Abstract
Urinary tract infections (UTIs) are common in the elderly, and cover a range of conditions from asymptomatic bacteriuria to urosepsis. Risk factors for developing symptomatic UTIs include immunosenescence, exposure to nosocomial pathogens, multiple comorbidities, and a history of UTIs. European guidelines on urological infections recommend antimicrobial treatment only for symptomatic UTIs. Non-antimicrobial options to treat and prevent UTIs include among others cranberry products, OM-89 Escherichia coli bacterial lysate vaccine, and estrogen therapy in postmenopausal women, although evidence for their efficacy is weak. Another non-antimicrobial option to control and prevent UTIs is a medical device (Utipro Plus®) containing xyloglucan, gelatin, propolis, and extracts of Hibiscus sabdariffa. The device acts in the intestine as a mechanical barrier to protect against invasion by uropathogenic E. coli strains. A randomized controlled trial of Utipro Plus® in patients with uncomplicated UTIs provided good-quality evidence of its efficacy compared with placebo. In an observational study of Utipro Plus® in patients with recurrent UTIs, more than 80% women reported a return to their pre-UTI clinical status and about 30% transitioned from symptomatic UTIs to asymptomatic bacteriuria. New treatment strategies that offer a safe and effective non-antimicrobial means of managing UTIs could have an important role in the elderly.
Keywords: elderly patients, medical device, non-antimicrobial treatment, urinary tract infections
Introduction
Urinary tract infections (UTIs) are common in the elderly, and cover a range of conditions from asymptomatic bacteriuria through to UTI-associated sepsis requiring hospitalization.1,2 Urinary growth of bacteria in the absence of urinary tract symptoms (i.e. asymptomatic bacteriuria) is most common and represents a commensal colonization.3 Asymptomatic bacteriuria has a prevalence of 1–5% in healthy premenopausal women, 4–19% in otherwise healthy elderly women and men, and 15–50% in institutionalized elderly individuals.4 As asymptomatic bacteriuria may protect against superinfecting symptomatic UTI, antimicrobial treatment is generally not indicated and may even be harmful.3,5 A diagnosis of symptomatic UTI in older adults generally requires the presence of localized genitourinary symptoms, pyuria, and a urine culture with an identified urinary pathogen.1,6 Antimicrobial therapy is indicated for symptomatic UTI.3
The classification system proposed by the European Association of Urology (EAU)3 and the EAU Section of Infections in Urology7 differentiates between low-risk uncomplicated UTIs and high-risk complicated UTIs based on the presence or absence of certain risk factors (Figure 1). EAU definitions for complicated, uncomplicated, recurrent, and catheter-associated UTIs are summarized in Table 1.3 Individuals at higher risk of a complicated UTI include postmenopausal women, patients with dysfunctional and/or reconstructed lower urinary tracts, patients with urinary tract catheters, all men, and elderly institutionalized patients,3 which describes a substantial proportion of the elderly population.
Figure 1.
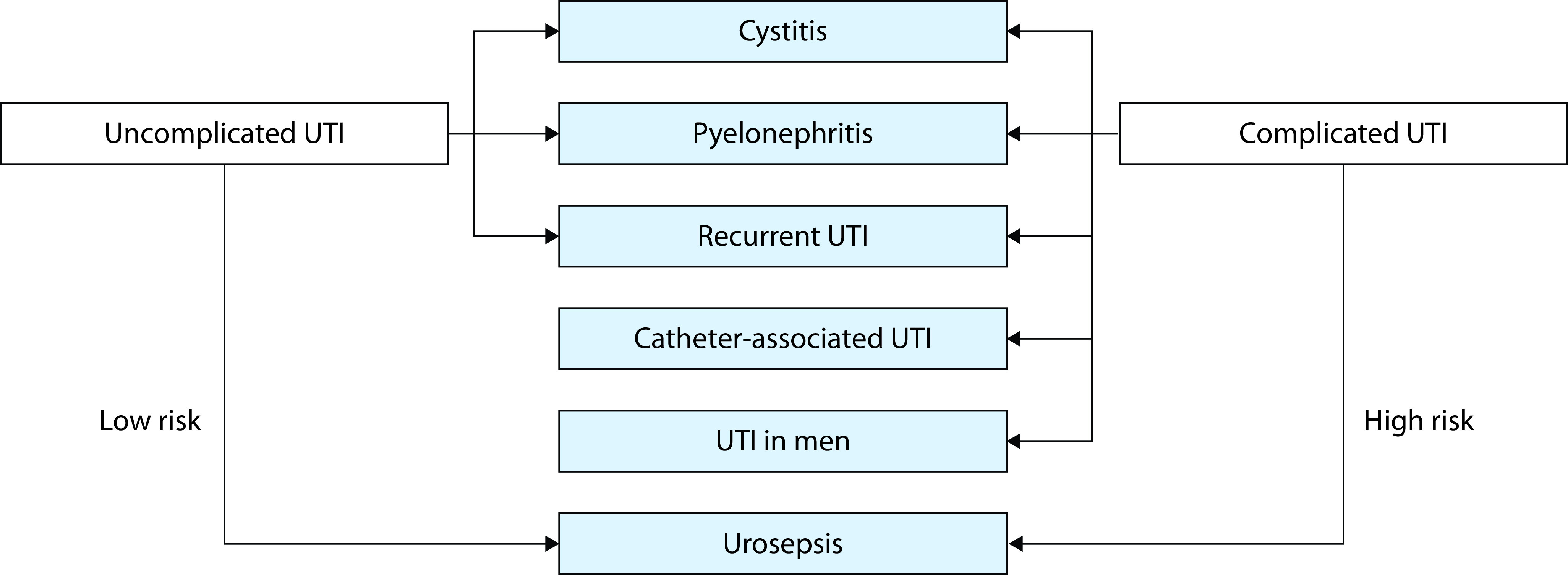
Uncomplicated and complicated urinary tract infection (UTI) defined by the European Association of Urology using ORENUC.
