Abstract
Background:
Today, the use of maggot therapy has become widespread due to the increase in chronic ulcers in the world. The recombinant production of secreted enzymes from these larvae is a novel non-invasive method for the treatment of chronic ulcers. Lucilia Sericata (L. sericata) collagenase (MMP-1) has been expressed in insect cells. Collagenase is an enzyme that is widely used in clinical therapy and industry. It has been indicated that collagenase is expressed and secreted in salivary glands of L. sericata while using for maggot debridement therapy.
Objectives:
In the present study we decided to produce the recombinant form of collagenase enzyme in Spodoptera frugiperda (SF9) insect cells using the baculovirus expression system (Bac-to-Bac).
Materials and Methods:
cloned the coding sequences (residues 494-1705) of L. sericata collagenase into the pFastBacHTA as donor plasmid. After transposition in the bacmid of DH10Bac host, the bacmid was transfected into the Sf9 cell line, then the expressed recombinant collagenase (MMP-1) was purified using the Ni-NTA agarose.
Results:
The recombinant protein was verified by Western blotting. Furthermore, the biological activity of purified protein was measured in the presence of its specific substrate and its inhibitor, which was 67 IU.mL-1 based on our results, it was revealed that the characterized gene in our previous study codes L. sericata collagenesa enzyme.
Conclusion:
Considering to the broad applications of collagenase in medical sciences, for the first time, we cloned the L. sericata collagenase (MMP-1) gene into the insect cell line to establish a method for the expression and purification of L. sericata collagenase (MMP-1). The result help for preparing and designing a safe and versatile recombinant drug in future.
Keywords: Collagenase, MMP-1, Lucilia Sericata, SF9 insect cell, Recombinant Protein
1. Background
Collagenase is a promising enzyme in view of their extensive industrial and biological applications ( 1 ). Today, maggot therapy, as an old method, is one of the most appropriate choices for treatment of chronic wounds and also the diseases induced subsequent to deposited collagen ( 2 ). Lucilia sericata (Meigen) is often used for maggot therapy ( 3 - 5 ). It has been confirmed that L. sericata’s salivary gland secretions play a main role in debridement and wound healing ( 6 - 7 ). Various studies have analyzed salivary gland proteome to find out its effect or the enzyms involved in wound healing process ( 7 - 10 ). Recently, the activity of five types of proteases in salivary gland secretions of L. sericata Meigen’s (Diptera: Calliphoridae) have been detected ( 11 ). Collagenase is one of these proteases belonging to matrix metalloproteinase (MMP) family. MMPs are a family of endopeptidases that have a
significant function in cellular matrix remodeling ( 12 ). Collagenases have directly been employed in clinical therapy such as debridement of burns, wound healing, Dupuytren’s and Peyronie’s diseases, sciatica, retained placenta, lumbar disc herniation, chronic total occlusions treatment, as well as in adenovirus-mediated cancer genetic therapy or electro-genetic therapy ( 1 , 13 ). Collagenases are used in various sectors such as food, tannery, fur, fish processing, brewing, meat processing, beer clarification and stabilization, medicine, cosmetics, fish silage, fish sauce, fish meal, animal feedstuffs processing, as well as in scientific and analytical research ( 14 , 15 ). The only approved collagenase in the market is related to Clostridium histolyticum ( 16 ). With respect to the benefits of maggot therapy and effectiveness of L. sericata collagenase during wound healing process, we decided to carry out molecular characterization, recombinant production, and enzymatic activity evaluation of L. sericata collagenase (MMP-1). In our previous study (unpublished), the coding sequence of L. sericata collagenase (MMP- 1) was determined. Therefore, regarding the broad applications of collagenase enzyme in medicine and its non-medical benefits, the recombinant production of L. sericata collagenase would improve different medical processes and contribute to the treatment of diseases. For instance, it has been estimated that the cost of wound care is about multibillion dollar in the world; in the United State, it affects about six million people each year with a cost about 20 billion dollars ( 17 ). The cost of wound care seems to be raised with the elevation of the number of non-healing surgical wounds due to the antibiotics resistance, ageing population, growing complexity of the surgeries, as well as increasing the accidents and hospital infections.
Removing the necrotic tissues of a target wound is one of the necessities for starting wound healing ( 18 ). Surgical methods or biological approaches, such as the use of collagenase, can be applied for debridement of the necrotic tissues. It has been demonstrated that using the first stage of larvae from L. sericata (green bottle fly), which secrets different types of proteolytic enzymes, is an effective method of wound debridement ( 19 ). Consequently, it seems that the combination of the L. sericata enzymes, which are involved in wound debridement, can be considered for designing and developing a new generation of debridement agents ( 20 ). Recently, microbial collagenase has been applied alone or in combination with other proteolytic enzymes for debridement in patients who are suffering from accumulation of collagen in their different organs. The first commercially useable collagenase was isolated from C. histolyticum in 195913. Due to the fact that L. sericata is an insect, we selected baculovirus expression system for recombinant production of collagenase and proper folding and post-translational modifications. Different expression vectors have been developed and improved for insect cell lines ( 21 ). The genomic structure of Baculoviridae order of insect cell viruses includes a large, double-stranded DNA (dsD), circular DNA and these viruses have been applied widely for recombinant protein expression in SF9, SF21 and High five insect cells ( 22 ). In most baculovirus expression vector systems, Autographa californica multiple nuclear polyhedrosis virus is used as the prototype baculovirus. Since the size of the AcMNPV genome is about 134 kb, a transposition is needed to put the target gene into the baculovirus genome ( 23 ). In practice, the target gene is cloned into the pFastBacHTA (as a transfer vector) to perform recombination with the baculovirus genome. Transfer vector includes an appropriate promoter that is joined to a non-basic location segment of the baculovirus genome such as the polyhedron gene of AcMNPV. This configuration of baculovirus DNA, which can be conserved and amplified in both Escherichia coli and insect cell lines, is nominated bacmid, a vector using for insect cell line transfection. Furthermore, manipulated bacmids have recently been demonstrated to possess other specifications such as the power to carry out site-specific transposition for quick insertion of the target gene based on Tn7. In addition, using the SV40 poly-adenylation signal to form a mini Tn7 and easy selection by adding the lacZ into the bacmid have been performed ( 24 ). Bacmid is used for insect cell line transfection and are usually transported by DH10Bac ( 25 ). Baculovirus expression system has different advantages, including high expression level based on the polyhedrin or p10 promoters, support of the post-translational modifications, ability in simultaneous expression of certain genes, no size limitation for the expressed proteins, and capability of producing the cytotoxic proteins ( 26 ).
