Abstract
Adiponectin is one of the most widely studied adipokines to date. First described in the mid-1990’s, studying its regulation, biogenesis and physiological effects has proven to be extremely insightful and improved our understanding of the mechanisms that ensure systemic metabolic homeostasis. Here, we provide a brief overview of the current state of the field with respect to adiponectin, its history, sites and mechanisms of action, and the critical questions that will need to be addressed in the future.
Introduction
Produced and secreted predominantly by fat cells in adipose tissue, adiponectin exerts pleiotropic effects on numerous tissues, including the liver 1,2, kidney 3, pancreatic β–cells 4, blood vessels 5, brain 6, bone 7 and immune cells 8, prior to its clearance in hepatocytes 9. The gene encoding adiponectin and its protein product has been extensively studied over decades in almost 20,000 publications. It would be an overwhelming task to summarize all the published data regarding adiponectin in a short overview. We will therefore limit ourselves to a brief synopsis of the history of the discovery of adiponectin, the regulation of its production and the critical effects on its target cells and tissues 10-12.
The discovery of adiponectin’s function
In 1995, a subtractive cloning approach targeted at enriching for cDNAs present in 3T3-L1 adipocytes (compared to 3T3-L1 fibroblasts), led to the identification of Adipose Complement Related Protein of 30 kDa (Acrp30) (Fig. 1)13. Shortly thereafter, other groups independently cloned the same gene using other approaches, referring to it as AdipoQ 14, apM1 15 or GBP28 16. A consensus name subsequently emerged, adiponectin, a name proposed by Matsuzawa and colleagues 17.
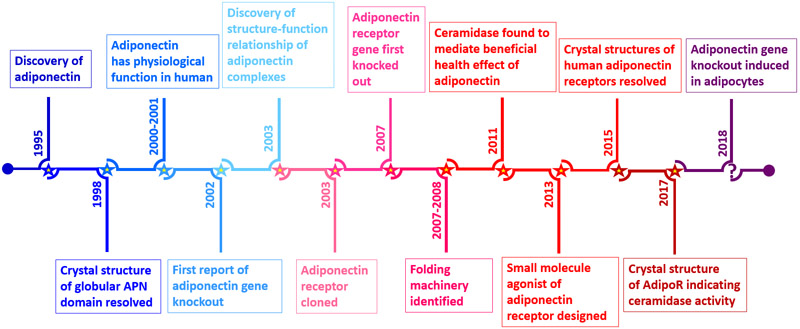
Figure 1: Timeline of the discovery of adiponectin.
First discovered in 1995, adiponectin’s physiological function in humans became soon apparent (2000-2001). Its receptor was discovered in 2003 and important signaling pathways were established. Not only were the genetic deletions of both adiponectin (2002 and 2018) and its receptors AdipoR1/2 (2007) published, but the pleiotropic effects of adiponectin was shown to be mediated by ceramidase function. The small molecule AdipoRon was the first AdipoR agonist (2013). The initial crystal structure of the AdipoR published in 2015 was further refined in 2017, which revealed that it contains an active site reminiscent of an enzymatic function consistent with a ceramidase activity within the receptor itself.
Adiponectin contains four major domains, including an N-terminal signal peptide domain, a hypervariable domain, a collagenous domain, and a carboxy-terminal globular domain 18. Upon solving the structure of adiponectin’s globular domain, an unexpected structural homology with protein members of the tumor necrosis factor family became apparent 19, which could not have been predicted based on the primary amino acid sequence.
Several congenital genetic deletions of the adiponectin gene were reported in rodents. Adiponectin knockout (KO) mice displayed a deterioration of insulin sensitivity upon feeding of chow or high-fat diets 20,21. Conversely, one group reported only a rather moderate phenotype, which in some aspects contradicted the previous findings 22. The phenotype of an inducible adipose tissue-specific KO allele was recently documented, which was very much consistent with the original diabetic phenotypes apparent in the congenital systemic KO mice, albeit even more pronounced 23. Collectively, this suggests that some of the compensatory mechanisms may mask the phenotype when mice are missing adiponectin developmentally. In several different genetically obese and diabetic mouse models, injecting adiponectin can improve diabetic symptoms, primarily by improving lipid homeostasis 1,2. Importantly, these potent gluco- and lipo-regulatory effects of adiponectin are conserved between mice, non-human primates and humans 24,25.