ORENUC: O, NO known factors; R, Risk for Recurrent UTI; E, Extra urogenital risk factors; N, Nephropathy; U, Urological risk factors that can be resolved by therapy; C, catheter and risk factors that cannot be resolved by therapy.
Reproduced from Bonkat et al. with permission.3
Table 1.
European Association of Urology urological infection guidelines classification of urinary tract infections.
| Term | Definition |
|---|---|
| Uncomplicated UTIs | Acute, sporadic, or recurrent lower (uncomplicated cystitis) and/or upper (uncomplicated pyelonephritis) UTIs, limited to non-pregnant, premenopausal women with no known relevant anatomical and functional abnormalities within the urinary tract or comorbidities. |
| Complicated UTIs | All UTIs which are not defined as uncomplicated: in a narrower sense, UTIs with an increased chance of a complicated course; that is, all men, pregnant women, patients with relevant anatomical or functional abnormalities of the urinary tract, indwelling urinary catheters, renal diseases, and/or with other concomitant immunocompromising diseases for example, diabetes. |
| Recurrent UTIs | Recurrence of uncomplicated and/or complicated UTIs, with a frequency of at least three UTIs/year or two UTIs in the last 6 months. |
| Catheter-associated UTIs | UTIs occurring in a person whose urinary tract is currently catheterized or has had a catheter in place within the past 48 hours. |
| Urosepsis | Life-threatening organ dysfunction caused by a dysregulated host response to infection originating from the urinary tract and/or male genital organs. |
UTI, urinary tract infections.
Reproduced from Bonkat et al. with permission.3
This narrative review considers the characteristics of UTIs – prevalence and incidence, disease burden, risk factors, and clinical presentation – in the elderly, and examines current treatment options to manage UTIs in the community. Relevant articles for inclusion were identified through literature searches using the PubMed database.
Prevalence and incidence
UTIs are significantly more common in adult women than men, possibly because of their shorter urethra, which permits easier passage of bacteria from the intestine.8,9 UTI is the second-most common infection in elderly women living in the community, and the most common cause of infection in hospitalized elderly women or those in long-term care.10 A prospective cohort study of postmenopausal community-dwelling women (aged 55–75 years) reported an overall incidence of UTI at 7 cases per 100 person-years.11 By comparison, a study from the United States conducted between 1988 and 1994 in men aged 65–74 years estimated the incidence at 5 cases per 100 person-years.12 A Dutch study of UTIs in subjects older than 85 years reported a 1.7-fold higher risk in women (incidence 12.8 per 100 person-years) than men (incidence 7.8 per 100 person-years).13
Irrespective of gender, the incidence of clinically diagnosed UTI increases with age. A large observational study of UTIs in older adults (aged ≥65 years) conducted from 2004 to 2014, in the United Kingdom (UK), showed that, in women, the incidence increased from 9–11 cases per 100 person-years in subjects aged 65–74 years, to 11.4–14.3 cases and 14.7–19.8 cases per 100 person-years in subjects aged 75–84 and >85 years, respectively. Corresponding values in men were 2.8–3.0, 5.9–6.1, and 8.1–10.5 cases per 100 person-years.14
Social and economic burden
Recurrent UTIs carry a substantial social and economic burden, and have a detrimental effect on patients’ quality of life (QoL).15–17 The economic cost of UTIs to healthcare systems is considerable. In the United States, the cost of UTIs is estimated to be at least US$2–3 billion per annum.18 An economic study of 20 hospitals in eight European countries with a high prevalence of multidrug resistant Gram-negative bacteria estimated that the mean cost per case of complicated UTIs was €5700, ranging from €4028 to €7740 per case. Higher patient costs were associated with admission, infection source and severity, comorbidity, and the presence of multidrug resistant bacteria.19
Risk factors for symptomatic UTI and asymptomatic bacteriuria
Risk factors for symptomatic UTI in the elderly differ from those in the younger population. Factors that increase the likelihood of developing UTIs include age-related changes in immune function (immunosenescence), exposure to nosocomial pathogens, and a higher number of comorbidities,20,21 although the strongest and most consistent risk factor for UTIs, namely a history of UTIs, is common to all age groups. Individuals with previous symptomatic UTIs have a 4–7 fold greater risk for future UTIs compared to those with no prior history.21,22
