Abstract
Background:
Quality of bread baking is affected by gluten genes and balance between their expressions. Hence, it is necessary for a comprehensive research to study and compare all gluten genes and their regulating elements simultaneously.
Objectives:
The aim of this study was to evaluate the molecular mechanism of bread quality at the level of coding genes and regulating elements via comparative transcriptome analysis of two extreme wheat cultivars.
Materials and Methods:
RNAs were extracted from the grain of two wheat cultivars with high (Pishtaz) and low (Navid) bread making qualities, collected during endosperm development at five stages. mRNAs were sequenced and gluten transcripts were assessed to find differentially expressed genes. Then, transcription factors interacting with gluten genes were detected and evaluated for expression.
Results:
Results showed that Ɣ-gliadin and LMW-GS genes had a higher expression in Pishtaz and Navid, respectively. Most identified transcription factors were active at the early stage of growth and it seemed that NAC and ERF transcription factors had significant roles in regulating genes with different expressions. There was no significant difference in the expression level of NACs between two cultivars. It is proposed that the ERF transcription factor which classified as BREB2C transcription factor could control the expression of LMW-GS genes in two cultivars and functionally act as a repressor for their target genes.
Conclusion:
The priority of Pishtaz wheat cultivar in bread quality originated from high expression levels of Ɣ-gliadin gene and ERF transcription factor.
Keywords: DREB2C, ERF, Gliadin, Glutenin, Triticum aestivum
1. Background
Wheat (Triticum aestivum L.) is one of the three most important crops ( 1 ), which is widely consumed as the major source of carbohydrates for its dough’s typical viscoelastic feature, which, is utilized in various baking products ( 2 ). The existence of gluten proteins makes wheat flour unique among other crops; therefore, the quality of these dough forming proteins with acceptable properties is important for researchers with respect to various aspects ( 3 ).
Glutens, as the main group of wheat storage proteins, are divided into two groups, gliadins and glutenins. Gliadins are generally in monomeric forms and include α, γ- and ω-gliadins, while glutenins are the polymeric fraction of gluten and are divided into high molecular weight glutenin (HMW-GS) and low molecular weight glutenin subunits (LMW-GS) ( 4 ). Although protein’s quality is a complicated trait to characterize and quantify, dough strength and elasticity are assumed to be affected by viscosity and extensibility produced by gliadins and glutenins ( 5 ). Gliadins are encoded by a considerable number of genes such as Gli-A1, Gli-B1, Gli-D1, Gli-A2, Gli-B2, and Gli-D2, which are located on the short arms of chromosome groups 1 and 6 ( 6 , 7 ).HMW-GS are encoded by the Glu-1 loci. Each of these genes can code two subunits named x- and y-type with different molecular weights. LMW-GS is encoded by the greater number of genes as follows: Glu-A3, Glu-B3, and Glu-D3 (on the short arms of group 1 chromosomes) and loci Glu-2, Glu-4 and Glu-5 located on chromosomes 1B, 1D, and 7D, respectively ( 8 ). There are a large number of genes encoding gliadins and glutenins and thus it is necessary to understand which genes have high/low expression level or have substantial effects on the bread making quality. Comparison of two contrasting wheat cultivars provides us with an overview of the candidate genes with high effectiveness on the bread quality. As well, it makes the role of gluten genes on the bread quality clearer by reducing the number of putative genes. Focusing on the major transcription factors that have major effects on gluten gene expressions and studying them in detail will reduce the number of candidate genes and lead to more definitive results.
The main obstacle in the studying bread making quality in addition to the complexity of the wheat genome is that it is a quantitative trait and influenced by a large number of genes. There are so many reports on the bread making quality particularly with respect to the proteome aspect of quality, but the major question about its genetic control affecting the quality (such as regulatory element, the most important grain development stage and the major differences in gluten gene structures) remains unanswered. In establishing regulatory networks for controlling flour quality, one of the main challenges is the study of direct/indirect interaction between transcription factors (TFs) and cis-motifs of storage protein genes ( 9 - 14 ). The emergence of high-throughput sequencing such as RNA-Seq could represent a substantial improvement in the study on qualitative traits as they considerably increase the possibility for the simultaneous investigation of the myriad of genes.
2. Objectives
The purpose of the present study was to identify the mechanism that determines gluten content and quality of bread wheat by comparative transcriptome analysis of two extreme bread wheat cultivars in quality (high and low bread making qualities) via RNA-Seq method.
3. Materials and Methods
3.1. Plant Materials
Pishtaz (high quality) and Navid (low quality), two extreme Iranian wheat cultivars in the bread making quality, were selected based on previous studies. The cultivars were cultivated in three replications, and then spikes were tagged at anthesis stage, followed by collection of grain samples at 5, 10, 14, 21 and 28 day post anthesis (DPA) from middle-third of each spike. RNA of ten grains of each spike was isolated by TRIzol Reagent (Invitrogen) and the extracted RNAs of 10 spikes were pooled to obtain the final RNA sample for each stage ( 15 ). The quality of extracted RNAs was controlled by checking the existence of standard banding pattern for RNA on the agarose gels and RIN number, measuring the RNA concentration, and A260/230 and A260/280 ratios by NanoDrop spectrophotometer. RNA sequencing was conducted as paired-end with 150nt length using Illumina HiSeq 2500.
