Abstract
There is an extensive effort to identify new molecular targets that are relevant to cancer stem cells and tumor metastasis. There is growing evidence that several chemokine receptors including CXCR3 contribute to metastasis of breast and other cancers, however, in order to target CXCR3 effectively, it is critical to understand the relative contribution of each CXCR3 isoform. Furthermore, the possible contribution of either major CXCR3 isoform (CXCR3-A, CXCR3-B) to cancer stem cell behavior has not been reported. In malignant breast cancer cells, CXCR3-A is more highly expressed than CXCR3-B and this relationship is most clearly observed in basal-type breast cancers with the worst prognosis. Conversely, immortalized normal MCF10A cells express more CXCR3-B relative to CXCR3-A. Overexpression of CXCR3-B inhibits CXCR3 ligand-mediated proliferation. Likewise, metastatic capacity of MDA-MB-231 basal-like cells expressing high levels of CXCR3-B is reduced in vivo and migratory and invasive properties are inhibited in vitro but silencing of CXCR3-B enhances lung colonization. In contrast to the anti-metastatic and anti-proliferative roles of CXCR3-B in the non-stem cell population, this isoform supports tumor cells with a stem cell phenotype. CXCR3-B is markedly elevated in mammosphere-forming parental cells and overexpressing CXCR3-B further enhances mammosphere-forming potential and the ability to form colonies in soft agar; stem cell behavior is inhibited in MDA-MB-231shCXCR3-B cells. These studies illustrate that targeting of both CXCR3 isoforms may be important to block the stem-cell promoting actions of CXCR3-B while inhibiting the pro-proliferative and metastasis promoting functions of CXCR3-A.
Precis
It was generally thought that CXCR3-A was chiefly responsible for malignant behavior i.e., proliferation and metastasis and that CXCR3-B was protective, however, this study demonstrates that CXCR3-B supports breast cancer stem cells and that both isoforms should be considered as therapeutic targets.
Introduction
Chemokine receptors including CCR5, CCR7, CCR9, CXCR2, CXCR3 and CXCR4 contribute to tumor progression and metastasis in a variety of tumor types (1,2). The CXCR3 receptor is specific for CXC family chemokines: monokine activated by interferon-γ (Mig/CXCL9), interferon-inducible protein-10 (IP-10/CXCL10), and interferon-inducible T cell α chemoattracant (I-TAC/CXCL11) and receptor and ligands play important roles in breast cancer (3,4). Expression of CXCR3 is up-regulated in human breast cancer and is associated with a worse prognosis (5,6). Inhibiting CXCR3 with the small molecule CXCR3 antagonist AMG487 or shRNA gene-silencing reduces lung metastasis in a murine model of breast cancer (5,7). Increased CXCR3 receptor expression and its cognate ligands play a role in breast tumor cell proliferation and survival (8,9).
Receptor expression level is not the only determinant of function. The presence of three splice isoforms of CXCR3 has made the picture more complex (10). The two major isoforms CXCR3-A and CXCR3-B often behave in opposing ways. CXCR3 and the CXCR3-A isoform have been reported to promote migration and invasion of a range of tumor cells, including melanoma, colon, prostate, renal and breast whereas CXCR3-B either inhibits migration or has no chemotactic function (11–17). When expressed, CXCR3-B also mediates growth-inhibitory actions and induces apoptosis in breast cancer cells (16) but, in some cells, is down regulated by the Ras oncogene (8). ). In renal cell carcinoma, CXCR3-A is more highly expressed than CXCR3-B and this relationship correlates with migration and invasion in vitro (14). The contribution of each isoform to metastasis in vivo has not been demonstrated. We now show that CXCR3-B can reverse the prometastatic functions of CXCR3-A.
While the role of CXCR3-B as an inhibitor of proliferation in the general cancer population is emerging, the role of CXCR3 isoforms as a survival factor for candidate? breast cancer stem cells has not been investigated. We now report a novel role for CXCR3-B in cancer stem-like cells. CXCR3-B is up-regulated in cells with cancer stem cell properties and contributes to the survival of this population.
Materials and Methods
Cell lines
MCF-10A is an immortalized nontumorigenic epithelial cell line grown in DMEM/F12 (Gibco) supplemented with 5% horse serum, insulin (5 ug/ml), EGF (20ng/ml), hydrocortisone (500 ng/ml), penicillin-streptomycin (100 ug/ ml each) and L-glutamine (2mM). MCF-7, MDA-MB-231, MDA-MB-468 and T47D are tumor cell lines derived from pleural effusions of patients with breast adenocarcincomas. These cells lines were grown in Dulbecco’s Modified Eagle Medium (DMEM), supplemented with 10% fetal bovine serum (FBS; Gemini Bioproducts, Woodland, CA), 1.5 ml/ml sodium bicarbonate?, 2 mM L-glutamine, 100 mM nonessential amino acids, 100 units/ ml penicillin and 100 units/ml streptomycin under 5% CO2 atmosphere. Murine mammary tumor cell line 66.1 was derived from a spontaneously occurring mammary adenocarcinoma in a Balb/cfC3H mouse. It is highly tumorigenic and metastatic in syngeneic Balb/cByJ mice. Cells were grown in the same media as malignant human breast cancer cells lines but maintained in a 10% CO2 atmosphere.
Real-time PCR
For cell lines, total RNA was extracted using TRIZOL. (no longer have tissue sample). SYBR Green dye (BIO-RAD) PCR was performed following the manufacturer’s protocol. The following primers were used: CXCR3-A: forward 5’-CAACCACAAGCACCAAAGC-3’; reverse 5’-AACCTCGGCGTCATTT AGC-3’. CXCR3-B: forward 5’- GCGGATGGAGTTGAGGAAG-3’; reverse 5’- TGTGATTGA GTCTGATTTAGTCTG-3’. As an internal control, glyceraldehyde-3-phosphate dehydrogenase (GADPH) cDNA was amplified and analyzed under identical conditions using specific primers.