Relative to elderly subjects living in the community, institutionalized adults generally have more comorbidities and functional impairments, and a higher incidence of cognitive deficits, which predispose them to higher rates of asymptomatic bacteriuria and UTIs. The presence of a urinary catheter in institutionalized elderly individuals is a significant risk factor for UTIs.21 In institutionalized elderly women without a urinary catheter, the presence of bowel and/or bladder incontinence, functional disability, and dementia were significantly associated with persistent asymptomatic bacteriuria. In non-catheterized institutionalized elderly men, the only significant risk factor for persistent asymptomatic bacteriuria was cancer.23
Clinical presentation
Localized genitourinary symptoms such as dysuria, urinary frequency, and urgency are classic symptoms of UTIs. However, many patients with complicated UTIs including elderly and catheterized patients do not present these symptoms. UTIs in elderly patients may instead manifest as confusion or delirium, increased lethargy, blunted fever response, new-onset incontinence, and anorexia.1,10,24 Distinguishing symptomatic UTI from asymptomatic bacteriuria in elderly patients can be difficult,1,24 but it is essential to ensure appropriate use of antimicrobials.1–3,22,25 Antibiotic stewardship is especially critical in older populations to reduce their risk of acquiring difficult-to-treat multidrug-resistant organisms and to avoid the common sequalae of antibiotic therapy on the vaginal and gastrointestinal tracts.6
Therapeutic options
Antimicrobial treatment for symptomatic UTIs
Antimicrobial treatment is appropriate for symptomatic UTIs but not for asymptomatic bacteriuria.1–3,25 A meta-analysis of six randomized controlled trials (RCTs) involving 328 elderly patients with asymptomatic bacteriuria showed no significant benefit for antimicrobial treatment over placebo in the resolution of bacteriuria (risk ratio [RR]: 1.33; 95% confidence interval [CI]: 0.63–2.79).3
The 2018 EAU guidelines on urological infections3 recommend fosfomycin, pivmecillinam, or nitrofurantoin as first-line treatment for uncomplicated cystitis in adult women. Combination antimicrobial therapy with amoxicillin plus an aminoglycoside, or a second-generation cephalosporin plus an aminoglycoside, is recommended for treatment of complicated UTIs. For complicated UTI with systemic symptoms, empirical intravenous treatment with a third-generation cephalosporin is recommended. Although EAU guidelines state that fluoroquinolones may be considered for use in certain circumstances,3 the European Medical Agency (EMA) has suspended or restricted their use due to disabling and potentially permanent side effects involving muscles, tendons or joints, and the nervous system. The EMA advises special caution if using quinolones or fluoroquinolones in the elderly due to their higher risk of tendon injury.26
Elderly patients with UTIs are at high risk for developing urosepsis, especially those who are frail, depend on assistance for daily living, suffer from dementia, or are bedridden. Guidelines recommend immediate and empirical antimicrobial therapy with broad antimicrobial coverage against all likely causative pathogens. Antimicrobial treatment can be adapted once culture results become available.3
The 2018 EAU guidelines state that antimicrobials may be given as continuous low-dose prophylaxis for 3–6 months to prevent recurrent UTIs; regimens include nitrofurantoin, fosfomycin, cephalexin, or cefaclor.3 A large retrospective cohort study from the UK reported on antibiotic prophylaxis for recurrent UTIs in 19,696 adults (79% women) aged ≥ 65 years.14 Prescription records were used to confirm ≥3 months’ prophylaxis with trimethoprim, cephalexin, or nitrofurantoin. Antibiotic prophylaxis was associated with reduced risk of clinical recurrence of UTIs (men: hazard ratio [HR]: 0.49, 95% CI: 0.45–0.54; women: HR: 0.57, 95% CI: 0.55–0.59) and acute antibiotic prescribing (men: HR: 0.54, 95% CI: 0.51–0.57; women: HR: 0.61, 95% CI: 0.59–0.62), but the authors called for further research to better understand the implications of prophylaxis on treatment-related adverse events, development of resistance, and QoL in this population.
