Abstract
Patients with peripheral artery disease (PAD) are at a high risk not only for the classical cardiovascular (CV) outcomes (major adverse cardiovascular events; MACE) but also for vascular limb events (major adverse limb events; MALE). Therefore, a comprehensive approach for these patients should include both goals. However, the traditional antithrombotic approach with only antiplatelet agents (single or dual antiplatelet therapy) does not sufficiently reduce the risk of recurrent thrombotic events. Importantly, the underlying cause of atherosclerosis in patients with PAD implies both platelet activation and the initiation and promotion of coagulation cascade, in which Factor Xa plays a key role. Therefore, to reduce residual vascular risk, it is necessary to address both targets. In the Cardiovascular Outcomes for People Using Anticoagulation Strategies (COMPASS) trial that included patients with stable atherosclerotic vascular disease, the rivaroxaban plus aspirin strategy (versus aspirin) markedly reduced the risk of both CV and limb outcomes, and related complications, with a good safety profile. In fact, the net clinical benefit outcome composed of MACE; MALE, including major amputation, and fatal or critical organ bleeding was significantly reduced by 28% with the COMPASS strategy, (hazard ratio: 0.72; 95% confidence interval: 0.59–0.87). Therefore, the rivaroxaban plus aspirin approach provides comprehensive protection and should be considered for most patients with PAD at high risk of such events.
Keywords: COMPASS, peripheral artery disease, residual risk, rivaroxaban
Introduction
Peripheral artery disease (PAD) is very common in clinical practice, although underdiagnosed.1,2 It has been estimated that more than 200 million people worldwide are affected by lower extremity PAD.3 The prevalence of PAD markedly increases with age, from approximately 5% in patients aged 45–49 years to around 10% in patients aged 65–74 years and nearly 20% by the age of 80 years.3,4 In Spain, in a cross-sectional analysis of 1568 subjects, aged 45–74 years, the prevalence of PAD was 3.8%.5 In the MERITO study, the prevalence of PAD evaluated by the ankle–brachial index in patients with metabolic syndrome was 28% and in patients with a previous coronary event, cerebrovascular disease, or both, it was 34, 32, and 54%, respectively.6,7
In addition, it is expected that these numbers will increase in the following years due to the aging of the population and the increased rates of diabetes, hypertension, and smoking.3 Importantly, the majority of patients with PAD do not refer specific symptoms. Thus, according to different studies, up to one-third of patients with an ankle–brachial index <0.90 have typical intermittent claudication.4,8,9
Atherosclerotic disease is the underlying cause of PAD.1,2 As atherosclerosis is often generalized, patients with PAD have usually more vascular beds affected. Thus, in the Reduction of Atherothrombosis for Continued Health (REACH) registry, 61.5% of patients with PAD had concomitant disease in other vascular beds (versus 24.7% of patients with coronary artery disease [CAD]).10 Patients with polyvascular disease have an increased risk of morbidity and mortality, being greater as a higher number of vascular beds are affected.11–14
Patients with symptomatic or asymptomatic PAD have an increased risk of cardiovascular (CV) death, myocardial infarction, and stroke (major adverse cardiovascular events; MACE).15 Compared with patients without PAD, the risk of CV death is increased among patients with asymptomatic and symptomatic PAD, by 5 and 11%, respectively.16 In addition, approximately one-fifth of patients with symptomatic PAD have myocardial infarction, and around 10–15% die after a 5-year period of follow-up.17 Of the one hand, the risk of CV death, myocardial infarction, or stroke is even higher in patients with PAD than in patients with CAD.12 On the other hand, in patients with asymptomatic carotid stenosis, approximately two-thirds of late deaths are related to cardiac events, with an annual mortality rate of 2.9%.18
Patients with PAD are not only at high risk of MACE, but also of major adverse limb events (MALE), including severe limb ischemia and amputation.19 Thus, it has been calculated that the annual incidence of major amputation ranges between 120 and 500 per million, and in Western Europe, the PAD-related mortality reached 3.5 per 100,000 individuals (excluding stroke and myocardial infarction) in 2010, and the years of life lost due to PAD were estimated at 31.7 years per 100,000 inhabitants.1,20,21 Therefore, in patients with PAD, it is not only mandatory to reduce MACE, but also MALE.
In a specific substudy of the Cardiovascular Outcomes for People Using Anticoagulation Strategies (COMPASS) trial, which analyzed the impact of MALE on the prognosis of patients with lower extremity PAD, showed that after MALE, the 1-year cumulative risk of subsequent hospitalization, vascular amputations, death, and MACE were 61.5; 20.5; 8.3; and 3.7%, respectively. Importantly, experiencing MALE dramatically increased the risk of subsequent amputations (hazard ratio [HR]: 197.5; p<0.0001), and death (HR: 3.23; p<0.001) (Figure 1).22
Figure 1.

Outcomes in patients before and after MALE in the COMPASS study.22
MACE: cardiovascular death, myocardial infarction, stroke.
As a result, PAD is a very common condition in routine practice, with very high rates of MACE and MALE. Reducing both and the related complications with the appropriate prophylaxis is mandatory. In this narrative review, data about how to reduce residual risk through the best antithrombotic approach among patients with PAD, with a particular focus on the COMPASS trial, are updated in order to provide some practical recommendations about its use in this population. For this purpose, a search was performed (up to March 2020) on PubMed (MEDLINE), using the MeSH terms [peripheral artery disease] + [COMPASS] + [rivaroxaban] + [treatment]. Original data from clinical trials, prospective and retrospective studies, and more useful reviews were selected.
Antithrombotic treatment in patients with PAD
Progressive atherosclerosis is the underlying cause of PAD and results in a spectrum of symptoms that, in the case of lower extremity PAD, ranges from asymptomatic to intermittent claudication, ischemic rest pain, and major and minor trophic changes. On the other hand, acute limb ischemia (ALI) is caused by native atherosclerotic plaque disruption and thrombus formation, and in the case of patients previously revascularized, thrombosis of the stent or graft.1,2,20,23 After atherosclerotic plaque disruption, the thrombogenic subendothelial matrix is exposed to circulating blood, leading to activation of circulating platelets and the coagulation cascade and lastly to thrombus formation.24–28 As a result, to reduce CV outcomes and the risk of PAD-related complications, it is necessary not only to control CV risk factors, but also follow a comprehensive antithrombotic approach that includes not only acting on platelets but also on coagulation inhibition.29
With regard to antithrombotic therapy, in patients with lower extremity PAD, the European guidelines recommend the use of long-term single antiplatelet therapy in symptomatic patients (recommendation I A) and in all patients who have undergone revascularization (recommendation I C). Furthermore, clopidogrel may be preferred over aspirin (recommendation IIb B). In addition, dual antiplatelet therapy with aspirin and clopidogrel for at least 1 month should be considered after infra-inguinal stent implantation (recommendation IIa C) and may be considered in below-the-knee bypass with a prosthetic graft (recommendation IIb B). Importantly, antiplatelet therapy is not routinely indicated in patients with asymptomatic PAD (recommendation III A).1 On the other hand, long-term single antiplatelet therapy is also recommended in patients with symptomatic carotid stenosis (recommendation I A), and dual antiplatelet therapy with aspirin and clopidogrel for at least 1 month after carotid artery stenting (recommendation I B).1
Despite these indications, patients with PAD treated with current evidence-based recommended care have an unacceptably high residual CV risk.29,30 This is not surprising as the current only antiplatelet-based antithrombotic approach is insufficient to effectively reduce not only MACE but also MALE in PAD patients.31,32 In addition, the 2017 European guidelines recognize that the results of the COMPASS trial were not included at the time of publication but should be updated after a critical analysis.1
Thus, the evidence supporting the use of aspirin or clopidogrel to prevent MACE in PAD patients remains insufficient. In a meta-analysis of 18 clinical trials involving 5269 individuals with PAD, treatment with aspirin alone resulted in a statistically nonsignificant reduction in MACE, and only a significant decrease in nonfatal stroke (Table 1).33 In another meta-analysis of 287 studies, aspirin reduced the risk of MACE among patients with atherosclerotic vascular disease, including those with PAD.34 In patients with symptomatic PAD and no history of stroke/transient ischemic attack included in the REACH registry, despite 61% of patients treated with aspirin and 83% with at least one antiplatelet agent, MACE rates reached 4.7% during the first year and increased continuously by 4–5% per year; the limb ischemic event rate was 5.7% at 2 years.35 In the Clopidogrel versus Aspirin in Patients at Risk of Ischemic Events (CAPRIE) trial, among 19,185 patients with atherosclerotic vascular disease, including recent ischemic stroke, recent myocardial infarction, or symptomatic PAD, after a mean follow-up of 1.91 years, compared with aspirin, clopidogrel significantly reduced the risk of MACE, including the subgroup of patients with PAD (Table 1).36 In the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management and Avoidance (CHARISMA) study, in the subgroup of patients with prior myocardial infarction, ischemic stroke, or symptomatic PAD, while the combination of clopidogrel plus aspirin (versus aspirin alone) significantly reduced the risk of MACE by 17%, there was a nonsignificant reduction of 13% in the PAD subpopulation. No reduction of mortality was observed. Moderate bleeding, but not severe bleeding, was more common with the combination (Table 1).37
Table 1.