Owing to the side effects of SantylTM and its pathogenic origin, the benefits of L. sericata collagenase and the advantages of baculovirus expression system, we decided to characterize molecularly and to express L. sericata collagenase for the evaluation of its potential power and its advantages in medical and non-medical applications.
2. Objectives
The aim of this study was to produce recombinant collagenase protein of L. sericata in SF9 insect cell as a Potential for Wound Healing.
3. Material and Methods
3.1. Primer Designing
For cloning and expression of the MMP-1, primers were designed by the Gene Runner version 4.0.9.68 and Oligo 7.0 softwares (Table 1). Non cutting Restriction enzymes were selected based on the CDS sequence of the L. sericata collagenase (MMP-1) (accession number KY612612) by Oligo7 software that it had been identified by authors previously ( 8 ).
Table 1.
The specific and universal primer sets used in this study
| Primer | Sequence |
|---|---|
| SpeF | 5’-CGGGATCCACCATGTCCCTGCTGACCGAGG-3’ |
| SphR | 5’-CCCAAGCTTAGCCATCGCTGCTGCCATT-3’ |
| pfastF | 5’-TAGTGGTTGGCTACGTATACTCC-3’ |
| pfastR | 5’-GCAAGTAAAACCTCTACAAATGTGGTA-3’ |
| pUC/M13F | 5’-CCCAGTCACGACGTTGTAAAACG-3’ |
| pUC/M13R | 5’-AGCGGATAACAATTTCACACAGG-3’ |
3.2. Polymerase Chain Reactions
The MMP-1 coding sequence was amplified from the recombinant plasmid (pTG19), which had been constructed in our previous study8 as a template. CDS was amplified by PCR using Pfu DNA polymerase (Thermo Fisher Scientific, USA). PCR was performed to amplify the coding sequence with specific cloning primers (SpeF and SphR) containing SpeI and SphI restriction sites at their 5’-ends (Table 1).
Amplification was performed with the FlexCycler PCR machine (Analyticjena, Germany) using the following program: initial denaturation at 94 °C for 5 min, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 58 °C for 30 s, and extension at 72 °C for 80 s, as well as a final extension at 72 °C for 10 min.
3.3. Gel Extraction
After PCR amplification, the amplicon was gel purified (GeneJET Gel Extraction Kit, Thermo Fisher Scientific USA), and the double digestion of the PCR product and pFastBacHTA plasmid was done according to the manufacture’s instruction (Fermentas, USA). Then the digested PCR product and vector were recovered from the agarose gel via a gel extraction kit (Vivantis, Malaysia).
3.4. Ligation of CDS L. sericata Collagen Gene and Linear pFastBacHTA
Subsequently, the linearized pFastBacHTA plasmid and the target gene (CDS of L. sericata collagenase) were ligated by using T4 DNA ligase (Invitrogen, Carlsbad, CA, USA) to generate the recombinant transfer vector (pFastBacHTA). In the next step, ligation product was transformed into DH5α competent cells (Invitrogen, USA) by a chemical method described before ( 27 ). The recombinant plasmid was isolated from DH5α using the QIAprep Spin Miniprep Kit (Qiagen, Germany) and subjected to PCR analysis by using plasmid specific primers to verify the insertion and correct orientation of the target gene within the pFastBac HTA vector based on frame of mentioned vector. The correct orientation and the presence of the cloned cassette were confirmed by PCR assay with the universal primers of pFastBacHTA sequencing (Table 1).
3.5. Transformation of pFastBac HTA to DH10Bac
The recombinant bacmids were generated according to the Bac-to-Bac manufacturer’s instruction (Invitrogen, USA). The pFastBacHTA recombinant plasmid was transformed into DH10Bac (Invitrogen, USA) competent cells containing the bacmid DNA and a helper plasmid encoding transposase enzyme. Transposase is necessary for generation of the recombinant bacmids through site-specific transposition (Invitrogen, USA) (Fig. 1). After the confirmation of transposition with M13-F and M13-R primers (Table 1), the recombinant bacmid was isolated according to the extraction of bacmid protocol based on molecular cloning book ( 28 ). The schematic map of vectors has been show in Figure 1. Finally, L. sericata collagenase gene was inserted in to the pFastBacHTA and then constructed vector transfected into the DH10Bac containing a helper plasmid to assist transposition of the recombinant Tn7 sequence into the bacmid DNA.
Figure 1.
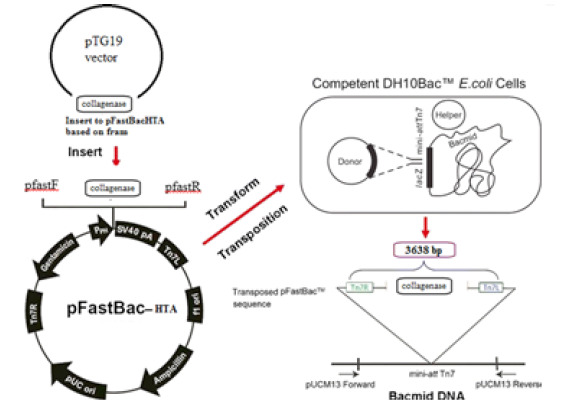
schematic of recombinant Bacmid DNA of L. sericata collagenase based on the mRNA coding sequence was synthesized by PCR amplification. L. sericata collagenase gene was inserted into the pFastBacHTA and then constructed vector transfected into the DH10Bac containing a helper plasmid to assist transposition of the recombinant Tn7 sequence into the bacmid DNA.