Overuse and misuse of antimicrobials have contributed to the continued development of resistance, which is a serious public health threat.27–29 The classic example is methicillin-resistant Staphylococcus aureus, which is responsible for numerous difficult-to-treat infections in humans.30 Escherichia coli accounts for the majority of all UTIs, followed by Klebsiella pneumoniae, Proteus mirabilis, Enterococcus faecalis, and Pseudomonas aeruginosa.6 Persistent intestinal colonization of drug-resistant E. coli, K. pneumoniae, and P. mirabilis isolates has been implicated in the pathophysiology of UTIs, particularly in patients who experience recurrent UTIs.31 A meta-analysis of antimicrobials prescribed for bacterial UTIs in primary care (five studies, 14,348 participants) showed that development of antimicrobial resistance was greatest within the first month post-treatment, and that the effect could be maintained for up to 1 year. Odds ratios for resistance were 4.40 (95% CI: 3.78–5.12) within 1 month and 1.33 (95% CI: 1.2–1.5) within 12 months of antimicrobial treatment.32 A Norwegian study that compared antimicrobial resistance patterns of bacteria causing UTIs in the elderly living in the community with those living in nursing homes found no significant difference in resistance rates between the two groups. The most common urinary bacterial isolate was E. coli, detected in nearly two-thirds of patients (64% in each group).33
The increasing antimicrobial resistance of uropathogens is challenging the paradigm of empirical antibiotic therapy for symptomatic UTIs, underscoring the need for alternative treatment strategies.
Non-antimicrobial and prophylactic treatment
Cranberry products
Cranberry products have been used widely for many years to treat and prevent UTIs, although the mechanism of action is unclear and disputed. A putative mechanism of action is preventing the adherence of P-fimbriated E. coli to uroepithelial cells on the bladder wall by proanthocyanidins contained in cranberries.25,34
Meta-analyses of 24 studies involving 4473 participants did not support use of cranberry products for UTIs. Compared with placebo, water, or no treatment, cranberry products did not significantly reduce the overall occurrence of symptomatic UTIs (RR: 0.86; 95% CI: 0.71–1.04), or the occurrence of symptomatic UTIs in subgroups including women with recurrent UTIs (RR: 0.74; 95% CI: 0.42–1.31) and older people (RR: 0.75; 95% CI: 0.39–1.44).34
A RCT of cranberry capsules administered to elderly women (n=185) with bacteriuria plus pyuria in residential care showed no significant difference compared with placebo in the presence of bacteriuria plus pyuria over 1 year (29.1 versus 29.0%). Similarly, there was no significant difference between cranberry capsules and placebo over 1 year in secondary measures, including symptomatic UTIs, mortality rate, hospitalizations, total antimicrobial utilization, and antimicrobials administered for suspected UTIs.35
Collectively, the results from meta-analyses34 and a subsequent placebo-controlled RCT35 do not support the use of cranberry products for prevention of UTI.36
OM-89 E. coli bacterial lysate vaccine
An oral non-antimicrobial prophylactic treatment for recurrent UTIs, based on lyophilized E. coli bacterial lysate (OM-89 vaccine), was developed more than 30 years ago. A systematic review and meta-analysis have described early studies of OM-89 (1985–2005) as being of low quality, with variable definitions of bacteriuria and UTI and with efficacy assessment limited to 6 months.37 A more recent 6-month observational study of OM-89, orally administered to 543 adults with recurrent lower UTIs for 3 months followed by a 3-month treatment-free period, reported a significant (p<0.0001) 59.3% decrease from baseline to 6 months in the mean number of UTIs. OM-89 also significantly improved QoL measures from baseline.15 In a small prospective observational study of adult women with uncomplicated, recurrent UTIs (n=21), OM-89 administered for 3 months significantly reduced the number of infections and improved QoL.17 RCTs are required before any definitive conclusions can be drawn about the efficacy of OM-89 in UTI.
Estrogen therapy
A decrease in estrogen is associated with several conditions that may promote recurrent UTIs in postmenopausal women: urinary incontinence, vesical prolapse, cystocoele, and post-voidal residue. As such, estrogen therapy has been used in postmenopausal women to prevent recurrent UTIs. However, a meta-analysis of 4 studies involving 2798 women found that oral estrogens failed to prevent UTIs compared to placebo (RR: 1.08; 95% CI: 0.88–1.33).38 Two small studies reported that vaginal estrogen reduced UTIs compared with placebo, with calculated RRs of 0.25 (95% CI: 0.13–0.50) and 0.64 (95% CI: 0.47–0.86), respectively.39,40 As with OM-89, additional well-controlled studies of estrogen in UTI are required before any conclusions can be drawn.