| Study | Treatment | Study population | Results | |
|---|---|---|---|---|
| Berger, et al33 | Aspirin versus control | Meta-analysis of randomized trials (5269 patients with PAD) |
|
|
| CAPRIE36 | Clopidogrel versus aspirin | 19,185 patients with atherosclerotic vascular disease (ischemic stroke, recent myocardial infarction, or symptomatic PAD) |
|
|
| CHARISMA37 | Clopidogrel + aspirin versus aspirin | 9478 patients with documented prior MI, ischemic stroke, or symptomatic PAD |
|
|
| TRA 2P–TIMI 5038 | Vorapaxar +aspirin versus aspirin | 3787 patients with a history of claudication and an ankle–brachial index of <0.85 or prior revascularization for limb ischemia |
|
|
| EUCLID39 | Ticagrelor (90 mg) versus clopidogrel | 13,885 patients with symptomatic PAD |
|
|
| PEGASUS–TIMI 5440 | Ticagrelor, 60/90 mg, + aspirin versus aspirin | 1143 patients with PAD and prior myocardial infarction (1–3 years) |
|
Ticagrelor 90 mg:
|
| COMPASS41 | Rivaroxaban, 2.5 mg, + aspirin versus aspirin | 7470 patients with chronic PAD |
|
|
ALI, acute limb ischemia; CV, cardiovascular;, PAD, peripheral artery disease; MACE: CV death, myocardial infarction, or stroke; MALE, major adverse limb event.
This limited efficacy of antiplatelet agents in patients with PAD has not only been limited to the traditional antiplatelet agents, such as aspirin or clopidogrel, but also with the newer ones. The Trial to Assess the Effects of Vorapaxar in Preventing Heart Attack and Stroke in Patients with Atherosclerosis–Thrombolysis in Myocardial Infarction 50 (TRA 2P–TIMI 50) study compared vorapaxar plus aspirin versus aspirin in patients with chronic atherosclerotic vascular disease. In the subgroup of 3787 patients with chronic symptomatic PAD, vorapaxar did not reduce the risk of MACE, but significantly reduced the rates of hospitalization for ALI and peripheral artery revascularization by 42 and 16%, respectively. Major bleeding was more common with the combination (Table 1).38 Ticagrelor, 90 mg, twice daily was compared with clopidogrel, 75 mg, once daily in 13,885 patients with symptomatic PAD in the Effects of Ticagrelor and Clopidogrel in Patients with Peripheral Artery Disease (EUCLID) trial. After a median follow-up of 30 months, rates of MACE, ALI, CV death, and major bleeding were similar between both groups (Table 1).39 In the Prevention of Cardiovascular Events in Patients with Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin–Thrombolysis in Myocardial Infarction 54 (PEGASUS–TIMI 54) trial, 21,162 patients with prior myocardial infarction (1–3 years) were randomized to receive ticagrelor, 90 mg, twice daily plus aspirin and ticagrelor, 60 mg, twice daily plus aspirin or placebo plus aspirin. Compared with aspirin in monotherapy, the combination of ticagrelor, 90 mg, and aspirin did not significantly reduce the risk of MACE, ischemic stroke, or CV death, but significantly reduced the risk of MALE by 51%. By contrast, ticagrelor, 60 mg, plus aspirin significantly reduced the risk of MACE and CV deaths by 31 and 53%, respectively, but not the risk of MALE or ischemic stroke (Table 1).40 All these data clearly indicate that treating PAD patients with antiplatelets (single or dual antiplatelet therapy) does not provide a comprehensive approach with the double target of reducing MACE and MALE.41
On the other hand, full anticoagulation with vitamin K antagonists has not been shown to provide further protection compared with single antiplatelet therapy in patients with PAD.42,43
Finding an antithrombotic regimen that targets both platelet and anticoagulation cascade activation may be more effective in preventing MACE and MALE in patients with PAD.25,26,44–47 In addition, experimental data have shown that the inhibition of Factor Xa decreases inflammation and platelet-dependent thrombin generation; attenuates neointima formation after vascular injury; has protective, repairing, and fibrinolytic effects on vascular endothelium; and stabilizes atherosclerotic plaque and reduces its progression. Of note, most of these positive data on experimental models have been obtained specifically with rivaroxaban.48–52 All these data strongly suggest that an inhibition of Factor Xa, as rivaroxaban, could exert a positive impact on patients with PAD. This hypothesis has been tested in the COMPASS study.
The COMPASS study was a double-blind superiority trial that included a total of 27,395 subjects with chronic atherosclerotic vascular disease (CAD or PAD). Patients were randomly allocated to receive rivaroxaban, 2.5 mg, twice daily combined with aspirin, 100 mg, once daily, rivaroxaban, 5 mg, twice daily or aspirin, 100 mg, once daily. At baseline, mean age was 68 years, 91% of patients had CAD (62% previous myocardial infarction; 73% only CAD; 18% CAD and PAD), and 27% had PAD (9% only PAD). The study was prematurely stopped due to the beneficial effects found with rivaroxaban, 2.5 mg, twice daily (vascular dose of rivaroxaban) plus aspirin over aspirin after a mean follow-up of only 23 months.53,54 This is very relevant as the Kaplan–Meier survival curves between rivaroxaban plus aspirin and aspirin alone separated over time, mainly after the first year of treatment, but the excess of bleeding risk dramatically fell after 1 year of therapy.53,54 As a result, compared with the traditional approach of aspirin alone in patients with chronic atherosclerotic vascular disease, the net clinical benefit of the rivaroxaban plus aspirin approach increases over time.
