Abstract
Western blotting is a widely used protein assay platform, but the technique requires long analysis times and multiple manual steps. Microfluidic systems are currently being explored for increased automation and reduction of analysis times, sample volumes, and reagent consumption for western blots. Previous work has demonstrated that proteins separated by microchip electrophoresis can be captured on membranes by dragging the microchip outlet across the membrane. This process reduces the separation and transfer time of a western blot to a few minutes. To further improve the speed and miniaturization of a complete western blot, a microscale immunoassay with direct deposition of immunoassay reagents has been developed. Flow deposition of antibodies is used to overcome diffusion limited binding kinetics so that the entire immunoassay can be completed in 1 h with detection sensitivity comparable to incubation steps requiring 20 h. The use of low microliter/min flow rates with antibody reagents applied directly and locally to the membrane where the target proteins have been captured, reduced antibody consumption ~30-fold. The complete western blot was applied to the detection of GAPDH and β-Tubulin from A431 cell lysate.
Keywords: Western blot, microchip electrophoresis, immunoassay, microfluidic, proteins
Graphical abstract

Introduction
Western blotting is one of the most widely used analytical techniques for determination of proteins in biological samples. It combines size-based separation with immunoaffinity binding to achieve selective and robust protein assays.1,2 Despite its popularity, the technique as commonly practiced requires long analysis times (8–24 h), has large sample and reagent requirements (10–50 μg total protein and 2–5 μg of antibodies), minimal automation, and limited ability to multiplex.3–5 Over the last decade, improvements in increasing sensitivity and automation have been made to western blotting as recently reviewed.2 To address the limitations of the immunoassay portion of the western, some commercialized systems have been developed to automate6–8 and accelerate9,10 different aspects of the process, but often do not address the entire western blot, nor do they reduce antibody consumption.
Interest in employing microfluidics for western blotting to reduce sample and reagent volumes, decrease analysis time, and increase automation is growing.2,4,11–24 In some systems, the separation and immunoassay are integrated directly into a microchip or capillary. For example, proteins can be captured on a photoactive gel or wall after separation in a microchannel via photoinduced cross-linking of proteins to allow in-situ immunoprobing.18,20–24 This approach completes assays in just over 1 h with a 103-fold reduction in antibody and reagent requirements. Extensive parallelization to a thousand channels with a 0.5 mm separation channel length on one glass slide has also been demonstrated.18,19
For conventional western blotting membranes, microfluidics has been employed in several ways to speed up the immunoassay and reduce reagent consumption. These methods generally mitigate diffusion-limited kinetics of conventional western immunoassays to reduce incubation times.25–32 A thin-film direct coating approach has been developed yielding a ~1.5 h method with 102 to 104-fold reduction in antibody consumption depending on the coating width from left to right down the membrane. This method gave a comparable result to a 4 h conventional immunoassay.33 In another approach, a microfluidic PDMS chip with seven parallel microchannels was clamped onto a conventional western blot membrane so that reagents could be applied through the microfluidic network. This arrangement enabled parallel immunoassays in 1 h, with a ~7-fold reduction in antibody consumption, and multiplexed detection.34 Sensitivity comparisons to conventional immunoassay methods were not reported. A PDMS-glass microfluidic device has been developed with three microchannels per blotting lane for a miniaturized immunoassay for conventional western blotting membranes.35 The method requires 4 h for the immunoassay; therefore, it does not speed up interactions greatly, but reduces antibody consumption 5380-fold. In another approach, a rotational glass tube incubation chamber was developed with a cyclic draining and replenishing method to better facilitate mixing of the antibody depletion layer.36 Results showed higher signal in 20 min of primary antibody binding in the chamber compared to 60 min on a conventional shaker allowing for a shorter method with 2–3-fold less antibody consumption.
Our lab developed an approach for separation and transfer steps of a western blot wherein proteins are separated by microchip gel electrophoresis while the outlet of the microchip is dragged across a membrane. Proteins are captured as they exit the separation channel so that separation and transfer is completed in 2–8 min.13,14 The separation media is an entangled polymer solution which can be easily replaced allowing long term operation of the microchip for multiple injections.37,38 In this method, proteins are deposited on the surface of the binding membrane in a strip that is less than 700-μm wide. The immunoassay step was performed using traditional methods;13 however, this approach is relatively slow and uses much more reagent than necessary for the small protein tracks made by the method. Here, we report a method of directly applying immunoassay reagents using syringe-driven flow that reduces the area within which the antibodies must diffuse to bind with target proteins. The flow deposition approach has some similarity to vertical flow assay methods that have been used to decrease assay time for sandwich immunoassays.39,40 In these vertical flow assays, the reagents are applied perpendicular to the membrane by pressure driven flow, decreasing assays to 10-min.
In the work presented here, the flow immunoassay method reduces immunoassay time to 1 h while maintaining the detection sensitivity of an overnight, diffusion-only based immunoassay. Antibody consumption is reduced ~30-fold in comparison to a traditional western blot immunoassay. In conventional western blotting, cross-linking has previously been shown to increase protein retention on the binding membrane and improve binding signal for many targets that were poorly detected.41–48 Such cross-linkers, formaldehyde or glutaraldehyde, have not been reported to interfere with antibody-target binding in western blotting immunoassays. Cross-linking is used after microchip western blotting deposition to increase the retention of the proteins that can be lost during the washing steps of the flow-driven assay. Combining the microchip electrophoresis blotting method with this fast immunoassay method, yields a microfluidic western blot that detects proteins in less than 1.5 h with reduced sample and reagent consumption and potential for multiplexing.