Xyloglucan-based medical devices
Xyloglucan is a hemicellulose extracted from tamarind seeds that is used to restore the physiological function of mucosal epithelial cells. By forming a bio-protective film, xyloglucan prevents contact of mucosal cells with pathogens and their products, allergens, and pro-inflammatory compounds.41
A formulation containing xyloglucan 100 mg, gelatin 50 mg, propolis 100 mg, and extracts of Hibiscus sabdariffa 100 mg (Utipro Plus®; Noventure, Barcelona, Spain) is a class III medical device approved in the European Union for control and prevention of UTIs. In in vitro studies, the device was shown to create a protective physical barrier on human intestinal epithelial cells, which protected against E. coli intracellular invasion.42 In another in vitro study in intestinal and uroepithelial cell models, the device prevented contact of uropathogenic E. coli strains on cell walls, without altering E. coli cell integrity, and in the absence of demonstrable antibacterial activity.43 In experimental rat models of acute infectious gastroenteritis and UTI, preventive treatment with oral xyloglucan–gelose prior to induction of infection with Salmonella enterica and Enterococcus hirae significantly reduced associated intestinal morphological changes, tight junctions permeability, and neutrophil infiltration. Treatment with oral xyloglucan–gelose also decreased bacterial growth in the urinary tract, suggesting that it protects against ascending infection of uropathogens from fecal flora to urinary tract and against infection by the hematogenous route.44
The efficacy and safety of the xyloglucan–gelose medical device has been demonstrated in clinical trials. In a multicenter, double-blind, phase IV study, patients with uncomplicated UTIs were randomized to receive xyloglucan + gelose (n=20) or placebo (n=20) in combination with an antimicrobial agent for 5 days, as monotherapy for 5 days and, from Day 30 of the study, for 15 days per month for 2 months.45 Uroculture positivity (defined as a bacterial count ≥103 CFU/mL) decreased from 100% of patients at baseline to 0% at Day 11 with xyloglucan + gelose, with recurrence in 3 patients (15%) by Day 76; and from 100% patients at baseline to 45% at Day 11 with placebo, with recurrence in 14 patients (70%) by Day 76. Compared with placebo, xyloglucan + gelose significantly reduced the frequency of urinary incontinence and urgency of micturition (both p<0.05), with symptom resolution in all patients by Day 90. All adverse events reported during the study were unrelated to treatment. The efficacy of xyloglucan + gelatin to manage recurrent UTIs was evaluated in a prospective observational study in which 61 women received one capsule daily for 15 days each month for 6 months.46 At 1, 3, and 6 months from the start of treatment, the numbers of women reporting improvement in QoL and return to pre-UTI clinical status were 41, 47, and 51, respectively. At 6 months, 29.5% of women had transitioned from symptomatic UTI to asymptomatic bacteriuria. No adverse events were reported during the study period.
Increasing microbial resistance is a compelling reason to seek alternative treatment and prevention strategies for UTIs.6 Utipro Plus shows early promise in addressing this treatment gap. Complementing the protective barrier effect of xyloglucan on mucous membranes, hibiscus and propolis appear to have bacterial anti-adhesive effects at the urinary level.47,48 The ability of Utipro Plus to transition patients from symptomatic UTI to asymptomatic bacteriuria, which may protect against symptomatic recurrence, is an interesting finding and indicative of its lack of effect on normal microbiota.
Other non-antibiotic approaches
A systematic review evaluated a range of non-antibiotic approaches to manage uncomplicated UTIs including cranberry products, Canephron N (a phytodrug), probiotics (Lactobacillus spp.), non-steroidal anti-inflammatory drugs (ibuprofen, diclofenac), D-mannose, estrogens, vitamins (C and D), and immunotherapy.49 The review captured RCTs and observational studies published from 1999 to 2019, which involved generally healthy adult non-pregnant women with no risk factors for recurrent UTIs. The overall conclusion was that the evidence was insufficiently conclusive to recommend non-antibiotic options in place of antibiotic usage, although incorporating some of these measures in the management strategy of UTIs may contribute to avoidance of antimicrobial resistance. Evidence for the role of these non-antibiotic approaches in the elderly population is currently lacking.