A major breakthrough in the study of the molecular mechanism of adiponectin action was accomplished when Yamauchi and colleagues discovered the genes of the adiponectin receptors 1 and 2 (Adipor1 and Adipor2) 26. Between rodents and humans, the receptors are strongly conserved. Congenital deletions of Adipor1 and Adipor2 resulted in a disruption of the main adiponectin signaling events in target cells 27, thereby leading to insulin resistance and glucose intolerance. On the other side the ADIPOR agonist- called AdipoRon- improves diabetic symptoms 28. The crystal structure of human ADIPORs revealed that the seven transmembrane spanning domains of ADIPOR1 and 2 form a cavity that directs three histidine residues to coordinate a zinc ion. The structure of these adiponectin receptors is however distinct from G-protein coupled receptors (GPCRs), as the N-terminus is cytoplasmic and the C-terminus is extracellular 29. A more refined structural analysis revealed a ceramidase domain present within the receptors 30, confirming previous reports demonstrating potent ceramide lowering effects associated with adiponectin action 31,32. In fact, the anti-apoptotic and anti-lipotoxic effects of adiponectin on cardiac myocytes and pancreatic β-cells, in addition to its insulin sensitizing properties on hepatocytes, relate to the ceramidase activity that adiponectin triggers within target cells 31.
Adiponectin in Disease
Clinical studies have implicated adiponectin as a possible causative factor in the etiology of multiple diseases. In 1999, adiponectin drew much attention as the first (and so far only) adipocyte-derived marker in plasma that shows an inverse correlation with fat mass (Fig. 1) 17; thus distinguishing it from all other adipokines (including leptin) which display a positive correlation with fat mass 33. Numerous additional studies further established inverse correlations of plasma adiponectin with several clinical pathophysiological disease states, such as type 2 diabetes (T2D), both prospectively and cross-sectionally 34,35, as well as coronary artery disease 36 and myocardial infarction 37.
Beneficial actions of adiponectin were also directly shown in rodent models. It became apparent that an increase in adiponectin levels could be therapeutically useful 38. One highly effective approach to increase the circulating levels of adiponectin is through the exposure to the anti-diabetic class of agents referred to as thiazolidinediones (TZDs), which serve as agonists of the transcription factor peroxisome proliferator activated receptor γ (PPARγ). In fact, the anti-diabetic actions of these TZDs are critically dependent on their ability to induce adiponectin 1. Similar effects of TZDs have also been observed in clinical studies 39.
A high degree of local fibrosis and inflammation are at the root of metabolic disorders of insulin resistance. These detrimental effects go hand-in-hand with a significant reduction in adiponectin production and secretion. As such, the circulating levels of adiponectin can serve as a critical marker of adipose tissue health and reflect the tissue’s overall metabolic flexibility during metabolic pertubations. While healthy adipose tissue secretes more Adiponectin 40, unhealthy adipose tissue, as in the case of fibrotic or inflamed adipose tissue, secretes less adiponectin. Adiponectin is widely thought to be the major hormonal factor mediating the beneficial health effects of adipose tissue, because a genetically-driven upregulation of circulating adiponectin levels can effectively offset any negative consequences of obesity 41.
Adiponectin as marker gene for mature adipocytes
Adiponectin can also serve as an excellent marker gene to distinguish mature adipocytes from other cell-types. While the gene regulatory elements of Fabp4 (also termed aP2) mediate the gene expression pattern in macrophages 42, endothelial cells 43 and adipocyte precursors 44, adiponectin regulatory regions on the other hand, display a greater selectivity for mature adipocytes. However, despite its high degree of selectivity for mature adipocytes, adiponectin has also been identified in cell types other than adipocytes; albeit at much lower levels and only under specific conditions. These additional cell-types include cardiomyocytes 45, quiescent hepatic stellate cells 46,47, as well as specific subsets of kidney cells 48. Developmentally, the mRNA for adiponectin can be detected as early as 15-17 days of gestation 49; primarily during the development of the inguinal fat-pad. This key finding can be taken advantage of to generate mouse models that eliminate gene products exclusively in the inguinal fat pad, through transient activation of adipocyte-specific inducible Cre models in utero that maintain their knock out phenotype for the rest of the rodent’s life due to low turn-over of inguinal adipocytes 50.