Materials and Methods
Reagents
All buffers were made using 18 MΩ water deionized by a Series 1090 E-pure system (Barnstead Thermolyne, Dubuque, IA). Actin from rabbit muscle was from Millipore Sigma (St Louis, MO), and A431 cell lysate was from LI-COR Bioscience (Lincoln, NE). FITC-protein ladder containing 7 proteins with masses from 11 kDa-155 kDa with a total protein concentration of 1 mg/mL was from Invitrogen (LC5928, Grand Island, NY). Anti-actin and anti-β-tubulin antibodies were from Millipore Sigma (St Louis, MO). Anti-GAPDH antibody was from Santa Cruz Biotechnology (Dallas, TX). Goat anti-rabbit secondary antibody, goat anti-mouse secondary antibody, nitrocellulose membranes, and blocking buffers were from LI-COR Bioscience (Lincoln, NE). Sodium chloride was from Fisher Scientific (Hampton, NH) and tris was from Bio-Rad Laboratories (Hercules, CA). Radioimmunoprecipitation assay (RIPA) lysis buffer with a composition of 25 mM Tris, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS, and adjusted to pH 7.6 with HCl was from Thermo Scientific (Waltham, MA). All other reagents were from Millipore Sigma (St Louis, MO). Tris buffered saline with Tween 20 (TBST) was made with 137 mM NaCl, 2.7 mM KCl, 190 mM Tris, and 1% (v/v) Tween 20.
Sample Preparation
Samples were denatured and prepared as previously described13 with the following exceptions. A final concentration of 1 mg/mL was prepared with actin. A431 cell lysate was lysed with RIPA buffer. After denaturation, the cell lysate was further diluted 100-fold in water and then filtered by centrifuging at 12,100 × g for 10 min using an Amicon® Ultra Centrifugal Filters with a 10 kDa cut off from Millipore Sigma (St Louis, MO). The concentrated filtrate protein was then collected from the filter and the final concentration was measured using a Bradford assay (Fischer Scientific).
Dot Blotting
For dot blotting, after sample preparation as described above, denatured actin was diluted 300-fold in water for a concentration of 3.3 μg/mL. Dot blots of actin were prepared by spotting 0.2 μL of protein sample directly onto a nitrocellulose membrane strip. The membrane was dry before starting an immunoassay.
Microchip Western Blotting
For microchip western blotting, denatured actin was diluted 10-fold in water. Then, 1 μL of the diluted actin was mixed with 1 μL of FITC-protein ladder and 28 μL of water for a final concentration of 3.3 μg/mL of actin. For A431 cells, 2 μL of the denatured and filtered lysate was mixed with 1 μL of FITC-protein ladder diluted in 27 μL of water for a final concentration of 100 μg/mL total protein.
Glass microchips were fabricated and conditioned as previously described13, and microchip conditioning solutions were filtered through a 0.22 μm syringe filter. The electrophoresis gel sieving matrix used was a dextran based entangled polymer solution. Figure 4 displays a schematic and image of the microchip. As shown, flow through sheath channels was used to carry eluted proteins to the membrane. A second set of sheath channels was used to provide electrical contact for grounding the end of the separation channel. This arrangement allowed the microchip to be interfaced directly with a strip of nitrocellulose membrane. Microchips with a 4-cm long separation channel were used where −2.0 kV was applied at the gating reservoir and −1.5 kV was applied at the sample reservoir with a separation field of 315 V/cm. A gated injection49 of 10 s for actin and 60 s for A431 cell lysate was used. During separation, sieving gel was pumped through the inner sheath flow channels at 30 nL/min and the stage was moved a 4 mm/min across the membrane surface.
Figure 4.
Representation of microchip western blotting onto nitrocellulose membranes. As shown, the microchip is in separation mode with normally closed (NC) relays. When the relays are switched the gating and waste channels are floated for injection mode. A second set of sheath channels at the outlet of the separation channel are used for grounding of the microchip. In this case, proteins can be deposited onto dry nitrocellulose. A bridged Pt electrode is used to ground the second set of sheath channels/reservoirs.
Immunoassays
Traditional immunoassays were completed at room temperature unless otherwise described. The following steps were used: 1) block with Odyssey® blocking buffer (TBS) for 1 h, 2) incubate overnight at 4 °C with primary antibody diluted 1000-fold in Odyssey® blocking buffer (TBS) and 0.05% Tween 20, 3) wash with TBST four times at 5 min each, 4) incubate for 1 h with secondary antibody diluted 5000-fold in Odyssey® blocking buffer (TBS) and 0.05% Tween 20, and 5) repeat step 3. During incubation steps the solutions were gently shaken.
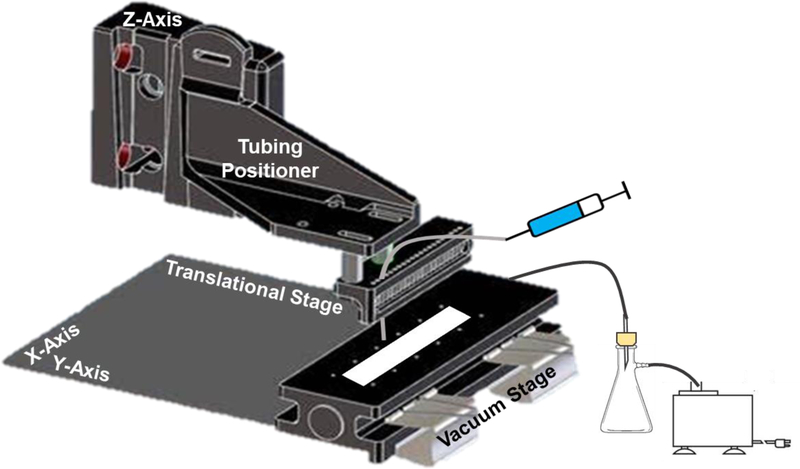
Flow immunoassays were completed, unless otherwise stated, as depicted in Figure 2 at room temperature. Membranes were placed on a 3D printed stage (Figure 1) that allowed vacuum to be applied to pull the solutions through the membrane. Syringe pumps (IDEX Health & Science, LLC, Oak Harbor, WA), connected to 500 μm i.d. fluorinated ethylene propylene (FEP) tubing, were used to deliver antibody, blocking, and washing solutions. The outlet of the tubing was mounted on an x-y-z positioner to allow the tubing to be swept across the membrane during solution delivery.