CXCR3-B over-expressing and CXCR3-B gene-silenced MDA-MB-231 cells
CXCR3-B overexpression cell lines were generated by transfecting CXCR3-B retroviral expression plasmid (gift from Dr. Steven G. Kelsen, Temple University, Philadelphia, PA) with Lipofectamine-2000 (Invitrogen, Carlsbad, CA) and stable clones were selected using G418 (1000 μg/ml). Multiple clones were characterized by quantitative real-time PCR and Western blots for mRNA and protein expression, respectively. Two CXCR3-B over-expressing clones were selected for further characterization. CXCR3-B silenced cell lines were generated by transfecting four different lentiviral CXCR3-B shRNA plasmids, vector and non-silencing scramble plasmids (Origene, Rockville, MD) into 293T-derived Phoenix cells (Allele Biotechnology, San Diego, CA). At 48 hours and 72 hours after transfection, virus containing supernatants were collected, filtered and added to MDA-MB-231 cells. At 72 hours post-infection, cells were placed under selection using puromycin (1μg/ml) and single-cell clones from each construct were generated. Multiple clones were characterized by quantitative real-time PCR and Western blots for mRNA and protein expression. Two CXCR3-B gene-silenced clones (from two different constructs) were selected for further characterization.
Xenogen/ Metastasis Assay
Luciferase-expressing MDA-MB-231 tumor cells were detected by bioluminescence imaging (IVIS 200, Xenogen, Alameda, CA) of anesthetized mice injected i.p. with 100 ul of 7.5 mg/ml D-luciferein (PerkinElmer, Waltham, MA). Bioluminescence from the regions of interest was defined manually and the data were expressed as photon-flux (photons/s/cm2/steradian) and analyzed by IVIS Software.
Proliferation assay
Prestoblue cell viability reagent (Life Technologies, Grande Island, NY) was used to measure the proliferation of MDA-MB-231 CXCR3-B overexpression or silenced cell lines in response to CXCL10. Fluorescence was measured in quintuplicate after cell attachment (Day 0) and 48 hours (Day 2) on a Beckman Coulter DTX 880 microplate reader.
Western blot analysis
Protein extracts were analyzed by standard methods and antibodies to CXCR3 (R&D Systems, MN), CXCR3-B (Creative Biomart, NY) and β-actin (Sigma-Aldrich, MO) as loading control. Densitometry was performed using ImageJ software.
Colony formation assay
Two thousand five hundred cells were plated in quadruplicate in 0.4 ml of 0.33% low-melting agarose. Twelve days later, colonies consisting of >50 cells were stained and counted by an automatic colony counter.
Transwell Boyden chamber migration and invasion assays
Migration and invasion studies were performed using BD migration or Matrigel invasion chambers (8.0 μm pore size, BD Biosciences) in 24-well plates, with the lower well containing DMEM supplemented with 0.5% FBS alone, plus CXCL9, CXCL10 or CXCL11. Five percent FBS was used as the positive control. Cells were serum-starved overnight before the assay, and were seeded at 5×104 cells/well in triplicate. After 24 hours, cells in the lower chamber were labeled with calcein AM and fluorescence at 485 nm determined.
Mammosphere formation assay
Mammosphere formation assay as previously described (18) in serum-free MammoCult® medium (Stem Cell Technology, Vancouver, Canada)and plated at 1×104 cells per well of a six-well ultra low-attachment plate (Corning, Lowell, MA). Experiments were done in triplicate. The mammospheres were cultured for 10 days and harvested for analysis.
Results
Human breast cancers express two CXCR3 isoforms
Human CXCR3 is expressed as two major splice isoforms CXCR3-A and CXCR3-B (Fig. 1A). CXCR3-A is considered the classical isoform consisting of a 368 amino acid protein. CXCR3-B is generated through alternative splicing and results in a protein with a longer N-terminal domain (415 amino acids) (10). We examined CXCR3-A and CXCR3-B mRNA expression patterns in four human breast cancer cell lines (luminal MCF7 and T47D and basal-type MDA-MB-231 and MDA-MB-468) and compared them to an immortalized normal human mammary epithelial cell line (MCF10A) by quantitative real-time PCR. All four malignant cell lines showed a trend of elevated CXCR3-A expression compared to MCF-10A cells (Fig. 1B). In contrast, CXCR3-B expression was somewhat decreased in MDA-MB-231 and significantly reduced in MDA-MB-468 but not in luminal MCF-7 or T47D cells as compared to MCF-10A cells (Fig. 1C). Because CXCR3-A and CXCR3-B sometimes have opposing functions, i.e., in regulating cell proliferation, the relative expression of CXCR3-A versus CXCR3-B in the same cell may be a more important determinant of cell behavior than the absolute expression of either isoform. Calculation of the CXCR3-B/CXCR3-A ratio indicated that immortalized normal MCF-10A cells expressed more CXCR3-B than CXCR3-A mRNA (CXCR3-B/A ratio: 1.88), but in the four malignant cell lines, B was significantly less abundant than A (p<0.05), with ratios of 0.57, 0.43, 0.13 and 0.38 for MCF-7, T47D, MDA-MB-231 and MDA-MB-468, respectively (Figure 1D). In four of five cell lines, the pattern of CXCR3-B protein expression corresponds to the mRNA expression patterns (Fig. 1E). We conclude that CXCR3-A expression is dominant in malignant cell lines but CXCR3-B is dominant in non-malignant MCF10A cells and more highly expressed in luminal versus basal-type malignant cells.
Figure 1.