3.2. Expression Analysis of Gluten Genes
Quality control of sequenced reads was performed by FastQC software (V0.11.5). Then the parameters that reduced the quality were trimmed by Trimmomatic software (V0.32). After each trimming, the quality of the trimmed reads was checked again by FastQC software and the trimmed sequence with higher quality was selected. Sequenced reads were aligned against T. aestivum assembly (TGACv1) with Tophat2 program ( 16 ). Differential gene expression analyses were carried out by Cufflinks ( 17 ) and CuffDiff2 ( 18 ) packages. An adjusted p-value ≤0.0001 was used to find the differentially expressed genes (DEGs) and DEGs with log2fold change ≥ 3 were selected for downstream analysis.
3.3. Gluten Transcripts Identification
To detect gluten genes, DEGs of all stages were aligned against gluten protein sequences using BLASTX (ncbi-blast-2.7.1) ( 19 ). Aligned wheat genes were used to find their corresponding genomic coordinates as 5 prime UTR+2kb upstream flank, cDNA+2kb upstream flank and unspliced (gene) sequences through the Ensembl BioMart (Ensembl Genomes release 37) ( 20 ). The upstream sequences were utilized to discover TFs interaction sites in their target genes. The investigation of TFs genes was performed by employing TF Enrichment tool of Plant Transcription Factor Database, PlantRegMap (v4.0) ( 21 ) for searching T. aestivum database with the threshold p-value ≤0.05. Then, experimental validation of the results was performed for the existence of cis-acting regulatory motifs relevant to TFs in the upstream of desired genes using PlantCARE database ( 22 ).
3.4. Expression Analysis of TF Genes
BLASTX (ncbi-blast-2.7.1) (19) was used to find the equivalent TF sequences of RNA samples; hence, the detected TF protein sequences were set as database and wheat grain transcripts were selected as queries, and their expressions were retrieved from differential expression analysis results. NCBI BLASTP ( 19 ) was used for determining the subfamily classification of the important TFs. Clustal Omega multiple sequence alignment program ( 23 ) was used for sequence comparison between TFs and BLASTP results; Moreover, the domain of each TF protein was retrieved from the Pfam database (31.0) ( 24 ).
3.5. Flour Test
Flour test (NIR test (moisture and Protein content, Zeleny value, hardness, water absorption, baking volume) and gluten index) was carried out on grains at the harvest time. This primary flour test was performed before RNA-Seq to be certain that the two wheat cultivars had low and high bread making quality. As well, their qualities did not affect by environmental condition.
4. Results
4.1. Gluten Genes Expression
In five sampling stages, starting from 5 DPA to 28 DPA, 34, 58, 71, 65, 62 gluten genes were expressed respectively. Among these, 27, 5, 1, 7 and 7 genes were differentially expressed at 5, 10, 14, 21, and 28 DPA, respectively. The status of all expressed genes is presented in Figure 1, however, only DEGs are discussed as regulatory elements in detail. The differential gene expression analysis showed that all gluten genes had significantly higher expressions in Navid compared to Pishtaz at 5 DPA (Table 1). The expression level of LMW-GS (A1A0071580) and Ɣ-gliadin (AA0070097) in Pishtaz were increased by grain development compared to Navid, but other DEGs had higher levels in Navid at 10 DPA. Interestingly, at 14 DPA, two cultivars had similar expression trends except for the Ɣ-gliadin (AA0070097) that predominantly increased in Pishtaz. Additionally, the expression of Ɣ-gliadin gene (AA0070097) was significantly increased merely in Pishtaz at 21 DPA and increased in all other genes predominant in Navid. Expression of the most genes was increased in Pishtaz rather than Navid at the end of growth, especially for Ɣ-gliadin (AA0070097) (Table 1).
Figure 1.
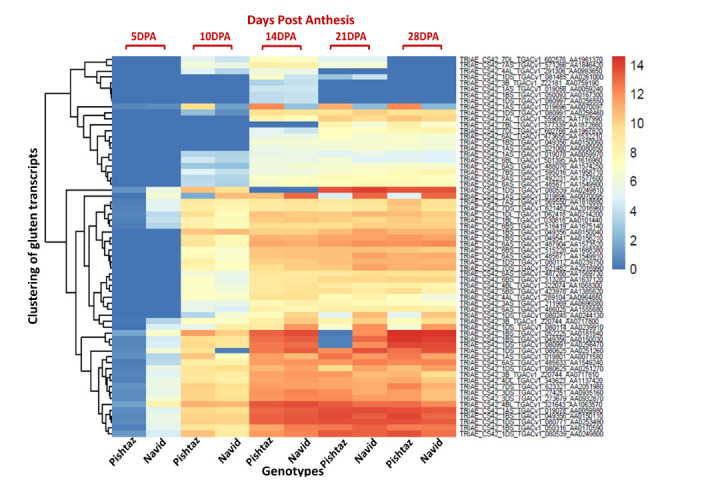
The cluster heat map of the expressed gluten genes in Pishtaz and Navid cultivars during grain development. Genes expression are presented as log2 (fpkm+1) for each gene. The color spectrum from red to blue indicates the high to low expression (from 14 to 0).
Table 1.
