OVERVIEW
The PACIFIC trial of giving durvalumab for 1 year to patients with stage III lung cancers has set a new care standard. PACIFIC has established the role of immune checkpoint inhibitors (ICIs) for individuals with inoperable and unresectable locally advanced lung cancers that achieve disease control from concurrent chemoradiation. For resectable and operable patients, ICIs given before surgery, either alone (JHU/MSK, LCMC3, and NEOSTAR) or in combination with chemotherapy (Columbia/MGH) and NADIM), have yielded high rates of major pathologic response in resection specimens, an outcome measure that correlates with improved progression-free and overall survival. These results have surfaced the dilemma of how to choose the optimal local therapy (either definitive concurrent chemoradiation or surgery) to use with an ICI for patients with stage III lung cancers that are both operable and resectable. Here, we review the data supporting the use of each local therapy. Recent successes have also raised the possibility using ICIs in persons with earlier stages of lung cancer will enhance curability. Randomized trials are underway, but, until they read out, physicians must choose local and systemic therapies based on the information we have today. Research demonstrates that using surgery, radiation, chemotherapy, and ICIs improve all efficacy outcomes and curability. All modalities should be considered in every patient with locally advanced lung cancer. It is imperative that a multimodality discussion including the possible addition of ICIs takes place to choose the best modality and sequence of therapies for each patient.
TWEET
Adding immune checkpoint inhibitors to multimodality treatment for early stage lung cancers – a review of advances from specialists in the field #lcsm
Introduction
For the field of thoracic oncology, the treatment of patients with locally advanced lung cancers marks both one of our greatest successes and greatest disappointments. Combining surgery, radiation, and chemotherapy, we can cure some but not all individuals with stages II-III lung cancers. Despite precise imaging studies, the availability of advanced radiation techniques and minimally invasive complete resections, both delivered with utmost precision, we fail to achieve cure in the majority of patients. We attempt to enhance individual cure rates of local therapies with systemic ones, usually cytotoxic chemotherapy given before, during, or after the “definitive local therapy”. Cisplatin-based chemotherapy enhances the curability of locally advanced cancers to a degree comparable to or exceeding those with the use of perioperative therapies in breast or colorectal cancers. However, in the minds of many physicians and patients, the benefits are too small to justify the disruption in lifestyle brought on by multimodality lung cancer therapies. How can we build on our successes and turn our frustration into a path to progress?
We can begin by rethinking our basic assumptions concerning early stage lung cancers. We can acknowledge that all stages of lung cancers are potentially deadly and routinely explore opportunities to improve outcomes. Clinically or pathologically staged, 1-centimeter primary lung cancers carry a five-year survival of only 92%.(1) Many physicians caring for patients with lung cancers consider this a “good prognosis”. Current treatment guidelines recommend no additional interventions even for patients with tumor characteristics that suggest a higher risk of recurrence (discussed further below). In contrast, patients with HER2-driven breast cancers with the same recurrence risk are offered surgery, radiation, chemotherapy, one year of trastuzumab, and an additional year of neratinib. (2) If individuals with lung cancers have clinical evidence of mediastinal nodal spread, even with a 1 cm primary tumor, their 5-year survival estimates fall to 32%. (1) Additional treatment modalities are recommended with clinical stage IIIA disease, but they still fall short, especially for patients with large primary tumors and mediastinal nodal spread. Better staging can define the risk of recurrence, but regardless of disease extent, our best therapies are unable to lead to cures in many patients.
Improved Results with Multimodality Approaches for Locally Advanced Lung Cancers
In the last 2 decades, we have made tremendous strides in surgery and radiation, both in terms of oncologic success and limiting treatment related disability. Local failure alone rarely occurs with optimally delivered local therapies. While continued advancements in these areas can be anticipated, they cannot be expected to make a major impact in the curability of lung cancers where relapse occurs systemically in most patients destined to relapse. Guideline-recommended chemotherapy (ideally cisplatin-based, given before or after surgery) consistently improves curability beyond surgery alone in patients with any nodal spread. Guidelines recommend routine chemotherapy use and further, consideration in patients with larger primary tumors with no nodal spread. (3),(4) A recent study suggests an even greater degree of adjuvant therapy benefit among individuals with tumors with certain high risk features including lymphatic, vascular, or visceral pleural invasion, and invasive size >2 cm.(5) However, chemotherapy benefits have generally plateaued, few chemotherapeutic agents are under study, and as the population of patients with lung cancers ages, many drugs (especially cisplatin) are difficult or impossible to deliver safely. Targeted therapies paired with surgery or radiation in patients with tumors with oncogenic drivers hold promise, but they have yet to become part of routine management. Concurrent chemotherapy with definitive thoracic radiation is now a worldwide standard of care for individuals with inoperable or unresectable stage III disease and a comparable approach to surgery in patient with operable and resectable stage IIIA lung cancers in many cases. How the optimal local modality is chosen to pair with cytotoxic chemotherapy is an area of scientific inquiry and daily debate at the world’s multimodality tumor boards. With the tools in hand, choosing the optimal type and sequence of each will undoubtedly result in further incremental gains, but not the transformative improvements necessary to make cure the norm and not the exception.
Immunotherapeutics Provide and Additional Benefits Beyond Surgery, Radiation, and Chemotherapy
After more than a century of research, we have now realized the dream that therapeutics can harness a person’s immune system to fight their cancers. This breakthrough has the potential to overcome the barriers we have faced to cure patients with locally advanced lung cancers. The experience in patients with metastatic lung cancers demonstrates that modulating T cell function using immune checkpoint inhibitors (ICIs) targeting PD-1, PD-L1, and CTLA4 can lead to durable tumor regressions, better overall survival (6), (7), (8) and 5 year disease free survival “off therapy”. (9) ICIs provide an additional modality that enhances a person’s immune system to target cancer and can work in concert with surgery, radiation, and cytotoxic chemotherapy to achieve results beyond those with other modalities given alone or in combination. The platform has been set for a new wave of clinical research to define the optimal systemic agents, dosing, scheduling, and sequencing of both systemic and local therapies, all attempting to increase the curability of locally advanced lung cancers.
