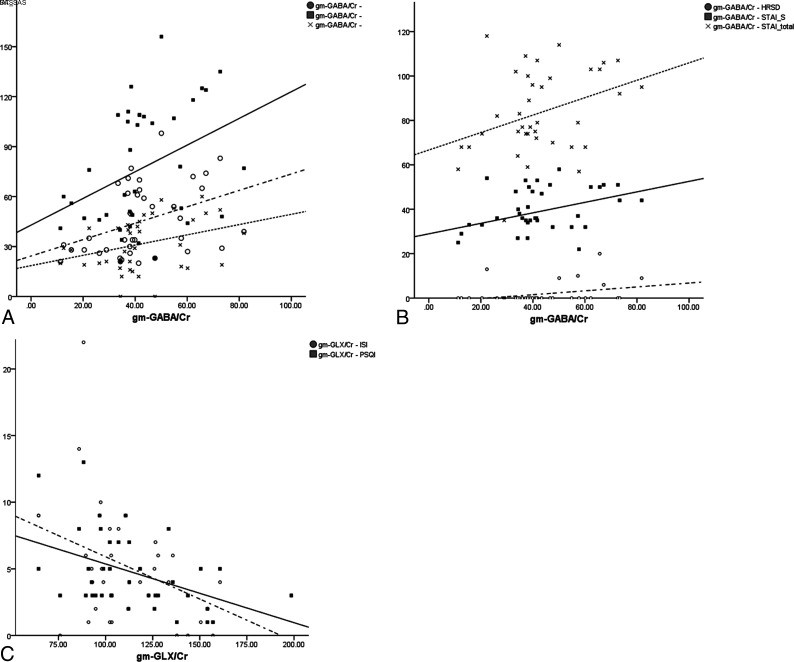
The results of correlations between neurotransmitters and psychological tests are described in Table 4. The GABA/Cr adjusted with gray-matter volume fraction (gm-GABA/Cr) positively correlated with the Internet Addiction Test and the Smartphone Addiction Scale as well as the sum of both scales. (Fig 4A) The bp-GABA/Cr correlated with the Internet Addiction Test but was not significantly correlated with Smartphone Addiction Scale.
Table 4:
Correlation between neurotransmitter and psychological tests
| bp-GABA/Cr | gm-GABA/Cr | wm-GABA/Cr | bp-GLX/Cr | gm-GLX/Cr | wm-GLX/Cr | ||
|---|---|---|---|---|---|---|---|
| IAT | r | 0.345 | 0.387 | 0.252 | –0.088 | –0.029 | –0.192 |
| P value | .034a | .016a | .127 | .599 | .863 | .249 | |
| SAS | r | 0.300 | 0.323 | 0.250 | –0.209 | –0.172 | –0.240 |
| P value | .067 | .048a | .129 | .209 | .302 | .146 | |
| IAT+SAS | r | 0.336 | 0.372 | 0.260 | –0.144 | –0.093 | –0.219 |
| P value | .039a | .022a | .116 | .388 | .578 | .186 | |
| HRSD | r | 0.312 | 0.326 | 0.298 | –0.201 | –0.17 | –0.201 |
| P value | .057 | .046a | .069 | .227 | .308 | .227 | |
| State STAI | r | 0.327 | 0.348 | 0.274 | 0.075 | 0.096 | 0.028 |
| P value | .045a | .032a | .095 | .655 | .566 | .867 | |
| Trait STAI | r | 0.294 | 0.305 | 0.263 | –0.197 | –0.173 | –0.212 |
| P value | .073 | .062 | .111 | .235 | .300 | .202 | |
| Total STAI | r | 0.335 | 0.350 | 0.291 | –0.071 | –0.048 | –0.097 |
| P value | .040a | .031a | .077 | .674 | .770 | .564 | |
| BIS | r | 0.132 | 0.145 | 0.109 | –0.06 | –0.042 | –0.079 |
| P value | .429 | .386 | .513 | .72 | .800 | .637 | |
| ISI | r | 0.073 | 0.037 | 0.139 | –0.386 | –0.419 | –0.255 |
| P value | .664 | .824 | .405 | .017a | .009a | .123 | |
| PSQI | r | 0.235 | 0.194 | 0.305 | –0.405 | –0.439 | –0.264 |
| P value | .155 | .242 | .063 | .012a | .006a | .109 |
Note:—PreTx indicates precognitive behavioral therapy; PostTx, postcognitive behavioral therapy; IAT, Internet Addiction Test; SAS, Smartphone Addiction Scale; HRSD, Hamilton Rating Scale for Depression; STAI, State-Trait Anxiety Inventory; BIS, Barratt Impulsiveness Scale; ISI, Insomnia Severity Index; PSQI, Pittsburgh Sleep Quality Assessment.