Differentially expressed gluten genes and regulating TFs of these genes between Navid and Pishtaz.
| TF ID | TF expression comparisons (fpkm) | Function | Gene ID | Gene expression comparisons (fpkm) |
|---|---|---|---|---|
| Stage one: 5 DPA | ||||
| N.A. | - | HMW-GS | TRIAE_CS42_1BL_TGACv1_030818_AA0101440 | N>P (14.62>0.39) |
| N.A. | - | TRIAE_CS42_1DL_TGACv1_062418_AA0214200 | N>P (11.97>0.56) | |
| N.A. | - | LMW-GS | TRIAE_CS42_1AS_TGACv1_019801_AA0071580 | N>P (11.24> 0.91) |
| NAC (39FF03C5D) | N=P | TRIAE_CS42_1BS_TGACv1_050316_AA0170590 | N>P (35.14>0.39) | |
| ERF (B397F2CE3) | N=P | |||
| GATA (F258582BB) | N>P(23.53>11.69) | |||
| NAC (39FF03C5D) | N=P | TRIAE_CS42_1BS_TGACv1_052225_AA0181540 | N>P (112.75>2.21) | |
| NAC (0924913F8) | N=P | |||
| ERF (B397F2CE3) | N=P | |||
| GATA (F258582BB) | N>P(23.53>11.69) | |||
| NAC (39FF03C5D0 | N=P | TRIAE_CS42_1DS_TGACv1_080114_AA0239910 | N>P (26.35>0.01) | |
| NAC (0924913F8) | N=P | |||
| ERF (B397F2CE3) | N=P | |||
| GATA (F258582BB) | N>P(23.53>11.69) | |||
| N.A. | - | TRIAE_CS42_1DS_TGACv1_080539_AA0249810 | N>P (52.42>1.16) | |
| NAC (39FF03C5D0 | N=P | TRIAE_CS42_1DS_TGACv1_080539_AA0249800 | N>P (23.86>1.26) | |
| NAC (0924913F8) | N=P | |||
| ERF (B397F2CE3) | N=P | |||
| GATA (F258582BB) | N>P(23.53>11.69) | |||
| N.A. | - | Ɣ-gliadin | TRIAE_CS42_1AS_TGACv1_019078_AA0059980 | N>P (31.14>0.66) |
| N.A. | - | TRIAE_CS42_1BS_TGACv1_049356_AA0150030 | N>P (22.67>0.55) | |
| N.A. | - | TRIAE_CS42_1DS_TGACv1_080991_AA0256470 | N>P (25.75>0.57) | |
| N.A. | - | TRIAE_CS42_1DS_TGACv1_080625_AA0251270 | N>P (49.07>0.39) | |
| N.A. | - | TRIAE_CS42_1BS_TGACv1_049356_AA0150110 | N>P (55.08>0.62> | |
| N.A. | - | TRIAE_CS42_1DS_TGACv1_080771_AA0253490 | N>P (50.05>0.76) | |
| N.A. | - | TRIAE_CS42_1DS_TGACv1_080625_AA0251260 | N>P (91.87>0.71) | |
| N.A. | - | TRIAE_CS42_1AS_TGACv1_019696_AA0070095 | N>P (88.25>0.00) | |
| N.A. | - | TRIAE_CS42_3B_TGACv1_220744_AA0717810 | N>P (30.28>0.61) | |
| MYB (12B53AB09) | N<P(9.0<12.4) | TRIAE_CS42_3B_TGACv1_220744_AA0717800 | N>P (10.55>0.15) | |
| MYB (1868E2A6C) | N>P (2.7>1.8) | |||
| MYB (40FA27AE7) | N>P (2.8>0.0) | |||
| MYB (D39684C41) | N=P | |||
| N.A. | - | TRIAE_CS42_3DS_TGACv1_274251_AA0935160 | N>P (42.89>0.70) | |
| N.A. | - | TRIAE_CS42_3DS_TGACv1_273679_AA0932870 | N>P (77.04>0.67) | |
| B3 (TRAES3BF066400010CFD_g) | N>P(7.09>0.17) | TRIAE_CS42_4BL_TGACv1_321643_AA1063570 | N>P (295.47>1.79) | |
| HD-ZIP (9C32B27E2) | N<P(11.35<11.36) | |||
| HD-ZIP (96F9EED93) | N>P(10.39>10.38) | |||
| Dof (2CAAB9D4A) | N<P (3.0<5.9) | |||
| C3H (70AD4B0D5) | N<P (6.29<6.43) | |||
| B3 (TRAES3BF066400010CFD_g) | N>P (7.09>0.17) | TRIAE_CS42_4DL_TGACv1_343623_AA1137420 | N>P (50.84>0.49) | |
| HD-ZIP (9C32B27E2) | N<P(11.35<11.36) | |||
| HD-ZIP (96F9EED930 | N>P(10.39>10.38) | |||
| N.A. | - | TRIAE_CS42_7DS_TGACv1_623321_AA2051980 | N>P (54.36>1.12) | |
| MYB (D39684C41) | N=P | TRIAE_CS42_7AS_TGACv1_569550_AA1818880 | N>P (36.87>0.35) | |
| MYB (D39684C41) | N=P | TRIAE_CS42_7DS_TGACv1_621482_AA2016960 | N>P (31.94>0.45) | |
| GATA (F258582BB) | N>P(23.53>11.69) | α-gliadin | TRIAE_CS42_6AS_TGACv1_485633_AA1549240 | N>P (12.22>0.33) |
| C3H (70AD4B0D5) | N<P (6.29<6.43) | TRIAE_CS42_6BS_TGACv1_516419_AA1675140 | N>P (10.83>0.27) | |
| Stage two: 10 DPA | ||||
| N.A. | - | Ɣ-gliadin | TRIAE_CS42_1AS_TGACv1_019696_AA0070095 | N>P(1605.08>2.60) |
| N.A. | - | TRIAE_CS42_1AS_TGACv1_019696_AA0070097 | N<P (2.00<722.61) | |
| N.A. | - | LMW-GS | TRIAE_CS42_1AS_TGACv1_019801_AA0071580 | N<P (164.63<1317.99) |
| NAC (39FF03C5D) | N=P | TRIAE_CS42_1DS_TGACv1_080114_AA0239910 | N>P (374.69>43.71) | |