Trials utilizing neoadjuvant ICIs represent the greatest amount of data available today. Five studies that have released data on ICIs used alone or with cytotoxic chemotherapy prior to surgery are reported in Table 1. (10),(11), (12), (13), (14), (15) In neoadjuvant trials in patients with lung cancers, the primary endpoint is major pathologic response (MPR) defined as less than 10% viable tumor cells in the surgical resection specimen. (16) MPR has been found to predict both progression free and overall survival. Preclinical data has suggested that ICIs given before chemotherapy in preclinical lung cancer models result in better outcomes than the same therapies administered after surgery. (13, 17),(18) Investigators have theorized that an “intact” primary tumor and draining lymph nodes, sites where key processes of immune response initiation and antigen encounter occur, may achieve the optimal results with ICIs. Neoadjuvant anti PD-1 (nivolumab) and anti-PD-L1 (atezolizumab) targeted monoclonal antibodies alone produced surprisingly better results compared to those seen in patients with ICIs given to patients with patients with metastatic lung cancers. (10), (12), (13) When neoadjuvant ICIs were combined with cytotoxic chemotherapies, results were nothing short of astonishing with MPR rates of 57 and 80%.(11),(14) This data has led to multiple phase III trials comparing chemotherapy alone with the combination of chemotherapy plus atezolizumab, durvalumab, nivolumab, and pembrolizumab, all agents given before surgery. Trials giving the same agents after chemotherapy post-operatively (adjuvantly) are also in progress. By design, no early results are available, and the readouts will take longer. No trials comparing neoadjuvant to adjuvant use of the same agent have been attempted. In addition to the preclinical data favoring neoadjuvant use of ICIs, there are many advantages of neoadjuvant approaches listed in Table 2, not the least of which is a years earlier demonstration of long-term benefit based on major pathologic response rates.(16),(19) In all the trials of neoadjuvant ICIs, no new perioperative toxicities emerged. The accumulated data detailed below demonstrates that ICIs given alone or with chemotherapy lead to high rates of Major Pathologic Response (historically associated with better progression-free and overall survival) in patients with stages IB to IIIB lung cancers. We are years away from determining whether adjuvant ICIs are a more effective perioperative strategy than their neoadjuvant use.
Table 1.
Phase II trials to determine pCR and MPR after neoadjuvant ICI or ICI plus chemotherapy in patients with resectable and operable stage I-III lung cancers.
| Author(s) | Agent(s) | MPR% (95% CI) | pCR% (95% CI) | |
|---|---|---|---|---|
| Johns Hopkins University/Memorial Sloan Kettering N=21 |
Forde and Chaft(10) | Nivolumab | 43% (21 to 66) | 14% (4 to 34) |
|
LCMC3 Lung Cancer Mutation Consortium - USA N=82 |
Kwiatkowski(12) | Atezolizumab | 18% (11 to 28) | 5% (2 to 12) |
|
NEOSTAR MD Anderson N=44 |
Cascone(13) | Nivolumab + Ipilimumab | 25% (14 to 40) | 18% (9 to 32) |
|
NADIM Spanish Lung Cancer Group N=30 |
Provencio(14) | Nivolumab + Paclitaxel Carboplatin | 80% (64 to 91) | 75% (4 to 76) |
| Columbia University New York/MGH N=30 |
Shu(11) | Atezolizumab + Paclitaxel Carboplatin | 57% (36 to 76) | 33% (18 to 52) |
| Duke/Dartmouth/Mayo N=25 |
Ready(15) | Pembrolizumab | 28% (12 to 49) | 8% (1 to 26) |
Table 2.
Factors favoring neoadjuvant therapy
| • Attacks micrometastases, the primary cause of relapse, at the earliest time |
| • Preservation of primary tumor and draining nodes can provide an intact immune environment when using checkpoint inhibitors |
| • Ability to assess sensitivity to agents planned for adjuvant use for individual patients and as part of drug development |
| • Opportunity to assess remaining tumor cells at maximal response (persisters) |
| • Ability to assess sensitivity of agents used in induction. You can change the regimen. |
| • Complete and Major Pathologic Response and outcome surrogate |
| • Better chemotherapy drug delivery and tolerability |
| • Better compliance with subsequent therapies |
| • Time to identify unsuspected metastases and comorbidities before local therapy |
Combining ICIs and Surgery
Much of the challenge of determining the optimal treatment for Stage III disease is the heterogeneity of the disease. Available approaches highlighting those that include surgery are listed in Table 3. Additionally, the definition of “resectable” vs “unresectable” is variable, making comparisons between trials difficult. Numerous randomized trials over the last 20 years have tried to address the optimal approach to Stage III patients (CRT (Concurrent Chemotherapy and Irradiation (CRT) alone, CRT→ Surgery (S) or C→S→±RT). For the most part, these trials have not demonstrated clear benefit to adding surgery to CRT except in a subset of patients with stage III cancers referred to as “resectable” (T3/4N0-1 or N2 non-bulky single station) where improved locoregional control may be important.(20) The dramatic results of the PACIFIC trial with one year of durvalumab after CRT compared to CRT alone has created a new standard of care for “unresectable” stage III patients who do not progress after CRT.(21),(22) Of interest, most of the benefit in the PACIFIC trial appeared to address the greatest need in the field ….the reduction of systemic relapse …with lower time to death or new metastases.(21) Given the landmark results of PACIFIC, the role of surgery in individuals with stage III NSCLCs needs to be re-evaluated.
Table 3.
Current and future strategies for patients with stage III adenocarcinomas and squamous cell carcinomas (boxed strategies include surgery).