A) Schematic of Human CXCR3. The CXCR3 gene is composed of three exons. Transcription of CXCR3-A is from exons one and three whereas CXCR3-B utilizes exon two instead of one resulting in a protein with a longer N terminus. B) CXCR3-A gene expression in human malignant mammary cell lines MCF-7, T47D, MDA-MB-231 and MDA-MB-468 compared to normal human mammary epithelial cell line MCF-10A C) CXCR3-B gene expression in human malignant mammary cells MCF-7, T47D, MDA-MB-231 and MDA-MB-468 compared to normal human mammary epithelial cell line MCF-10A D) The ratio of CXCR3-B:CXCR3-A mRNA expression in MCF-7, T47D, MDA-MB-231 and MDA-MB-468 cells versus MCF-10A cell lines. Experiment performed in triplicate. Data represent mean ± SE. * p<0.05 vs MCF-10A by Student t test. E) CXCR3-B protein expression levels in MCF-10A, MCF-7, T47D, MDA-MB-231 and MDA-MB-468. β-actin is shown as a loading control. Western blot shown is representative of triplicate experiment.
Generation and characterization of CXCR3-B overexpression and gene-silenced cell lines
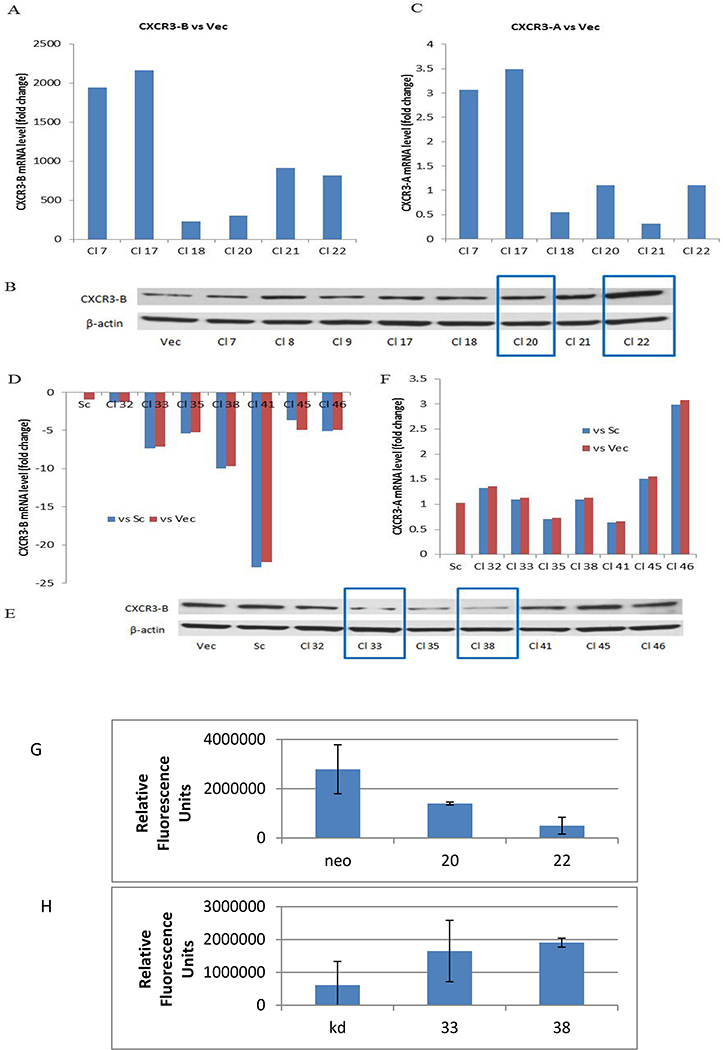
MDA-MB-231 is a highly metastatic human basal-type breast cancer cell line expressing both CXCR3-A and CXCR3-B, and based on our data, expression is markedly shifted to CXCR3-A. To determine the contribution of each isoform to stem cell behavior, we engineered MDA-MB-231 cells to overexpress CXCR3-B. CXCR3-B was successfully increased in all six clones shown (Fig. 2A). In clones 20 and 22, CXCR3-B was increased by 300-fold and 800-fold, respectively, at the RNA level (Fig. 2A) and by 2-fold and 4-fold, respectively at the protein level (Fig. 2B). Furthermore, CXCR3-A mRNA expression was not altered in clones 20 and 22 (Fig. 2C) so these clones were employed for further studies. Protein expression for CXCR3-A isoform could not be determined due to lack of CXCR3-A specific protein sequence in which to target an antibody.
Figure 2.
Generation of stable CXCR3-B overexpression and silenced MDA-MB-231 cell lines. MDA-MB-231 cells were transfected with CXCR3-B retroviral overexpressing plasmid (pcDNA3-CXCR3-B), or CXCR3-B lentiviral shRNA plasmids or respective vector/scramble controls. A) Real-time quantitative PCR measurement of CXCR3-B mRNA expression in different stable CXCR3-B over-expressing MDA-MB-231 clones as compared with vector control. B) CXCR3-B protein expression in different CXCR3-B overexpressing MDA-MB-231clones. C) CXCR3-A mRNA expression in same cells as in A. D,F) Real-time quantitative PCR measurement of CXCR3-B and CXCR3-A mRNA expression in different stable CXCR3-B gene-silenced MDA-MB-231 clones as compared with vector control. E) CXCR3-B protein expression in different CXCR3-B gene-silenced clones. One of three replicates. F,G) Effect of CXCL10 on proliferation of CXCR3-B overexpressing and silenced MDA-MB-231 cells by prestoblue assay. The change in proliferation with and without the addition of 100 ng/ml CXCL10 over 48 hrs.