| NAC (0924913F8) | N=P | |||
| ERF (B397F2CE3) | N<P (0.48<0.93) | |||
| NAC (39FF03C5D) | N=P | TRIAE_CS42_1DS_TGACv1_080245_AA0244130 | N>P (92.44>10.75) | |
| NAC (0924913F8) | N=P | |||
| ERF (B397F2CE3) | N<P (0.48<0.93) | |||
| Stage three: 14 DPA | ||||
| N.A. | - | Ɣ-gliadin | TRIAE_CS42_1AS_TGACv1_019696_AA0070097 | N<P (5.02<4697.81) |
| Stage four: 21 DAP | ||||
| N.A. | - | Ɣ-gliadin | TRIAE_CS42_1BS_TGACv1_049356_AA0150030 | N>P (8405.70>046) |
| N.A. | - | TRIAE_CS42_1DS_TGACv1_080991_AA0256470 | N>P(4000.7>0.35) | |
| N.A. | - | TRIAE_CS42_1AS_TGACv1_019696_AA0070095 | N>P (8117.06>25.70) | |
| N.A. | - | TRIAE_CS42_1AS_TGACv1_019696_AA0070097 | N<P(4.54<2193.12) | |
| NAC (39FF03C5D) | N=P | LMW-GS | TRIAE_CS42_1BS_TGACv1_052225_AA0181540 | N>P (4072.90>0.19) |
| NAC (0924913F8) | N=P | |||
| ERF (B397F2CE3) | N<P (0.92<3.87) | |||
| NAC (39FF03C5D) | N=P | TRIAE_CS42_1DS_TGACv1_080114_AA0239910 | N>P (4283.01>101.90) | |
| NAC (0924913F8) | N=P | |||
| ERF (B397F2CE3) | N<P (0.92<3.87) | |||
| NAC (39FF03C5D) | N=P | TRIAE_CS42_1DS_TGACv1_080245_AA0244130 | N>P (1671.94>34.44) | |
| NAC (0924913F8) | N=P | |||
| ERF (B397F2CE3) | N<P (0.92<3.87) | |||
| Stage five: 28 DPA | ||||
| N.A. | - | Ɣ-gliadin | TRIAE_CS42_1BS_TGACv1_049356_AA0150030 | N<P(17628.6<20485.6) |
| N.A. | - | TRIAE_CS42_1DS_TGACv1_080991_AA0256470 | N<P(13732.7<15041.8) | |
| N.A. | - | TRIAE_CS42_1AS_TGACv1_019696_AA0070095 | N>P (8318.35>29.79) | |
| N.A. | - | TRIAE_CS42_1AS_TGACv1_019696_AA0070097 | N<P (3.70<4501.26) | |
| NAC (39FF03C5D) | N=P | LMW-GS | TRIAE_CS42_1BS_TGACv1_052225_AA0181540 | N<P (21669.6<24557) |
| NAC (0924913F8) | N=P | |||
| ERF (B397F2CE3) | N<P (0.90<1.54) | |||
| NAC (39FF03C5D) | N=P | TRIAE_CS42_1DS_TGACv1_080114_AA0239910 | N>P(3541.46>89.5951) | |
| NAC (0924913F8) | N=P | |||
| ERF (B397F2CE3) | N<P (0.90<1.54) | |||
| NAC (39FF03C5D) | N=P | TRIAE_CS42_1DS_TGACv1_080245_AA0244130 | N>P (3541.46>89.59) | |
| NAC (0924913F8) | N=P | |||
| ERF (B397F2CE3) | N<P (0.90<1.54) | |||
N: Navid cultivar; P: Pishtaz cultivar; fpkm: Fragments Per Kilobase of transcript per Million mapped reads; N.A. (Not Assigned): It did not experimentally identified TF for related gene.
4.2. TF Identification and Their Expression Profile
The identified TFs belonging to MYB, NAC, HD-ZIP, B3, Dof, C3H, ERF, and GATA families are presented in Table 1. There was no significant difference in NAC expression between two cultivars in all stages, while the expression of ERFs in Pishtaz was increased significantly in comparison to Navid, particularly at 21 and 28 DPA. The expression of other TFs at 5 DPA varied depending on the gene. ERF regulates the genes that had higher expression in Navid, except AA0181540 (28 DPA). The trend of TF expression changes during grain filling was compared to make an assessment of the most critical TF that had a significant effect on the gluten gene expression (Fig. 2). As shown in Figure 2, MYB, Dof, ERF and B3 had significant fold changes compared to the other TFs at 5 DPA, while by increasing grain development, only ERF (B397F2CE3) had significant fold changes. This was a predictable result as there were large number of significant gluten genes at 5 DPA, and it has been expected to have more TFs with significant expression. BLASTP showed that ERF (B397F2CE3) has a high sequence homology with the three predicted dehydration-responsive element-binding proteins (DREB2C) in rice; EAZ08049.1, XP_015651102.1 and Q84ZA1.1, suggesting that ERF (B397F2CE3) belongs to DREB2C TF subfamily with a single AP2 domain (Fig. 3), the distinguishing characteristic of ERF group.