Overall, compared with aspirin, the rivaroxaban plus aspirin combination reduced the risk of MACE by 24% (HR: 0.76; 95% CI: 0.66–0.86; p<0.001). This beneficial effect was independent of the presence of PAD (p for interaction was 0.61). The risk of ischemic stroke, myocardial infarction, ALI, or death from CAD was also reduced by 28% with the combination (HR: 0.72; 95% CI: 0.63–0.83; p<0.001), and also the risk of MACE or ALI by 26% (HR: 0.74; 95% CI: 0.65–0.85; p<0.001). The risk of stroke and hospitalization for CV causes was also significantly reduced by the rivaroxaban plus aspirin approach. But more importantly, there was a trend toward a lower risk of both CV death and death from any cause with the combination of rivaroxaban plus aspirin compared with aspirin. Although the risk of major bleeding was increased with the combination (HR: 1.70; 95% CI: 1.40–2.05; p<0.001), the risk of fatal bleeding or intracranial bleeding did not significantly differ between the groups.54
Importantly, while there was an absolute risk reduction of 0.5% in CV death between aspirin alone and the rivaroxaban plus aspirin approach (from 2.2 to 1.7%; p=0.02) and an absolute risk reduction of 0.7% in death from any cause (from 4.1 to 3.4%; p=0.01), there was a nonsignificant absolute increase of 0.1% in fatal bleeding (from 0.1 to 0.2%; p=0.32), without any difference in the risk of nonfatal symptomatic intracranial hemorrhage (0.2% in both groups; p=0.77).54 In summary, in patients with chronic atherosclerotic vascular disease, the beneficial effect of the combination of rivaroxaban plus aspirin on MACE is much higher than the possible increase in bleeding risk.
Efficacy and safety of the vascular dose of rivaroxaban in patients with established PAD
The COMPASS substudy included a total of 7470 patients with PAD. The definitions of PAD, according to the COMPASS criteria, are summarized in Table 2. Briefly, to be included, patients should have a history of PAD of the lower extremities (previous revascularization, amputation, or intermittent claudication), of the carotid arteries (previous revascularization or asymptomatic ≥50% carotid artery stenosis), or patients with ischemic heart disease and an ankle–brachial index <0.90. Although all these patients were included according to PAD criteria, there were relevant differences in the clinical profile of these subgroups of patients. With regard to the exclusion criteria, patients who required dual antiplatelet therapy for any clinical condition or with a high risk of bleeding were excluded from the study (Table 2).41,53
Table 2.
|
|
eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; PAD, peripheral artery disease.
Mean age of patients with PAD was 68 years, 81% had symptomatic PAD, 55% symptomatic PAD of lower extremities, 26% previous carotid revascularization or ≥50% carotid stenosis, 20% CAD and an ankle–brachial index <0.90, and 4.5% prior limb or foot amputation. In addition, 66% of patients with PAD had concomitant CAD and 28% renal insufficiency (Table 3).41
Table 3.
Baseline clinical characteristics of patients with peripheral artery disease (lower extremities or carotid) included in the COMPASS trial.41
| Total population | |
|
| |
| Patients with PAD (n) | 7470 |
| Symptomatic PAD of the lower extremities (n) | 4129 |
| Previous carotid revascularization or carotid stenosis ≥50% (n) | 1919 |
| CAD who had an ABI <0.90 (n) | 1422 |
|
| |
| Biodemographic data | |
|
| |
| Mean age, years | 68 |
|
| |
| Men (%) | 71 |
|
| |
| Cardiovascular risk factors | |
|
| |
| Hypertension (%) | 80 |
|
| |
| Diabetes (%) | 44 |
|
| |
| Smoking status (%) | |
| Current | 27 |
| Former | 46 |
| Never | 27 |
|
| |
| Vascular disease | |
|
| |
| CAD (%) | 66 |
|
| |
| Renal insufficiency (%) | 28 |
|
| |
| Previous stroke (%) | 7 |
|
| |
| Concomitant medications | |
|
| |
| Antiplatelets (%) | 87 |
|
| |
| Lipid-lowering drugs (%) | 83 |
|
| |
| Renin–angiotensin system inhibitors (%) | 70 |
ABI, ankle–brachial index; CAD, coronary artery disease; PAD, peripheral artery disease.
Among PAD patients, compared with aspirin, the rivaroxaban plus aspirin approach significantly reduced the risk of MACE by 28%; the risk of ischemic heart disease death, myocardial infarction, ischemic stroke, and ALI by 32%; the risk of CV death, myocardial infarction, ischemic stroke, and ALI by 29%; and the risk of MACE plus MALE by 31% (HR: 0.69; 95% CI: 0.56–0.85; p=0.0004). In addition, the risk of stroke was significantly reduced by the combination of rivaroxaban plus aspirin (HR: 0.54; 95% CI: 0.33–0.87), and there was a trend toward a lower risk of CV death (HR: 0.82; 95% CI: 0.59–1.14) and death for any cause (HR: 0.91; 95% CI: 0.72–1.16) (Figure 2).41 Of note, the effects of low-dose rivaroxaban plus aspirin versus aspirin alone on the combined outcome of MACE and MALE including major amputation were consistent regardless the presence of CAD (p for interaction was 0.33).41
Figure 2.

Effects of treatment on MACE, MACE or MALE including major amputation, MACE plus acute limb ischemia, death and fatal bleeding in patients with chronic peripheral artery disease from the COMPASS study.41
MACE: Cardiovascular death, myocardial infarction, MACE1: Cardiovascular death, myocardial infarction, ischemic stroke; MALE: major adverse limb event; ALI: acute limb ischemia.
Compared with the traditional approach of aspirin alone, the combination of rivaroxaban plus aspirin markedly reduced the prespecified limb outcomes. Thus, the rivaroxaban plus aspirin approach significantly reduced the risk of ALI by 44%, the risk of MALE by 46%, the risk of all vascular amputations by 60%, and the risk of major amputations by 70% (Figure 3).41
Figure 3.