We also generated CXCR3-B silenced MDA-MB-231 cells. Four different lentiviral CXCR3-B shRNA plasmids, vector and scramble plasmid were transduced into MDA-MB-231 cells and multiple clones were characterized. Clones 32, 33 and 35 were derived from shRNA construct B, and clones 38, 41, 45 and 46 were derived from shRNA construct C. As shown in Figure 2D, the majority of the clones showed a 1.5 to 22-fold decrease in expression of CXCR3-B as compared with the vector and scramble controls (hereafter named MDA-MB-231 shR3B Vec and MDA-MB-231 shR3B Sc, respectively). Clones 33 (derived from construct B) and 38 (derived from construct C) had reduced CXCR3-B mRNA and protein expression (Fig. 2E) but unchanged CXCR3-A mRNA expression compared with vector and scramble control (Fig. 2F) and, on this basis were selected for further experiments. Based on the literature, it was not surprising that overexpression of CXCR3-B significantly decreased CXCL10-induced proliferation of MDA-MB-231 cells (Fig. 2G) consistent with the reported inhibitory effect of CXCR3-B on proliferation of other cells. Conversely, silencing of CXCR3-B enhanced the proliferative response (Fig. 2H).
CXCL10 and CXCR3-B promote soft agar growth and mammosphere formation
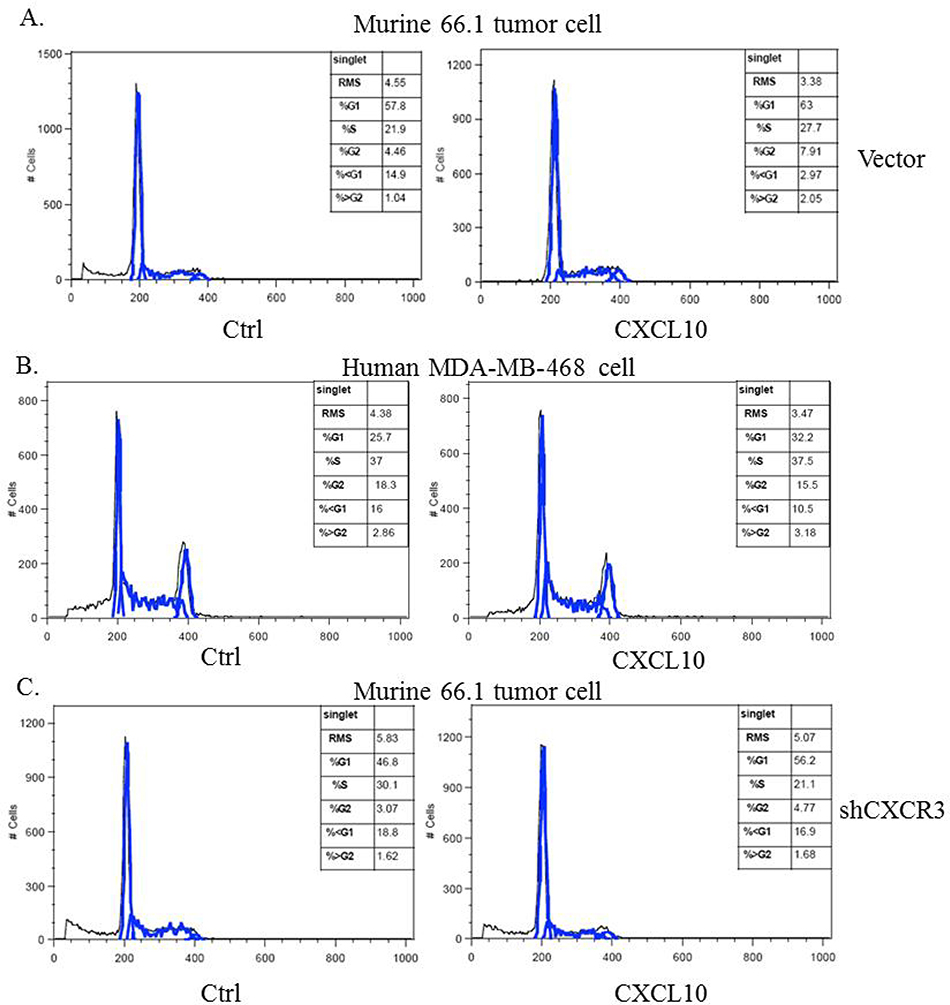
Malignant cells acquire resistance to apoptosis induced by loss of attachment to biological substrates. CXCR4 enhances colony formation of cancer cells in soft agar (19) but CXCR3 has not been examined in this regard. Nothing is known regarding the role of either CXCR3 isoform in cells with cancer stem cell-like properties. Murine 66.1 cells with or without CXCR3 gene-silencing or human MDA-MB-468 cells were plated into a low-attachment dish coated with a hydrogel layer that effectively inhibits cellular attachment and forces cells into suspension. (maybe mention here that 66.1 does not have isoform and mention again later?) Cells were grown in serum-free medium with or without CXCL10 and 48 hr later, the sub-G1 population was examined by flow cytometry as an indicator of apoptotic cells. CXCL10 treatment protected cells from anoikis-induced apoptosis as indicated by the lower percentage of cells in sub-G1 in both 66.1 and MDA-MB-468 cells. In vector transfected 66.1 cells, 14.9% of the cells were in the sub-G1 population, but in the presence of CXCL10, the apoptotic fraction was reduced to 2.97% (Fig. 3A). Likewise, the sub-G1 population in MDA-MB-468 cells was reduced from 16% to 10.5% by CXCL10 treatment (Fig. 3B). Line 66.1 cells with reduced CXCR3 expression (66.1shCXCR3) failed to respond to CXCL10 treatment, indicating that the protective effect of CXCL10 was through CXCR3 (Fig. 3C).
Figure 3.
The protective effect of CXCR3 on suspension-induced apoptosis. A) Line 66.1 or B) MDA-MB-468 or C) 66.1shCXCR3 cells added to Ultra-Low attachment plases and treated with 100 ng/ml CXCL10. At 48 hrs, cells were stained with propidium iodide and cell cycle analysis carried out by flow cytometry.
Overexpression of CXCR3-B significantly increased both the number and size of tumor cell colonies in soft agar (Fig. 4A,B). Conversely, reduced CXCR3-B expression resulted in fewer and smaller colonies (Figure 4C,D). The growth promoting effects of CXCR3-B in this context are the opposite of the growth-inhibitory effects of CXCR3-B seen in the attached monolayer cell culture and provided the first hint that CXCR3-B plays divergent roles in cell behavior that are context dependent. We hypothesized that this observation might be relevant to breast cancer stem cells.