3.6. Cells and Transfection
Spodoptera frugiperda (Sf9) insect cells, donated by Dr. Behdani from Pasteur Institute of Iran, were cultured in a 6-well plate. Sf9 monolayer cells were transfected with the recombinant bacmids using the Cellfectin® II (Thermo Fisher Scientific USA), as the cationic lipid reagent, according to the manufacturer’s instruction. Transfection was performed with recombinant baculovirus bacmid with insert and without insert (as a negative control) simultaneously. The cells were then incubated at 27 °C for 3-5 hours in the Grace’s insect medium (Gibco, UK) supplemented with 10% (v/v) fetal bovine serum (Gibco, UK) and 1% pen/strep antibiotic (50 U.mL-1 penicillin and 100 g.mL-1 streptomycin; both from Sigma-Aldrich, USA). Briefly, it was initially verified that the Sf9 cells are in the log phase (1.5–2.5×106 cells.mL-1) with greater than 95% viability. Subsequently, 10 mL plating medium was prepared by mixing 1.5 mL supplemented Grace’s insect medium containing 10% FBS (without antibiotics) and 8.5 mL unsupplemented Grace’s insect medium (without FBS and antibiotics). Next, 8×105 Sf9 cells from the previous step were plated per well and allowed to attach at room temperature for 15 minutes. Medium was removed, and 2.5 mL plating medium from the step 1 was added per well. After that, for each transfection, 8 μL of Cellfectin® II (Thermo Fisher Scientific USA) was diluted in 100 μL unsupplemented Grace’s medium. This mixture was vortexed briefly, and 1–2 μg baculovirus DNA was added to the unsupplemented Grace’s medium. These gradients were mixed gently, and the diluted DNA was combined with diluted Cellfectin® II (total volume ~210 μL), mixed gently and incubated at room temperature for 15–30 minutes. In the next step, ~210 μL DNA-lipid mixture or transfection mixture, prepared in the previous step, was dropwised onto the cells, and cells were incubated at 27 °C for 3–5 hours. Subsequently, the transfection mixture was removed and replaced with 2 mL complete growth medium (e.g., Grace’s insect medium supplemented with 10% FBS and 1% pen/strep). Cells were then incubated at 27 °C for 72 hours or until the signs of viral infection were seen. Transfected cells were checked daily by the observation of the cytopathic effects, and after the observation of the transfection signs, the recombinant baculoviral particles were harvested from the cell culture lysate at 72 h post transfection. Then the harvested lysates was centrifuged at 12,000 ×g for 5 min, collected and stored at 4 °C. This is the P1 viral stock used in the next steps for large-scale expression. Furthermore, P2 and P3 viral stocks were produced according to the manufacture’s instruction (Invitrogen, USA). Generally, the large-scale expression is performed in a total volume of 150 mL (cells at a density of 2×106cells.mL-1), and infection is done with the high titer of viral stock at a multiplicity of infection of 10 pfu/cell. At the point of maximal expression, cells were harvested. To determine the solubility and insolubility, 1 ×106 of transfected cells were centrifuged as mentioned above. Cells were then resuspended with 50 μLPBS 1× and centrifuged again, and the pellet and supernatant were then analyzed separately by SDS-PAGE to select the proper purification method.
3.7. SDS-PAGE, Western Blotting Analysis, and Recombinant Protein Purification
The expression of recombinant L. sericata collagenase (MMP-1) under the transcriptional control of the polyhedrin promoter was evaluated using SDS-PAGE, followed by Western blotting analysis. Recombinant baculovirus-infected and No Template Control(NTC) Sf9 monolayer cells were harvested by centrifugation at 12,000 ×g for 5 min, and the cell pellets were lysed via freeze-thaw with liquid nitrogen for 3 to 4 times and then resuspended in 50 μL PBS ( 29 ). Next, cell lysate proteins (10-20 μg) from both groups of Sf9 cells were separated on 12% SDS-PAGE. After the confirmation of target gene expression based on its size, the presence of His-tag was evaluated by performing the Western blotting according to the Raz et al. method ( 30 ). After large-scale recombinant protein production, the cell lysate was subjected to purification. Recombinant protein was purified based on the provided protocol by Life Technologies according to the hybrid conditions procedure. For certain insoluble proteins, this protocol can be used to restore protein activity and to bind under denaturing conditions after cell lysis. After purification in hybrid condition, the amount of collagenase was measured by Bradford assay ( 31 ). Crystalline bovine serum albumin (Sigma-Aldrich, USA) was used as a standard for depicting the standard curve.
3.8. Evaluation of Biological Activity
The biological activity of the expressed L. sericata collagenase (MMP-1) was assessed using collagenase Activity Colorimetric Assay Kit (BioVision, Inc, Country, USA) according to the manufacturer’s instruction. The assay measures the degradation ability of collagenase enzyme on specific synthetic substrate. A synthetic peptide, 2-furanacryloyl-L-leucylglycyl-L-prolyl-L-alanine (FALGPA) that mimics the collagen structure was used to perform this biological assay. It is an standard assay for measuring the biological activity of collagenases enzyme ( 14 , 32 ). Furthermore, it can be used for screening/characterizing the collagenase inhibitors ( 33 ). The limit of detection for this assay is 0.02 mU of collagenase. The assay was performed according to the manufacturer’s instruction. In brief, 10 μL purified recombinant protein was added to the desired well(s) in a 96-well plate, and the volume was adjusted to 100 μL with collagenase assay buffer. For positive control, 10 μL provided collagenase (0.35 U.mL-1) was used. In addition, for inhibitor assay, 10 μL provided collagenase (0.35 U.mL-1) and 2 μL inhibitor (1, 10-Phenanthroline) were used. Finally, the absorbance of the tests was read at 345 nm, and the activity of the sample was determined according to that of the standard curve.