Conclusions
Despite the high prevalence of UTIs in the elderly, treatment options are limited. Although the medical community may agree in principle about the need for rational antibiotic usage, the absence of effective alternatives to treat UTIs can be a genuine barrier to change. Utipro Plus is a promising non-antimicrobial option for control and prevention of UTIs. A RCT of Utipro Plus conducted in patients with uncomplicated UTIs provided good-quality evidence of its efficacy compared with placebo. In an observational study in patients with recurrent UTIs, 83.6% of women reported a return to their pre-UTI clinical status and about 30% transitioned to asymptomatic bacteriuria within 6 months. Elderly patients are not only more prone to UTIs but are also more likely to have comorbidities and require multiple medications. The option to use a device that acts in a physical manner (barrier effect) without pharmacological properties, and with potential to reduce antimicrobial use, has obvious appeal in this patient population. Although additional studies are required to fully ascertain the role of Utipro Plus in elderly patients with UTI in the community or under institutional care, early evidence suggests benefit.
Acknowledgements
Medical writing assistance was provided by Robert Furlong and Kerry Dechant of behalf of Content Ed Net (Madrid, Spain), with funding by Noventure SL, Barcelona, Spain. This manuscript is a review article and does not involve a research protocol requiring approval by the relevant institutional review board or ethics committee.
Footnotes
Contributions: The named author meets the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, takes responsibility for the integrity of the work as a whole, and has given their approval for this version to be published.
Disclosure and potential conflicts of interest: The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2020/06/dic.2020-4-13-COI.pdf
Funding declaration: Writing assistance was funded by Noventure SL, Barcelona, Spain.
Correct attribution: Copyright © 2020 Rodriguez-Mañas L. https://doi.org/10.7573/dic.2020-4-13. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Provenance: submitted; externally peer reviewed.
Peer review comments to author: 18 May 2020
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: Plaza Building, Lee High Road, London, England, SE13 5PT.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editorial office editorial@drugsincontext.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
References
- 1.Rowe TA, Juthani-Mehta M. Diagnosis and management of urinary tract infection in older adults. Infect Dis Clin North Am. 2014;28(1):75–89. doi: 10.1016/j.idc.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mody L, Juthani-Mehta M. Urinary tract infections in older women: a clinical review. JAMA. 2014;311(8):844–854. doi: 10.1001/jama.2014.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonkat G, Pickard R, Bartoletti R, et al. EAU guidelines on urological infections. [Accessed 28 May 2020]. https://uroweb.org/wp-content/uploads/EAU-Guidelines-on-Urological-Infections-2018-large-text.pdf. Published 2018.
- 4.Nicolle LE. Asymptomatic bacteriuria in the elderly. Infect Dis Clin North Am. 1997;11:647–662. doi: 10.1016/s0891-5520(05)70378-0. [DOI] [PubMed] [Google Scholar]
- 5.Trautner BW. Asymptomatic bacteriuria: when the treatment is worse than the disease. Nat Rev Urol. 2011;9(2):85–93. doi: 10.1038/nrurol.2011.192. [DOI] [PubMed] [Google Scholar]
- 6.Foxman B. The epidemiology of urinary tract infection. Nat Rev Urol. 2010;7(12):653–660. doi: 10.1038/nrurol.2010.190. [DOI] [PubMed] [Google Scholar]
- 7.Johansen TE, Botto H, Cek M, et al. Critical review of current definitions of urinary tract infections and proposal of an EAU/ESIU classification system. Int J Antimicrob Agents. 2011;38(Suppl):64–70. doi: 10.1016/j.ijantimicag.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 8.Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Dis Mon. 2003;49:53–70. doi: 10.1067/mda.2003.7. [DOI] [PubMed] [Google Scholar]
- 9.Guglietta A. Recurrent urinary tract infections in women: risk factors, etiology, pathogenesis and prophylaxis. Future Microbiol. 2017;12:239–246. doi: 10.2217/fmb-2016-0145. [DOI] [PubMed] [Google Scholar]
- 10.Matthews SJ, Lancaster JW. Urinary tract infections in the elderly population. Am J Geriatr Pharmacother. 2011;9(5):286–309. doi: 10.1016/j.amjopharm.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Jackson SL, Boyko EJ, Scholes D, Abraham L, Gupta K, Fihn SD. Predictors of urinary tract infection after menopause: a prospective study. Am J Med. 2004;117(12):903–911. doi: 10.1016/j.amjmed.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 12.Griebling TL. Urologic diseases in America project: trends in resource use for urinary tract infections in men. J Urol. 2005;173(4):1288–1294. doi: 10.1097/01.ju.0000155595.98120.8e. [DOI] [PubMed] [Google Scholar]