Figure 4.
CXCR3-B expression changes growth in soft agar. A,B) Two CXCR3-B overexpressing or C,D) shCXCR3-B clones with respective vector control clones were grown in soft agar and colony number and size were enumerated. Colonies consisting of >50 cells were automatically counted by a colony counter, and the density and size of the colonies were analyzed. * p˂0.05 vs vector. E) CXCR3-A or F) CXCR3-B gene expression levels in mammosphere forming MDA-MB-231 cells after culture in low adhesion plates in mammocult media for 7 or 10 days. Fold change in gene expression was calculated compared to attached MDA-MB-231 cells (bulk). *p<0.05 vs bulk control by Student t test. One of four replicates.
There is considerable interest in identifying the mechanisms by which cancer stem cell survival is enhanced with the aim of identifying new therapeutic targets. Among chemokine receptors, CXCR4 is implicated in the biology of breast cancer stem cells. CXCR4 activation maintains a stem cell population in tamoxifen-resistant MCF-7 breast cancer cells and in parental MDA-MB-231 cells (20, 21). Whether CXCR3 plays a role in breast cancer cell stem cell properties remains unknown. Breast cancer stem-like cells are identified by their ability to survive under low attachment conditions and to form three dimensional spheres (mammospheres). We compared CXCR3-A and CXCR3-B expression in mammosphere (MS) forming versus the bulk, monolayer population of MDA-MB-231 cells. We harvested mammospheres after 7 and 10 days of culture and mRNA was analyzed by qPCR for expression of CXCR3 isoforms and compared to expression in bulk MDA-MB-231 cells. Both CXCR3-A and CXCR3-B expression levels were increased in mammosphere forming cells relative to the bulk population (Fig. 4E,F). CXCR3-A mRNA levels were increased by 4~8-fold; however, a much more pronounced induction of CXCR3-B was observed (50~200-fold). The very high expression of CXCR3-B in the stem cell population is very different than observed in the bulk population cells in which CXCR3-A is more highly expressed than CXCR3-B (Fig. 1D)
Based on these data, we hypothesized that CXCR3-B supports breast cancer stem cells. We compared the mammosphere forming capacity of CXCR3-B over-expressing and CXCR3-B gene-silenced MDA-MB-231 cells. CXCR3-B over-expression enhanced mammosphere formation as indicated by both number and size of mammospheres (Fig. 5A,B). In contrast, reduced CXCR3-B expression inhibited mammosphere number and size (Figure 5C,D). The enhancing effect of CXCR3-B overexpression on mammosphere formation and the reduced mammosphere forming capacity of CXCR3-B gene-silencing was maintained throughout serial passage of primary mammospheres (MS1) into secondary (MS2) and tertiary (MS3) cultures and was consistent with the growth supportive role of CXCR3-B in soft agar colony formation.
Figure 5.
Effect of CXCR3-B on mammosphere formation in MDA-MB-231 cells. A,B) Mammosphere number and size in CXCR3-B over-expressing MDA-MB-231 cells compared to their respective vector control cell line in primary (MS1) or serially passaged (MS2, MS3) cultures. C,D) Mammosphere number and size in shCXCR3 cells compared to vector controls. Mammospheres were counted manually and photomicrographs were taken after 7 days. *p<0.05 vs vector control by Student t test. One of three replicates.
CXCR3-A supports migration and invasion that are blunted by CXCR3-B
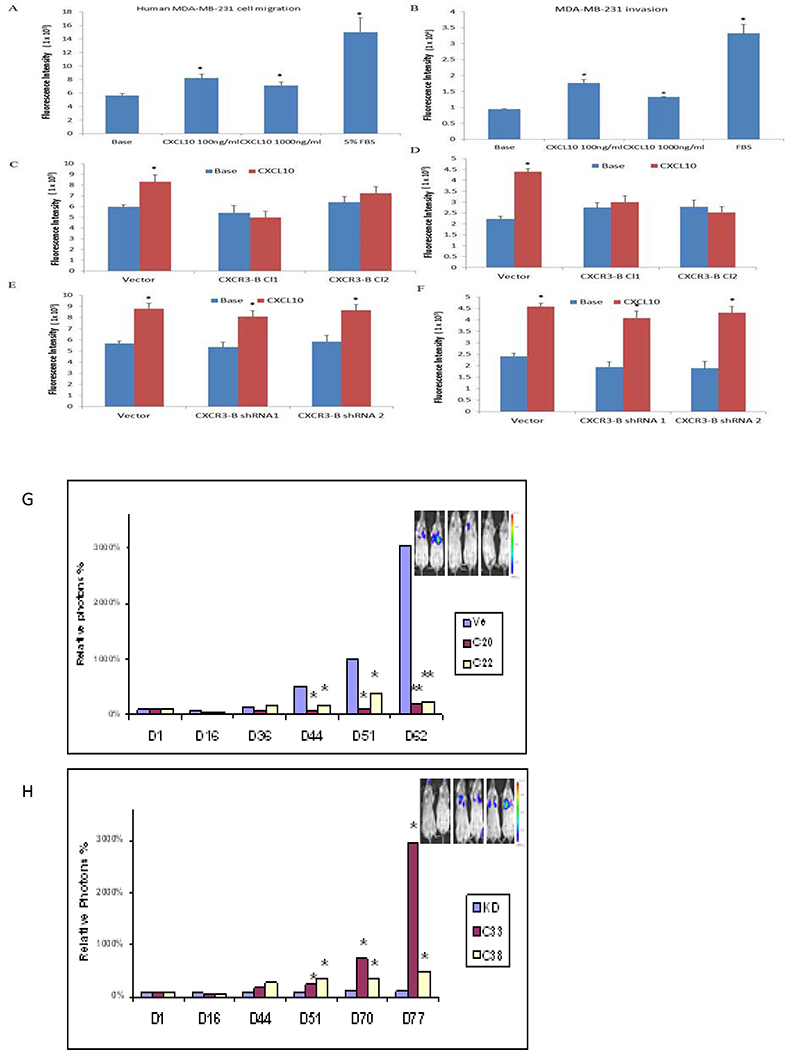
CXCR3 blockade in a murine model of metastatic breast cancer by either genetic or pharmacologic approaches inhibits metastasis however mice express only one CXCR3 isoform (7). Likewise, we previously linked the upregulation of CXCR3 to a worse breast cancer prognosis (5), however, that study could not distinguish the contribution of CXCR3-A versus CXCR3-B to survival. To address whether the change of balance between CXCR3-A and CXCR3-B could affect metastatic properties, we first compared migration, invasive and wound–healing capacity in CXCR3-B over-expressing and gene-silenced MDA-MB-231 cells. In Boyden chamber migration assays, parental MDA-MB-231 cells responded to CXCL10 by modestly increased migration and invasion when compared to base media alone (Figure 6A,B). Overexpressing CXCR3-B did not affect basal migration but blunted the migratory and invasive responses to CXCL10 (Fig. 6C,D). Wound-healing capacity was also delayed by high levels of CXCR3-B (data not shown). CXCR3-B silencing had no discernible effect on migratory (Fig. 6E) or invasive (Fig. 6F) properties. This is likely due to the fact that basal CXCR3-B levels are already very low in parental cells. Taken together these results support a pro-migratory and pro-invasive function of CXCR3-A which can be over-ridden by excess CXCR3-B.