P value ≤ .05.
FIG 4.
The correlations between neurotransmitters and clinical scales of Internet addiction and psychological tests. A, The gm-GABA/Cr is significantly correlated with the Internet Addiction Test (circle and dashed line) and the Smartphone Addiction Scale (x and dotted line) as well as the sum of both scales (square and solid line). B, The gm-GABA/Cr was significantly correlated with the depression scale (circle and dashed line), total anxiety score (x and dotted line), and state anxiety score (square and solid line). C, The gm-Glx/Cr was correlated with insomnia (circle and dashed line) and sleep quality scores (square and solid line). IAT indicates Internet Addiction Test; SAS, Smartphone Addiction Scale; HRSD, Hamilton Rating Scale for Depression; STAI-S, State-Trait Anxiety Inventory to measure state component of anxiety; PSQI, Pittsburgh Sleep Quality Assessment.
The gm-GABA/Cr significantly correlated with the depression score (P = .046), state anxiety (P = .032), and total anxiety (P = .031; Fig 4B). The bp-GABA/Cr significantly correlated with state anxiety (P = .045) and total anxiety (P = .040).
Glx/Cr did not correlate with Internet and smartphone addictions scores. The bp-Glx/Cr and gm-Glx/Cr significantly correlated with insomnia (P = .017 and P = .009, respectively) and sleep quality (P = .012 and .006, respectively; Fig 4C). Impulsivity did not correlate with GABA or Glx levels.
DISCUSSION
The results of this study showed that GABA levels were higher in the ACC in young subjects with Internet and smartphone addictions and decreased after 9 weeks of cognitive behavioral therapy. The bp-GABA and gm-GABA correlated with depression and anxiety scores as well as Internet and smartphone addiction scores. The bp-Glx and gm-Glx negatively correlated with insomnia severity and sleep quality.
Recently, the high accessibility of smartphones has led to severe functional impairments compared with conventional Internet addiction. Smartphone addiction is a behavioral or technological addiction based on Internet use and shares core symptoms and risk factors with Internet addiction.22,23 Therefore, smartphone addiction could be considered a category of Internet addiction;23 thus, Internet and smartphone addictions were not separated in this study.
GABA is the main inhibitory neurotransmitter and is present at approximately one-third of all synapses.24 The GABA concentration in the human brain is approximately 1 mM.24 GABA is present in inhibitory local interneurons and is approximately 7-fold more concentrated in gray matter than in white matter.24,25 Therefore, the gm-GABA level was statistically more significant than wm-GABA in this study (Tables 2–4). Glutamate is the main component in Glx based on MR spectroscopy. Glutamate is the major excitatory neurotransmitter, and GABA is mostly synthesized from glutamate via decarboxylation.26 The concentration of glutamate was also approximately 2-fold higher in gray matter.25 GABA and glutamate are also key opposite modulators of dopamine in mesocorticolimbic pathways, which are closely associated with addiction4,27,28
In previous in vivo MR spectroscopy studies, the decreased GABA level was associated with depression and autism spectrum disorders.29 Schizophrenia did not show statistical significance in the meta-analysis but tended to exhibit lower GABA levels. In several studies, a low GABA level was reported in subjects with attention deficit/hyperactivity disorder and panic disorder.30-33 GABAA receptor subunit expression has also been shown to exert significant influence on substance and gambling addictions.28,34 A high signal-to-noise ratio is a technical challenge when using MR spectroscopy to measure GABA levels. However, the occipital or parietal lobes have been evaluated in many GABA MR spectroscopy studies without being implicated in the etiology of psychiatric disorders, though homogeneous magnetic field and increased signal-to-noise ratios were obtained.29
The ACC is the dopamine pathway associated with Internet and substance addictions. The ACC also provides a higher signal-to-noise ratio and a homogeneous magnetic field because the ACC is distant from the skull and relatively close to the MR imaging receiver compared with other regions of the mesocorticolimbic system such as the ventral tegmental area, nucleus accumbens, insula, and prefrontal cortex. Therefore, the ACC was thought to satisfy the hypothesis-driven and technical approaches when using MR spectroscopy to study Internet addiction.