Human adiponectin gene regulatory elements have been systematically studied and shown to be conserved between humans and mice 51. The most important regulatory sequences within the adiponectin gene 49 can be combined within a 5.4 kb transgenic cassette used to drive expression of various constructs in a mature adipocyte-specific fashion 52. To date, there is an ample amount of in vitro evidence identifying the key transcription factors that regulate adiponectin gene expression 53-56. In fact, PPARγ agonist treatment has been shown to increase adiponectin transcription both in vitro and in vivo 57. Insulin has also shown to regulate adiponectin levels 58,59. At the protein level, adiponectin is multimerized within the secretory pathway of the adipocyte. As such, the protein is secreted in multimeric forms, and multimerization is heavily dependent upon post-translational modifications 60,61. The smallest form of secreted adiponectin is a trimer, the intermediate form is a hexamer (LMW), as well as a high molecular weight (HMW) form with 12-18 subunits. The different multimers show varying binding affinities for the AdipoRs and their effects on a particular target tissue depends on the receptor and specific multimer bound to the receptor 62. Specifically, the HMW form has been shown to be a better correlate to insulin sensitivity, at least under some circumstances 63.
The key sites of adiponectin action
Circulating adiponectin has a plethora of effects on many different target tissues by signaling through its receptors (Fig. 2). The over-expression of either adiponectin receptor (ADIPOR1 or 2) in hepatocytes or adipocytes, results in a potent insulin sensitizing and anti-lipotoxic phenotype 65. Importantly, such effects were not observed upon overexpression of the receptors in an adiponectin null background; further substantiating a ligand / receptor interaction between adiponectin and its receptors 65. In terms of endogenous regulation, under fasted conditions, transcription of both Adipor genes is up-regulated ubiquitously, whereas refeeding has the opposite effect 66. With respect to additional potential receptors, T-cadherin is another molecule with affinity for adiponectin 67, and thus may serve as a co-receptor. T-cadherin itself is a cell surface glycoprotein with a glycosylphosphatidylinositol anchor that lacks signaling capacity, because it neither contains a transmembrane nor a cytoplasmic signaling domain 68. The tissue distribution of T-cadherin- also called CDH13 in humans- overlaps widely with that of the Adiponectin receptors 69. Interestingly, a genetic deletion of T-cadherin leads to an accumulation of adiponectin in circulation 70, a phenomenon not observed for the individual adiponectin receptor deletions.
Figure 2: Target tissues and biological activity of adiponectin:
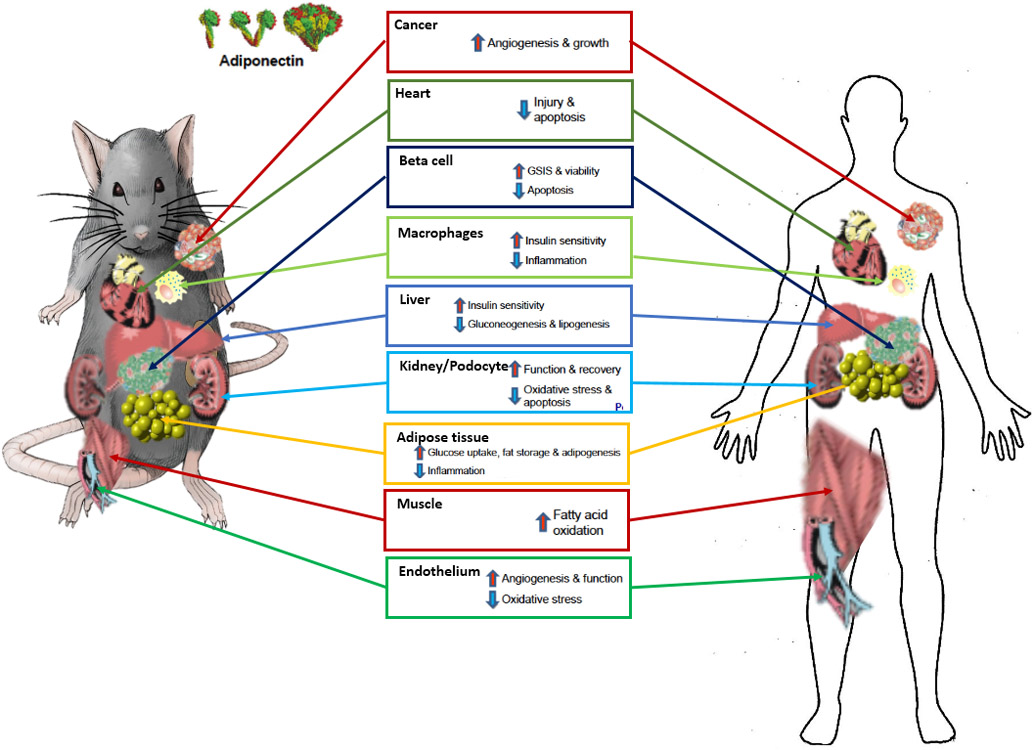