Figure 6.
Effect of CXCR3-B on metastasis. A,B) Migration of parental MDA-MB-231 cells loaded with the fluorescent indicator calcein AM in response to stimulation with CXCL10. C,D) Migration and invasion in modified Boyden chambers with or without matrigel of CXCR3-B overexpressing cells. E,F) Migration and invasion of CXCR3-B gene silenced cells. The intensity of fluorescence is proportional to the number of migrated cells. Each experiment was done at least three times. Data represent mean ± SE. *P˂0.05 vs base control. G) Two clones of MDA-MB-231-luc cells overexpressing CXCR3-B or vector control or H) shCXCR3-B or vector control were injected i.v. into Balb/SCID mice (5 mice/group) and analyzed by bioluminescent imaging at the days indicated. *p<0.05; **p<0.01 by Student t test.
CXCR3-B inhibits metastasis
Based on the migration and invasion properties in vitro, we predicted that metastatic potential of cells expressing high levels of CXCR3-B would be reduced in vivo. CXCR3-B overexpression and silenced MDA-MB-231 clones along with their respective vector controls were engineered to stably express luciferase and these cell lines were injected via the tail vein into Balb/cSCID mice and monitored using whole body bioluminescence imaging. Overexpression of CXCR3-B significantly inhibited metastatic capacity relative to vector control cells (Fig. 6G) consistent with the reduced migratory and invasive potential in vitro. Conversely, silencing of CXCR3-B enhanced lung colonizing capacity (Fig. 6H). In contrast to the differences in luciferase signal at later time points, the initial seeding in the lungs at day 1 did not vary in the different cell lines.
Discussion
CXCR3 is detected in many epithelial malignancies and is usually associated with more aggressive disease but the role of individual isoforms in humans remains to be completely defined (1,2). Among chemokine receptors, CXCR4 and CXCR1 are implicated in breast cancer stem cell biology (20–22); CXCR4 activation maintains a stem cell population in MDA-MB-231 and in tamoxifen-resistant MCF-7 breast cancer cells. CXCR4 antagonists are under evaluation in cancer clinical trials. Our central finding is that, like CXCR4 and CXCR1, CXCR3-B supports a cell with cancer stem cell properties but this same isoform inhibits metastasis in the bulk population. We found that CXCR3-B and to a lesser extent CXCR3-A mRNA levels were up-regulated in mammosphere-forming cells, compared with bulk MDA-MB-231 cells. We have shown previously that mammosphere forming cells behave as cancer stem cells in vivo with heightened tumorigenic capacity in orthotopic models (18) consistent with a cancer stem cell phenotype. Forced CXCR3-B over-expression further enhanced mammosphere formation while reduced CXCR3-B expression inhibited mammosphere-forming ability. Consistent with these data, the ability to form tumor cell colonies in soft agar was enhanced by CXCR3-B overexpression and reduced in cells expressing lower levels of CXCR3-B. Taken together, these data provide the first evidence that CXCR3-B contributes to a breast cancer stem cell phenotype and that CXCR3-B may represent an additional therapeutic target with particular relevance to the elimination of cells with stem-like properties.
CXCR3 is usually associated with more aggressive disease. Our previous studies in a murine model of metastatic breast cancer establish a pro-metastatic role for CXCR3, however, only one isoform of murine CXCR3 is known. Consistent with these preclinical data, we demonstrated that high CXCR3 expression at the time of diagnosis is associated with poor long term and overall survival in women with breast cancer (5). The positive correlation of CXCR3 status and poor prognosis breast cancer was recently confirmed by others (6), however neither of these studies could determine the relative contribution of each CXCR3 isoform to outcome. Primary renal cell carcinomas express more CXCR3-A than CXCR3-B (14) and we observed the same relationship in breast cancer. CXCR3 promotes chemotaxis in vitro of MDA-MB-231 cells (17) but the relevant isoform was not identified. We have now demonstrated that ectopic expression of CXCR3-B in MDA-MB-231 cells negatively regulates experimental metastasis in vivo. Thus, overexpression of CXCR3-B is able to overcome the metastasis-promoting function of endogenously expressed CXCR3-A. Conversely, metastasis is enhanced in cells with reduced CXCR3-B.