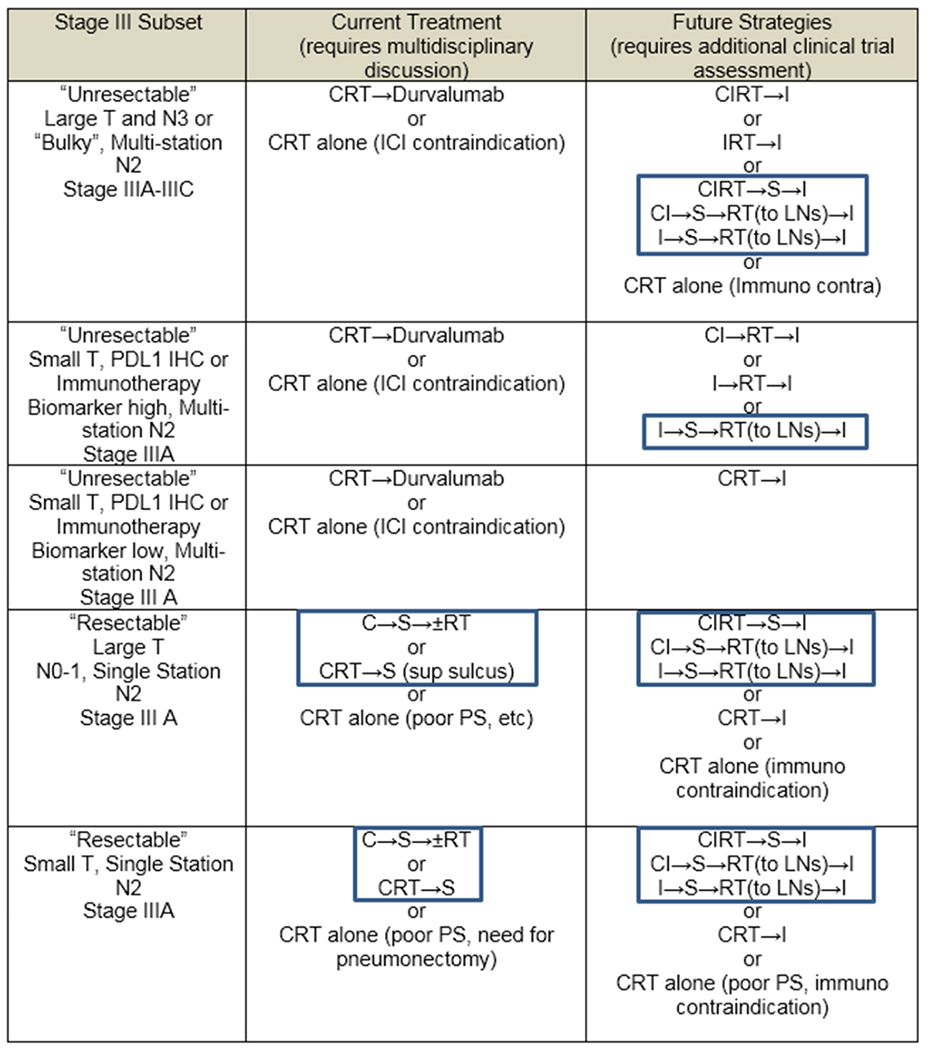 |
CRT= concurrent chemoradiation; CI=chemoimmunotherapy; CIRT=concurrent chemoradiation and immunotherapy; ICI-immune checkpoint inhibitor
The Role of Surgery to Enhance Locoregional Control in Patients with Stage III NSCLCs
Investigators at the University of Texas MD Anderson Cancer Center recently reviewed their experience with stage IIIA (N2) lung cancers treated from 2004 to 2014 in which 159 patients with “resectable” lung cancers with documented N2 nodes underwent induction chemotherapy followed by surgery ± adjuvant radiation therapy (40% of patients). The 5 year overall survival was 50% with 15% locoregional and 44% distant recurrences.(23) During that same period, 336 individuals with “unresectable” stage IIIA disease underwent definitive CRT with 5 year overall survival of 29%, with 30% locoregional and 41% distant recurrence.(23) While these two groups represent distinct patient populations, the data suggests that better locoregional control can be obtained by incorporating surgery with chemotherapy ± radiation therapy. Ongoing clinical trials will demonstrate whether the addition of immunotherapy to a surgical strategy can improve distant control as demonstrated in patients treated with concurrent CTRT in the PACIFIC study.
The Role of Surgery with Immunotherapy
Success with immunotherapy in metastatic disease and in the PACIFIC trial have led to efforts to improve survival in earlier stage NSCLCs through the incorporation of ICIs with surgery. These efforts have focused on adjuvant and neoadjuvant approaches with surgery. Adjuvant approaches to date include four randomized trials utilizing immunotherapy. The ANVIL trial (EA5142) in ALCHEMIST randomized patients with resected stages IB-IIIA NSCLCs to adjuvant nivolumab (q 4 wk for one year) or observation after standard adjuvant therapy (NCT02595944, n=903). This trial has completed enrollment in 2019 but is not expected to “read out” its disease-free survival primary endpoint for several years, highlighting the challenge with adjuvant studies that endpoints of overall survival and progression free survival take up to a decade to assess. Additionally, the subset of patients with each stage of the disease may not be large enough to make clear determinations of benefit of adjuvant ICIs by stage.
The neoadjuvant approach represents another strategy to incorporate ICIs with surgery. Biologically, this approach may be more effective than the adjuvant strategy because the presence of intact tumor “draining” lymph nodes at the time of ICI treatment may allow better neoantigen presentation to dendritic cells and development of immunoreactive T cells through dendritic antigen presentation at the organ site of antigen presentation.(18),(24) In preclinical models, Cascone et al. and others demonstrated that neoadjuvant immunotherapy was more effective in mice than adjuvant treatment. (17), (13) Surrogate endpoints such as pathological complete response (pCR) in resection specimens or MPR (<10% viable tumor) may also allow a more rapid read-out of long term benefits than can be anticipated in adjuvant studies.(16),(25) Several phase II studies listed in Table 2 have evaluated this neoadjuvant approach. Forde et al. noted no increase in adverse events after surgery with MPR rates of 45% after two cycles of nivolumab. There was also evidence of biologic effect with increased T cell infiltration in the tumor and peripheral blood and increased neoantigen-specific T-cell clones from the primary tumor. (10) NEOSTAR, conducted at MD Anderson, randomized patients with stages IB-IIIA NSCLCs to 3 cycles of nivolumab or nivolumab and ipilimumab. MPR rates were higher with the combination treatment (44% vs 19%) as was T cell infiltration in the tumor.(13) LCMC3 is a 180 patient phase II trial evaluating neoadjuvant atezolizumab for 2 cycles in patients with resectable stage IB-IIIA NSCLCs. Preliminary results have suggested no increased toxicity. The MPR rate in the initial 82 patients was 18% with pCR rate of 4%.(12) The range of MPR rates from these neoadjuvant studies is 18%-43% which is encouraging when compared to MPR rates of 19% reported in earlier trials of neoadjuvant chemotherapy alone.(16),(25, 26)
Role of Surgery with Chemoimmunotherapy
Chemoimmunotherapy has demonstrated superiority over chemotherapy alone in patients with metastatic lung cancers.(7) This has led to interest in assessing the role of chemoimmunotherapy with surgery in earlier stage NSCLC. In the adjuvant setting, ALCHEMIST is launching a new trial designed to evaluate adjuvant chemoimmunotherapy. This study (NCT04267848, N=1263, DFS endpoint) set to open in the USA in 2020, will randomize patients with resected stage IB-IIIA NSCLCs of any histology with one of three arms: standard adjuvant therapy with a platinum doublet, standard adjuvant therapy with a platinum doublet followed by pembrolizumab for (17 cycles total), or a standard adjuvant platinum doublet with concurrent pembrolizumab for 4 cycles followed by 12 additional cycles of pembrolizumab (17 doses total).