In this study, GABA levels were increased in Internet- and smartphone-addicted youth compared with other psychiatric disorders and substance addictions. Two mechanisms can be postulated for the increased GABA levels in Internet and smartphone addiction. One involves the different neurobiology of Internet or behavioral addiction, and the other involves tolerance or an antireward mechanism. GABA inhibits synaptic signal transmission in the central nervous system. Activation of GABAA and GABAB receptors hyperpolarizes neurons and inhibits action potential generation and neurotransmission.24 The inhibition of GABA at the synapse attenuates the function of involved neural networks. Therefore, the increased GABA levels in subjects with Internet and smartphone addiction may be associated with the down-regulation of ACC functions, including impulsiveness control during the decision-making process under conditions of risk.18
The ACC is also important for input integration and regulation of processing in cognitive and emotional neural networks.35 In many neuroimaging and animal studies, the ACC was shown to be associated with affective disorders (Fig 1).35-37 ACC functional loss caused by lesions produces symptoms of emotional instability, inattention, and decreased social interaction.35,37 The emotional and personal traits caused by neurotransmitter derangement include risk factors for Internet and smartphone addictions and may be shared with the pathophysiology of psychiatric comorbidities in Internet and smartphone addiction, such as depression, attention deficit/hyperactivity disorder, and hostility.1,2 In a meta-analysis, Internet addiction was strongly associated with comorbid psychopathology, though the causal interaction could not be defined because of the insufficient longitudinal study.38 In this study, the risk factors and psychiatric comorbidities of Internet and smartphone addiction were possibly associated with decreased ACC function due to increased GABA levels (Table 4 and Fig 4).
Glutamate was shown to enhance the ACC functions in emotion processing and personal traits in previous studies.13-15 The ACC glutamate concentration was positively correlated with impulsivity.14 Conversely, reduced ACC glutamate concentration increased the risk of exposure to noxious stimulation, concerns regarding potential problems, and the fear associated with uncertainty.13,15 The negative correlations of Glx with insomnia severity and sleep quality in this study may be explained by decreased emotional ACC functions. In this study, the change in GABA levels after cognitive behavioral treatment showed that the GABA change was not the structural change of GABAergic interneurons but a functional change of GABAergic inhibition.29 The neurotransmitters can be reversed and normalized with improvement regarding Internet and smartphone addiction and comorbidities.
This study had several limitations. First is the reproducibility issue of GABA MR spectroscopy. GABA levels can be affected by age, sex, and circadian rhythm. In a previous study, the GABA level was lower in older subjects on the basis of the age-related gray matter loss; however, the tissue-corrected GABA analysis could correct the age effect.28,39 Although the neurotransmitters in youth can be changed by the developing brain volume before and after puberty, the change of neurotransmitters in healthy youth has not yet been reported. Males showed higher specific GABA receptor subunits in tissue analysis,40 and GABA levels were increased at the time of ovulation in females.41 However, in this study, tissue-corrected GABA analysis and exact sex- and age-matched case and control groups were used to reduce the biologic variability. We tried to improve the technical reproducibility of MR spectroscopy as much as possible with the use of large voxel volume and careful positioning in the ACC, as well as preparing very low first- and second-order shimming values.