When proliferation of bulk populations is considered, CXCR3-A and CXCR3-B have opposing functions. CXCR3-A promotes cell proliferation; whereas CXCR3-B is inhibitory to cell growth (8,13,16). CXCR3-B inhibits growth of MCF7 and T47D breast cancer cells and induces apoptosis through inhibition of ERK1/2 activation (16). The growth-inhibitory actions of CXCR3-B corresponded to nuclear translocation of the transcription factor Bach-1 and nuclear export of Nrf2 resulting in down-regulation of the anti-apoptotic molecule heme oxygenase HO-1. We have confirmed that CXCR3-B overexpression inhibits proliferation and prevents ERK activation (not shown) of the bulk tumor cell population. These results suggest that the proliferative response to CXCL10 in bulk MDA-MB-231cells is mediated by CXCR3-A. Without altering CXCR3-A, reduced CXCR3-B alone can enhance proliferation and migration of tumor cells, indicating that the relative expression of the two CXCR3 splice variants determines the final functional response to CXCR3 ligands.
Consistent with the opposing roles of CXCR3-A versus CXCR3-B in the growth of stem and non-stem cell populations, the relative levels of each isoform differ in these two populations. CXCR3-A is more highly expressed relative to CXCR3-B in metastatic breast cells relative to nonmetastatic or benign populations. Likewise, invasive and metastatic prostate cancers display increased CXCR3-A and decreased CXCR3-B expression relative to benign populations (13). Others have shown that activation of Ras led to down-regulated CXCR3-B in breast cancer cells which could be one mechanism to explain the relative increase in CXCR3-A and loss of CXCR3-B observed in breast and other malignancies relative to normal tissues (8). We observed the opposite ratios in stem cell populations, where CXCR3-B is up-regulated to a much greater extent than is CXCR3-A.
It is perhaps not surprising that CXCR3-B promotes stem cell survival but inhibits proliferation of the bulk population. In contrast to the general malignant cell population, stem cells typically have low proliferation rates which contribute to the relative insensitivity of these cells to chemotherapy and radiation that targets proliferating cells. MDA-MB-231cells grown as monolayers have a doubling time of 24–30 hours; under unlimited growth and space conditions, these cells would be able to multiply by 250–500-fold in ten days. In contrast, MDA-MB-231 mammosphere forming cells increase by approximately 24-fold during the same 10 days consistent with an anti-proliferative role for CXCR3-B. In this regard, breast cancer stem cells resemble benign mammary cells that also express relatively high levels of CXCR3-B and are relatively quiescent. The mechanism by which CXCR3-B supports stem cells remains to be elucidated. Stat3 levels are markedly increased in mammospheres and Stat3 inhibitors reduce mammosphere-forming potential (unpublished). CXCL11 induces Stat3 in T cells (23) so it is possible that the stem cell phenotype is maintained by CXCL11/CXCR3-B mediated induction of Stat3. Ligand-mediated activation of ERK1/2 and p38 are inhibited by high levels of CXCR3-B in the bulk population (not shown) suggesting that CXCR3-B competes for ligand and is linked to different signaling pathways that remain to be elucidated in stem cell populations. These mechanisms are likely to be complex. The ability of CXCR4 to support breast cancer stem cells was associated with changes in the expression of >300 phosphoproteins (21) and the ability of CXCR3 to support cancer stem cells is likely to be equally complex. Regardless of the mechanism, it is clear that CXCR3-B plays an additional positive and supportive role in stem cells since knockdown of CXCR3-B inhibits mammosphere-forming capacity.
Stem cell properties are sometimes linked to enhanced metastatic potential, therefore, it was interesting that while CXCR3-B inhibited metastasis, it promotes cells with properties of cancer stem cells. While it might be anticipated that cancer stem cells also contribute to metastatic capacity, the current results would suggest that these processes are not always linked and that the contribution of CXCR3 may diverge in these processes. The current results suggest that preferential expression of CXCR3-A in highly metastatic and basal-type breast cancers supports pro-proliferative, migratory, invasive and metastatic responses while a rarer stem-like population up-regulates CXCR3-B allowing for improved anchorage independent survival and mammosphere forming capacity and a lower proliferative rate resulting in resistance to conventional therapies that target proliferating cells. (may want to mention that parental cells alter CXCR3 expression based on environment, cell line plasticity – isoform switching, etc?)
In summary, we have demonstrated, for the first time, a unique role for the CXCR3-B isoform in which CXCR3-B supports anchorage independent growth and stem-like properties. We also have confirmed previously reported anti-proliferative, anti-migratory and anti-invasive effects of CXCR3-B expression and have demonstrated that metastasis in vivo is inhibited by the B isoform. The results of this study would suggest that targeting both isoforms would be beneficial to inhibit cancer stem cells while decreasing metastasis.
Acknowledgement
We thank Dr. Steven Kelsen, Temple University for the provision of a CXCR3-B retroviral expression plasmid.
Grant Support: Supported by the U.S. Department of Defense Breast Cancer Research Program.