4. Results
4.1. PCR Amplification and Cloning
After transformation of ligation product of the digested pFastBacHTA and target gene with the 1212 bp amplicon (Fig. 2A) into DH5-α, clones were checked by colony PCR, and one of the positive clones was selected for the next steps. Colony PCR was performed using the pfastF and pfastR primers, and a 1622-bp amplicon confirmed positive clones (Fig. 2B). After transposition in DH10, different clones were evaluated by colony PCR. Colony PCR was performed with universal pUC/ M13 and gene-specific primers. An amplicon with the size of about 3638 bp confirmed the performance of the desired transposition (Figs. 2 and 3C). Finally, one of the positive clones was selected for bacmid extraction and transfection of SF9 cell line.
Figure 2.
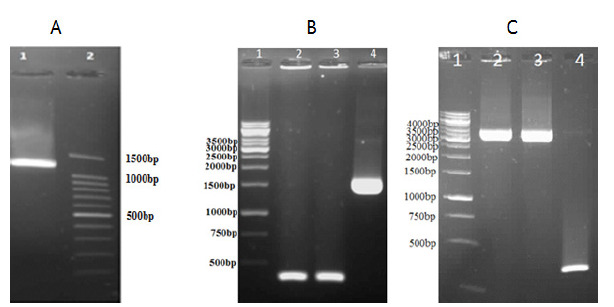
Polymerase chain reactions for ligation confirmation of the target gene into pFastBacHTA vector and transposition in DH10 (A) lane 1; The 1212-bp amplicon of the SpeF and SphR primers on the cloned cDNA of L. sericata collagenase (MMP-1) lane 2; DNA Ladder 100bp. (B); lane 2& 3, self-ligated clones; lane 3, Ligation confirmation of the target gene into the pFastBacHTA by pfastF and pfastR primers with a 1622-bp amplicon(C) Lane 1, 1-kb DNA ladder, lane 2 & 3, to the amplicon with 3662-bp length confirms the performance of correct transposition; lane 4 no-transposition(300bp).
Figure 3.
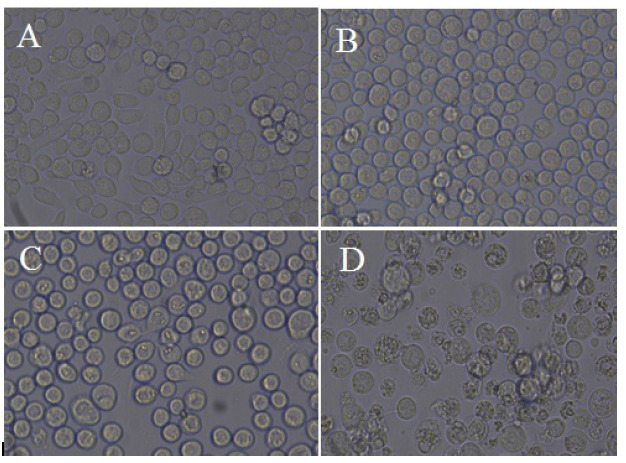
Transfection of Sf9 cell line. Control cells (A), cells transfected with bacmid after 24 hours (B), after 48 hours (C), and after 72 hours (D) (magnification ×40)
4.2. Production of Stable Insect Cells that Express L. sericata Collagenase
Following the transfection of Sf9 cells with 1 μg bacmid plasmid, clones were continuously expanded to the wells of 6-well cell culture plates. Primary assessment of concentrated lysate of the cells using SDS-PAGE and Western blotting confirmed their ability to express the L. sericata collagenas (Fig. 4). Finally, P2 viral stock was subjected to high-yield expression of the recombinant protein in a large scale. Total infected cells were 500 million.
Figure 4.
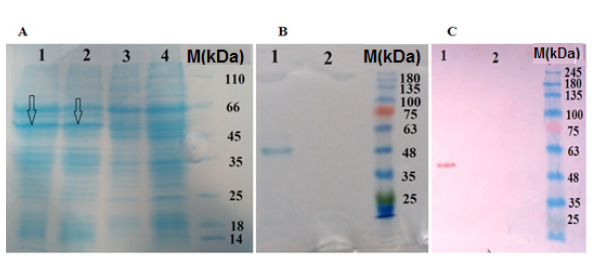
SDS-PAGE and Western blotting assays of L. sericata collagenase (MMP-1). (A) SDS-PAGE analysis of recombinant collagenase (MMP-1) in the cell lysate. Lane 1, 48 h after transfection ( The arrows show the target protein); lane 2, 72 h after transfection; lane 3, bacmid control (without target gene); lane 4, normal cells (B) SDS-PAGE analysis of the purified recombinant collagenase (MMP-1) Lane 1, elution 1; lane 2, NTC. (C) Western blotting of the purified recombinant collagenase (MMP-1). Lane 1, elution 1; lane 2, NTC (cell lysate of the transfected cell with bacmid-without target gene)
4.3. Expression of Collagenase
Expression of collagenase (MMP-1) was carried out by previously authenticated stable lines of Sf9 cells in T25 and T75 cell culture flasks. Western blotting analysis of the cell lysate confirmed the expression of the collagenase protein by revealing a band of about 52 kDa (Fig. 5). The concentration of L. sericata collagenase (MMP-1), which was produced by the insect cells, was determined by Bradford assay, and our result revealed that its concentration is 68.5 μg.mL.
Figure 5.