Figure 2.
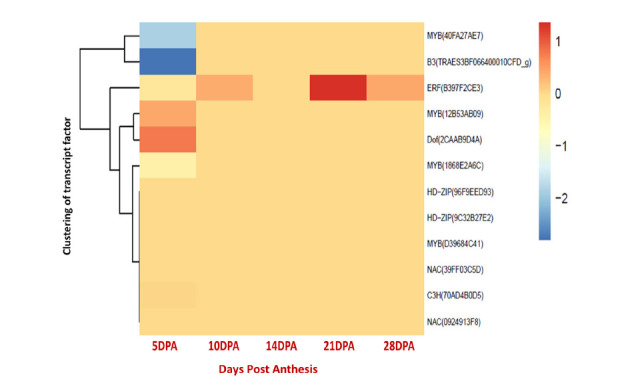
Fold changes expression of TFs from 5 DPA to 28 DPA. Values are expressed as log2fold change (fpkm+1). Red or blue colors indicate the differentially up or down regulated TF genes, respectively.
Figure 3.
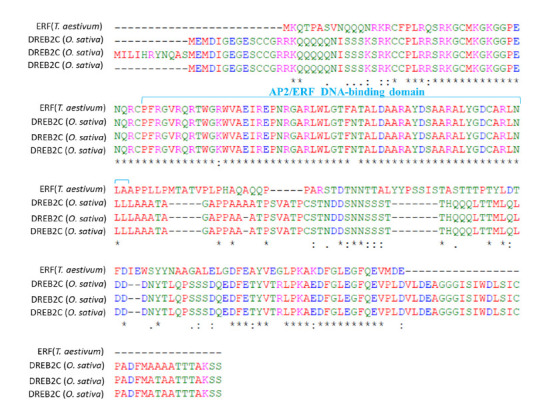
The Global Multiple Sequence Alignment for the ERF (B397F2CE3) using Clustal Omega.
4.3. Flour Test Results
Results of flour testing particularly gluten index confirmed the high and low bread making quality of obtained flour from the cultivation of Pishtaz and Navid cultivars.
5. Discussion
In the current study, our focus was on the genes that might explain the bread quality differences between the two cultivars at different stages of grain development. Gluten test showed that Pishtaz was more elastic and has a better quality. Two cultivars had a somewhat similar gluten gene expression level, except at 5 DPA. This suggests that differences in their gluten contents have resulted from variation in a limited number of genes. Overall, these results indicate that Pishtaz had a noticeable Ɣ-gliadin gene expression (AA0070097) compared to Navid from 10 DPA to the end, whereas Navid lacked such a gene during seed development. Navid had a variable gene expression in DEGs during this period and there was no gene with a consistent higher expression compared to Pishtaz. This evidence suggests that comparison of the promoter elements of AA0070097 gene with others in Navid may help to understand the mechanism of gluten gene expression. The results of 10 DPA showed that ERF may have a potential role, since it had a higher expression level in Pishtaz, while their target genes (LMW-GS) had lower expressions.
The homology alignment between the ERF (B397F2CE3) protein and DREB2C TFs showed that both proteins had a similar domain. This result showed that ERF is a member of AP2/ERF superfamily genes encoding several proteins ( 25 ). The existence of at least one conserved AP2 DNA binding domain is the common feature of this superfamily, which consists of four families named AP2, ERF, RAV and Soloist, based on their sequence similarity and domain number ( 26 ). ERF TFs which have a single AP2 domain with few introns ( 25 ) are subdivided into twelve groups. The dehydration responsive element binding proteins (DREBs) are one of ERF subfamily members that bind into GCC-box (A/GCCGAC element) located in the promoter region of its target gene. DREBs which act as an activator or a repressor are involved in the abiotic responsive gene expression such as drought, salinity, dehydration, heat shock, and cold ( 27 ).
Results also showed that there is a negative correlation between ERF (B397F2CE3) TFs and lower expression of gluten genes in Pishtaz as LMW-GS expression decreased by an increase in TF expression (Fig. 4). Also, there are many studies about the function of AP2/ERF genes acting as activators of the genes involved in stress induced conditions ( 28 - 30 ), but there are few studies on their repressive roles for expression of gluten genes in wheat and under normal growth conditions, particularly.
Figure 4.
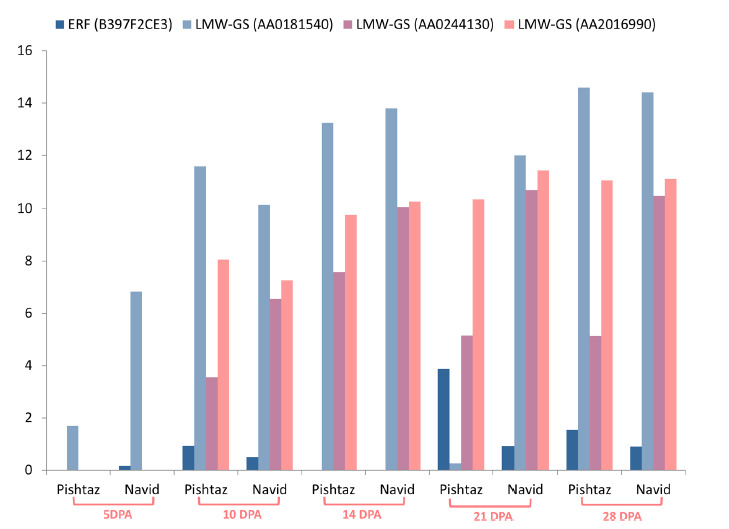
Expression pattern of ERF transcription factor gene (B397F2CE3) and differentially expressed LMW-GS genes during grain filling in Pishtaz and Navid cultivars. The vertical axis represents the LMW-GS and ERF gene expression values during 5, 10, 14, 21 and 28 DPA in two wheat cultivars. Values are expressed as gene expression (fpkm).