Both adiponectin as well as its receptors are highly conserved between mouse and human. Most observations were made in rodents, but are supported by strong correlational data in the clinic. The physiological effects of adiponectin are therefore strongly preserved between rodents and humans. Adiponectin forms higher order structures through multimerization. The high molecular weight multimer (HMW) of adiponectin is the most biologically active form, targeting a diverse set of tissues and cell types and regulating important metabolic processes. Adiponectin’s effects range from anti-inflammatory and anti-apoptotic to insulin sensitizing.
Adiponectin’s main functions can be categorized as anti-apoptotic, anti-inflammatory / anti-fibrotic and insulin-sensitizing. Although the key sites of adiponectin’s action are adipose tissue, heart, kidney, liver and pancreas, the ubiquitous expression of the adiponectin receptors suggests that the beneficial effects that adiponectin exerts are not restricted to a limited number of tissues (Fig. 2).
The anti-apoptotic effects of adiponectin are considerable. When cells are genetically programmed to die by activation of caspase 8, adiponectin powerfully exerts anti-apoptotic activity in diverse cells, such as in cardiomyocytes and in pancreatic β cells 31. An important question is whether adiponectin could possibly trigger the formation of cancer lesions. This is unlikely, since adiponectin is the only factor secreted by the adipose tissue that shows an inverse correlation with obesity, while obesity significantly elevates cancer risk 71,72. For breast cancer, the anti-metastatic effects of adiponectin have been attributed to the inhibition of adhesion, invasion and migration of cancer cells, which is regulated through the AMPK-S6K cell signaling axis 73. Adiponectin’s pro-angiogenic effects can however lead to enhanced tumor growth, but this effect is limited to already established tumors 74,75. As a member of the C1q/TNF superfamily, adiponectin shows not only structural homology to tumor necrosis factor alpha (TNFα), but also acts upon the immune system and the bone marrow 76. Unlike TNFα, adiponectin antagonizes inflammation by reprogramming immune cells 8. For example, adiponectin can shift Kupffer cells and other macrophages towards an anti-inflammatory phenotype 77,78.
The actions of adiponectin as an anti-fibrotic factor are seen in many tissues, particularly in the liver, kidney and adipose tissue itself. Elevated adiponectin levels protect from hepatic and kidney fibrosis 80. Furthermore, skin fibrosis is reduced as a consequence of increased adiponectin levels, while the absence of adiponectin exaggerates dermal fibrosis 81. Another key role adiponectin exerts systemically is tissue regeneration 3. Podocytes are key functional constituents in the kidney. While podocyte ablation in adiponectin deficient mice causes irreversible renal failure, the overexpression of adiponectin leads to a rapid recovery of kidney function. These regenerative effects extend to several other tissues, including pancreatic β cells where adiponectin supports β cell reconstitution after an apoptotic insult 4.