- 13.Caljouw MA, Den Elzen WP, Cools HJ, Gussekloo J. Predictive factors of urinary tract infections among the oldest old in the general population. A population-based prospective follow-up study. BMC Med. 2011;9:57. doi: 10.1186/1741-7015-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmed H, Farewell D, Jones HM, Francis NA, Paranjothy S, Butler CC. Incidence and antibiotic prescribing for clinically diagnosed urinary tract infection in older adults in UK primary care, 2004–2014. PLoS One. 2018;13(1):e0190521. doi: 10.1371/journal.pone.0190521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Renard J, Ballarini S, Mascarenhas T, et al. Recurrent lower urinary tract infections have a detrimental effect on patient quality of life: a prospective, observational study. Infect Dis Ther. 2015;4:125–135. doi: 10.1007/s40121-014-0054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wagenlehner F, Wullt B, Ballarini S, Zingg D, Naber KG. Social and economic burden of recurrent urinary tract infections and quality of life: a patient web-based study (GESPRIT) Expert Rev Pharmacoecon Outcomes Res. 2018;18(1):107–117. doi: 10.1080/14737167.2017.1359543. [DOI] [PubMed] [Google Scholar]
- 17.López-Martín L, Alcover-Díaz J, Charry-Gónima P, et al. Prospective observational cohort study of the efficacy of bacterial immune prophylaxis in the prevention of uncomplicated, recurrent urinary tract infections. Urol Int. 2019;27:1–7. doi: 10.1159/000497107. [DOI] [PubMed] [Google Scholar]
- 18.Simmering JE, Tang F, Cavanaugh JE, Polgreen LA, Polgreen PM. The increase in hospitalizations for urinary tract infections and the associated costs in the United States, 1998–2011. Open Forum Infect Dis. 2017;4(1):ofw281. doi: 10.1093/ofid/ofw281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vallejo-Torres L, Pujol M, Shaw E, et al. Cost of hospitalised patients due to complicated urinary tract infections: a retrospective observational study in countries with high prevalence of multidrug-resistant Gram-negative bacteria: the COMBACTE-MAGNET, RESCUING study. BMJ Open. 2018;8(4):e020251. doi: 10.1136/bmjopen-2017-020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pawelec G, Larbi A. Immunity and ageing in man: Annual Review 2006/2007. Exp Gerontol. 2008;43(1):34–38. doi: 10.1016/j.exger.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 21.Rowe TA, Juthani-Mehta M. Urinary tract infection in older adults. Aging Health . 2013;9(5) doi: 10.2217/ahe.13.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Detweiler K, Mayers D, Fletcher SG. Bacteruria and urinary tract infections in the elderly. Urol Clin North Am. 2015;42(4):561–568. doi: 10.1016/j.ucl.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Eberle CM, Winsemius D, Garibaldi RA. Risk factors and consequences of bacteriuria in non-catheterized nursing home residents. J Gerontol. 1993;48(6):M266–M271. doi: 10.1093/geronj/48.6.m266. [DOI] [PubMed] [Google Scholar]
- 24.Bader MS, Loeb M, Brooks AA. An update on the management of urinary tract infections in the era of antimicrobial resistance. Postgrad Med. 2017;129(2):242–258. doi: 10.1080/00325481.2017.1246055. [DOI] [PubMed] [Google Scholar]
- 25.Beveridge LA, Davey PG, Phillips G, McMurdo ME. Optimal management of urinary tract infections in older people. Clin Interv Aging. 2011;6:173–180. doi: 10.2147/CIA.S13423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.European Medical Agency (EMA) Quinolone- and fluoroquinolone-containing medicinal products. [Accessed 28 May 2020]. https://www.ema.europa.eu/en/medicines/human/referrals/quinolone-fluoroquinolone-containing-medicinal-products. Published 2018.
- 27.Hulscher ME, Grol RP, van der Meer JW. Antibiotic prescribing in hospitals: a social and behavioural scientific approach. Lancet Infect Dis. 2010;10(3):167–175. doi: 10.1016/S1473-3099(10)70027-X. [DOI] [PubMed] [Google Scholar]
- 28.Bell BG, Schellevis F, Stobberingh E, Goossens H, Pringle M. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect Dis. 2014;14:13. doi: 10.1186/1471-2334-14-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization (WHO) Antimicrobial resistance: global report on surveillance. 2014. [Accessed 28 May 2020]. http://www.who.int/drugresistance/documents/surveillancereport/en/. Published 2014.