Another limitation was the relatively small sample size of the study population, particularly in cognitive behavioral therapy. A future study with a larger sample size could clarify the roles of neurotransmitters in subjects with Internet and smartphone addiction. Last, the MR spectroscopy analysis used did not separate the glutamate peak from the glutamine peak. MEGA-PRESS MR spectroscopy with a 1.9-ppm editing pulse can separate the Glx signal at 3.7 ppm as well as GABA at 2.01 ppm,42 and the Glx concentration detected on MR spectroscopy was very likely associated with excitatory neurotransmission.43
CONCLUSIONS
A higher GABA level in the ACC was associated with Internet and smartphone addiction. The high GABA level in Internet and smartphone addiction was normalized after cognitive behavioral therapy. In addition, GABA and Glx levels were correlated with clinical scores obtained using standard psychological tests. The abnormal GABA level or disrupted balance between GABA and Glx including glutamate in the ACC may contribute to understanding the biochemical and molecular basis of the Internet and smartphone addiction and the associated comorbidities that could be used to devise appropriate treatments.
ABBREVIATIONS:
- ACC
anterior cingulate cortex
- bp-
brain parenchymal volume-adjusted
- GABA
γ-aminobutyric acid
- gm-
gray matter volume-adjusted
- MEGA-PRESS
Meshcher-Garwood point-resolved spectroscopy
- wm-
white matter volume-adjusted
Footnotes
Disclosures: Hyung Suk Seo—RELATED: Grant: National Research Foundation of Korea (2013R1A1A1012361).
This study was supported by the National Research Foundation of Korea (NRF-2013R1A1A1012361).
Paper previously presented at: Annual Meeting of the Radiological Society of North America, November 26–December 1, 2017; Chicago, Illinois.
References
- 1.Weinstein A, Lejoyeux M. Internet addiction or excessive internet use. Am J Drug Alcohol Abuse 2010;36:277–83 10.3109/00952990.2010.491880 [DOI] [PubMed] [Google Scholar]
- 2.Jorgenson AG, Hsiao RC, Yen CF. Internet addiction and other behavioral addictions. Child Adolesc Psychiatr Clin N Am 2016;25:509–20 10.1016/j.chc.2016.03.004 [DOI] [PubMed] [Google Scholar]
- 3.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association; 2013 [Google Scholar]
- 4.Jasinska AJ, Stein EA, Kaiser J, et al. Factors modulating neural reactivity to drug cues in addiction: a survey of human neuroimaging studies. Neurosci Biobehav Rev 2014;38:1–16 10.1016/j.neubiorev.2013.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayes DJ, Jupp B, Sawiak SJ, et al. Brain gamma-aminobutyric acid: a neglected role in impulsivity. Eur J Neurosci 2014;39:1921–32 10.1111/ejn.12485 [DOI] [PubMed] [Google Scholar]
- 6.Kim SH, Baik SH, Park CS, et al. Reduced striatal dopamine D2 receptors in people with Internet addiction. Neuroreport 2011;22:407–11 10.1097/WNR.0b013e328346e16e [DOI] [PubMed] [Google Scholar]
- 7.Hou H, Jia S, Hu S, et al. Reduced striatal dopamine transporters in people with internet addiction disorder. J Biomed Biotechnol 2012;2012:854524 10.1155/2012/854524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Littel M, van den Berg I, Luijten M, et al. Error processing and response inhibition in excessive computer game players: an event-related potential study. Addict Biol 2012;17:934–47 10.1111/j.1369-1600.2012.00467.x [DOI] [PubMed] [Google Scholar]
- 9.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry 2005;162:1403–13 10.1176/appi.ajp.162.8.1403 [DOI] [PubMed] [Google Scholar]