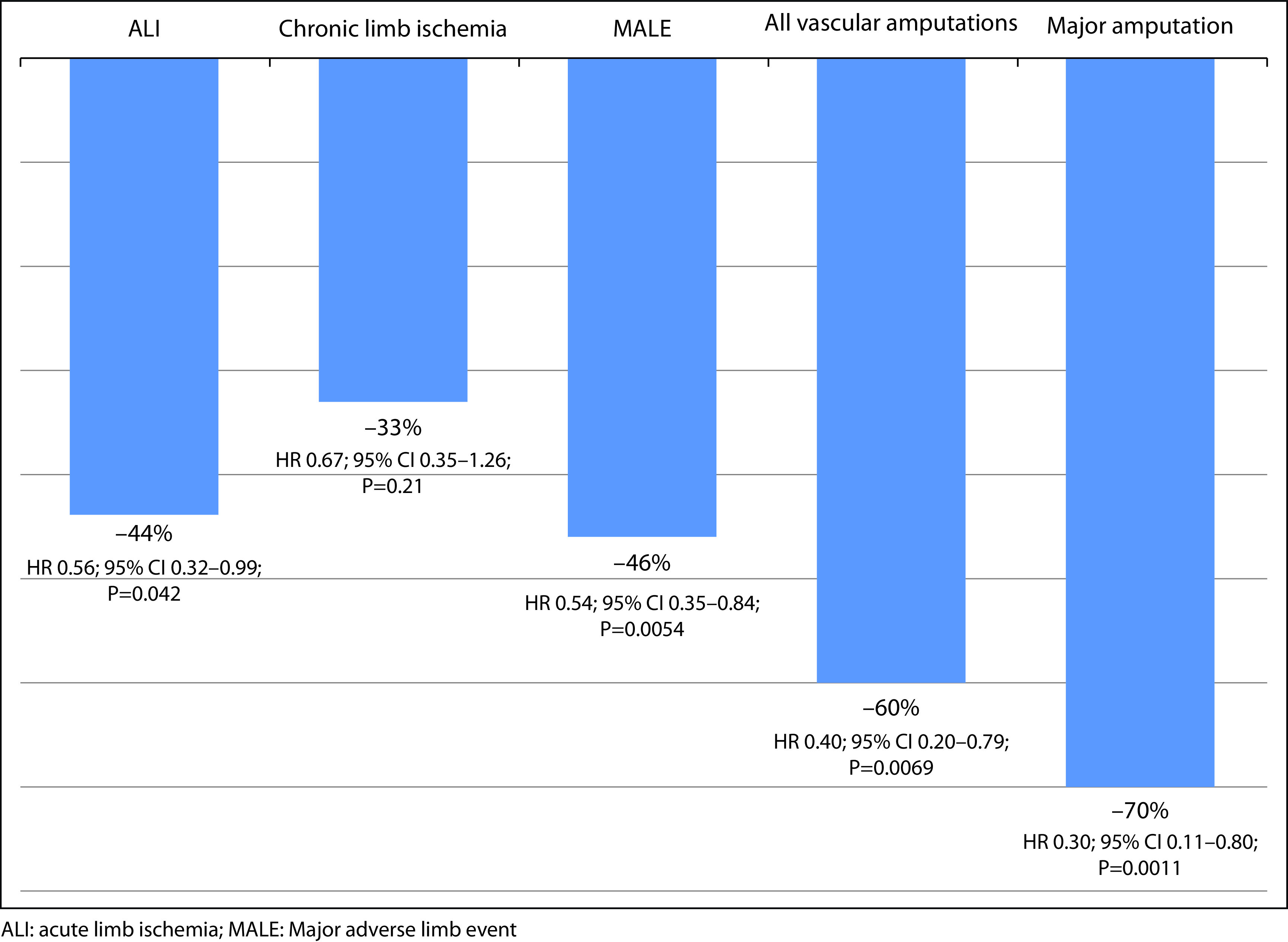
Effects of treatment on prespecified limb outcomes in patients with chronic peripheral artery disease from the COMPASS study.41
ALI: acute limb ischemia; MALE: Major adverse limb event
Major bleeding occurred more frequently with the combined therapy than with aspirin alone (3 versus 2%; HR: 1.61; 95% CI: 1.12–2.31; p=0.0089), mainly gastrointestinal. Importantly, fatal bleeding and non-fatal symptomatic intracranial hemorrhage occurred in less than 1% of patients, regardless of the assigned group (Figure 2).41
As a result, while the proportion of patients with MACE plus MALE decreased from 9 to 6% and the risk of death from 6 to 5% with the combined therapy, the risk of fatal bleeding was <1% in both groups (Figure 2). In addition, the net clinical benefit outcome composed of MACE, MALE, including major amputation, and fatal or critical organ bleeding was reduced by 28% with the rivaroxaban plus aspirin strategy compared to aspirin alone (HR: 0.72; 95% CI: 0.59–0.87; p=0.0008). As a result, during a 21-month period, for every 1000 patients treated with the COMPASS strategy, 27 events of MACE or MALE, including major amputation, would be prevented, but 1 fatal and 1 critical organ hemorrhage would occur.41
Beneficial effects of the vascular dose of rivaroxaban in patients with established PAD and other clinical conditions
Many patients with PAD have other vascular beds affected, thus increasing the risk of CV morbidity and mortality.10–14 In the COMPASS trial, two-thirds of patients with PAD had concomitant CAD,41 and among patients with CAD, 20% of patients had also PAD, defined according to COMPASS inclusion criteria.55 In those patients with ischemic heart disease, the presence of PAD did not modify the effects of the rivaroxaban plus aspirin approach on the primary outcome (p for interaction was 0.37), major bleeding (p for interaction was 0.46), and net clinical benefit (p for interaction 0.24).55
Chronic kidney disease increases both the risk of bleeding and MACE.56,57 In the COMPASS study, rates of MACE and major bleeding were more common in those patients with renal insufficiency than in those individuals with normal function. Importantly, while the beneficial effect of the rivaroxaban plus aspirin approach over aspirin on MACE was independent of renal function, there was no excess hazard of major bleeding.58
Approximately one-third of patients with symptomatic PAD have heart failure (HF).59 In fact, the risk of HF is doubled in patients with PAD60 or even more in elderly patients.61 In the COMPASS trial, although patients with severe HF were excluded from the study, 22% of patients had a history of mild-to-moderate HF. In the COMPASS trial, in patients with chronic ischemic heart disease or PAD and a history of mild or moderate HF, the rivaroxaban plus aspirin approach (versus aspirin) resulted in a similar relative but higher absolute risk reduction in MACE and mortality compared to those patients without HF, with a similar excess hazard for major bleeding, independently of the presence of HF.62
Discussion
Patients with PAD are at a very high risk of both MACE and MALE, and treatment should be necessarily focused on both targets.19,63,64 Thus, although CV death is the main cause of mortality in this population,15–17 it should not be forgotten that in patients with PAD who experience MALE, the risk of death increases by 3 and the risk of subsequent vascular amputation by 200.22 The traditional approach with antiplatelet treatment with a single or dual antiplatelet therapy has failed in providing complete protection of patients with PAD. For example, in the PEGASUS–TIMI 54 study, while ticagrelor, 60 mg, reduced the risk of MACE, no beneficial effect was obtained on limb outcomes.40 In the CHARISMA trial, the combination of clopidogrel plus aspirin did not reduce the risk of MACE compared with aspirin in monotherapy.36 Full anticoagulation with vitamin K antagonists has not been shown to provide further protection over antiplatelet therapy in PAD patients.42,43 By contrast, the COMPASS trial demonstrated in the PAD population that compared with aspirin, 100 mg, once daily in monotherapy, the rivaroxaban, 2.5 mg, twice daily plus aspirin, 100 mg, once daily approach significantly reduced the risk of MACE by 28% and the risk of MALE by 46%. Even more, the combination of vascular doses of rivaroxaban (2.5 mg twice daily) plus aspirin reduced by 60% the risk of all vascular amputations and by 70% the risk of major amputations (Table 1).41 The development of atherosclerosis in patients with PAD includes both platelet and coagulation cascade activation, with Factor Xa playing a key role. The good results of the COMPASS trial are in line with the underlying cause of atherosclerosis.24–28,48–52 Importantly, a great proportion of patients with PAD in clinical practice meet the inclusion criteria of the COMPASS trial, indicating that the results of this study can be extended to this population.65–67 As a result, all these data strongly suggest that the rivaroxaban plus aspirin approach should be considered as the first choice for the treatment of patients with PAD to achieve the double target of MACE and MALE. In fact, current guidelines recommend the use of the combination of aspirin plus rivaroxaban, 2.5 mg, twice daily in different clinical settings. Thus, the 2019 ESC Guidelines of chronic coronary syndromes recommend the use of the rivaroxaban plus aspirin approach in those patients at high or moderate risk of ischemic events, but without a high bleeding risk (recommendation IIb).68 However, despite the COMPASS trial showing a trend toward a reduction of mortality rates in this population, but not other antithrombotic approaches (i.e. clopidogrel, ticagrelor, or prasugrel), these guidelines performed the same recommendation for all these drugs. Nonetheless, in the light of the results of clinical trials, net clinical benefit clearly favors rivaroxaban over other antithrombotic strategies, and the recommendations of use should not have been the same. On the other hand, the 2019 ESC Guidelines on diabetes, prediabetes, and CV diseases indicate that the low-dose rivaroxaban, 2.5 mg, twice daily plus aspirin, 100 mg, daily may be considered in patients with diabetes and symptomatic lower extremity artery disease (recommendation IIa).69 The global vascular guidelines on the management of chronic limb-threatening ischemia state that low-dose aspirin and rivaroxaban, 2.5 mg, twice daily should be considered to reduce adverse CV events and lower extremity ischemic events in patients with chronic limb-threatening ischemia (grade 2; level of evidence B).70
The COMPASS trial showed that the rivaroxaban plus aspirin approach had a higher risk of major bleeding than aspirin alone. However, there was no excess in fatal or intracranial bleeds. Of note, as the risk of MACE or MALE (7 and 2%, in the aspirin group, respectively) was much higher than the risk of fatal or intracranial bleeding (<1%), the reduction in the risk of these outcomes by the combination of rivaroxaban plus aspirin was greater than the possible risk of fatal or intracranial bleeding. In addition, most of the major bleedings had a gastrointestinal origin and rarely are fatal or leave permanent sequelae.41 In addition, compared with aspirin alone, the risk of major bleeding was increased with the rivaroxaban plus aspirin approach only during the first year of treatment, but not during the following years.55 Therefore, the concern about the risk of bleeding should not be to avoid the use of the combination of the vascular dose of rivaroxaban plus aspirin in patients with PAD at high risk of MACE or MALE. However, it should be emphasized that as these data are provided from post-hoc subgroup analysis, the generalization of the results may be limited.