Insights into ADIPOR signaling explains how adiponectin can maintain this broad range of effects (Fig. 3). Effects on ceramide turnover constitutes the most receptor-proximal signaling events of the adiponectin receptors 30,31,82. ADIPORs were co-crystalized with a ceramide moiety. The structure of ADIPOR reveals a strong similarity to the 7 transmembrane alkaline ceramidases (ACERs) 83. In ceramidase-deficient yeast, the human ADIPOR can promote ceramidase activity 84. Ceramidases deacetylate ceramide to sphingosine, which in turn can be phosphorylated by sphingosine kinase (SphK) to S1P 85. An increased S1P:Ceramide ratio suffices to potently inhibit apoptosis and even induces proliferation. Treatment with S1P or its pharmacological mimetic FTY720 can rescue apoptosis-prone cells 31. The actions of the adiponectin receptors lead to an increase in S1P, thereby activating the S1P receptors (S1PRs). Downstream of S1PRs, the heterotrimeric G-protein Gαq mediates the ADIPOR-triggered calcium signaling by inducing phospholipase C (PLC) function. One of the products of PLC is inositol (1,4,5) triphosphate (IP3), the ligand of the IP3 receptor. This signal elicits Ca2+ release from the endoplasmic reticulum. Insulin resistant livers display a dysregulated lipogenesis that eventually leads to lipotoxicity. Insulin sensitivity is impacted by the hepatic ADIPOR signaling 1. Since high ceramide concentrations can inhibit insulin signaling 86, the reduction of hepatic ceramide concentrations reverts insulin resistance 87. Ceramides act on the insulin signal transduction cascade at several distinct levels, inhibiting protein kinase B (PKB) by activating protein kinase C ζ (PKCζ) and protein phosphatase 2A (PP2A) 87. Consistent with this model, adiponectin receptor signaling mediates translocation of GLUT4 to the plasma membrane 88, thereby leading to an increase in glucose uptake in muscle and adipose tissue. The de-repression of PKB can lead to inhibition of forkhead box O family members (FoxO’s), which positively regulate gene expression of gluconeogenic enzymes such as G6Pase and PEPCK. In addition, APPL1 (adaptor protein phosphotyrosine interacting with PH domain and leucine zipper 1) is a scaffold protein that interacts with important signaling proteins and thereby potentially links ADIPOR with insulin receptor (INSR) signaling 89,90.
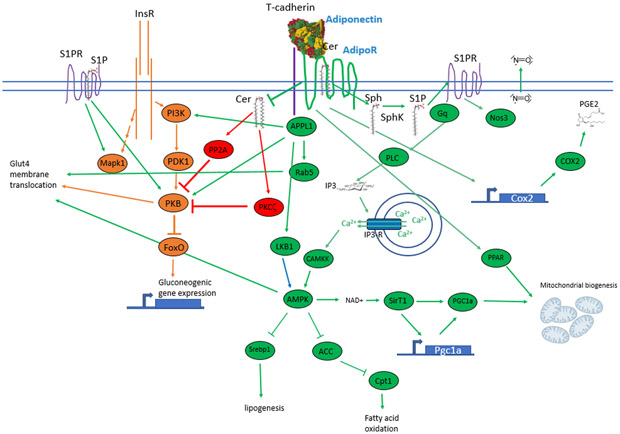
Figure 3: Downstream signaling cascade of AdipoRs:
Adiponectin binds to the AdipoR, and its binding may be enhanced by T-cadherin. AdipoR signaling targets the cellular metabolic pathways through regulation of mitochondrial biogenesis, lipogenesis and fatty acid oxidation. AdipoR signaling (green) interfaces with insulin receptor signaling (InsR) (orange), which is mediated by sphingosine-1-phosphate receptor (S1PR) and ceramide. High ceramide levels suppress insulin signaling mainly through the inactivation of serine/threonine protein phosphatase 2A (PP2A). By hydrolyzing Ceramide (Cer) to Sphingosine (Sph), AdipoR reduces Cer levels that de-repress PKB via PKCζ. De-repressed PKB inhibits FOXO and thereby downregulates gluconeogenic gene expression. Sph can be phosphorylated to sphingosine-1-phosphate (S1P) that activates S1PR which can induce the downstream mediators of InsR signaling Mapk1 and PKB. S1PR also initiates a PLC mediated IP3 downstream signal that triggers Calcium (Ca2+) resulting in activation of AMPK by CAMKK. AMPK spreads the signal across many downstream factors e.g. SirT1, Srebp1 and ACC. AdipoR induces PPARs through a yet to be elucidated pathway. The localization of glucose transporter to the plasma membrane can be impacted by Rab5, AMPK and PKB. The scaffold protein APPL1 binds important signaling mediators and thereby contributes to the crosstalk of AdipoR and InsR as well. ADIPOR also regulates the expression of Cox2 that produces prostaglandin E2 (PGE2).