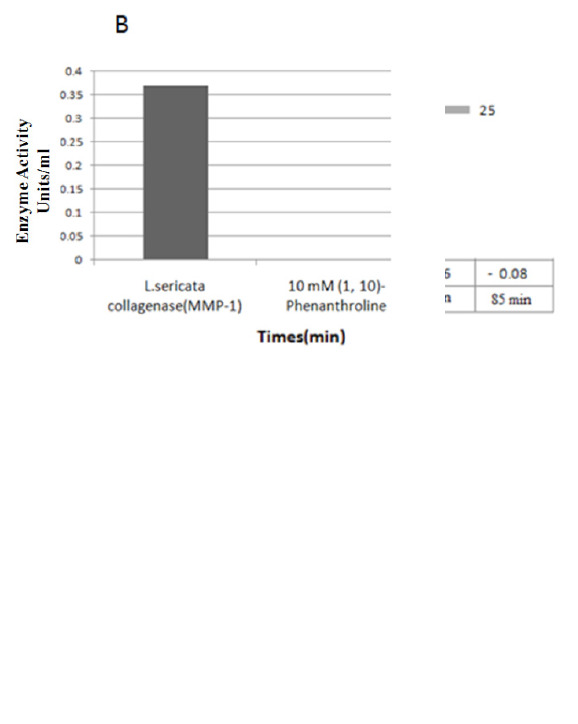
L. sericata collagenase (MMP-1) biological activity assay. (A) Evaluation of the biological activity of L .sericata collagenase in different time frames. The result revealed that by increasing the time, the biological activity of the enzyme was raised, and its activity was started after one hour. (B) Evaluation the efficacy of 1,10-phenanthroline on biological activity of L. sericata collagenase (MMP-1)
4.4 . Purification of the Recombinant L. sericata Collagenase (MMP-1)
In vitro expression of L. sericata collagenase (MMP-1) in SF9 cells resulted in the production of a recombinant protein with a molecular weight of about 52 kDa (without signal peptide) (Fig. 4). Bradford assay revealed purified collagenase of 68.5 μg.mL-1.
4.5. Assessment of Biological Activity
Following the preparation of samples according to the manufacture’s instruction, the optical density (OD) of the tests was measured at 345 nm wave length at 37 °C for 5-15 min. Furthermore, the biological activity was monitored in different time frames till 60 min, but no change was observed in the OD of the samples. After 1 hour, the enzyme activity was started, which can be related to the test situation. Data comparison showed that the OD of the test collagenase (positive control) and recombinant L. sericata collagenase (MMP-1) at 345 nm was decreased by increasing the time (Fig. 5). The enzyme activity of recombinant collagenase (MMP-1) was measured, and the result revealed that it is equal to 3.7 unit.mL-1 according to the formula introduced in the biovision kit protocol (Fig. 5B). Collagenase-specific activity against synthetic collagen (FALGPA) at 0, 60, 65, 70, 75, 80, and 85 min were 0.0, -0.01, -.0.02, -0.04, -0.06 and -0.08 unit.mg-1, respectively (Fig. 5A). In addition, the effect of the general zinc protease inhibitor, 1, 10-phenanthroline, was determined on L. sericata collagenase MMP-1 activity, and our results showed that the biological activity of our recombinant enzyme is blocked by its specific inhibitor (Fig. 5B).
The result indicated that the biological activity of L. sericata collagenase in the presence of 10 mM of 1, 10-phenanthroline, as its specific inhibitor, was blocked completely.
5. Conclusions
Conclusions
In recent years, collagenase has been used in invasive therapeutical methods in medical sciences; therefore, the production of its recombinant protein is very important and can be a help to treatment of diseases resulted from the accumulation of collagen in tissue ( 1 ). The recombinant production of secreted enzymes from L.sericata larvae is very important due to non-pathogenesis as a novel method in the treatment of chronic ulcers. In the present study, for the first time, we cloned the L. sericata collagenase (MMP-1) gene into the insect cell line (SF9) to establish a method for the expression and purification of L. sericata collagenase (MMP-1). The expression of collagenase by the E. coli expression system has been reported by various research groups ( 34 , 35 ). In 1996, the colH gene collagenase from C. histolyticum was cloned into an E. coli-Bacillus subtilis shuttle vector to develop a method for purification of recombinant collagenase ( 36 ). Purification of collagenase has been complicated due to the presence of multiple forms of collagenase and other proteases with similar physical and chemical characteristics ( 37 ). The purified form of collagenase is required to study the biochemical properties, enzyme structure, catalytic mechanism, structure-function relationships, and their biotechnological and medical applications ( 38 ). These findings could provide a deeper insight into the function and mechanism of bacterial collagenases that are used in medical and biotechnological applications ( 35 ). Because of disadvantages of the prokaryotic expression system and the lack of report on the collagenase expression of C. histolitycom by the baculovirus expression system, in the present study, we have evaluated the expression of the recombinant collagenase by a Bac-to-Ba insect cell expression system. This system takes the advantage of a strong immediate-early baculovirus promoter, which is activated independent of viral infection. In addition, the expression of the recombinant protein could be obtained continuously following the insertion of the coding sequence into the genomic DNA of the host cells. Expression of L. sericata collagenase (MMP- 1) was finally confirmed by revealing a band of about 52 kDa in Western blot analysis. Theoretically, the predicted size of the expressed protein was 45.1 kDa; however, the presence of the N-terminal 6× His-tag and the recognition site for the AcTEV™ protease will increase the size of protein by at least 3 kDa. It seems that this increase in molecular weight is due to the occurrence of post-translational modification; insect cells perform almost all post-translational modifications performed by mammalian cells ( 39 ). This kind of weight variations have also been reported by other researchers for various proteins expressed by the baculovirus-insect cell expression systems ( 40 - 44 ).