Figure 2.
Representation of the fast immunoassay method from a side-view perspective where the reagents are applied directly to the mm-wide protein trace on a nitrocellulose membrane. The nitrocellulose membrane strip is placed on a 3D printed vacuum stage, and reagents are applied sequentially as shown. The vacuum is turned on during the washing steps. The entire fast immunoassay is completed in 1 h.
Figure 1.
Representation of the 3D printed parts that are used for the fast immunoassay. A tubing holder is used for precise positioning of the deposition, and the membrane is placed on the vacuum stage. The immunoassay solutions are flowed directly on and over the protein trace that is deposited on the membrane from microchip western blotting.
Unless otherwise stated, membranes were treated with 40% (v/v) glutaraldehyde for 10 s to cross-link proteins and then rinsed for 1 min and 50 s with water prior to beginning the immunoassay. After cross-linking, the following procedure was used: 1) block with Intercept™ protein-free blocking buffer (TBS) with a 0.6 mL/min deposition flow rate with stage speed for 8 mm/min back and forth for 15 min, 2) deposit primary antibody diluted 1000-fold in Intercept™ protein-free blocking buffer (TBS) 0.05% Tween 20 with a 20 μL/min deposition flow rate and 4 mm/min stage speed for 25 min, 3) wash with TBST with a 6.0 mL/min deposition flow rate and stage at 130 mm/min back and forth for 2.5 min with −0.2 bar vacuum applied to the stage to pull wash solution through the membrane, 4) deposit secondary antibody diluted 1000-fold diluted in Intercept™ protein-free blocking buffer (TBS) 0.05% Tween 20 with a 5 μL/min deposition flow rate while moving the stage at 70 mm/min back and forth for 15 min, and 5) repeat step 3. Tests revealed that blocking with Intercept™ protein-free blocking buffer (TBS) could also be applied with gentle shaking for 15 min. Washing with TBST could also be achieved by using a pipette to spray on.
For some experiments no cross-linking was used. This method follows that of the flow method described above except that washing was done four times at 2.5 min each with TBST and secondary antibody diluted 1000-fold in Intercept™ protein-free blocking buffer (TBS) and 0.05% Tween 20 was incubated for 1 h.
Detection on membrane
After separation and transfer of the sample mixed with FITC-protein ladder, the fluorescent ladder was detected on the membrane using an Amersham Typhoon NIR Plus (GE Healthcare, Pittsburgh, PA) with 488 nm fluorescence measurement. After the immunoassay, the membrane was dried at room temperature and imaged on an Odyssey® near-IR fluorescence imager at 700 nm (LI-COR Bioscience, Lincoln, NE). Line scans were generated using ImageJ (NIH) and imported into Cutter 7.0 software50 where a median filter at 4.0 pixels was used. After a baseline correction, the noise was selected from the baseline in between peaks. Peak heights were measured for calculating signal-to-noise (S/N) ratio. Data was plotted in GraphPad Prism 7 where an average and standard deviation were calculated. A relative standard deviation (RSD) was plotted as an error bar. For dot blots, the average and RSDs were generated from multiple protein spots on the same membrane, minimally n = 3 protein spots. For microchip western blotting, data was averaged from three injections of the same sample. All statistical analyses were performed in GraphPad Prism 7. For statistical analysis between traditional and flow immunoassay data of the same sample, an unpaired Student’s t test was applied where differences deemed significant if P < 0.01.
Results and Discussion
Microscale immunoassay method
In surface-based immunoassay methods, antibody diffusion is the rate determining step of the assay; as a result, long incubation times are used to achieve maximum detection sensitivity.25–32 Mechanisms of mass transport for analytes in a solution reacting with surface binding targets include diffusion based on a concentration gradient, diffusion in a temperature gradient (thermal), diffusion in a pressure gradient, and diffusion aided by external forces acting on a chemical species/analytes including free and forced convection.51–53 We used flow deposition to achieve convective transport and reduced diffusion distance resulting in shorter time scales for antibody binding. Figure 1 shows the 3D printed tubing mount and vacuum stage that was used to perform fast immunoassays. The membrane is placed on the vacuum stage and flow deposition of the immunoassay solutions occurs through a syringe connected to tubing as depicted in Figure 2.
We developed conditions for the assay by performing dot blots of 3.3 μg/mL actin dissolved in water and varying immunoassay solution deposition conditions. We found that depositing primary antibody at 20 μL/min with a stage speed of 4 mm/min gave comparable signal intensity to a traditional overnight immunoassay method (Figure S1). The total primary antibody binding time was 25 min and total primary antibody used was 175 ng, a 40-fold and 30-fold reduction respectively over the conventional method.