One potential benefit of chemoimmunotherapy may be an increase in immunogenic tumor-specific peptides, or neoantigens, released from chemotherapy induced cell death of the primary tumor. This might then lead to increases in primed neoantigen-specific T cells and a more robust immunologic response targeting micrometastases.(18),(24) From this standpoint, a neoadjuvant approach may be more efficacious than adjuvant because of the presence of primary tumor during treatment with chemoimmunotherapy (Table 2). The NADIM trial evaluated neoadjuvant nivolumab, paclitaxel and carboplatin in patients with stage IIIA “resectable” NSCLCs followed by surgical resection and adjuvant nivolumab for one year. Preliminary observations include no increased toxicity and a high rate of MPR (80%) and complete pathologic response (75%) in resected specimens.(14) Another study from Columbia University utilizing neoadjuvant atezolizumab and chemotherapy has also found encouraging MPR rates of 57% and pCR rates of 33% in resected specimens.(11) These dramatic phase II pathological response results have led to worldwide interest and the development of phase III trials: IMpower 030 is evaluating atezolizumab and chemotherapy vs chemotherapy alone (NCT03456063, n=374, MPR, EFS), the Aegean trial is evaluating durvalumab with chemotherapy vs chemotherapy alone (NCT03800134, n=300, MPR). Keynote-671 is evaluating pembrolizumab and chemotherapy vs chemotherapy alone (NCT03425643, n=786, EFS, OS). The Lung Cancer Mutation Consortium is initiating a 1000 patient screening trial (LCMC4) to detect 8 oncogenic drivers (EGFR, ALK, MET, BRAF, RET, NTRK, ROS1, HER2) in patients who are being evaluated for neoadjuvant therapies. (19) Individuals identified with early stage tumors with oncogenic drivers (approximately one-third of patients) will be directed to industry-sponsored trials of targeted therapies matched to the oncogenic driver detected.
Role of Surgery with Immunotherapy and Concurrent Chemoradiation Therapy
Ultimately, the effectiveness of checkpoint inhibition may also be dependent on the immunogenic environment in and around the primary tumor. Non-immunogenic environments or “cold” tumors may be less likely to respond to ICIs. These environments could potentially be enhanced not only with chemotherapy but also with the addition of radiation therapy to increase the neoantigen load in the tumor microenvironment. This concept has led to a neoadjuvant strategy combining immunotherapy with concurrent chemoradiation followed by surgery and consolidative immunotherapy. The optimal timing sequence and potential morbidity of such a strategy is being evaluated in several ongoing trials. The Hoosier Cancer Research Network is evaluating the feasibility of durvalumab and CRT followed by surgery and adjuvant durvalumab (NCT03871153, n=25, safety, pCR) while Case Comprehensive Cancer Center is evaluating pembrolizumab and CRT followed by surgery and adjuvant pembrolizumab (NCT029879998, n=20, safety).
Role of Surgery with Immunotherapy and Novel Immune Enhancing Agents
Novel immune enhancing agents are also being evaluated in the neoadjuvant setting in the hopes of rapidly identifying effective combinations. NEOCOAST is evaluating neoadjuvant durvalumab alone or with oleclumab, danvatirsen or monalizumab (NCT03794544, n=160, MPR). Canopy N is evaluating neoadjuvant Pembrolizumab and Canakinumab vs Canakinumab alone, an anti-IL-1B antibody, (27) (NCT03968419, n=110, MPR). Neoadjuvant SHR1210 (anti PD-1 antibody) is also being evaluated with apatinib, a TKI inhibitor with selective inhibition of VEGFR2 (28), (NCT04133337, n=20, MPR). The neoadjuvant platform provides the opportunity to rapidly test many combinations using MPR or pCR as a surrogate. (16) In the future, many investigators have proposed using ctDNA to quantitate minimal residual disease and its presence can serve a trigger to intensify therapy if ctDNA is still detectable after the completion of multimodality therapy. (29)
Personalized Approach to Stage III
Although the PACIFIC trial established a new standard of care for patients with “unresectable” stage III lung cancers in general, surgery remains a critical component of multimodality care for many individuals, especially those with stage IIIA disease. Patients with ECOG 0 or 1 performance status, T3–4N0–1, or single station non-bulky N2 tumors, should be considered for CRT→S or C→S→±RT after multi-disciplinary review. In the future, if the impressive results seen in the completed phase 2 trials (Table 1) are confirmed in larger series and eventually phase III studies, patients with stage III lung cancers may routinely receive ICIs in addition to surgery. The neoadjuvant approach provides the advantage of assessing tumor response at the time of surgery, permitting the use of different treatments in the adjuvant setting for “non-responders” or responding patients with less than complete regressions. This success strategy has recently been demonstrated in patients with HER2-driven breast cancers with present disease after trastuzumab. (30) One could even imagine adding in an adjuvant fashion different immune enhancing drugs, CAR T cells, CAR NK cells or TILs derived from the resected primary tumor in ICI “non-responders”. Many groups are pursuing development of blood or tissue biomarkers to better select optimal therapies in stage III including surgical resection.
Patient Case 1 below demonstrates the concepts surrounding the place of surgery in the management of patients with locally advanced lung cancers.