Rivaroxaban, 2.5 mg, twice daily, coadministered with 75–100 mg aspirin, once daily is currently indicated for the prevention of atherothrombotic events in adult patients with CAD or symptomatic PAD at high risk of ischemic events.71 However, it is important to ascertain which patients with chronic atherosclerotic vascular may benefit more from the COMPASS strategy.24,31,44,64,72,73 In patients with PAD, not only those patients at high risk of MACE but also at high risk of MALE should be taken into account.22,74–76 A recent analysis of the COMPASS trial showed that patients with ≥2 vascular beds affected, HF, renal insufficiency, diabetes, or having a high REACH score had the highest risk of recurrent vascular events.74 Other recent analysis of the COMPASS trial showed that those patients with PAD and Fontaine classification III or IV ischemia, history of peripheral revascularization, prior amputation, or patients with MALE, treated with aspirin alone as antithrombotic therapy, had the highest risk of recurrent limb events.22 Considering these studies and other recommendations, excepting for those patients with any of the main exclusion criteria of the COMPASS trial (high risk of bleeding, recent stroke, severe HF or renal insufficiency, and need for dual antiplatelet therapy or anticoagulation), those patients with PAD who meet any of the criteria for high risk of MACE or MALE may be considered for the treatment with the rivaroxaban plus aspirin approach (Table 4). On the other hand, the Vascular Outcomes studY of ASA along with rivaroxaban in Endovascular or surgical limb Revascularization for PAD (VOYAGER PAD) study has shown in patients with PAD who have undergone lower-extremity revascularization that rivaroxaban, 2.5 mg, twice daily plus aspirin was associated with a significantly lower incidence of the composite outcome of ALI, major amputation for vascular causes, or MACE compared with aspirin alone. In addition, the incidence of TIMI major bleeding was similar between both groups, but the incidence of International Society on Thrombosis and Haemostasis (ISTH) major bleeding was significantly higher with the combination.77
Table 4.
| To reduce MACE | To reduce MALE |
|---|---|
|
|
MACE, major adverse cardiovascular events (cardiovascular death, stroke, myocardial infarction); MALE, major adverse limb events: acute or chronic limb ischemia, including all major vascular amputations.
A number of studies have analyzed whether the combination of the vascular dose of rivaroxaban plus aspirin is a cost-effective approach according to different National Healthcare Systems. All of them have shown that in those patients with lower extremity PAD or carotid artery disease, rivaroxaban plus aspirin therapy is effective and cost effective in the prevention of recurrent CV disease compared to the traditional approach of aspirin therapy alone.78–80 These data emphasize its use in clinical practice for the majority of patients with PAD at high risk of vascular or limb events.
In light of all these data, more efforts are required to implement the COMPASS strategy in patients with PAD in routine practice. Thus, guidelines should be updated including the information of the COMPASS trial and its substudies, indicating those patients who may benefit more from this approach. In addition, specific educational activities for general practitioners, vascular specialists, cardiologists, and internists would be very helpful to better understand the benefits and risks of the rivaroxaban plus aspirin approach for PAD patients. Importantly, screening of PAD should be emphasized not only by general practitioners but also by other specialists who manage patients with vascular disease, regardless of the vascular bed affected. Moreover, more cost-effective studies are mandatory in order to facilitate a potential reimbursement by the authorities.
Conclusions
Patients with PAD have a high risk of both adverse vascular and limb outcomes. The underlying cause of atherosclerosis in PAD patients implies not only platelet activation but also the initiation and promotion of coagulation cascade, in which Factor Xa plays a key role. The traditional antithrombotic approach with only antiplatelet agents (single or dual antiplatelet therapy) has failed to provide complete protection in PAD patients. By contrast, the rivaroxaban plus aspirin strategy markedly reduces the risk of both MACE and MALE, and related complications, with a low risk of bleeding. Therefore, the rivaroxaban plus aspirin approach provides a comprehensive protection and should be considered for many patients with PAD at high risk of such events.
Acknowledgements
Writing and editorial assistance was provided by Content Ed Net, Madrid, Spain.
Footnotes
Contributions: All authors contributed equally to the preparation of this review. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosure and potential conflicts of interest: JLH reports consulting fees and/or lectures honoraria from Bayer, Daiichi Sankyo, Sanofi, Abbie, and AMGEN. FSL received compensation for advisory-board membership from Boehringer-Ingelheim, Bayer Health Care, Daiichi Sankyo, Rovi, and Sanofi-Aventis; and lectures fees from Bayer Health Care, Daiichi Sankyo, Glaxo Smith Kline, Leo Pharma, Menarini, Rovi, and Sanofi-Aventis. VR reports consulting fees from Bayer, Sanofi, and AstraZeneca. MAD reports consulting fees and/or lectures honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb/Pfizer, Eli Lilly, GlaxoSmithKline, Daiichi-Sankyo, Rovi Pharmaceuticals, and Sanofi Aventis, and grants support from AstraZeneca. JCS reports consulting fees and/or lectures honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Daiichi Sankyo, and Pfizer-BMS. SBM reports consulting fees from Bayer. JGA reports consulting fees and/or lectures honoraria from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, and Daiichi Sankyo. XGM reports consulting fees and/or lectures honoraria and/or advisory-board membership with Astra-Zeneca, Bayer, Boehringer-Ingelheim, Daiichi Sankyo, and Bristol-Myers Squibb/Pfizer. JJGD reports consulting fees and/or lectures honoraria from Bayer, Boehringer Ingelheim, AstraZeneca, and Daiichi Sankyo. JRGJ reports honoraria for lectures and advisory board from Bayer. CSF reports consulting fees and/or lectures honoraria from Bayer, Daiichi Sankyo, Pfizer-BMS and SBM. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2020/06/dic.2020-5-5-COI.pdf
Funding declaration: Writing and editorial assistance was funded by Bayer Hispania.