We next examined the secondary antibody deposition step. Flow deposition at 20 μL/min with stage speeds from 2–8 mm/min, resulted in poor signal and high background and noise levels. Specifically, under these conditions we observed formation of large fluorescent spots on the binding membrane that could not easily be removed. It was determined that using lower flow rates (3.5–7.5 μL/min) while the stage moved back and forth at 70 mm/min so that the membrane and protein samples pass through the secondary antibody several times, resulted in much less formation of these background spots and comparable sensitivity to conventional immunoassays (Figure S2). Using 5 μL/min, the total secondary antibody deposition time was 15 min and total secondary antibody used was 75 ng, a 4-fold and 27-fold reduction respectively over conventional method.
It is unclear why different conditions were required for the primary and secondary antibody deposition; however, differences are also seen in traditional immunoassay method that seem to correlate with our observations. Incubation times for secondary antibodies are typically much shorter (1 h) than primary antibodies (overnight). Fluorescent secondary antibodies are typically used at more dilute concentrations than primary antibody as well. Several factors are likely to contribute to the differences primary and secondary antibody binding. The binding kinetics of secondary to primary antibody may differ from those of primary antibody to surface-bound antigen. The presence of fluorescent tags provides hydrophobic groups on the secondary antibody that may also affect the potential for non-specific binding and aggregation to alter background signals.
Regardless of the reason for the differences, using these deposition methods, and rinsing in between each deposition while with using vacuum to pull solutions through the membrane as shown in Figure 2, a complete dot blot immunoassay is completed in 1 h (Table 1). S/N ratios obtained by this abbreviated procedure are comparable to those obtained using a 20 h analysis time (Figure 3).
Table 1.
Comparison of traditional and fast immunoassay method analysis times and antibody consumption for dot blotting membranes.
| Traditional Immunoassay | Flow Immunoassay | |
|---|---|---|
| Time Per Step | ||
| Blocking | 1 h | 15 min |
| Primary Antibody | 17 h | 25 min |
| Washing | 20 min | 2.5 min |
| Secondary Antibody | 1 h | 15 min |
| Washing | 20 min | 2.5 min |
| Total Time | 20 h | 1 h |
| Antibody Consumption | ||
| Primary Antibody | 5 μg | 175 ng |
| Secondary Antibody | 2 μg | 75 ng |
These values are based on a membrane trace that is 7-cm in length.
Figure 3.
Comparison between a 20 h, traditional immunoassay (a) and the developed 1 h, fast immunoassay (b) for dot blots of 3.3 μg/mL actin. The S/N (c), signal (d), and noise (e) values calculated and plotted with n=3 protein spots are displayed showing comparable detection between the two immunoassay methods. Unpaired two-tailed Student’s t-test statistics were performed with p < 0.01 to compare the S/N (c) of the traditional and flow immunoassay results in which the two data sets are not significantly different.
Microchip western blotting with direct deposition immunoassay
The flow immunoassay method was then applied to proteins deposited by microchip gel electrophoresis. Figure 4 displays a schematic and image of microchip western blotting. The sample is introduced using a gated, timed injection after which proteins migrate through the separation channel. In this work, an outer set of sheath flow are used to ground the end of the separation channel while the inner sheath flow channels are used to deposit separated proteins onto a nitrocellulose membrane strip. Pilot experiments using this system to deposit actin onto a member unexpectedly resulted in low or no signal when using the fast immunoassay described above. To determine cause of this loss in signal, we performed the flow process for all steps except secondary antibody addition, which was performed on a shaker for 1 h. This modified flow method allowed detection of proteins from microchip western blotting but with a reduced S/N compared to the traditional, 20 h immunoassay (Figure S3). These results suggested that the protein was not captured effectively with the flow deposition system in the presence of gel and was hypothesized to be due to the effect of gel on the interactions of proteins with the binding membrane. To test this hypothesis, experiments comparing dot blots of protein diluted in water and gel solution were performed. These experiments showed lower signal intensity for the gel dot blots with the fast immunoassay approach (Figure S4) supporting the hypothesis that the gel contributed to loss of signal.
To address the loss of proteins co-deposited with gel sieving matrix, glutaraldehyde was used to cross-link proteins for membrane fixation, a method used in some cases with conventional western blotting.41,42,45,46 Cross-linking for the improvement of protein retention on binding membranes is attributed to result from multi-point attachments of the cross-linked proteins.43,46,48 In prior western blot methods, treatment with 0.5% (v/v)-2.5% (v/v) glutaraldehyde for 15–60 min was used to improve protein retention on blotting membranes.42,43,45,46 We have recently used 40% (v/v) glutaraldehyde to achieve cross-linking in 10 s for protein-protein interaction (PPI) studies.54 Inspired by this approach, we found that exposing the blotted proteins and membrane to 40% (v/v) glutaraldehyde for 10 s and rinsing with water for ~ 2 min prior to the microscale immunoassay mitigated protein loss issues so that results for microchip western blotting membranes were comparable between a traditional and a flow immunoassay (Figure 5). Figure S3 compares the initial S/N level achieved with a modified (1.75 h) immunoassay, with a non-cross-linked (1 h) method, and a cross-linked (1 h) flow method with a traditional (20 h) immunoassay showing that glutaraldehyde cross-linking is effective at preventing loss of proteins from the gel trace of microchip western blotting.
Figure 5.