Patient Case 1: Stage IIIA, RUL squamous cell lung cancer with a single “bulky” ipsilateral mediastinal lymph node (N2)
| 64 yr old male with history of smoking |
| Presented with RUL mass noted on CXR |
| Past medical history – high blood pressure, no CAD |
| WHO PS1 |
| PFT- FEV1 81% predicted; DLCO 86% predicted |
| CT thorax abdomen – right upper lobe tumor & enlarged station 4R (25.6 mm x 17.4 mm) |
| EBUS – positive 4R, 11R; negative 7, 4L, 10R |
| PET-CT - FDG right hilar adenopathy lymph node T2 N2 M0 |
| MR Brain – no metastases |
| Treatment – IMRT 66 Gy, concurrently with paclitaxel and carboplatin |
| 4 weeks post CRT |
| Decision to start with durvalumab 5 weeks after completion of CRT for a duration of 12 months |
| 2 months post CRT (on durvalumab) |
| PET-CT – Decreased avidity RUL mass and R hilar adenopathy |
| 8 months post CTRT (on durvalumab) |
| PET-CT- No FDG avidity in RUL mass, increased avidity R hilar adenopathy, no metastases |
| EBUS- positive 4R; negative 7, 4L |
| Referred for surgical evaluation |
|
PFT- FEV1 71% predicted; DLCO 74% predicted MRI Brain – no metastases Treatment - Robotic resection |
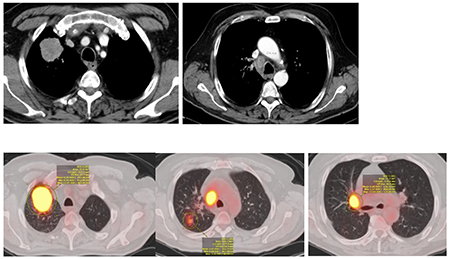
Case 1- Initial CT thorax and CT-PET
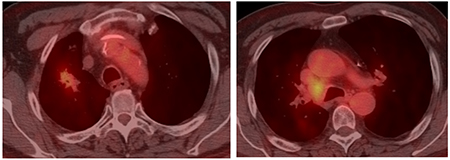
Case 1- CT-PET - 2-month post CTRT on Durvalumab
Case 1- CT-PET - 8-month post CTRT on Durvalumab
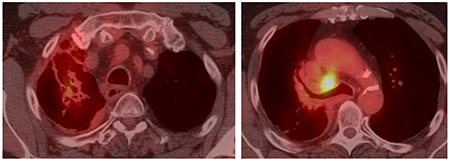
This case is not straightforward with regards to the initial decision to not include surgery and to proceed with concurrent chemoradiation followed by durvalumab. The single N2 station is considered by many as “borderline bulky” with a short axis of 18 mm and long axis 26 mm. The locoregional recurrence highlights the challenge to choose the best local therapy if ICIs are able to control distant recurrences. The decision to proceed with resection 8 months after CT while still on durvalumab is likely associated with increased risk of pulmonary toxicity following chemotherapy, radiation, and durvalumab. The increased fibrosis at the hilum and RUL can be appreciated on the CT-PET (8 months post-CRT) scan.
Combining ICIs and Radiotherapy
There is a strong rationale for combining immunotherapy and radiotherapy (RT). Over one hundred years following the discovery of ionizing radiation, we now know that RT elicits immune interactions that can synergize with systemic therapies, particularly immunotherapeutics. Our knowledge and understanding of how RT affects the tumor microenvironment is growing - its ability to induce immunogenic cell death, release tumor antigens, induce the cGAS-STING pathway, upregulate MHC-I expression, stimulate type I interferons, and promote CD8+ T-cell infiltration (31), (32), (33). These immune effects provide a rationale that RT can deliver the ‘kick start’ required to improve immune-mediated tumor control with ICIs. Furthermore, the ability of RT to induce PD-L1 expression on tumor cells provides an additional benefit: the combination with ICI may negate ‘adaptive resistance’ mechanisms. (34)
In locally advanced NSCLCs, CRT forms the mainstay of treatment. Combining ICIs with CRT in this patient population is an attractive strategy. Preclinical studies of RT-ICI combinations have demonstrated improved outcomes, primarily in the setting of the ICI being administered concurrently with RT (34), (35), (36), (37). However, we are coming to realize that RT can also have negative immune consequences in unselected patients. These include the ability of RT to promote introduction of myeloid-derived suppressor cells (MDSCs) and regulatory T-cells (Tregs) in the tumor microenvironment (TME), and to induce release of undesirable cytokines and chemokines that further recruit suppressive immune effector cells or impair T-cell trafficking.(38),(39) It is prudent to consider that these negative immune consequences may outweigh the positive leading to radioresistant disease. Biomarkers are needed to help in patient selection for ICIs in combination.
The PACIFIC results mark the first change in the management of inoperable and unresectable stage III lung cancers in over two decades. (40) The addition of induction or consolidation chemotherapy to CRT and dose escalation did not lead to improved outcomes. (41) The Phase III PACIFIC trial has demonstrated that the ICI durvalumab, administered for one year after concurrent CTRT, improves both the median progression free (by 12 months to 17.2 months) and 3-year overall survival (by 13% to 57%) in patients with inoperable or unresectable stage III lung cancers. (21, 42) Furthermore the addition of durvalumab after chemotherapy was well tolerated. This trial was the first demonstration of improved survival over concurrent chemotherapy and radiation in over two decades and has established durvalumab after CRT as a standard of care. It is important to highlight that patients in PACIFIC were eligible if they had unresectable disease, had responded to CRT, had recovered from side effects (CTCAE grade ≤2) and had a performance status of 0-1. An important consideration is therefore to use state of the art radiation treatment to reduce the dose delivered to normal tissues and consequently the side effects of CRT. This can be achieved by using 1) 4DCT scanning for radiotherapy planning resulting in the use of smaller margins around the gross tumor volume, 2) intensity modulated radiotherapy (IMRT) for treatment delivery technique that adds fluence modulation to beam shaping, which improves radiotherapy dose conformity around the tumor and spares surrounding normal structures) and 3) daily online imaging for treatment verification.
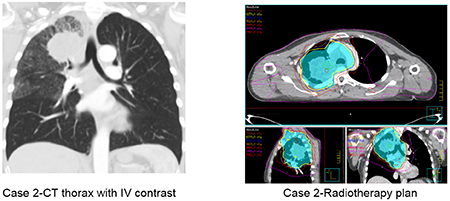
A typical case of patient suitable for durvalumab after CRT is presented in Case 2. This case is straightforward with regards to the decision to treat with durvalumab since the patient would have met the inclusion criteria of the PACIFIC study. However, a number of questions remain unanswered regarding the application of this treatment listed in Table 4.
Table 4.