- 10.Lin F, Zhou Y, Du Y, et al. Abnormal white matter integrity in adolescents with internet addiction disorder: a tract-based spatial statistics study. PLoS One 2012;7:e30253 10.1371/journal.pone.0030253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stan AD, Schirda CV, Bertocci MA, et al. Glutamate and GABA contributions to medial prefrontal cortical activity to emotion: implications for mood disorders. Psychiatry Res 2014;223:253–60 10.1016/j.pscychresns.2014.05.016 [DOI] [PubMed] [Google Scholar]
- 12.Graham BM, Milad MR. The study of fear extinction: implications for anxiety disorders. Am J Psychiatry 2011;168:1255–65 10.1176/appi.ajp.2011.11040557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallinat J, Kunz D, Lang UE, et al. Association between cerebral glutamate and human behaviour: the sensation seeking personality trait. Neuroimage 2007;34:671–78 10.1016/j.neuroimage.2006.10.004 [DOI] [PubMed] [Google Scholar]
- 14.Hoerst M, Weber-Fahr W, Tunc-Skarka N, et al. Correlation of glutamate levels in the anterior cingulate cortex with self-reported impulsivity in patients with borderline personality disorder and healthy controls. Arch Gen Psychiatry 2010;67:946–54 10.1001/archgenpsychiatry.2010.93 [DOI] [PubMed] [Google Scholar]
- 15.Kim HJ, Kim JE, Cho G, et al. Associations between anterior cingulate cortex glutamate and gamma-aminobutyric acid concentrations and the harm avoidance temperament. Neurosci Lett 2009;464:103–07 10.1016/j.neulet.2009.07.087 [DOI] [PubMed] [Google Scholar]
- 16.Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron 2002;33:905–19 10.1016/s0896-6273(02)00625-6 [DOI] [PubMed] [Google Scholar]
- 17.Mescher M, Merkle H, Kirsch J, et al. Simultaneous in vivo spectral editing and water suppression. NMR Biomed 1998;11:266–72 [DOI] [PubMed] [Google Scholar]
- 18.Fujihara K, Narita K, Suzuki Y, et al. Relationship of gamma-aminobutyric acid and glutamate+glutamine concentrations in the perigenual anterior cingulate cortex with performance of Cambridge Gambling Task. Neuroimage 2015;109:102–08 10.1016/j.neuroimage.2015.01.014 [DOI] [PubMed] [Google Scholar]
- 19.Beard KW, Wolf EM. Modification in the proposed diagnostic criteria for Internet addiction. Cyberpsychol Behav 2001;4:377–83 10.1089/109493101300210286 [DOI] [PubMed] [Google Scholar]
- 20.Kwon M, Kim DJ, Cho H, et al. The smartphone addiction scale: development and validation of a short version for adolescents. PLoS One 2013;8:e83558 10.1371/journal.pone.0083558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee HC, Ahn CI. Study on the development and effectiveness of cognitive-behavioral therapy for internet addiction. Korean Journal of Psychology 2002;12:463–86 [Google Scholar]
- 22.Choi SW, Kim DJ, Choi JS, et al. Comparison of risk and protective factors associated with smartphone addiction and Internet addiction. J Behav Addict 2015;4:308–14 10.1556/2006.4.2015.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin YH, Chiang CL, Lin PH, et al. Proposed diagnostic criteria for smartphone addiction. PLoS One 2016;11:e0163010 10.1371/journal.pone.0163010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Owens DF, Kriegstein AR. Is there more to GABA than synaptic inhibition? Nat Rev Neurosci 2002;3:715–27 10.1038/nrn919 [DOI] [PubMed] [Google Scholar]
- 25.Ganji SK, An Z, Banerjee A, et al. Measurement of regional variation of GABA in the human brain by optimized point-resolved spectroscopy at 7 T in vivo. NMR Biomed 2014;27:1167–75 10.1002/nbm.3170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Danbolt NC. Glutamate uptake. Prog Neurobiol 2001;65:1–105 10.1016/s0301-0082(00)00067-8 [DOI] [PubMed] [Google Scholar]