Comparison between a traditional immunoassay (a) and the cross-linked (X-link) flow immunoassay (b) for microchip western blotting of 3.3 μg/mL actin. Membrane images and line scans from ImageJ are shown (black trace), where the line scan of the FITC-protein ladder (red trace) are overlaid. The arbitrary units of the signal intensities of the FITC-protein ladder were divided by 250 to scale the ladder for the plot above. The error bars represent the standard deviation of the protein detected by the various immunoassay methods for repeated injections, separations, and depositions of the protein sample from the microchip. The S/N (c), signal (d), and noise (e) values calculated and plotted with n=3 injected and detected protein peaks are displayed showing comparable detection between the two immunoassay methods. The relationship between S/N and actin concentration for a traditional immunoassay and X-link flow immunoassay gives an R2 equal to 0.9995 and 0.996, respectively (f). Limits of detection were calculated accounting for limit of the blank with an LOD of 2 nM for traditional immunoassay and a LOD of 7 nM for X-link flow immunoassay. Unpaired two-tailed Student’s t-test statistics were performed with p < 0.01 to compare the S/N (c) of the traditional and flow immunoassay results in which the two data sets are not significantly different.
A calibration curve of microchip western blotting for actin by the traditional and cross-linked flow immunoassays yields a linear response (Figure 5). From these data, concentration limits of detection (LODs) were calculated as 2 nM for the traditional immunoassay and 7 nM for the cross-linked flow immunoassay. Since we estimate 4.5 nL were injected, this concentration corresponds to a mass LOD of 9 amol or 400 fg for the traditional immunoassay and 30 amol or 1 pg for the cross-linked flow immunoassay. Of course, 20 μL were used to fill the reservoir, so more sample was used for the assay. Miniaturized sample preparation and loading methods would be needed to take full advantage of the mass LOD that is possible.
Table 2 summarizes the cross-linked, flow immunoassay method. Further experiments revealed that if some speed could be sacrificed, the processing steps that impact the protein loss from reduced adherence of proteins from the microchip deposition gel trace can be modified so that a 2 h immunoassay method is used for protein detection without cross-linking, as summarized in Table 2. In this case, the secondary antibody is incubated for 1 h with shaking similar to a traditional immunoassay. These different flow immunoassay methods allow for the flexibility of either using a cross-linking step prior to the immunoassay or not. The non-cross-linked method could be useful if cross-linking inhibits antibody binding, although this has not been seen or previously reported by other cross-linking western techniques.48–55
Table 2.
Comparison of traditional, modified microscale, and fast immunoassay methods analysis times and antibody consumption for microchip western blotting membranes.
| Traditional Immunoassay | Modified Flow Immunoassay | X-link Flow Immunoassay | |
|---|---|---|---|
| Time Per Step | |||
| Cross-Linking | - | - | 10 s |
| Rinsing | - | - | 110 s |
| Blocking | 1 h | 15 min | 15 min |
| Primary Antibody | 17 h | 25 min | 25 min |
| Washing | 20 min | 10 min | 2.5 min |
| Secondary Antibody | 1 h | 1 h | 15 min |
| Washing | 20 min | 10 min | 2.5 min |
| Total Time | 20 h | 2 h | 1 h 2 min |
| Antibody Consumption | |||
| Primary Antibody | 5 μg | 175 ng | 175 ng |
| Secondary Antibody | 2 μg | 10 μg | 75 ng |
These values are based on a membrane trace that is 7-cm in length.
We further tested the method on different proteins. A comparison of microchip western blots for detection of GAPDH from A431 cell lysate using the traditional (20 h) immunoassay, the microscale, non-cross-linked (2 h) immunoassay, and the cross-linked, flow (1 h) immunoassay are shown in Figure 6. As shown, comparable S/N is achieved for all methods; but the cross-linked flow method reduces assay time by 20-fold and reagent usage by ~30-fold. We also compared results to a 20 h immunoassay method for the detection of β-Tubulin in A431 cell lysate (Figure 7). As shown, comparable results were obtained for these proteins from lysate as well. These results suggest that the flow method can be reliably used for different proteins with minimal method development.
Figure 6.
Microchip western blotting of 100 μg/mL total protein A431 cell lysate was done for the detection of GAPDH. A comparison between a traditional immunoassay (a), a modified flow immunoassay (b) and the cross-linked (X-link) flow immunoassay (c) for detection of GAPDH are displayed. Membrane images and line scans from ImageJ are shown (black trace), where the line scan of the FITC-protein ladder (red trace) are overlaid. The arbitrary units of the signal intensities of the FITC-protein ladder were divided by 2500 to scale the ladder for the plot above. The error bars represent the standard deviation of the protein detected by the various immunoassay methods for repeated injections, separations, and depositions of the protein sample from the microchip. The FITC-protein ladder molecule weight labels 1–7 stand for: 11, 21, 32, 40, 63, 96, and 155 kDa. The S/N (d), signal (e), and noise (f) values calculated and plotted with n=3 injected and detected protein peaks are displayed showing comparable detection between the two immunoassay methods. Unpaired two-tailed Student’s t-test statistics were performed with p < 0.01 to compare the S/N (d) of the traditional and flow immunoassay results in which the 20 h and 1 h data sets are not significantly different.
Figure 7.
Microchip western blotting of 100 μg/mL total protein A431 cell lysate was done for the detection of β-Tubulin. A comparison between a traditional immunoassay (a), a modified flow immunoassay (b) and the cross-linked (X-link) flow immunoassay (c) for detection of β-Tubulin are displayed. Membrane images and line scans from ImageJ are shown (black trace), where the line scan of the FITC-protein ladder (red trace) are overlaid. The arbitrary units of the signal intensities of the FITC-protein ladder were divided by 2500 to scale the ladder for the plot above. The error bars represent the standard deviation of the protein detected by the various immunoassay methods for repeated injections, separations, and depositions of the protein sample from the microchip. The FITC-protein ladder molecule weight labels 1–7 stand for: 11, 21, 32, 40, 63, 96, and 155 kDa. The S/N (c), signal (d), and noise (e) values calculated and plotted with n=3 injected and detected protein peaks are displayed showing comparable detection between the two immunoassay methods. Unpaired two-tailed Student’s t-test statistics were performed with p < 0.01 to compare the S/N (c) of the traditional and flow immunoassay results in which the two data sets are not significantly different.