More downstream signaling events of the ADIPOR include the Ca2+/calmodulin-dependent protein kinase (CAMKK) and AMP-activated protein kinase (AMPK) cascades 91. Other aspects of adiponectin’s anti-lipotoxic effects may be explained by enhanced fatty acid oxidation, which the receptors induce through enhanced activity of PPARα and PGC1α 92,93. Adiponectin’s main suppressive effects on lipogenesis in the liver are mediated by AMPK through inhibition of sterol regulatory element binding transcription factor 1 (SREBF1) and acetyl-CoA carboxylase (ACC) 26,94. Beyond AMPK signaling, prostaglandin-endoperoxide synthase 2 (Ptgs2 or Cox2) can be regulated by ADIPORs and are involved in protecting the heart from ischemia-reperfusion injury 95.
Critical questions for future research
Several questions regarding adiponectin remain to be answered. The sheer abundance of adiponectin mRNA at any given time puts the spotlight on the study of how post-transcriptional mechanism regulate adiponectin secretion. How does the metabolic state of the adipocyte, particularly with respect to the functional integrity of its mitochondria, affect adiponectin production? With respect to ADIPOR signaling, its hydrolase activity might also affect other lipid substrates beyond the established action on ceramides. More generally, does the ADIPOR hydrolase also act upon other lipid species and potentially generate activating ligands for PPARs? What is the impact of adiponectin production and its degradation on the overall protein homeostasis? How does adiponectin expressed in kidney, heart and hepatic stellate cells contribute to the physiological responses within these tissues? Finally, how is the secretion of adiponectin orchestrated with that of other adipokines? Particularly with respect to leptin, would the assessment of the combined actions of adiponectin and leptin, rather than examining these factors individually, provide better insights into how these adipokines work? Finding answers to these critical questions will certainly provide us with new insights, not only into adipose tissue physiology as a whole, but also into the critical whole-body signaling axis that maintains systemic metabolic homeostasis during obesity and insulin resistance. The quest for adiponectin receptor agonists has begun and promises to yield new pharmacological tools for anti-lipotoxic and anti-diabetic treatment regimens.
Acknowledgements
The authors were supported by US National Institutes of Health grants R01-DK55758, R01-DK099110, P01-DK088761 and P01-AG051459 as well as by a Novo Nordisk Foundation grant (to P.E.S).
Footnotes
Competing Interests
The authors report no conflicts of interest
References
- 1.Berg AH, Combs T, Du X, Brownlee M & Scherer PE The Adipocyte-Secreted Protein Acrp30 Enhances Hepatic Insulin Action. Nat Med 7, 947–953 (2001). [DOI] [PubMed] [Google Scholar]
- 2.Yamauchi T, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med 7, 941–946 (2001). [DOI] [PubMed] [Google Scholar]
- 3.Rutkowski JM, et al. Adiponectin promotes functional recovery after podocyte ablation. J Am Soc Nephrol 24, 268–282 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ye R, et al. Adiponectin is essential for lipid homeostasis and survival under insulin deficiency and promotes beta-cell regeneration. Elife 3(2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okamoto Y, et al. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation 106, 2767–2770 (2002). [DOI] [PubMed] [Google Scholar]
- 6.Qi Y, et al. Adiponectin acts in the brain to decrease body weight. Nat Med 10, 524–529 (2004). [DOI] [PubMed] [Google Scholar]
- 7.Oshima K, et al. Adiponectin increases bone mass by suppressing osteoclast and activating osteoblast. Biochem Biophys Res Commun 331, 520–526 (2005). [DOI] [PubMed] [Google Scholar]
- 8.Takemura Y, Walsh K & Ouchi N Adiponectin and cardiovascular inflammatory responses. Curr Atheroscler Rep 9, 238–243 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Halberg N, et al. Systemic Fate of the Adipocyte-Derived Factor Adiponectin. Diabetes (2009). [DOI] [PMC free article] [PubMed]