Questions not answered by the PACIFIC trial (testing durvalumab after the completion of concurrent chemoradiation in patients with unresectable and inoperable stage III lung cancers)
| • Applicability of the PACIFIC data to other population of patients? |
| • Resectable disease |
| • PS2 |
| • Elderly |
| • Individuals receiving sequential chemoradiotherapy |
| • Patients with EFGR L858R and exon 19 deletion mutations |
| • Can durvalumab be safely prescribed in patients who have received a high radiation dose to thoracic organs at risk (e.g. lung)? |
| • Can we create precise definitions of “operable and resectable”? How is this assessment best obtained? |
| • How soon should durvalumab start after CTRT? |
| • What is the optimal duration of durvalumab? |
| • What is the optimal timing durvalumab and CTRT: concomitant or sequential? |
| • Should we treat patients with durvalumab if PDL1 <1%? |
| • Do biomarkers exist to better select patients? |
Patient Case 2
| 71 yr old female |
| Presented with shortness of breath |
| Past medical history – high blood pressure, ischemic heart disease 10 years ago |
| WHO PS1 |
| PFT- FEV1 80% predicted; KCO 105% predicted |
| CT thorax abdomen – right upper lobe tumor & enlarged station 4R, 7 lymph nodes T3 N2 M0 |
| Mediastinoscopy - Station 4R adenocarcinoma PDL1 10% |
| PET-CT - FDG avid right supraclavicular lymph node T3 N3 M0 |
| MR Brain - Clear |
| Treatment – IMRT 66 Gy in 33 fractions, concurrently with cisplatin etoposide x 2 cycles starting day 1 of radiotherapy |
| 2 weeks post CTRT |
| CTCAE v5.0 Grade 3 oesophagitis, PS2 |
| 4 weeks post CTRT |
| CTCAE v5.0 Grade 2 oesophagitis, PS1 |
| Decision to start durvalumab 5 weeks after completion of CRT for a duration of 12 months |
PFT: pulmonary function test, IMRT: intensity modulated radiotherapy, CTRT: chemoradiotherapy, PS: performance status, CTCAE: Common Terminology Criteria for Adverse Events
Several important points should be taken into consideration when interpreting the results of the PACIFIC trial. First, the PACIFIC population may not be fully representative of the ‘real world’ since only 45% of the patients were above age 65. Second, no data was collected at the time of randomization on disease volume, radiation dose delivered to organs at risk, and radiotherapy techniques used. There is uncertainty as to whether patients presenting with large volume disease and with radiation doses to the organs at risk at the limit of tolerance can be safely given durvalumab. Third, subgroup analyses suggested that PFS and OS were superior if durvalumab was delivered <14 days after completion of CRT. However, this does not mean that patient should be treated as soon as possible after completion of CRT in routine practice. It is likely that these patients who had recovered from the side effects of CRT within 2 weeks had small volume disease at some distance from the mediastinum. Finally, tissue collection was not mandatory for trial entry. Consequently, tissue samples were available for only 63% of patients with only 148 patients out of 713 having PD-L1 expression <1%. There is therefore no guarantee that the tumor samples are missing completely at random, potentially biasing the interpretation of the results bases on PD-L1 expression.(43). PACIFIC also left many questions unanswered (listed in Table 4) for individuals with stage III lung cancers.
Other trials have evaluated the administration of ICIs pembrolizumab, atezolizumab and nivolumab with the concurrent chemoradiation as outlined in Table 5. (35),(36) Investigators have theorized that cellular apoptosis from concurrent chemotherapy and radiation lead to increased tumor-derived neoantigens, increased antigen presentation and improved response to ICIs. Preclinical data suggests that PD-1 blockade delivered either concurrently with RT or just afterwards is superior to sequential PD-1blockade.(34) Both these studies have demonstrated that the approach is feasible, with no dramatic increase in the rate of treatment-related pneumonitis over chemoradiation alone as shown in Table 5. Whether this latter approach improves outcomes over the ICIs given after radiation requires further study. The benefit of adding ICI in patients treated with sequential chemotherapy followed by RT also remains to be determined and is currently under investigation in PACIFIC-6 (NCT NCT03693300).
Table 5.
Trials with immune checkpoint inhibitors (ICIs) during or after concurrent chemoradiation (CRT) in stage III lung cancers
| Author Trial Title | Antonia(22) PACIFIC | Antonia(22) PACIFIC | Durm(47) HCRN | Peters(48) ETOP NICHOLAS | Lin(49) DETERRED |
|---|---|---|---|---|---|
| Agent | Durvalumab | Placebo | Pembrolizumab | Nivolumab | Atezolizumab |
| Patients | 473 | 236 | 93 | 80 | 40 |
| Timing of ICI | After CRT | - - - | After CRT | During CRT | During CRT |
| 18 Month Progression Free Survival |
44% |
27% |
50% |
Not Reported | Not Reported |
| Any Pneumonitis | 34% | 25% | Not Reported | 43% | 25% |
| ≥Gr 3 Pneumonitis | 3% | 3% | 6% | 10% | 3% |
| Deaths on Study | 4% | 6% | 3% | 9% | 5% |
To make further progress, we need a better understanding of the biology of lung cancers to identify which patients will benefit from CRT-ICI combinations. One key issue to address is the timing of RT. When is RT is likely to have a detrimental immune-priming effect, preventing appropriate T-cell infiltration into the tissue microenvironment and instead promoting a suppressive immunophenotype? Perhaps in patients whose tumor biopsies suggest an already suppressive tissue microenvironment (low T-cell and high myeloid infiltrate), an alternative RT-ICI approach is needed where ICIs help to ‘reprogram’ immune effector cells to allow improved T-cell infiltration and response to ICIs (e.g. TLR and CD40 agonist antibodies).
To explore these issues, biomarker evaluations should be incorporated into future clinical trials. Minimal residual disease (MRD) assessed by circulating tumor DNA (ctDNA) can predict survival in patients undergoing curative treatment and has also been linked to improved response to ICIs. (44, 45) Additionally, while we know that tumor mutational burden correlates to response to ICIs, the ratio of clonal to subclonal mutations may be important for long term response. RT is capable of generating tumor antigens and immunogenic cell death, but recent data suggests that the generation of potential subclonal versus clonal mutations following RT needs careful study. (46)
Conclusions
The exciting data that has emerged clearly establishes a role for ICIs with surgery, radiation, and chemotherapy. In individuals where data supports the use of either surgical resection or concurrent chemoradiation, we find ourselves in a virtually “data free zone” with no direct comparisons between the two local approaches. In addition to the local therapy questions, we have only limited data to assist practitioners caring for patients with locally advanced lung cancers on the selection of specific chemotherapeutic agents, the selection of ICIs, and where surgery is the chosen modality, which treatments should be given adjuvantly or neoadjuvantly. Additional questions remain in after deciding between surgery or chemoradiation such as the extent of surgical resection (lobectomy or limited resection), radiation technique (IMRT vs protons), and radiation dose. Our best advice is to consider all approaches in a frank and open multimodality discussion that includes a careful assessment of individual patient attributes and preferences.
PRACTICAL APPLICATIONS.
Patients with locally advanced lung cancers (Stage III) can be cured by combining surgery, radiation, and systemic therapy in a multimodal plan of care.
Even with optimal timing and delivery of surgery, radiation, and chemotherapy, relapse can occur, primarily at distant sites. To cure more, we need better systemic therapies. Adding immune checkpoint inhibitors to multimodal regimens can fill that need.
For individuals with unresectable and inoperable stage III NSCLC that remain controlled after concurrent chemotherapy and irradiation, one year of durvalumab improves both progression-free and overall survival (57% at 3 years, a 13% improvement)
Neoadjuvant approaches offer many advantages, both for the care of individual patients and for research. Preoperative opportunities should be considered in patients where surgery and adjuvant therapy are appropriate based on stage.