The biological activity of the preliminary expressed L. sericata collagenase (MMP-1) protein was measured and revealed to be almost similar to that of the standard of the collagenase assay (Biovision) kit. This result shows that the expressed collagenage (MMP- 1) successfully underwent a proper post-translational modification and structural folding. George et al (45) have reported the expression of human collagenase in insect cell. Yihyung and co-workers ( 46 ) have successfully expressed the full-size MT1-MMP (fMT1- MMP) and a transmembrane -domain-deleted soluble MT1-MMP (sMT1-MMP) in the Sf9. These results confirm that L. sericata collagenase MMP-1 encodes an active enzyme, which are consistent with other collagenase reports ( 1 , 47 , 48 ).
FALGPA is clearly a more specific substrate for collagenase because FALGPA is not able to cleave any other proteinases tested ( 35 ). The recombinant protein has been shown to have good proteolytic activity with a FALGPA substrate and inhibition profile characteristic of collagenase. Most of the collagenase assays are time-consuming, which takes at least 3-18 h to show their effectiveness ( 49 ). If the these assays conducted at a temperature higher than 37 oC, a temperature above denaturation temperature of collagen, result would not be desirable ( 13 ).
At high temperature, collagen is hydrolyzed, and collagen hydrolysate or gelatin is certainly occurred. Therefore, various synthetic peptides such as 4 phenylazobenzyloxycarbonyl-L-Pro-L-Leu-Gly-L-Pro-D-Arg (Pz-peptide), FALGPA, and Azo dye-impregnated collagen are required to be developed as substrates for the efficient quantification of collagenase activity ( 50 - 52 ). L. sericata larvae, at the time of exposure to the infected wounds, secretes collagenase protein that has a critical role in wound healing. Furthermore, production and development of this collagenase, which is obtained from a useful and non-pathogenic organism, can be an appropriate alternative to a similar drug that is produced from a pathogenic organism. L. sericata is reared in insectarium of school of health ( 53 , 54 ), Shiraz University of medical sciences.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- 1.Alipour H, Raz A, Zakeri S, Djadid ND. Therapeutic applications of collagenase (metalloproteases): A review. Asian Pac J Trop Biomed. 2016;6(11):975–981. doi: 10.1016/j.apjtb.2016.07.017. [DOI] [Google Scholar]
- 2.Minniti CP, Kato GJ. Critical Reviews: How we treat sickle cell patients with leg ulcers. AM J HEMATOL. 2016;91(1):22–30. doi: 10.1002/ajh.24134. [DOI] [PubMed] [Google Scholar]
- 3.Rafinejad J, Akbarzadeh K, Rassi Y, Nozari J, Sedaghat MM, Hosseini M, Alipour H, et al. Traumatic myiasis agents in Iran with introducing of new dominant species, Wohlfahrtia magnifica (Diptera: Sarcophagidae) Asian Pac J Trop Biomed. 2014;4(6):451–455. doi: 10.12980/apjtb.4.2014c1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akbarzadeh K, Rafinejad J, Alipour H, Biglarian A. Human myiasis in Fars province, Iran. SE ASIAN J TROP MED. 2012;43(5):1205. [PubMed] [Google Scholar]
- 5.Pickles S, Pritchard D. Quality control of a medicinal larval (Lucilia sericata) debridement device based on released gelatinase activity. MED VET ENTOMOL. 2017;31(2):200–206. doi: 10.1111/mve.12220. [DOI] [PubMed] [Google Scholar]
- 6.Alipour H, Raz A, Djadid ND, Zakeri S. Lucilia sericata collagenase: Google Patents; 2017 [Google Scholar]
- 7.Pöppel A-K, Kahl M, Baumann A, et al. A Jonah-like chymotrypsin from the therapeutic maggot Lucilia sericata plays a role in wound debridement and coagulation. Insect Biochem, Mol. 2016;70:138–147. doi: 10.1016/j.ibmb.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Alipour H, Raz A, Zakeri S, Djadid ND. Molecular characterization of matrix metalloproteinase-1 (MMP-1) in Lucilia sericata larvae for potential therapeutic applications. ELECTRON J BIOTECHN. 2017;29:47–56. doi: 10.1016/j.ejbt.2017.06.007. [DOI] [Google Scholar]
- 9.Franta Z, Vogel H, Lehmann R, Rupp O, Goesmann A, Vilcinskas A. Next Generation Sequencing Identifies Five Major Classes of Potentially Therapeutic Enzymes Secreted by Lucilia sericata Medical Maggots. BioMed research international. 2016:27. doi: 10.1155/2016/8285428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ratcliffe NA, Mello CB, Garcia ES, Butt TM, Azambuja P. Insect natural products and processes: new treatments for human disease. Insect Biochem. Mol. 2011;41(10):747–769. doi: 10.1016/j.ibmb.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Valachova I, Majtan T, Takac P, Majtan J. Identification and characterisation of different proteases in Lucilia sericata medicinal maggots involved in maggot debridement therapy. J Appl Biomed. 2014;12(3):171–177. doi: 10.1016/j.jab.2014.01.001. [DOI] [Google Scholar]
- 12.Chou J, Chan MF, Werb Z. Metalloproteinases: A functional pathway for myeloid cells. Microbiol Spectr. 2016;4(2) doi: 10.1128/microbiolspec.mchd-0002-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duarte AS, Correia A, Esteves AC. Bacterial collagenases–a review. Crit. 2016;42(1):106–126. doi: 10.3109/1040841x.2014.904270. [DOI] [PubMed] [Google Scholar]