- 27.Gardner EL. Addiction and brain reward and antireward pathways. Adv Psychosom Med 2011;30:22–60 10.1159/000324065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mick I, Ramos AC, Myers J, et al. Evidence for GABA-A receptor dysregulation in gambling disorder: correlation with impulsivity. Addict Biol 2017;22:1601–09 10.1111/adb.12457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schur RR, Draisma LW, Wijnen JP, et al. Brain GABA levels across psychiatric disorders: a systematic literature review and meta-analysis of (1) H-MRS studies. Hum Brain Mapp 2016;37:3337–52 10.1002/hbm.23244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edden RA, Crocetti D, Zhu H, et al. Reduced GABA concentration in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 2012;69:750–53 10.1001/archgenpsychiatry.2011.2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ende G, Cackowski S, Van Eijk J, et al. Impulsivity and aggression in female BPD and ADHD patients: association with ACC glutamate and GABA concentrations. Neuropsychopharmacology 2016;41:410–18 10.1038/npp.2015.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goddard AW, Mason GF, Almai A, et al. Reductions in occipital cortex GABA levels in panic disorder detected with 1H-magnetic resonance spectroscopy. Arch Gen Psychiatry 2001;58:556–61 10.1001/archpsyc.58.6.556 [DOI] [PubMed] [Google Scholar]
- 33.Long Z, Medlock C, Dzemidzic M, et al. Decreased GABA levels in anterior cingulate cortex/medial prefrontal cortex in panic disorder. Prog Neuropsychopharmacol Biol Psychiatry 2013;44:131–35 10.1016/j.pnpbp.2013.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stephens DN, King SL, Lambert JJ, et al. GABAA receptor subtype involvement in addictive behaviour. Genes Brain Behav 2017;16:149–84 10.1111/gbb.12321 [DOI] [PubMed] [Google Scholar]
- 35.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci 2000;4:215–22 10.1016/S1364-6613(00)01483-2 [DOI] [PubMed] [Google Scholar]
- 36.Baskin-Sommers AR, Foti D. Abnormal reward functioning across substance use disorders and major depressive disorder: considering reward as a transdiagnostic mechanism. Int J Psychophysiol 2015;98:227–39 10.1016/j.ijpsycho.2015.01.011 [DOI] [PubMed] [Google Scholar]
- 37.Hadland KA, Rushworth MF, Gaffan D, et al. The effect of cingulate lesions on social behaviour and emotion. Neuropsychologia 2003;41:919–31 10.1016/s0028-3932(02)00325-1 [DOI] [PubMed] [Google Scholar]
- 38.Carli V, Durkee T, Wasserman D, et al. The association between pathological internet use and comorbid psychopathology: a systematic review. Psychopathology 2013;46:1–13 10.1159/000337971 [DOI] [PubMed] [Google Scholar]
- 39.Hermans L, Levin O, Maes C, et al. GABA levels and measures of intracortical and interhemispheric excitability in healthy young and older adults: an MRS-TMS study. Neurobiol Aging 2018;65:168–77 10.1016/j.neurobiolaging.2018.01.023 [DOI] [PubMed] [Google Scholar]
- 40.Pandya M, Palpagama TH, Turner C, et al. Sex- and age-related changes in GABA signaling components in the human cortex. Biol Sex Differ 2019;10:5 10.1186/s13293-018-0214-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Bondt T, De Belder F, Vanhevel F, et al. Prefrontal GABA concentration changes in women-Influence of menstrual cycle phase, hormonal contraceptive use, and correlation with premenstrual symptoms. Brain Res 2015;1597:129–38 10.1016/j.brainres.2014.11.051 [DOI] [PubMed] [Google Scholar]
- 42.Edden RA, Barker PB. Spatial effects in the detection of gamma-aminobutyric acid: improved sensitivity at high fields using inner volume saturation. Magn Reson Med 2007;58:1276–82 10.1002/mrm.21383 [DOI] [PubMed] [Google Scholar]
- 43.Bauer J, Pedersen A, Scherbaum N, et al. Craving in alcohol-dependent patients after detoxification is related to glutamatergic dysfunction in the nucleus accumbens and the anterior cingulate cortex. Neuropsychopharmacology 2013;38:1401–08 10.1038/npp.2013.45 [DOI] [PMC free article] [PubMed] [Google Scholar]