Conclusions
We have developed a microscale immunoassay method that achieves comparable S/N for the detection of proteins from microchip western blotting. This development has resulted in a microfluidic western blotting system that can detect a protein in under 1.5 h with 100-fold reduction in sample requirements and ~30-fold reduction in antibody reagents, while using conventional blotting materials and reagents. Further adaptations of this work could include parallelization of the fast, direct deposition immunoassay. This could easily be achieved in the current set-up to allow for four parallel immunoassays to be run simultaneously. Altogether, our microfluidic western blotting methods will allow for improved analysis throughput and ease for multiplexed detection of proteins. The investigation and development of microfluidic techniques for western blotting brings forth a next generation of techniques for protein detection to overcome the limitations of conventional western blotting methods. With the use of glutaraldehyde cross-linking, it may be necessary to find antibody that can bind with cross-linked antigens. Thus far, this issue has not been reported. Further development of the method would involve application to other biological sources, targets, and a broader range of molecular weights.
Supplementary Material
Acknowledgements
This work is supported by NIH grant R37 DK046960 and 1R43GM112289–01. We thank Lakshmi Pillai (LI-COR Biosciences) for supplying cell lysate samples.
Footnotes
Conflict of interest
The authors declare the following financial interest(s): We have a patent on the technology described and an industrial partner may commercialize this technology. Therefore, some financial gain is possible from the development of this technology.
References
- (1).Towbin H; Staehelin T; Gordon J. Electrophoretic Transfer of Proteins from Polyacrylamide Gels to Nitrocellulose Sheets: Procedure and Some Applications. Proc. Natl. Acad. Sci 1979, 76 (9), 4350–4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Mishra M; Tiwari S; Gomes AV Protein Purification and Analysis: Next Generation Western Blotting Techniques. Expert Rev. Proteomics 2017, 14 (11), 1037–1053. 10.1080/14789450.2017.1388167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Anderson GJ; M. Cipolla C; Kennedy RT Western Blotting Using Capillary Electrophoresis. Anal. Chem 2011, 83 (4), 1350–1355. 10.1021/ac102671n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Castro ER; Manz A. Present State of Microchip Electrophoresis: State of the Art and Routine Applications. J. Chromatogr. A 2015, 1382, 66–85. 10.1016/j.chroma.2014.11.034. [DOI] [PubMed] [Google Scholar]
- (5).Kurien BT; Scofield RH Western Blotting. Methods San Diego Calif 2006, 38 (4), 283–293. 10.1016/j.ymeth.2005.11.007. [DOI] [PubMed] [Google Scholar]
- (6).iBindTM Western Systems. https://www.thermofisher.com/us/en/home/life-science/protein-biology/protein-assays-analysis/western-blotting/detect-proteins-western-blot/ibind-western-system.html.
- (7).BlotCyclerTM Touch - Precision Biosystems-Automated, Reproducible Western Blot Processing and DNA, RNA Analysis. Precision Biosystems-Automated, Reproducible Western Blot Processing and DNA, RNA analysis. [Google Scholar]
- (8).GOBlot - The First Affordable Western Blot Processor https://www.cytoskeleton.com/western-blot-processor-goblot (accessed Mar 1, 2018).
- (9).SNAP i.d.® 2.0. Protein Detection System. http://www.emdmillipore.com/US/en/product/SNAP-i.d.−2.0-Protein-Detection-System,MM_NF-C73105.
- (10).Mazet F; Dunster JL; Jones CI; Vaiyapuri S; Tindall MJ; Fry MJ; Gibbins JM A High-Density Immunoblotting Methodology for Quantification of Total Protein Levels and Phosphorylation Modifications. Sci. Rep 2015, 5, 16995 10.1038/srep16995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Sanders BJ; Kim DC; Dunn RC Recent Advances in Microscale Western Blotting. Anal. Methods 2016, 8 (39), 7002–7013. 10.1039/C6AY01947A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Jin S; Kennedy RT New Developments in Western Blot Technology. Chin. Chem. Lett 2015, 26 (4), 416–418. 10.1016/j.cclet.2015.01.021. [DOI] [Google Scholar]
- (13).Jin S; Furtaw MD; Chen H; Lamb DT; Ferguson SA; Arvin NE; Dawod M; Kennedy RT Multiplexed Western Blotting Using Microchip Electrophoresis. Anal. Chem 2016, 88 (13), 6703–6710. 10.1021/acs.analchem.6b00705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Jin S; Anderson GJ; Kennedy RT Western Blotting Using Microchip Electrophoresis Interfaced to a Protein Capture Membrane. Anal. Chem 2013, 85 (12), 6073–6079. 10.1021/ac400940x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Rodríguez-Ruiz I; Babenko V; Martínez-Rodríguez S; Gavira JA Protein Separation under a Microfluidic Regime. Analyst 2017. 10.1039/C7AN01568B. [DOI] [PubMed] [Google Scholar]
- (16).Štěpánová S; Kašička V. Analysis of Proteins and Peptides by Electromigration Methods in Microchips. J. Sep. Sci 2017, 40 (1), 228–250. 10.1002/jssc.201600962. [DOI] [PubMed] [Google Scholar]
- (17).Dawod M; Arvin NE; Kennedy RT Recent Advances in Protein Analysis by Capillary and Microchip Electrophoresis. Analyst 2017, 142 (11), 1847–1866. 10.1039/C7AN00198C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Gerver RE; Herr AE Microfluidic Western Blotting of Low-Molecular-Mass Proteins. Anal. Chem 2014, 86 (21), 10625–10632. 10.1021/ac5024588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Hughes AJ; Spelke DP; Xu Z; Kang C-C; Schaffer DV; Herr AE Single-Cell Western Blotting. Nat. Methods 2014, 11 (7), 749–755. 10.1038/nmeth.2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Nguyen U; Squaglia N; Boge A; Fung PA The Simple WesternTM: A Gel-Free, Blot-Free, Hands-Free Western Blotting Reinvention. Nat. Methods 2011, 8 (11). 10.1038/nmeth.f.353. [DOI] [Google Scholar]
- (21).2100 Bioanalyzer Instruments. http://www.genomics.agilent.com/en/product.jsp?cid=AG-PT-106&_requestid=1285828.