- 10.Stern JH, Rutkowski JM & Scherer PE Adiponectin, Leptin, and Fatty Acids in the Maintenance of Metabolic Homeostasis through Adipose Tissue Crosstalk. Cell Metab 23, 770–784 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ye R & Scherer PE Adiponectin, driver or passenger on the road to insulin sensitivity? Molecular metabolism 2, 133–141 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scherer PE Adiponectin: basic and clinical aspects. Preface. Best practice & research. Clinical endocrinology & metabolism 28, 1–2 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Scherer PE, Williams S, Fogliano M, Baldini G & Lodish HF A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem 270, 26746–26749 (1995). [DOI] [PubMed] [Google Scholar]
- 14.Hu E, Liang P & Spiegelman BM AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem 271, 10697–10703 (1996). [DOI] [PubMed] [Google Scholar]
- 15.Maeda K, et al. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1). Biochem Biophys Res Commun 221, 286–289 (1996). [DOI] [PubMed] [Google Scholar]
- 16.Nakano Y, Tobe T, Choi-Miura NH, Mazda T & Tomita M Isolation and characterization of GBP28, a novel gelatin-binding protein purified from human plasma. J Biochem (Tokyo) 120, 803–812 (1996). [DOI] [PubMed] [Google Scholar]
- 17.Arita Y, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 257, 79–83 (1999). [DOI] [PubMed] [Google Scholar]
- 18.Berg AH, Combs TP & Scherer PE ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metab 13, 84–89 (2002). [DOI] [PubMed] [Google Scholar]
- 19.Shapiro L & Scherer PE The crystal structure of a complement-1q family protein suggests an evolutionary link to tumor necrosis factor. Curr Biol 8, 335–338 (1998). [DOI] [PubMed] [Google Scholar]
- 20.Maeda N, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med 8, 731–737 (2002). [DOI] [PubMed] [Google Scholar]
- 21.Kubota N, et al. Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem 277, 25863–25866 (2002). [DOI] [PubMed] [Google Scholar]
- 22.Ma K, et al. Increased beta -oxidation but no insulin resistance or glucose intolerance in mice lacking adiponectin. J Biol Chem 277, 34658–34661. (2002). [DOI] [PubMed] [Google Scholar]
- 23.Xia JY, et al. Acute loss of adipose tissue-derived adiponectin triggers immediate metabolic deterioration in mice. Diabetologia 61, 932–941 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hotta K, et al. Circulating concentrations of the adipocyte protein adiponectin are decreased in parallel with reduced insulin sensitivity during the progression to type 2 diabetes in rhesus monkeys. Diabetes 50, 1126–1133 (2001). [DOI] [PubMed] [Google Scholar]
- 25.Weyer C, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab 86, 1930–1935. (2001). [DOI] [PubMed] [Google Scholar]
- 26.Yamauchi T, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423, 762–769 (2003). [DOI] [PubMed] [Google Scholar]
- 27.Yamauchi T, et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med 13, 332–339 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Okada-Iwabu M, et al. A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature 503, 493–499 (2013). [DOI] [PubMed] [Google Scholar]
- 29.Tanabe H, et al. Crystal structures of the human adiponectin receptors. Nature 520, 312–316 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vasiliauskaite-Brooks I, et al. Structural insights into adiponectin receptors suggest ceramidase activity. Nature 544, 120-+ (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holland WL, et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med 17, 55–63 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holland WL & Scherer PE Structural biology: Receptors grease the metabolic wheels. Nature 544, 42–44 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Scherer PE The Multifaceted Roles of Adipose Tissue-Therapeutic Targets for Diabetes and Beyond: The 2015 Banting Lecture. Diabetes 65, 1452–1461 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hotta K, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol 20, 1595–1599 (2000). [DOI] [PubMed] [Google Scholar]
- 35.Spranger J, et al. Adiponectin and protection against type 2 diabetes mellitus. Lancet 361, 226–228 (2003). [DOI] [PubMed] [Google Scholar]
- 36.Ouchi N, et al. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation 100, 2473–2476 (1999). [DOI] [PubMed] [Google Scholar]
- 37.Pischon T, et al. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA 291, 1730–1737 (2004). [DOI] [PubMed] [Google Scholar]
- 38.Turer AT & Scherer PE Adiponectin: mechanistic insights and clinical implications. Diabetologia 55, 2319–2326 (2012). [DOI] [PubMed] [Google Scholar]