AP2 has different functions in plants, including regulation of grain number and weight, protein content, spike density ( 31 , 32 ), and accelerating phase transmission ( 33 ).
Jung et al., (2017) have recently reported that overexpression of ERF (OsERF48) resulted in a significantly higher root growth and grain yield under drought stress in the transgenic rice compared to non-transgenic ( 34 ). Lee et al., (2016) have shown that there is no difference between non-transgenic and transgenic rice plants (overexpression of OsERF71) under normal condition; however, employing drought stress could increase grain filling rate and whole grain weight in transgenic plants ( 35 ). All together, these results indicate that ERF could be identified as one of the regulating factors of tolerance to drought and it cannot increase the expression of target genes under normal conditions. Jofuku et al., (2005) pointed out that AP2 deactivation in Arabidopsis by mutation can cause a significant increase in the seed protein content (13-78%), seed mass (27- 104%), and seed weight (35%) in transgenic plants rather than wild types ( 31 ). They proposed that AP2 has a role in the control of final grain yield. AP2 activity affects source–sink relations as well as maintains the seed size uniformity. They suggested that AP2 has a negative control over cell size and number, and gibberellins function to regulate the metabolisms of the source and sink tissues. It is necessary to mention that AP2 usually performs its activity as AP2-ERF complex and ERF is an important subfamily with several roles ( 30 ).
According to the results, it could be inferred that LMW-GS protein content in the cultivars is regulated via two mechanisms:
a) The repressor activity of AP2-ERF: It seems that AP2- ERF TFs can reduce the transcription copy number of LMW-GS genes due to their negative roles in protein expression encoded by AP2 genes. As shown in Figure 5, ERF (B397F2CE3) binds to the cis-regulating elements (GCC-box) located in the upstream region of LMW-GS gene and significantly prevents RNA-polymerase activity and reducing LMW-GS transcripts; therefore, higher transcript numbers of ERF (B397F2CE3) in Pishtaz resulted in larger decrease in LMW-GS gene expression than Navid. We proposed that ERF (B397F2CE3) gene could be classified as DREB2C subfamily of TF and its activity consequences could be categorized as DREB2C TF.
b) The growth condition: As pointed out earlier, the results of the current research support the idea that unlike the role of ERF genes which act as an activator for their downstream genes under stress condition, an increase in ERF expression cannot affect the expression of its target genes (storage protein genes) under normal growth conditions; therefore, gluten gene expression differences could be caused by genetic differences or other regulating factors.
According to the results, it seems that the stable higher expression of Ɣ-gliadin gene (AA0070097) from 10 DPA and higher, could be assumed as the source of superior quality in Pishtaz. Data from several studies showed that gliadins contribute to extensibility and there is a close relation between gliadins and Zeleny sedimentation (an important criterion for prediction of the quality) ( 36 ). Indeed, Van Lonkhuijsen et al., (1992) have shown that gliadins are the common factors in determining the quality of wheat and their combination is the major source of 82% variation in the bread quality ( 37 ). Several studies have investigated the effects of gliadins on the quality not only for their direct influences but also for their interactions with glutenins especially LMW-GS either in the genome or proteome levels ( 4 , 38 - 42 ). It can be concluded that for quality, the interaction of Ɣ-gliadins with LMW-GS is more important than LMW-GS individually since Navid has poor quality with higher expressions of LMW-GS and lower expression of Ɣ-gliadins comparing to Pishtaz.
It seems that the high expression of Ɣ-gliadin gene was enough to increase bread making quality in Pishtaz, while high expression of LMW-GS could not recover bread quality in Navid cultivar. This shows the importance of Ɣ-gliadin in bread quality as Navid expected to have higher quality if LMW-GS which has more significant role compared to the Ɣ-gliadins. In addition, the aforementioned factors are not the only regulating mechanism for the gluten content. It is noteworthy that this trait as other traits is controlled in several levels. The gluten content in Pishtaz may have benefited more from post translational modification, especially from LMW proteins, comparing to Navid. This issue could be an important subject for further investigation in the future.
6. Conclusion
Regarding the delayed expression of the gluten genes in Pishtaz, it could be concluded that the higher quality is not necessarily a consequence of earlier expression of the gluten genes. Taken together, these findings suggest a special role for Ɣ-gliadin gene (TRIAE_CS42_1AS_ TGACv1_019696_AA0070097) in promoting protein quality of the bread wheat; moreover, the current data highlight the importance of regulating elements, especially ERF (B397F2CE3) TF that predicted as DREB2C, to regulate gluten gene expressions.
Acknowledgement
The financial support from the National Institute of Genetic Engineering and Biotechnology through the Wheat Mission-Driven Project, Grant No. 451M, and the Ferdowsi University of Mashhad are gratefully acknowledged.