- (22).Wenz C; Rüfer A. Microchip CGE Linked to Immunoprecipitation as an Alternative to Western Blotting. ELECTROPHORESIS 2009, 30 (24), 4264–4269. 10.1002/elps.200900347. [DOI] [PubMed] [Google Scholar]
- (23).Hamm M; Ha S; Rustandi RR Automated Capillary Western Dot Blot Method for the Identity of a 15-Valent Pneumococcal Conjugate Vaccine. Anal. Biochem 2015, 478, 33–39. 10.1016/j.ab.2015.03.021. [DOI] [PubMed] [Google Scholar]
- (24).Loughney JW; Ha S; Rustandi RR Quantitation of CRM197 Using Imaged Capillary Isoelectric Focusing with Fluorescence Detection and Capillary Western. Anal. Biochem 2017, 534, 19–23. 10.1016/j.ab.2017.06.013. [DOI] [PubMed] [Google Scholar]
- (25).Ahmad AL; Low SC; Shukor SRA; Fernando WJN; Ismail A. Hindered Diffusion in Lateral Flow Nitrocellulose Membrane: Experimental and Modeling Studies. J. Membr. Sci 2010, 357 (1), 178–184. 10.1016/j.memsci.2010.04.018. [DOI] [Google Scholar]
- (26).Kapur V; Charkoudian J; Anderson JL Transport of Proteins through Gel-Filled Porous Membranes. J. Membr. Sci 1997, 131 (1), 143–153. 10.1016/S0376-7388(97)00037–9. [DOI] [Google Scholar]
- (27).Charcosset C; Yousefian F; Thovert J-F; Adler PM Calculation of Flow and Solute Deposition through Three-Dimensional Reconstructed Model of Microporous Membranes. Desalination 2002, 145 (1), 133–138. 10.1016/S0011-9164(02)00398-3. [DOI] [Google Scholar]
- (28).Thomas N; Jones CN; Thomas PL Low Volume Processing of Protein Blots in Rolling Drums. Anal. Biochem 1988, 170 (2), 393–396. 10.1016/0003-2697(88)90650-1. [DOI] [PubMed] [Google Scholar]
- (29).Giri B; Pandey B; Neupane B; Ligler FS Signal Amplification Strategies for Microfluidic Immunoassays. TrAC Trends Anal. Chem 2016, 79, 326–334. 10.1016/j.trac.2015.10.021. [DOI] [Google Scholar]
- (30).Liu Y; Yu J; Du M; Wang W; Zhang W; Wang Z; Jiang X. Accelerating Microfluidic Immunoassays on Filter Membranes by Applying Vacuum. Biomed. Microdevices 2012, 14 (1), 17–23. 10.1007/s10544-011-9581-z. [DOI] [PubMed] [Google Scholar]
- (31).Pollard TD A Guide to Simple and Informative Binding Assays. Mol. Biol. Cell 2010, 21 (23), 4061–4067. 10.1091/mbc.E10-08-0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Kusnezow W; Syagailo YV; Rüffer S; Baudenstiel N; Gauer C; Hoheisel JD; Wild D; Goychuk I. Optimal Design of Microarray Immunoassays to Compensate for Kinetic Limitations: Theory and Experiment. Mol. Cell. Proteomics 2006, 5 (9), 1681–1696. 10.1074/mcp.T500035-MCP200. [DOI] [PubMed] [Google Scholar]
- (33).Liu C-Y; Lu D-C; Jiang Y-W; Yen Y-K; Chang S-C; Wang A-B Easy and Fast Western Blotting by Thin-Film Direct Coating with Suction. Anal. Chem 2016, 88 (12), 6349–6356. 10.1021/acs.analchem.6b00699. [DOI] [PubMed] [Google Scholar]
- (34).He S; Zhang Y; Wang P; Xu X; Zhu K; Pan W; Liu W; Cai K; Sun J; Zhang W; et al. Multiplexed Microfluidic Blotting of Proteins and Nucleic Acids by Parallel, Serpentine Microchannels. Lab. Chip 2014, 15 (1), 105–112. 10.1039/C4LC00901K. [DOI] [PubMed] [Google Scholar]
- (35).Chang H-N; Leroueil PR; Selwa K; Gasper CJ; Tsuchida RE; Wang JJ; McHugh WM; Cornell TT; Jr JRB; Goonewardena SN Profiling Inflammatory Responses with Microfluidic Immunoblotting. PLOS ONE 2013, 8 (11), e81889. 10.1371/journal.pone.0081889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Qi L-Y; Yin X-F; Liu J-H Rapid and Efficient Isotachophoretic Preconcentration in Free Solution Coupled with Gel Electrophoresis Separation on a Microchip Using a Negative Pressure Sampling Technique. J. Chromatogr. A 2009, 1216 (20), 4510–4516. 10.1016/j.chroma.2009.03.034. [DOI] [PubMed] [Google Scholar]
- (37).Heiger DN; Cohen AS; Karger BL Separation of DNA Restriction Fragments by High Performance Capillary Electrophoresis with Low and Zero Crosslinked Polyacrylamide Using Continuous and Pulsed Electric Fields. J. Chromatogr. A 1990, 516 (1), 33–48. 10.1016/S0021-9673(01)90202-X. [DOI] [PubMed] [Google Scholar]