- 14.Pal GK, Suresh P. Microbial collagenases: challenges and prospects in production and potential applications in food and nutrition. RSC Adv. 2016;6(40):33763–33780. doi: 10.1039/c5ra23316j. [DOI] [Google Scholar]
- 15.Preet Kaur S, Azmi W. Cost Effective Production of a Novel Collagenase from a Non-Pathogenic Isolate Bacillus tequilensis. Curr. Biotechnol. 2013;2(1):17–22. doi: 10.2174/2211550111302010004. [DOI] [Google Scholar]
- 16.Desai SS, Hentz VR. Collagenase clostridium histolyticum for Dupuytren’s contracture. Expert Opin Biol Ther. 2010;10(9):1395–1404. doi: 10.1517/14712598.2010.510509. [DOI] [PubMed] [Google Scholar]
- 17.Frykberg RG, Banks J. Challenges in the treatment of chronic wounds. ADV WOUND CARE. 2015;4(9):560–582. doi: 10.1089/wound.2015.0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pugliese DJ. Infection in venous leg ulcers: considerations for optimal management in the elderly. Drugs & aging. 2016;33(2):87–96. doi: 10.1007/s40266-016-0343-8. [DOI] [PubMed] [Google Scholar]
- 19.Wilson M, Nigam Y, Jung W, Knight J, Pritchard D. The impacts of larval density and protease inhibition on feeding in medicinal larvae of the greenbottle fly Lucilia sericata. MED VET ENTOMOL. 2016;30(1):1–7. doi: 10.1111/mve.12138. [DOI] [PubMed] [Google Scholar]
- 20.Han G, Ceilley R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv Ther. 2017:1–12. doi: 10.1007/s12325-017-0478-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lynn DE, Harrison RL. Available lepidopteran insect cell lines. Baculovirus and Insect Cell Expression Protocols. 2016:119–142. doi: 10.1007/978-1-4939-3043-2_6. [DOI] [PubMed] [Google Scholar]
- 22.Crook NE. Baculoviridae: subgroup B: comparative aspects of granulosis viruses. Virus of Invertebrates: Routledge . 2017:73–110. doi: 10.1201/9780203734322-3. [DOI] [Google Scholar]
- 23.Martínez-Solís M, Gómez-Sebastián S, Escribano JM, Jakubowska AK, Herrero S. A novel baculovirus-derived promoter with high activity in the baculovirus expression system. PeerJ. 2016;4:e2183. doi: 10.7287/peerj.preprints.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yazdani Y, Azari S, Kalhor HR. Expression of functional recombinant human tissue transglutaminase (TG2) using the bac-to-bac baculovirus expression system. Adv Pharm Bull. 2016;6(1):49. doi: 10.15171/apb.2016.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu X, Wei Y, Li Y, et al. A Highly Efficient and Simple Construction Strategy for Producing Recombinant Baculovirus Bombyx mori Nucleopolyhedrovirus. PLoS One. 2016;11(3):e0152140. doi: 10.1371/journal.pone.0152140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harrison RL, Jarvis DL. Transforming Lepidopteran Insect Cells for Improved Protein Processing and Expression. Baculovirus and Insect Cell Expression Protocols. 2016 :359–379. doi: 10.1007/978-1-4939-3043-2_18. [DOI] [PubMed] [Google Scholar]
- 27.Kumar B, Ghosh S. Laboratory Scale Production of Recombinant Haa86 Tick Protein in Pichia pastoris and in Escherichia coli System. Vaccine Design: Methods and Protocols, Volume 2: Vaccines for Veterinary Diseases. 2016:459–482. doi: 10.1007/978-1-4939-3389-1_30. [DOI] [PubMed] [Google Scholar]
- 28.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual: Cold spring harbor laboratory press; 1989. doi: 10.1002/biuz.19900200607. [DOI] [Google Scholar]
- 29.Ogiue-Ikeda M, Machida K. Expression and Purification of SH2 Domains Using Baculovirus Expression System. SH2 Domains: Methods and Protocols. 2017:183–198. doi: 10.1007/978-1-4939-6762-9_11. [DOI] [PubMed] [Google Scholar]
- 30.Raz A, Djadid ND, Zakeri S. Molecular characterization of the carboxypeptidase B1 of Anopheles stephensi and its evaluation as a target for transmission-blocking vaccines. Infec. immun. 2013;81(6):2206–2216. doi: 10.1128/iai.01331-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng Y, Wei H, Sun R, Tian Z, Zheng X. Rapid method for protein quantitation by Bradford assay after elimination of the interference of polysorbate 80. Analytical biochemistry. 2016;494:37–39. doi: 10.1016/j.ab.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 32.Bond MD, Van Wart HE. Purification and separation of individual collagenases of Clostridium histolyticum using red dye ligand chromatography. Biochemistry. 1984;23(13):3077– 3085. doi: 10.1021/bi00308a035. [DOI] [PubMed] [Google Scholar]
- 33.Safandowska M, Pietrucha K. Effect of fish collagen modification on its thermal and rheological properties. Int J Biol Macromol. 2013;53:32–37. doi: 10.1016/j.ijbiomac.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 34.Freije JM, Diez-Itza I, Balbín M, et al. Molecular cloning and expression of collagenase-3, a novel human matrix metalloproteinase produced by breast carcinomas. J BIOL CHEM. 1994;269(24):16766–16773. doi: 10.1677/joe.0.1490405. [DOI] [PubMed] [Google Scholar]
- 35.Abfalter CM, Schönauer E, Ponnuraj K, et al. Cloning, Purification and Characterization of the Collagenase ColA Expressed by Bacillus cereus ATCC 14579. PLoS One. 2016;11(9):e0162433. doi: 10.1371/journal.pone.0162433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jung C-M, Matsushita O, Katayama S, Minami J, Ohhira I, Okabe A. Expression of the colH gene encoding Clostridium histolyticum collagenase in Bacillus subtilis and its application to enzyme purification. MICROBIOL IMMUNOL. 1996;40(12):923–929. doi: 10.1111/j.1348-0421.1996.tb01161.x. [DOI] [PubMed] [Google Scholar]