References
- 1. FAOSTAT website 2017. Available from: http://faostat.fao.org/
- 2.Zhang X, Jin H, Zhang Y, Liu D, Li G, Xia X, et al. Composition and functional analysis of low-molecular-weight glutenin alleles with Aroona near-isogenic lines of bread wheat. BMC Plant Biol. 2012;12(1):243. doi: 10.1186/1471-2229-12-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He J, Penson S, Powers SJ, Hawes C, Shewry PR, Tosi P. Spatial patterns of gluten protein and polymer distribution in wheat grain. J Agric Food Chem. 2013;61(26):6207–6215. doi: 10.1021/jf401623d. [DOI] [PubMed] [Google Scholar]
- 4.Wieser H. Chemistry of gluten proteins. Food microbiology. 2007;24(2):115–9. doi: 10.1016/j.fm.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Barak S, Mudgil D, Khatkar B. Relationship of gliadin and glutenin proteins with dough rheology, flour pasting and bread making performance of wheat varieties. LWT - Food Sci Technol. 2013;51(1):211–217. doi: 10.1016/j.lwt.2012.09.011. [DOI] [Google Scholar]
- 6.Shewry PR, Halford NG, Lafiandra D. Genetics of wheat gluten proteins. Adv Genet. 2003;49:111–184. doi: 10.1016/S0065-2660(03)01003-4. [DOI] [PubMed] [Google Scholar]
- 7.Ribeiro M, Nunes-Miranda JlD, Branlard Gr, Carrillo JM, Rodriguez-Quijano M, Igrejas G. One hundred years of grain omics: identifying the glutens that feed the world. J Proteome Res. 2013;12(11):4702–4716. doi: 10.1021/pr400663t. [DOI] [PubMed] [Google Scholar]
- 8.Rasheed A, Xia X, Yan Y, Appels R, Mahmood T, He Z. Wheat seed storage proteins: Advances in molecular genetics, diversity and breeding applications. J Cereal Sci. 2014;60(1):11–24. doi: 10.1016/j.jcs.2014.01.020. [DOI] [Google Scholar]
- 9.Rubio‐Somoza I, Martinez M, Abraham Z, Diaz I, Carbonero P. Ternary complex formation between HvMYBS3 and other factors involved in transcriptional control in barley seeds. Plant J. 2006;47(2):269–281. doi: 10.1111/j.1365-313X.2006.02777.x. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto MP, Onodera Y, Touno SM, Takaiwa F. Synergism between RPBF Dof and RISBZ1 bZIP activators in the regulation of rice seed expression genes. Plant Physiol. 2006;141(4):1694– 707. doi: 10.1104/pp.106.082826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verdier J, Thompson RD. Transcriptional regulation of storage protein synthesis during dicotyledon seed filling. Plant Cell Physiol. 2008;49(9):1263–1271. doi: 10.1093/pcp/pcn116. [DOI] [PubMed] [Google Scholar]
- 12.Weber H, Sreenivasulu N, Weschke W. Molecular physiology of seed maturation and seed storage protein biosynthesis. InPlant Developmental Biology-Biotechnological Perspectives 2010 (pppp. 83-104). Springer, Berlin, Heidelberg. [Google Scholar]
- 13.Han R, Jian C, Lv J, Yan Y, Chi Q, Li Z, et al. Identification and characterization of microRNAs in the flag leaf and developing seed of wheat (Triticum aestivum L.) . BMC Genomics. 2014;15(1):289. doi: 10.1186/1471-2164-15-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun F, Guo G, Du J, Guo W, Peng H, Ni Z, et al. Whole-genome discovery of miRNAs and their targets in wheat (Triticum aestivum L.) . BMC Plant Biol . 2014;14(1):142. doi: 10.1186/1471-2229-14-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pellny T.K, Lovegrove A, Freeman J, Tosi P, Love C G, Knox J P, Shewry P R, Mitchell R A . Cell walls of developing wheat starchy endosperm: comparison of composition and RNA-Seq transcriptome. Plant physiology . 2012;158(2):612 –627. doi: 10.1104/pp.111.189191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14(4):R36. doi: 10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, Van Baren MJ, et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 2010;28 (5):511–515. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trapnell C, Hendrickson DG, Sauvageau M, Goff L, Rinn JL, Pachter L. Differential analysis of gene regulation at transcript resolution with RNA-Seq. Nat Biotechnol. 2013;31 (1):46–53. doi: 10.1038/nbt.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 20.Aken BL, Achuthan P, Akanni W, Amode MR, Bernsdorff F, Bhai J, et al. Ensembl 2017. Nucleic Acids Res. 2016;45 (D1):D635–D642. doi: 10.1093/nar/gkw1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin J. , Tian F, Yang D. C , Meng Y Q, Kong L, Luo J, Gao G. PlantTFDB 4.0: toward a central hub for transcription factors and regulatory interactions in plants . Nucleic Acids Res. 2016;45(D1):D1040–D1045. doi: 10.1093/nar/gkw982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rombauts S. , Déhais P , Van Montagu M , Rouzé P. PlantCARE, a plant cis-acting regulatory element database . Nucleic Acids Res . 1999 ; 27 (1):295–296. doi: 10.1093/nar/27.