- (38).Wu D; Regnier FE Sodium Dodecyl Sulfate-Capillary Gel Electrophoresis of Proteins Using Non-Cross-Linked Polyacrylamide. J. Chromatogr. A 1992, 608 (1), 349–356. 10.1016/0021-9673(92)87142-U. [DOI] [PubMed] [Google Scholar]
- (39).Chinnasamy T; Segerink LI; Nystrand M; Gantelius J; Svahn HA Point-of-Care Vertical Flow Allergen Microarray Assay: Proof of Concept. Clin. Chem 2014, 60 (9), 1209–1216. 10.1373/clinchem.2014.223230. [DOI] [PubMed] [Google Scholar]
- (40).Lin R; Skandarajah A; Gerver RE; Neira HD; Fletcher DA; Herr AE A Lateral Electrophoretic Flow Diagnostic Assay. Lab Chip 2015, 15 (6), 1488–1496. 10.1039/C4LC01370K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Karey KP; Sirbasku DA Glutaraldehyde Fixation Increases Retention of Low Molecular Weight Proteins (growth Factors) Transferred to Nylon Membranes for Western Blot Analysis. Anal. Biochem 1989, 178 (2), 255–259. 10.1016/0003-2697(89)90634-9. [DOI] [PubMed] [Google Scholar]
- (42).Mizzen CA; Cartel NJ; Yu WH; Fraser PE; McLachlan DR Sensitive Detection of Metallothioneins-1, −2 and −3 in Tissue Homogenates by Immunoblotting: A Method for Enhanced Membrane Transfer and Retention. J. Biochem. Biophys. Methods 1996, 32 (2), 77–83. [DOI] [PubMed] [Google Scholar]
- (43).Jeon J-H; Cho S-Y; Kim C-W; Shin D-M; Kwon J-C; Choi K-H; Kim I-G Improved Immunodetection of Human Papillomavirus E7. Exp. Mol. Med 2002, 34 (6), 496 10.1038/emm.2002.69. [DOI] [PubMed] [Google Scholar]
- (44).Suzuki Y; Takeda Y; Ikuta T. Immunoblotting Conditions for Human Hemoglobin Chains. Anal. Biochem 2008, 378 (2), 218–220. 10.1016/j.ab.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Van Eldik LJ; Wolchok SR Conditions for Reproducible Detection of Calmodulin and S100 Beta in Immunoblots. Biochem. Biophys. Res. Commun 1984, 124 (3), 752–759. [DOI] [PubMed] [Google Scholar]
- (46).Nestal de Moraes G; Carvalho É; Maia RC; Sternberg C. Immunodetection of Caspase-3 by Western Blot Using Glutaraldehyde. Anal. Biochem 2011, 415 (2), 203–205. 10.1016/j.ab.2011.04.032. [DOI] [PubMed] [Google Scholar]
- (47).Lee BR; Kamitani T. Improved Immunodetection of Endogenous α-Synuclein. PLOS ONE 2011, 6 (8), e23939. 10.1371/journal.pone.0023939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Newman AJ; Selkoe D; Dettmer U. A New Method for Quantitative Immunoblotting of Endogenous α-Synuclein. PLOS ONE 2013, 8 (11), e81314. 10.1371/journal.pone.0081314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Ermakov SV; Jacobson SC; Ramsey JM Computer Simulations of Electrokinetic Transport in Microfabricated Channel Structures. Anal. Chem 1998, 70 (21), 4494–4504. 10.1021/ac980551w. [DOI] [PubMed] [Google Scholar]
- (50).Shackman JG; Watson CJ; Kennedy RT High-Throughput Automated Post-Processing of Separation Data. J. Chromatogr. A 2004, 1040 (2), 273–282. 10.1016/j.chroma.2004.04.004. [DOI] [PubMed] [Google Scholar]
- (51).Chaiken I; Rosé S; Karlsson R. Analysis of Macromolecular Interactions Using Immobilized Ligands. Anal. Biochem 1992, 201 (2), 197–210. [DOI] [PubMed] [Google Scholar]
- (52).Modeling Micropatterned Antigen–antibody Binding Kinetics in a Microfluidic Chip. Biosens. Bioelectron 2007, 22 (7), 1403–1409. 10.1016/j.bios.2006.06.017. [DOI] [PubMed] [Google Scholar]
- (53).Bird RB; Lightfoot EN; Stewart WE Notes on Transport Phenomena. [By Bird RB, Stewart Warren E. and Lightfoot Edwin N.; New York; Chapman & Hall: London: John Wiley & Sons, 1958. [Google Scholar]
- (54).Ouimet CM; Dawod M; Grinias J; Assimon VA; Lodge J; Mapp AK; Gestwicki JE; Kennedy RT Protein Cross-Linking Capillary Electrophoresis at Increased Throughput for a Range of Protein–protein Interactions. Analyst 2018, 143 (8), 1805–1812. 10.1039/C7AN02098H. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.