In resectable stage IIIA NSCLCs (T4N0-1 or N2 “non-bulky” single station N2), the standard of care should include consideration of surgical resection after multidisciplinary review (CRT→S or C→S→±RT). Thoracic surgical oncologists are in the best position to determine operability and resectability. Based on research to date, it is likely that in the near future, immune checkpoint inhibitors will be added to multimodality regimens.
Acknowledgements -
Professor Corinne Faivre-Finn is supported by a grant from the NIHR Manchester Biomedical Research Centre. The research of Mark G. Kris, Jamie Chaft, and Jia Luo was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748
References
- 1.Rami-Porta R, Asamura H, Travis WD, Rusch VW. Lung cancer - major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA: a cancer journal for clinicians 2017. March;67(2):138–55. [DOI] [PubMed] [Google Scholar]
- 2.Chan A, Delaloge S, Holmes FA, Moy B, Iwata H, Harvey VJ, et al. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet Oncology 2016. March;17(3):367–77. [DOI] [PubMed] [Google Scholar]
- 3.Kris MG, Gaspar LE, Chaft JE, Kennedy EB, Azzoli CG, Ellis PM, et al. Adjuvant Systemic Therapy and Adjuvant Radiation Therapy for Stage I to IIIA Completely Resected Non-Small-Cell Lung Cancers: American Society of Clinical Oncology/Cancer Care Ontario Clinical Practice Guideline Update. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2017. September 1;35(25):2960–74. [DOI] [PubMed] [Google Scholar]
- 4.Ettinger DS, Wood DE, Aggarwal C, Aisner DL, Akerley W, Bauman JR, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 1.2020. J Natl Compr Canc Netw 2019. December;17(12):1464–72. [DOI] [PubMed] [Google Scholar]
- 5.Tsutani Y, Imai K, Ito H, Mimae T, Miyata Y, Ikeda N, et al. Adjuvant chemotherapy for pathological stage I non-small cell lung cancer with high-risk factors for recurrence: A multicenter study. Journal of clinical oncology : official journal of the American Society of Clinical OncologyASCO 2019;37(15_suppl):8500-. [Google Scholar]
- 6.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. The New England journal of medicine 2016. November 10;375(19):1823–33. [DOI] [PubMed] [Google Scholar]
- 7.Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. The New England journal of medicine 2018. May 31;378(22):2078–92. [DOI] [PubMed] [Google Scholar]
- 8.Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim SW, Carcereny Costa E, et al. Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. The New England journal of medicine 2019. November 21;381(21):2020–31. [DOI] [PubMed] [Google Scholar]
- 9.Garon EB, Hellmann MD, Rizvi NA, Carcereny E, Leighl NB, Ahn MJ, et al. Five-Year Overall Survival for Patients With Advanced NonSmall-Cell Lung Cancer Treated With Pembrolizumab: Results From the Phase I KEYNOTE-001 Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2019. October 1;37(28):2518–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forde PM, Chaft JE, Smith KN, Anagnostou V, Cottrell TR, Hellmann MD, et al. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. The New England journal of medicine 2018. May 24;378(21):1976–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shu CA, Gainor JF, Awad MM, Chiuzan C, Grigg CM, Pabani A, et al. Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small cell lung cancer: a single-arm, phase 2 trial. The Lancet Oncology 2020. (in press). [DOI] [PubMed] [Google Scholar]
- 12.Kwiatkowski DJ, Rusch VW, Chaft JE, Johnson BE, Nicholas A, Wistuba II, et al. Neoadjuvant atezolizumab in resectable non-small cell lung cancer (NSCLC): Interim analysis and biomarker data from a multicenter study (LCMC3). Journal of clinical oncology : official journal of the American Society of Clinical OncologyASCO 2019;37(15_suppl):8503-. [Google Scholar]
- 13.Cascone T, William WN, Weissferdt A, Lin HY, Leung CH, Carter BW, et al. Neoadjuvant nivolumab (N) or nivolumab plus ipilimumab (NI) for resectable non-small cell lung cancer (NSCLC): Clinical and correlative results from the NEOSTAR study. Journal of clinical oncology : official journal of the American Society of Clinical OncologyASCO 2019;37(15_suppl):8504-. [Google Scholar]
- 14.Provencio-Pulla M, Nadal-Alforja E, Cobo M, Insa A, Rivas MC, Majem M, et al. Neoadjuvant chemo/immunotherapy for the treatment of stages IIIA resectable non-small cell lung cancer (NSCLC): A phase II multicenter exploratory study—NADIM study-SLCG. Journal of clinical oncology : official journal of the American Society of Clinical OncologyWCLC 2018;36(15_suppl):8521-. [Google Scholar]
- 15.Ready N, Tong B, Clarke J, Gu L, Wigle D, Dragnev K, et al. P2.04-89 Neoadjuvant Pembrolizumab in Early Stage Non-Small Cell Lung Cancer (NSCLC): Toxicity, Efficacy, and Surgical Outcomes. Journal of Thoracic Oncology 2019;14(10):S745. [Google Scholar]
- 16.Hellmann MD, Chaft JE, William WN Jr., Rusch V, Pisters KM, Kalhor N, et al. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: proposal for the use of major pathological response as a surrogate endpoint. The Lancet Oncology 2014. January;15(1):e42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J, Blake SJ, Yong MC, Harjunpaa H, Ngiow SF, Takeda K, et al. Improved Efficacy of Neoadjuvant Compared to Adjuvant Immunotherapy to Eradicate Metastatic Disease. Cancer discovery 2016. December;6(12):1382–99. [DOI] [PubMed] [Google Scholar]
- 18.Topalian SL, Taube JM, Pardoll DM. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science (New York, NY) 2020. January 31;367(6477). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blumenthal GM, Bunn PA Jr., Chaft JE, McCoach CE, Perez EA, Scagliotti GV, et al. Current Status and Future Perspectives on Neoadjuvant Therapy in Lung Cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2018. December;13(12):1818–31. [DOI] [PubMed] [Google Scholar]
- 20.Majem M, Hernandez-Hernandez J, Hernando-Trancho F, Rodriguez de Dios N, Sotoca A, Trujillo-Reyes JC, et al. Multidisciplinary consensus statement on the clinical management of patients with stage III non-small cell lung cancer. Clinical & translational oncology : official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico 2020. January;22(1):21–36. [DOI] [PubMed] [Google Scholar]
- 21.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. The New England journal of medicine 2018. December 13;379(24):2342–50. [DOI] [PubMed] [Google Scholar]
- 22.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after Chemoradiotherapy in Stage III Non–Small-Cell Lung Cancer The New England journal of medicine 2017;377(20):1919–29. [DOI] [PubMed] [Google Scholar]