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McWilliam H, Li W, Uludag M, Squizzato S, Park YM, Buso N, et al. Analysis tool web services from the EMBL-EBI. Nucleic Acids Res. 2013;41(W1):W597–W600. doi: 10.1093/nar/gkt376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finn RD, Coggill P, Eberhardt RY, Eddy SR, Mistry J, Mitchell AL, et al. The Pfam protein family database: towards a more sustainable future. Nucleic Acids Res. 2016;44 (D1):D279–D285. doi: 10.1093/nar/gkv1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakano T, Suzuki K, Fujimura T, Shinshi H. Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiol. 2006;140(2):411–432. doi: 10.1104/pp.105.073783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Licausi F, Ohme‐Takagi M, Perata P. APETALA2/Ethylene Responsive Factor (AP2/ERF) transcription factors: mediators of stress responses and developmental programs. New Phytol. 2013;199 (3):639–649. doi: 10.1111/nph.12291. [DOI] [PubMed] [Google Scholar]
- 27.Akhtar M, Jaiswal A, Taj G, Jaiswal J, Qureshi M, Singh N. DREB1/CBF transcription factors: their structure, function and role in abiotic stress tolerance in plants. J. Genet 2012;91(3):385–395. doi: 10.1007/s12041-012-0201-3. [DOI] [PubMed] [Google Scholar]
- 28.Pan Y, Seymour GB, Lu C, Hu Z, Chen X, Chen G. An ethylene response factor (ERF5) promoting adaptation to drought and salt tolerance in tomato. Plant Cell Rep. 2012;31(2):349–360. doi: 10.1007/s00299-011-1170-3. [DOI] [PubMed] [Google Scholar]
- 29.Rong W, Qi L, Wang A, Ye X, Du L, Liang H, et al. The ERF transcription factor TaERF3 promotes tolerance to salt and drought stresses in wheat. Plant Biotechnol. J 2014;12(4):468– 479. doi: 10.1111/pbi.12153. [DOI] [PubMed] [Google Scholar]
- 30.Guo Z-H, Hao P-P, Wang G-M, Jin Z-M, Zhang S-L. Multiple regulatory roles of AP2/ERF transcription factor in angiosperm. Bot. Stud 2017;58 (1):6. doi: 10.1186/s40529-016-0159-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jofuku KD, Omidyar PK, Gee Z, Okamuro JK. Control of seed mass and seed yield by the floral homeotic gene APETALA2. Proc. Natl Acad Sci USA 2005;102(8):3117–3122. doi: 10.1073/pnas.0409893102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Houston K, McKim SM, Comadran J, Bonar N, Druka I, Uzrek N, et al. Variation in the interaction between alleles of HvAPETALA2 and microRNA172 determines the density of grains on the barley inflorescence. Proc. Natl Acad Sci 2013;110 (41):16675–16680. doi: 10.1073/pnas.1311681110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu Q-H, Helliwell CA. Regulation of flowering time and floral patterning by miR172. J. Exp Bot. 2010;62(2):487–495. doi: 10.1093/jxb/erq295. [DOI] [PubMed] [Google Scholar]
- 34.Jung H, Chung PJ, Park SH, Redillas MCFR, Kim YS, Suh JW, et al. Overexpression of OsERF48 causes regulation of OsCML16, a calmodulin‐like protein gene that enhances root growth and drought tolerance. Plant Biotechnol J. 2017 doi: 10.1111/pbi.12716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee D-K, Jung H, Jang G, Jeong JS, Kim YS, Ha S-H, et al. Overexpression of the OsERF71 transcription factor alters rice root structure and drought resistance. Plant Physiol. 2016:575–88. doi: 10.1104/pp.16.00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sozinov AA, Poperelya FA. Polymorphism of prolamins and variability of grain quality. Plant Foods for Hum Nutr. 1982;31(3):243–249. doi: 10.1007/BF01108633. [DOI] [Google Scholar]
- 37.Van Lonkhuijsen HJ, Hamer RJ, Schreuder C. Influence of specific gliadins on the bread making quality of wheat. Cereal Chem. 1992;69 (2):174–177. [Google Scholar]
- 38.Payne P, Jackson E, Holt L, Law C. Genetic linkage between endosperm storage protein genes on each of the short arms of chromosomes 1A and 1B in wheat. Theor Appl Genet. 1984;67 (2):235–243. doi: 10.1007/BF00317044. [DOI] [PubMed] [Google Scholar]
- 39.Payne P, Seekings J, Worland A, Jarvis M, Holt L. Allelic variation of glutenin subunits and gliadins and its effect on breadmaking quality in wheat: Analysis of F5 progeny from Chinese Spring× Chinese Spring (Hope 1A) J Cereal Sci. 1987;6(2):103–118. doi: 10.1016/S0733-5210(87)80047-4. [DOI] [Google Scholar]
- 40.Singh N, Shepherd K. Linkage mapping of genes controlling endosperm storage proteins in wheat. Theor Appl Genet. 1988;75(4):628–641. doi: 10.1007/BF00289132. [DOI] [Google Scholar]
- 41.Gupta R, Singh N, Shepherd K. The cumulative effect of allelic variation in LMW and HMW glutenin subunits on dough properties in the progeny of two bread wheats. Theor Appl Genet. 1989;77 (1):57–64. doi: 10.1007/BF00292316. [DOI] [PubMed] [Google Scholar]
- 42.Wieser H. Investigations on the extractability of gluten proteins from wheat bread in comparison with flour. Z Lebensm Unters Forsch. 1998;207(2):128–132. doi: 10.1007/s002170050306. [DOI] [Google Scholar]