Correct attribution: Copyright © 2020 Hernández JL, Lozano FS, Riambau V, Almendro-Delia M, Cosín-Sales J, Bellmunt-Montoya S, Garcia-Alegria J, Garcia-Moll X, Gomez-Doblas JJ, Gonzalez-Juanatey JR, Suarez Fernández C. https://doi.org/10.7573/dic.2020-5-5. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Provenance: submitted; externally peer reviewed.
Peer review comments to author: 20 May 2020
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: Plaza Building, Lee High Road, London, England, SE13 5PT.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editorial office editorial@drugsincontext.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
References
- 1.Aboyans V, Ricco JB, Bartelink MEL, et al. 2017 ESC Guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS): document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries endorsed by: the European Stroke Organization (ESO) the task force for the diagnosis and treatment of peripheral arterial diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS) Eur Heart J. 2018;39:763–816. doi: 10.1093/eurheartj/ehx095. [DOI] [PubMed] [Google Scholar]
- 2.Aboyans V, Ricco JB, Bartelink MEL, et al. Editor’s choice - 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS) Eur J Vasc Endovasc Surg. 2018;55:305–368. doi: 10.1016/j.ejvs.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 3.Fowkes FG, Rudan D, Rudan I, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–1340. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 4.Grondal N, Sogaard R, Lindholt JS. Baseline prevalence of abdominal aortic aneurysm, peripheral arterial disease and hypertension in men aged 65–74 years from a population screening study (VIVA trial) Br J Surg. 2015;102:902–906. doi: 10.1002/bjs.9825. [DOI] [PubMed] [Google Scholar]
- 5.Cornejo Del Río V, Mostaza J, Lahoz C, et al. Prevalence of peripheral artery disease (PAD) and factors associated: an epidemiological analysis from the population-based Screening PRE-diabetes and type 2 DIAbetes (SPREDIA-2) study. PLoS One. 2017;12:e0186220. doi: 10.1371/journal.pone.0186220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suárez C, Manzano L, Mostaza J, et al. Prevalence of peripheral artery disease evaluated by ankle brachial index in patients with metabolic syndrome. MERITO I study. Rev Clin Esp. 2007;207:228–233. doi: 10.1157/13102314. [DOI] [PubMed] [Google Scholar]
- 7.Mostaza JM, Manzano L, Suárez C, et al. Prevalence of asymptomatic peripheral artery disease detected by the ankle–brachial index in patients with cardiovascular disease. MERITO II study. Med Clin (Barc) 2008;131:561–565. doi: 10.1157/13128016. [DOI] [PubMed] [Google Scholar]
- 8.Diehm C, Schuster A, Allenberg JR, et al. High prevalence of peripheral arterial disease and co-morbidity in 6880 primary care patients: cross-sectional study. Atherosclerosis. 2004;172:95–105. doi: 10.1016/s0021-9150(03)00204-1. [DOI] [PubMed] [Google Scholar]
- 9.Sigvant B, Wiberg-Hedman K, Bergqvist D, et al. A population-based study of peripheral arterial disease prevalence with special focus on critical limb ischemia and sex differences. J Vasc Surg. 2007;45:1185–1191. doi: 10.1016/j.jvs.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Bhatt DL, Steg PG, Ohman EM, et al. REACH Registry Investigators. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA. 2006;295:180–189. doi: 10.1001/jama.295.2.180. [DOI] [PubMed] [Google Scholar]
- 11.van Kuijk JP, Flu WJ, Welten GM, et al. Long-term prognosis of patients with peripheral arterial disease with or without polyvascular atherosclerotic disease. Eur Heart J. 2010;31:992–999. doi: 10.1093/eurheartj/ehp553. [DOI] [PubMed] [Google Scholar]
- 12.Alberts MJ, Bhatt DL, Mas JL, et al. Three-year follow-up and event rates in the international REduction of Atherothrombosis for Continued Health Registry. Eur Heart J. 2009;30:2318–2326. doi: 10.1093/eurheartj/ehp355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steg PG, Bhatt DL, Wilson PW, et al. One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA. 2007;297:1197–1206. doi: 10.1001/jama.297.11.1197. [DOI] [PubMed] [Google Scholar]
- 14.Suárez C, Zeymer U, Limbourg T, et al. Influence of polyvascular disease on cardiovascular event rates. Insights from the REACH Registry. Vasc Med. 2010;15:259–265. doi: 10.1177/1358863X10373299. [DOI] [PubMed] [Google Scholar]
- 15.Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res. 2015;116:1509–1526. doi: 10.1161/CIRCRESAHA.116.303849. [DOI] [PubMed] [Google Scholar]
- 16.Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381–386. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 17.Weitz JI, Byrne J, Clagett GP, et al. Diagnosis and treatment of chronic arterial insufficiency of the lower extremities: a critical review. Circulation. 1996;94:3026–3049. doi: 10.1161/01.cir.94.11.3026. [DOI] [PubMed] [Google Scholar]
- 18.Giannopoulos A, Kakkos S, Abbott A, et al. Long-term mortality in patients with asymptomatic carotid stenosis: implications for statin therapy. Eur J Vasc Endovasc Surg. 2015;50:573–582. doi: 10.1016/j.ejvs.2015.06.115. [DOI] [PubMed] [Google Scholar]
- 19.Kruger PC, Anand SS, de Vries TAC, Eikelboom JW. Patients with peripheral artery disease in the COMPASS trial. Eur J Vasc Endovasc Surg. 2018;56:772–773. doi: 10.1016/j.ejvs.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 20.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Intersociety consensus for the management of peripheral arterial disease (TASC II) J Vasc Surg. 2007;45(Suppl S):S5–S67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 21.Sampson UK, Fowkes FG, McDermott MM, et al. Global and regional burden of death and disability from peripheral artery disease: 21 world regions, 1990 to 2010. Glob Heart. 2014;9:145–158. doi: 10.1016/j.gheart.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Anand SS, Caron F, Eikelboom JW, et al. Major adverse limb events and mortality in patients with peripheral artery disease: the COMPASS trial. J Am Coll Cardiol. 2018;71:2306–2315. doi: 10.1016/j.jacc.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 23.Hirsch AT, Van’t Hof JR, Bonaca M. The conundrum of ALI and systemic embolic events: seeing our way to improved vascular health. Vasc Med. 2016;21:535–538. doi: 10.1177/1358863X16673730. [DOI] [PubMed] [Google Scholar]
- 24.Bauersachs R, Zannad F. Rivaroxaban: a new treatment paradigm in the setting of vascular protection? Thromb Haemost. 2018;118(S 01):S12–S22. doi: 10.1055/s-0038-1636530. [DOI] [PubMed] [Google Scholar]
- 25.Coppens M, Weitz JI, Eikelboom JWA. Synergy of dual pathway inhibition in chronic cardiovascular disease. Circ Res. 2019;124:416–425. doi: 10.1161/CIRCRESAHA.118.313141. [DOI] [PubMed] [Google Scholar]