- 23.Rajaram R, Correa AM, Xu T, Nguyen Q-N, Antonoff MB, Rice D, et al. Locoregional Control, Overall Survival, and Disease-Free Survival in Stage IIIA (N2) Non–Small-Cell Lung Cancer: Analysis of Resected and Unresected Patients. Clinical Lung Cancer 2020. 2020/01/27/. [DOI] [PubMed] [Google Scholar]
- 24.Sharma P, Allison JP. The future of immune checkpoint therapy. Science (New York, NY) 2015. April 3;348(6230):56–61. [DOI] [PubMed] [Google Scholar]
- 25.Pataer A, Kalhor N, Correa AM, Raso MG, Erasmus JJ, Kim ES, et al. Histopathologic response criteria predict survival of patients with resected lung cancer after neoadjuvant chemotherapy. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2012. May;7(5):825–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.William WN Jr., Pataer A, Kalhor N, Correa AM, Rice DC, Wistuba II, et al. Computed tomography RECIST assessment of histopathologic response and prediction of survival in patients with resectable non-small-cell lung cancer after neoadjuvant chemotherapy. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2013. February;8(2):222–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ridker PM, MacFadyen JG, Thuren T, Everett BM, Libby P, Glynn RJ. Effect of interleukin-1beta inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet (London, England) 2017. October 21;390(10105):1833–42. [DOI] [PubMed] [Google Scholar]
- 28.Xu JM, Zhang Y, Jia R, Yue CY, Chang L, Liu R-R, et al. Anti-PD-1 Antibody SHR-1210 combined with Apatinib for Advanced Hepatocellular Carcinoma, Gastric or Esophagogastric Junction Cancer: An Open-label, Dose Escalation and Expansion Study. Clinical Cancer Research 2018:clincanres.2484.018. [DOI] [PubMed] [Google Scholar]
- 29.Chaudhuri AA, Chabon JJ, Lovejoy AF, Newman AM, Stehr H, Azad TD, et al. Early Detection of Molecular Residual Disease in Localized Lung Cancer by Circulating Tumor DNA Profiling. Cancer discovery 2017. December;7(12):1394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.von Minckwitz G, Huang CS, Mano MS, Loibl S, Mamounas EP, Untch M, et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. The New England journal of medicine 2019. February 14;380(7):617–28. [DOI] [PubMed] [Google Scholar]
- 31.Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, et al. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity 2014. November 20;41(5):843–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. The Journal of experimental medicine 2006. May 15;203(5):1259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta A, Probst HC, Vuong V, Landshammer A, Muth S, Yagita H, et al. Radiotherapy promotes tumor-specific effector CD8+ T cells via dendritic cell activation. Journal of immunology (Baltimore, Md : 1950) 2012. July 15;189(2):558–66. [DOI] [PubMed] [Google Scholar]
- 34.Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer research 2014. October 1;74(19):5458–68. [DOI] [PubMed] [Google Scholar]
- 35.Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015. April 16;520(7547):373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herter-Sprie GS, Koyama S, Korideck H, Hai J, Deng J, Li YY, et al. Synergy of radiotherapy and PD-1 blockade in Kras-mutant lung cancer. JCI insight 2016. June 16;1(9):e87415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gong X, Li X, Jiang T, Xie H, Zhu Z, Zhou F, et al. Combined Radiotherapy and Anti-PD-L1 Antibody Synergistically Enhances Antitumor Effect in Non-Small Cell Lung Cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2017. July;12(7):1085–97. [DOI] [PubMed] [Google Scholar]
- 38.Barker HE, Paget JT, Khan AA, Harrington KJ. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nature reviews Cancer 2015. July;15(7):409–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sampath S, Won H, Massarelli E, Li M, Frankel P, Vora N, et al. Combined modality radiation therapy promotes tolerogenic myeloid cell populations and STAT3-related gene expression in head and neck cancer patients. Oncotarget 2018. February 16;9(13):11279–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Auperin A, Le Pechoux C, Rolland E, Curran WJ, Furuse K, Fournel P, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2010. May 1;28(13):2181–90. [DOI] [PubMed] [Google Scholar]
- 41.Bradley JD, Paulus R, Komaki R, Masters G, Blumenschein G, Schild S, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. The Lancet Oncology 2015. February;16(2):187–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gray JE, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Three-Year Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC-Update from PACIFIC. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2019. October 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peters S, Dafni U, Boyer M, De Ruysscher D, Faivre-Finn C, Felip E, et al. Position of a panel of international lung cancer experts on the approval decision for use of durvalumab in stage III non-small-cell lung cancer (NSCLC) by the Committee for Medicinal Products for Human Use (CHMP). Annals of oncology : official journal of the European Society for Medical Oncology 2019. February 1;30(2):161–5. [DOI] [PubMed] [Google Scholar]
- 44.Moding EJ, Liu Y, Nabet BY, Chabon JJ, Chaudhuri AA, Hui AB, et al. Circulating tumor DNA dynamics predict benefit from consolidation immunotherapy in locally advanced non-small-cell lung cancer. Nature Cancer 2020. 2020/01/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hellmann MD, Nabet BY, Rizvi H, Chaudhuri AA, Wells DK, Dunphy MPS, et al. Circulating tumor DNA analysis to assess risk of progression after long-term response to PD-(L)1 blockade in NSCLC. Clinical Cancer Research 2020:clincanres.3418.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McGranahan N, Furness AJ, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science (New York, NY) 2016. March 25;351(6280):1463–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Durm GA, Jabbour S, Althouse SKL, Ziyue, Sadiq AA, Zon R, Jalal SI, et al. A Phase II Trial of Consolidation Pembrolizumab Following Concurrent Chemoradiation for Patients with Unresectable Stage III Non-Small Cell Lung Cancer (NSCLC): Hoosier Cancer Research Network (HCRN) LUN 14-179. 2020. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peters S, Felip E, Dafni U, Belka C, Guckenberger M, Irigoyen A, et al. Safety evaluation of nivolumab added concurrently to radiotherapy in a standard first line chemo-radiotherapy regimen in stage III non-small cell lung cancer-The ETOP NICOLAS trial. Lung cancer (Amsterdam, Netherlands) 2019. July;133:83–7. [DOI] [PubMed] [Google Scholar]
- 49.Lin SH, Lin Y, Yao L, Kalhor N, Carter BW, Altan M, et al. Phase II Trial of Concurrent Atezolizumab With Chemoradiation for Unresectable NSCLC. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2019. November 25. [DOI] [PubMed] [Google Scholar]