- 37.Matsushita O, Yoshihara K, Katayama S, Minami J, Okabe A. Purification and characterization of Clostridium perfringens 120-kilodalton collagenase and nucleotide sequence of the corresponding gene. J BACTERIOL. 1994;176(1):149–156. doi: 10.1128/jb.176.1.149-156.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thadathil N, Velappan SP. Recent developments in chitosanase research and its biotechnological applications: A review. Food Chem. 2014;150:392–399. doi: 10.1016/j.foodchem.2013.10.083. [DOI] [PubMed] [Google Scholar]
- 39.Altmann F, Staudacher E, Wilson IB, März L. Insect cells as hosts for the expression of recombinant glycoproteins. Glycotechnology: Springer. 1999:29–43. doi: 10.1007/978-1-4615-5257-4_3. [DOI] [PubMed] [Google Scholar]
- 40.Gouveia R, Kandzia S, Conradt HS, Costa J. Production and N-glycosylation of recombinant human cell adhesion molecule L1 from insect cells using the stable expression system. Effect of dimethyl sulfoxide. J Biotechnol. 2010;145(2):130–138. doi: 10.1016/j.jbiotec.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 41.Jahanian-Najafabadi A, Bouzari S, Oloomi M, Habibi Roudkenar M, Shokrgozar M. Assessment of selective toxicity of insect cell expressed recombinant A1-GMCSF protein toward GMCSF receptor bearing tumor cells. RPS. 2012;7(3):133–140. doi: 10.1271/bbb.110862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mohseni N, Jahanian-Najafabadi A, Kazemi-Lomedasht F, et al. Recombinant expression and purification of functional vascular endothelial growth factor-121 in the baculovirus expression system. Asian Pac J Trop Biomed. 2016;9(12):1195–1199. doi: 10.1016/j.apjtm.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 43.Shokrollahi N, Shahbazzadeh D, Pooshang-Bagheri K, Habibi- Anbouhi M, Jahanian-Najafabadi A, Behdani M. A model to study the phenotypic changes of insect cell transfection by copepod super green fluorescent protein (cop-GFP) in baculovirus expression system. Iran. Biomed J. 2016;20(3):182. doi: 10.1016/j.apjtm.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kazemi-Lomedasht F, Behdani M, Bagheri KP, et al. Expression and purification of functional human vascular endothelial growth factor-a121; the most important angiogenesis factor. Adv. Pharm Bull. 2014;4(4):323. doi: 10.1016/j.apjtm.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.George HJ, Marchand P, Murphy K, et al. Recombinant human 92-kDa type IV collagenase/gelatinase from baculovirus-infected insect cells: expression, purification, and characterization. PROTEIN EXPRES PURIF. 1997;10(1):154–161. doi: 10.1006/prep.1997.0725. [DOI] [PubMed] [Google Scholar]
- 46.Yihyung J, Jungheum Y, Hwa-Jung K, Seung-Taek L. Analysis of tissue inhibitor of metalloproteinases-2 effect on pro-matrix metalloproteinase-2 activation by membrane-type 1 matrix metalloproteinase using baculovirus/insect-cell expression system. BIOCHEM J. 2000;345(3):511–519. doi: 10.1042/bj3450511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuliopulos A, Koukos G. PAR-1 activation by metalloproteinase-1 (MMP-1): Google Patents; 2016 [Google Scholar]
- 48.He X, Dai J, Fan Y, Zhang C, Zhao X. Regulation function of MMP-1 downregulated by siRNA on migration of heat-denatured dermal fibroblasts. Bioengineered. 2017:1–7. doi: 10.1080/21655979.2016.1267885. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Zhang Y, Fu Y, Zhou S, Kang L, Li C. A straightforward ninhydrin-based method for collagenase activity and inhibitor screening of collagenase using spectrophotometry. Anal. Biochem. 2013;437(1):46–48. doi: 10.1016/j.ab.2013.02.030. [DOI] [PubMed] [Google Scholar]
- 50.Lima CA, Campos JF, Lima Filho JL, Converti A, da Cunha MGC, Porto AL. Antimicrobial and radical scavenging properties of bovine collagen hydrolysates produced by Penicillium aurantiogriseum URM 4622 collagenase. INT J FOOD SCI TECH. 2015;52(7):4459–4466. doi: 10.1007/s13197-014-1463-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Petrova DH, Shishkov SA, Vlahov SS. Novel thermostable serine collagenase from Thermoactinomyces sp.21E: purification and some properties. J Basic Microbiol . 2006;46(4):275–285. doi: 10.1002/jobm.200510063. [DOI] [PubMed] [Google Scholar]
- 52.Lima CA, Júnior ACF, Lima Filho JL, et al. Two-phase partitioning and partial characterization of a collagenase from Penicillium aurantiogriseum URM4622: Application to collagen hydrolysis. Biochem Eng J. 2013;75:64–71. doi: 10.1016/j.bej.2013.03.012. [DOI] [Google Scholar]
- 53.Alipour H, Shahriari-Namadi M, Raz A, Moemenbellah- Fard MD. Cold-Preservation of Lucilia sericata (DIPTERA: CALLIPHORIDAE) pupae and adult produvtion as a new venture to adult rearing . JEBAS. 2018;6(3):544–549. doi: 10.18006/2018.6(3).544.549. [DOI] [Google Scholar]
- 54.Saleh V, Soltani A, Dabaghmanesh T, Alipour H, Azizi K, Moemenbellah-Fard MD. Mass Rearing and Life Table Attributes of Two Cyclorrhaphan Flies, Lucilia sericata Meigen (Diptera: Calliphoridae) and Musca domestica (Diptera: Muscidae) under Laboratory Conditions. J Entomol. 2014;11 (5): 291–298. doi: 10.3923/je.2014.291.298. [DOI] [Google Scholar]