- 26.Al Said S, Bode C, Duerschmied D. Anticoagulation in atherosclerotic disease. Hamostaseologie. 2018;38:240–246. doi: 10.1055/s-0038-1673412. [DOI] [PubMed] [Google Scholar]
- 27.Hess CN, Norgren L, Ansel GM, et al. A structured review of antithrombotic therapy in peripheral artery disease with a focus on revascularization: a TASC (InterSociety Consensus for the Management of Peripheral Artery Disease) initiative. Circulation. 2017;135:2534–2555. doi: 10.1161/CIRCULATIONAHA.117.024469. [DOI] [PubMed] [Google Scholar]
- 28.Stachon P, Ahrens I, Bode C, Zirlik A. Dual pathway therapy in acute coronary syndrome. J Thromb Thrombolysis. 2016;42:254–260. doi: 10.1007/s11239-015-1306-3. [DOI] [PubMed] [Google Scholar]
- 29.Patel KV, Pandey A, de Lemos JA. Conceptual framework for addressing residual atherosclerotic cardiovascular disease risk in the era of precision medicine. Circulation. 2018;137:2551–2553. doi: 10.1161/CIRCULATIONAHA.118.035289. [DOI] [PubMed] [Google Scholar]
- 30.Vanuzzo D. The epidemiological concept of residual risk. Intern Emerg Med. 2011;6(Suppl 1):45–51. doi: 10.1007/s11739-011-0669-5. [DOI] [PubMed] [Google Scholar]
- 31.Hussain MA, Wheatcroft M, Nault P, et al. COMPASS for vascular surgeons: practical considerations. Curr Opin Cardiol. 2019;34:178–184. doi: 10.1097/HCO.0000000000000597. [DOI] [PubMed] [Google Scholar]
- 32.Jones WS, Patel MR. Antithrombotic therapy in peripheral artery disease: generating and translating evidence into practice. J Am Coll Cardiol. 2018;71:352–362. doi: 10.1016/j.jacc.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 33.Berger JS, Krantz MJ, Kittelson JM, Hiatt WR. Aspirin for the prevention of cardiovascular events in patients with peripheral artery disease: a meta-analysis of randomized trials. JAMA. 2009;301:1909–1919. doi: 10.1001/jama.2009.623. [DOI] [PubMed] [Google Scholar]
- 34.Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abtan J, Bhatt DL, Elbez Y, et al. Geographic variation and risk factors for systemic and limb ischemic events in patients with symptomatic peripheral artery disease: insights from the REACH registry. Clin Cardiol. 2017;40:710–718. doi: 10.1002/clc.22721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet. 1996;348:1329–1339. doi: 10.1016/s0140-6736(96)09457-3. [DOI] [PubMed] [Google Scholar]
- 37.Bhatt DL, Flather MD, Hacke W, et al. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol. 2007;49:1982–1988. doi: 10.1016/j.jacc.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 38.Bonaca MP, Scirica BM, Creager MA, et al. Vorapaxar in patients with peripheral artery disease: results from TRA2°P-TIMI 50. Circulation. 2013;127:1522–1529. doi: 10.1161/CIRCULATIONAHA.112.000679. [DOI] [PubMed] [Google Scholar]
- 39.Hiatt WR, Fowkes FG, Heizer G, et al. Ticagrelor versus clopidogrel in symptomatic peripheral artery disease. N Engl J Med. 2017;376:32–40. doi: 10.1056/NEJMoa1611688. [DOI] [PubMed] [Google Scholar]
- 40.Bonaca MP, Bhatt DL, Storey RF, et al. Ticagrelor for prevention of ischemic events after myocardial infarction in patients with peripheral artery disease. J Am Coll Cardiol. 2016;67:2719–2728. doi: 10.1016/j.jacc.2016.03.524. [DOI] [PubMed] [Google Scholar]
- 41.Anand SS, Bosch J, Eikelboom JW, et al. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet. 2018;391:219–229. doi: 10.1016/S0140-6736(17)32409-1. [DOI] [PubMed] [Google Scholar]
- 42.Anand S, Yusuf S, Xie C, et al. Oral anticoagulant and antiplatelet therapy and peripheral arterial disease. N Engl J Med. 2007;357:217–227. doi: 10.1056/NEJMoa065959. [DOI] [PubMed] [Google Scholar]
- 43.The Dutch Bypass Oral Anticoagulants or Aspirin Study Group. Efficacy of oral anticoagulants compared with aspirin after infrainguinal bypass surgery (The Dutch Bypass Oral Anticoagulants or Aspirin Study): a randomised trial. Lancet. 2000;355:346–351. doi: 10.1016/S0140-6736(99)07199-8. [DOI] [PubMed] [Google Scholar]
- 44.Sanmartín M, Bellmunt S, Cosín-Sales J, et al. Role of rivaroxaban in the prevention of atherosclerotic events. Expert Rev Clin Pharmacol. 2019;12:771–780. doi: 10.1080/17512433.2019.1637732. [DOI] [PubMed] [Google Scholar]
- 45.Thomas R. Does rivaroxaban have a role in treating patients with PAD? JAAPA. 2019;32:16–17. doi: 10.1097/01.JAA.0000558364.85986.ad. [DOI] [PubMed] [Google Scholar]
- 46.Witkowski A, Barylski M, Filipiak KJ, et al. Non-vitamin K antagonist oral anticoagulants in the treatment of coronary and peripheral atherosclerosis. Kardiol Pol. 2019;77:490–504. doi: 10.5603/KP.a2019.0033. [DOI] [PubMed] [Google Scholar]
- 47.Bonaca MP, Creager MA. Antithrombotic therapy and major adverse limb events in peripheral artery disease: a step forward. J Am Coll Cardiol. 2018;71:2316–2318. doi: 10.1016/j.jacc.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 48.Hara T, Fukuda D, Tanaka K, et al. Rivaroxaban, a novel oral anticoagulant, attenuates atherosclerotic plaque progression and destabilization in ApoE-deficient mice. Atherosclerosis. 2015;242:639–646. doi: 10.1016/j.atherosclerosis.2015.03.023. [DOI] [PubMed] [Google Scholar]
- 49.Wu TC, Chan JS, Lee CY, et al. Rivaroxaban, a factor Xa inhibitor, improves neovascularization in the ischemic hindlimb of streptozotocin-induced diabetic mice. Cardiovasc Diabetol. 2015;14:81. doi: 10.1186/s12933-015-0243-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanada F, Muratsu J, Otsu R, et al. Local production of activated factor X in atherosclerotic plaque induced vascular smooth muscle cell senescence. Sci Rep. 2017;7:17172. doi: 10.1038/s41598-017-17508-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Álvarez E, Paradela-Dobarro B, Raposeiras-Roubín S, González-Juanatey JR. Protective, repairing and fibrinolytic effects of rivaroxaban on vascular endothelium. Br J Clin Pharmacol. 2018;84:280–291. doi: 10.1111/bcp.13440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hara T, Fukuda D, Tanaka K, et al. Inhibition of activated factor X by rivaroxaban attenuates neointima formation after wire mediated vascular injury. Eur J Pharmacol. 2018;820:222–228. doi: 10.1016/j.ejphar.2017.12.037. [DOI] [PubMed] [Google Scholar]
- 53.Bosch J, Eikelboom JW, Connolly SJ, et al. Rationale, design and baseline characteristics of participants in the Cardiovascular Outcomes for People Using Anticoagulation Strategies (COMPASS) trial. Can J Cardiol. 2017;33:1027–1035. doi: 10.1016/j.cjca.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 54.Eikelboom JW, Connolly SJ, Bosch J, et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377:1319–1330. doi: 10.1056/NEJMoa1709118. [DOI] [PubMed] [Google Scholar]