Footnotes
Conflict: The authors disclose no potential conflicts of interest
Literature Cited
- 1.Fulton A Editor. Chemokine Receptors in Cancer, Springer Publisher, 2009. [Google Scholar]
- 2.Billottet C, Quemener C, Bikfalvi A. CXCR3, a double-edged sword in tumor progression and angiogenesis. Biochim. Biophys. Acta 2013; 1836:287–295. [DOI] [PubMed] [Google Scholar]
- 3.Li Y and Fulton AM CXCR3/CXCL3 Axis: Friend or Foe? In: Ben-Baruch A, Ed. The inflammatory milieu of tumors:cytokines and chemokines that affect tumor growth and metastasis. Bentham Science Pub., 2012, Pp. 88–98. [Google Scholar]
- 4.Goldberg-Bittman L, Neumark E, Sagi-Assif O, Azenshtein E, Meshel T, Witz IP, Ben-Baruch A. The expression of the chemokine receptor CXCR3 and its ligand, CXCL10, in human breast adenocarcinoma cell lines. Immunology Letters 2004; 92(1–2):171–178. [DOI] [PubMed] [Google Scholar]
- 5.Ma X, Norsworthy K, Kundu N, Rodgers WH, Gimotty PA, Goloubeva O, Lipsky M, Li Y, Holt D, Fulton A. CXCR3 expression is associated with poor survival in breast cancer and promotes metastasis in a murine model. Molecular Cancer Therapeutics 2009; 8(3):490–498. [DOI] [PubMed] [Google Scholar]
- 6.Hilborn E, Sivik T, Fornander T, Stal O, Nordenskjold B, Jansson A. CXC ligand 10 and CXC receptor 3 status can predict tamoxifen treatment response in breast cancer patients. Breast Cancer Res. Treat. 2014; 145:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walser T, Rifat S, Ma X, Kundu N, Ward C, Goloubeva O, Johnson M, Medina J, Collins T, Fulton A. Antagonism of CXCR3 inhibits lung metastasis in a murine model of metastatic breast cancer. Cancer Res 2006; 66:7701–7707. [DOI] [PubMed] [Google Scholar]
- 8.Datta D, Flaxenburg JA, Laxmanan S, Geehan C, Grimm M, Waaga-Gasser AM, Briscoe DM, Pal S. Ras-induced modulation of CXCL10 and its receptor splice variant CXCR3-B in MDA-MB-435 and MCF-7 cells: relevance for the development of human breast cancer. Cancer Res 2006; 66(19):9509–9518. [DOI] [PubMed] [Google Scholar]
- 9.Mulligan AM, Raitman I, Feeley L, Pinnaduwage D, Nguyen LT, O’Malley FP, Ohashi PS, Andrulis I. Tumoral lymphocytic infiltration and expression of the chemokine CXCL10 in breast cancers from the Ontario Familial Breast Cancer Registry. Clin. Cancer Res. 2013; 19:336–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lasagni L, Francalanci M, Annunziato F, Lazzeri E, Giannini S, Cosmi L, et al. An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC, and acts as functional receptor for platelet factor 4. J Exp Med 2003; 197(11):1537–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robledo MM, Bartolomé RA, Longo N, Rodríguez-Frade JM, Mellado M, Longo I, van Muijen GNP, Sánchez-Mateos P, Teixidó J. Expression of functional chemokine receptors CXCR3 and CXCR4 on human melanoma cells. Journal of Biological Chemistry 2001; 276(48):45098–45105. [DOI] [PubMed] [Google Scholar]
- 12.Kawada K, Hosogi H, Sonoshita M, Sakashita H, Manabe T, Shimahara Y, Sakai Y, Takabayashi A, Oshima M, Taketo MM. Chemokine receptor CXCR3 promotes colon cancer metastasis to lymph nodes. Oncogene 2007; 26(32):4679–4688. [DOI] [PubMed] [Google Scholar]
- 13.Wu Z, Dhir R, Wells A: Altered CXCR3 isoform expression regulates prostate cancer cell migration and invasion. Mol. Cancer 2012; 11:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Utsumi T, Suyama T, Imamura Y, Fuse M, Sakamoto S, Nihei N, Ueda T, Suzuki H, Seki N, Ichikawa T. The association of CXCR3 and renal cell carcinoma metastasis. 2014; J. Urol. Online February 8, 2014. [DOI] [PubMed] [Google Scholar]
- 15.Petrai I, Rombouts K, Lasagni L, Annunziato F, Cosmi L, Romanelli RG et al. Activation of p38MAPK mediates the angiostatic effect of the chemokine receptor CXCR3-B. The International Journal of Biochemistry & Cell Biology 2008; 40(9):1764–1774. [DOI] [PubMed] [Google Scholar]
- 16.Balan M, Pal S. A novel CXCR3-B chemokine receptor induced growth inhibitory signal in cancer cells is mediated through the regulation of Bach-1 protein and Nrf2 protein nuclear translocation. J. Biol. Chem. 2014; 289:3126–3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shin SY, Nam J-S, Lim Y, Lee YH. TNFa-exposed bone marrow derived mesenchumal stem cells promote locomotion of MDA-MB231 breast cancer cells through transcriptional activation of CXCR3 ligand chemokines. J. Biol. Chem. 2–10;285:30731–30740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kundu S, Ma X, Kochel T, Goloubeva O, Staats P, Thompson K, Martin S, Reader J, Take Y, Collin P and Fulton A. Prostaglandin E receptor EP4 is a therapeutic target in breast cancer cells with stem-like properties. Breast Cancer Res. Treatment 2–14;143:19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfeiffer M, Hartmann TN, Leick M, Catusse J, Schmitt-Graeff A and Burger M. Alternative implication of CXCR4 in JAK2/STAT3 activation in small cell lung cancer. Brit. J. Cancer 2009; 100:1949–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dubrovska A, Hartung A, Bouchez LC, Walker JR, Reddy VA, Cho CY, Schultz PG. CXCR4 activation maintains a stem cell population in tamoxifen-resistant breast cancer cells through AhR signalling. Br J Cancer 2012; 107(1):43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yi T, Zhai B, Yu Y, Kiyotsugu Y, Raschle T, Etzkorn M, Seo H-C et al. Quantitative phosphoproteomic analysis reveals system-wide signaling pathways downstream of SDF-1/CXCR4 in breast cancer stem cells. Proc. Natl. Acad. Sci. 2014; 111:E2182–E2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ginestier C, Liu S, Diebel ME, Korkaya H, Luo M, Brown M, Wicinski J et al. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J. Clin. Invest. 2010;120:485–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zohar Y, Wildbaum G, Novak R, Salzman AL, Thelen M, Alon R, Barsheshet Y et al. CXCL11-dependent induction of FOXP3-negative regulatory T cells suppresses autoimmune encephalomyelitis. J. Clin. Invest. 2014;124:2009–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]