- 30.Chambers HF, Deleo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol. 2009;7(9):629–641. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thänert R, Reske KA, Hink T, et al. Comparative genomics of antibiotic-resistant uropathogens implicates three routes for recurrence of urinary tract infections. mBio. 2019;10(4):e01977–19. doi: 10.1128/mBio.01977-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340:c2096. doi: 10.1136/bmj.c2096. [DOI] [PubMed] [Google Scholar]
- 33.Fagan M, Lindbæk M, Grude N, et al. Antibiotic resistance patterns of bacteria causing urinary tract infections in the elderly living in nursing homes versus the elderly living at home: an observational study. BMC Geriatr. 2015;15:98. doi: 10.1186/s12877-015-0097-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2012;10:CD001321. doi: 10.1002/14651858.CD001321.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Juthani-Mehta M, Van Ness PH, Bianco L, et al. Effect of cranberry capsules on bacteriuria plus pyuria among older women in nursing homes: a randomized clinical trial. JAMA. 2016;316(18):1879–1887. doi: 10.1001/jama.2016.16141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nicolle LE. Cranberry for prevention of urinary tract infection? Time to move on. JAMA. 2016;316(18):1873–1874. doi: 10.1001/jama.2016.16140. [DOI] [PubMed] [Google Scholar]
- 37.Taha Neto KA, Nogueira Castilho L, Reis LO. Oral vaccine (OM-89) in the recurrent urinary tract infection prophylaxis: a realistic systematic review with meta-analysis. Actas Urol Esp. 2016;40(4):203–208. doi: 10.1016/j.acuro.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 38.Perrotta C, Aznar M, Mejia R, Albert X, Ng CW. Oestrogens for preventing recurrent urinary tract infection in postmenopausal women. Cochrane Database Syst Rev. 2008;2:CD005131. doi: 10.1002/14651858.CD005131.pub2. [DOI] [PubMed] [Google Scholar]
- 39.Raz R, Stamm WE. A controlled trial of intravaginal estriol in postmenopausal women with recurrent urinary tract infections. N Engl J Med. 1993;329(11):753–756. doi: 10.1056/NEJM199309093291102. [DOI] [PubMed] [Google Scholar]
- 40.Eriksen B. A randomized, open, parallel-group study on the preventive effect of an estradiol-releasing vaginal ring (Estring) on recurrent urinary tract infections in postmenopausal women. Am J Obstet Gynecol. 1999;180(5):1072–1079. doi: 10.1016/s0002-9378(99)70597-1. [DOI] [PubMed] [Google Scholar]
- 41.Piqué N, Gómez-Guillén MDC, Montero MP. xyloglucan, a plant polymer with barrier protective properties over the mucous membranes: an overview. Int J Mol Sci. 2018;19(3):673. doi: 10.3390/ijms19030673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Servi B, Ranzini F, Piqué N. Effect of Utipro(®) (containing gelatin-xyloglucan) against Escherichia coli invasion of intestinal epithelial cells: results of an in vitro study. Future Microbiol. 2016;11:651–658. doi: 10.2217/fmb-2016-0022. [DOI] [PubMed] [Google Scholar]
- 43.Fraile B, Alcover J, Royuela M, et al. Xyloglucan, hibiscus and propolis for the prevention of urinary tract infections: results of in vitro studies. Future Microbiol. 2017;12:721–731. doi: 10.2217/fmb-2017-0015. [DOI] [PubMed] [Google Scholar]
- 44.Esposito E, Campolo M, Casili G, et al. Protective effects of xyloglucan in association with the polysaccharide gelose in an experimental model of gastroenteritis and urinary tract infections. Int J Mol Sci. 2018;19:1844. doi: 10.3390/ijms19071844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Costache RC, Novac B, Bardan TR, Agapie DN, Edu A. Xyloglucan + gelose combination versus placebo as adjuvant therapy to first-line antimicrobials for uncomplicated urinary tract infection in adults. Urol Int. 2019;102:468–475. doi: 10.1159/000497106. [DOI] [PubMed] [Google Scholar]
- 46.Cai T, Tamanini I, Cocci A, et al. Xyloglucan, hibiscus and propolis to reduce symptoms and antibiotics use in recurrent UTIs: a prospective study. Future Microbiol. 2019;14:1013–1021. doi: 10.2217/fmb-2019-0145. [DOI] [PubMed] [Google Scholar]
- 47.Alshami I, Alharbi AE. Hibiscus sabdariffa extract inhibits in vitro biofilm formation capacity of Candida albicans isolated from recurrent urinary tract infections. Asian Pac J Trop Biomed. 2014;4:104–108. doi: 10.1016/S2221-1691(14)60217-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lavigne JP, Vitrac X, Bernard L, Bernard L, Bruyère F, Sotto A. Propolis can potentialise the anti-adhesion activity of proanthocyanidins on uropathogenic Escherichia coli in the prevention of recurrent urinary tract infections. BMC Res Notes. 2011;4:522. doi: 10.1186/1756-0500-4-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wawrysiuk S, Naber K, Rechberger T, Miotla P. Prevention and treatment of uncomplicated lower urinary tract infections in the era of increasing antimicrobial resistance-non-antibiotic approaches: a systemic review. Arch Gynecol Obstet. 2019;300(4):821–828. doi: 10.1007/s00404-019-05256-z. [DOI] [PMC free article] [PubMed] [Google Scholar]