- 55.Connolly SJ, Eikelboom JW, Bosch J, et al. Rivaroxaban with or without aspirin in patients with stable coronary artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet. 2018;391:205–218. doi: 10.1016/S0140-6736(17)32458-3. [DOI] [PubMed] [Google Scholar]
- 56.Jun M, James MT, Manns BJ, et al. The association between kidney function and major bleeding in older adults with atrial fibrillation starting warfarin treatment: population based observational study. BMJ. 2015;350:h246. doi: 10.1136/bmj.h246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fox KAA, Antman EM, Montalescot G, et al. The impact of renal dysfunction on outcomes in the ExTRACT-TIMI 25 study. J Am Coll Cardiol. 2007;49:2249–2255. doi: 10.1016/j.jacc.2006.12.049. [DOI] [PubMed] [Google Scholar]
- 58.Fox KAA, Eikelboom JW, Shestakovska O, Connolly SJ, Metsarinne KP, Yusuf S. Rivaroxaban plus aspirin in patients with vascular disease and renal dysfunction: from the COMPASS trial. J Am Coll Cardiol. 2019;73:2243–2250. doi: 10.1016/j.jacc.2019.02.048. [DOI] [PubMed] [Google Scholar]
- 59.Ward RP, Goonewardena SN, Lammertin G, Lang RM. Comparison of the frequency of abnormal cardiac findings by echocardiography in patients with and without peripheral arterial disease. Am J Cardiol. 2007;99:499–503. doi: 10.1016/j.amjcard.2006.09.102. [DOI] [PubMed] [Google Scholar]
- 60.Hedberg P, Hammar C, Selmeryd J, et al. Left ventricular systolic dysfunction in outpatients with peripheral atherosclerotic vascular disease: prevalence and association with location of arterial disease. Eur J Heart Fail. 2014;16:625–632. doi: 10.1002/ejhf.95. [DOI] [PubMed] [Google Scholar]
- 61.Aronow WS, Ahmed MI, Ekundayo OJ, Allman RM, Ahmed A. A propensity matched study of the association of peripheral arterial disease with cardiovascular outcomes in community-dwelling older adults. Am J Cardiol. 2009;103:130–135. doi: 10.1016/j.amjcard.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Branch KR, Probstfield JL, Eikelboom JW, et al. Rivaroxaban with or without aspirin in patients with heart failure and chronic coronary or peripheral artery disease: the COMPASS trial. Circulation. 2019;140:529–537. doi: 10.1161/CIRCULATIONAHA.119.039609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bhagirath VC, Eikelboom JW, Anand SS. Low-dose rivaroxaban plus aspirin for the prevention of cardiovascular events: an evaluation of COMPASS. Future Cardiol. 2018;14:443–453. doi: 10.2217/fca-2018-0059. [DOI] [PubMed] [Google Scholar]
- 64.Barrios V, Almendro-Delia M, Facila L, et al. Rivaroxaban: searching the integral vascular protection. Expert Rev Clin Pharmacol. 2018;11:719–728. doi: 10.1080/17512433.2018.1495559. [DOI] [PubMed] [Google Scholar]
- 65.Kruger PC, Guzik TJ, Eikelboom JW. How can the results of the COMPASS trial benefit patients with coronary or peripheral artery disease in Poland. Kardiol Pol. 2019;77(7–8):661–669. doi: 10.33963/KP.14855. [DOI] [PubMed] [Google Scholar]
- 66.Würtz M, Olesen KKW, Thim T, Kristensen SD, Eikelboom JW, Maeng M. External applicability of the COMPASS trial: the Western Denmark heart registry. Eur Heart J Cardiovasc Pharmacother. 2019;5(4):192–199. doi: 10.1093/ehjcvp/pvz013. [DOI] [PubMed] [Google Scholar]
- 67.Kerkar P, Bose D, Nishandar T, et al. A critical analysis of the COMPASS trial with respect to benefit-risk assessment using the numbers needed to treat: applicability and relevance in Indian patients with stable cardiovascular disease. Indian Heart J. 2018;70:911–914. doi: 10.1016/j.ihj.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Knuuti J, Wijns W, Saraste A, et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407–477. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 69.Grant PJ, Cosentino F. The 2019 ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2019;40(39):3215–3217. doi: 10.1093/eurheartj/ehz687. [DOI] [PubMed] [Google Scholar]
- 70.Conte MS, Bradbury AW, Kolh P, et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. J Vasc Surg. 2019;69(6S):3S–125S.e40. doi: 10.1016/j.jvs.2019.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.European Medicines Agency (EMA) Xarelto®, “Summary of Product Characteristics”. Updated September 6, 2018 https://www.ema.europa.eu/en/documents/product-information/xarelto-epar-product-information_en.pdf.
- 72.Gradolí J, Vidal V, Brady AJ, Facila L. Anticoagulation in patients with ischaemic heart disease and peripheral arterial disease: clinical implications of COMPASS study. Eur Cardiol. 2018;13:115–118. doi: 10.15420/ecr.2018.12.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nicholls SJ, Nelson AJ. Rivaroxaban with or without aspirin for the secondary prevention of cardiovascular disease: clinical implications of the COMPASS trial. Am J Cardiovasc Drugs. 2019;19:343–348. doi: 10.1007/s40256-018-00322-4. [DOI] [PubMed] [Google Scholar]
- 74.Anand SS, Eikelboom JW, Dyal L, et al. Rivaroxaban plus aspirin versus aspirin in relation to vascular risk in the COMPASS trial. J Am Coll Cardiol. 2019;73:3271–3280. doi: 10.1016/j.jacc.2019.02.079. [DOI] [PubMed] [Google Scholar]
- 75.González-Juanatey JR, Almendro-Delia M, Cosín-Sales J. Residual risk reduction opportunities in patients with chronic coronary syndrome. Role of dual pathway inhibition. Expert Rev Clin Pharmacol. 2020 doi: 10.1080/17512433.2020.1772056. [DOI] [PubMed] [Google Scholar]
- 76.Kaplovitch E, Rannelli L, Anand SS. Antithrombotics in stable peripheral artery disease. Vasc Med. 2019;24:132–140. doi: 10.1177/1358863X18820123. [DOI] [PubMed] [Google Scholar]
- 77.Bonaca MP, Bauersachs RM, Anand SS, et al. Rivaroxaban in peripheral artery disease after revascularization. N Engl J Med. 2020 Mar 28; doi: 10.1056/NEJMoa2000052. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 78.Tsilimigras DI, Moris D, Karaolanis G, Kakkos SK, Filis K, Sigala F. Rivaroxaban versus clopidogrel for peripheral artery disease: a clinico-economic approach of the COMPASS trial. Curr Pharm Des. 2018;24:4516–4517. doi: 10.2174/1381612825666190101100832. [DOI] [PubMed] [Google Scholar]
- 79.Zomer E, Si S, Hird TR, et al. Cost-effectiveness of low-dose rivaroxaban and aspirin versus aspirin alone in people with peripheral or carotid artery disease: an Australian healthcare perspective. Eur J Prev Cardiol. 2019;26:858–868. doi: 10.1177/2047487318817910. [DOI] [PubMed] [Google Scholar]
- 80.Ademi Z, Zomer E, Tonkin A, Liew D. Cost-effectiveness of rivaroxaban and aspirin compared to aspirin alone in patients with stable cardiovascular disease: an Australian perspective. Int J Cardiol. 2018;270:54–59. doi: 10.1016/j.ijcard.2018.06.091. [DOI] [PubMed] [Google Scholar]