- 39.Maeda N, et al. PPARgamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes 50, 2094–2099. (2001). [DOI] [PubMed] [Google Scholar]
- 40.Kusminski CM, et al. MitoNEET-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity. Nat Med 18, 1539–U1144 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim JY, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest 117, 2621–2637 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Makowski L, et al. Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nat Med 7, 699–705 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elmasri H, et al. Fatty acid binding protein 4 is a target of VEGF and a regulator of cell proliferation in endothelial cells. Faseb J 23, 3865–3873 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shan T, Liu W & Kuang S Fatty acid binding protein 4 expression marks a population of adipocyte progenitors in white and brown adipose tissues. Faseb J 27, 277–287 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, et al. Cardiomyocyte-derived adiponectin is biologically active in protecting against myocardial ischemia-reperfusion injury. Am J Physiol Endocrinol Metab 298, E663–670 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shafiei MS, Shetty S, Scherer PE & Rockey DC Adiponectin regulation of stellate cell activation via PPARgamma-dependent and -independent mechanisms. Am J Pathol 178, 2690–2699 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ding X, et al. The roles of leptin and adiponectin: a novel paradigm in adipocytokine regulation of liver fibrosis and stellate cell biology. Am J Pathol 166, 1655–1669 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jasinski-Bergner S, Buttner M, Quandt D, Seliger B & Kielstein H Adiponectin and Its Receptors Are Differentially Expressed in Human Tissues and Cell Lines of Distinct Origin. Obes Facts 10, 569–583 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Das K, Lin Y, Widen E, Y., Z. & Scherer, P.E. Chromosomal localization, expression pattern and promoter analysis of the mouse gene encoding adipocyte-specific secretory protein Acrp30. Biochem. Biophys. Res. Comm 280, 1120–1129 (2001). [DOI] [PubMed] [Google Scholar]
- 50.Wang QA, et al. Distinct regulatory mechanisms governing embryonic versus adult adipocyte maturation. Nat Cell Biol 17, 1099–1111 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Segawa K, et al. Identification of a novel distal enhancer in human adiponectin gene. J Endocrinol 200, 107–116 (2009). [DOI] [PubMed] [Google Scholar]
- 52.Wang ZV, Deng Y, Wang QA, Sun K & Scherer PE Identification and characterization of a promoter cassette conferring adipocyte-specific gene expression. Endocrinology 151, 2933–2939 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim HB, et al. NFATc4 and ATF3 negatively regulate adiponectin gene expression in 3T3-L1 adipocytes. Diabetes 55, 1342–1352 (2006). [DOI] [PubMed] [Google Scholar]
- 54.Park SK, et al. CCAAT/enhancer binding protein and nuclear factor-Y regulate adiponectin gene expression in adipose tissue. Diabetes 53, 2757–2766 (2004). [DOI] [PubMed] [Google Scholar]
- 55.Iwaki M, et al. Induction of adiponectin, a fat-derived antidiabetic and antiatherogenic factor, by nuclear receptors. Diabetes 52, 1655–1663 (2003). [DOI] [PubMed] [Google Scholar]
- 56.Seo JB, et al. Adipocyte determination- and differentiation-dependent factor 1/sterol regulatory element-binding protein 1c regulates mouse adiponectin expression. J Biol Chem 279, 22108–22117 (2004). [DOI] [PubMed] [Google Scholar]
- 57.Combs TP, et al. Induction of adipocyte complement-related protein of 30 kilodaltons by PPARgamma agonists: a potential mechanism of insulin sensitization. Endocrinology 143, 998–1007 (2002). [DOI] [PubMed] [Google Scholar]
- 58.Fasshauer M, Klein J, Neumann S, Eszlinger M & Paschke R Hormonal regulation of adiponectin gene expression in 3T3-L1 adipocytes. Biochem Bioph Res Co 290, 1084–1089 (2002). [DOI] [PubMed] [Google Scholar]
- 59.Halleux CM, et al. Secretion of adiponectin and regulation of apM1 gene expression in human visceral adipose tissue. Biochem Biophys Res Commun 288, 1102–1107 (2001). [DOI] [PubMed] [Google Scholar]
- 60.Wang ZV & Scherer PE DsbA-L is a versatile player in adiponectin secretion. P Natl Acad Sci USA 105, 18077–18078 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kondo H, et al. Association of adiponectin mutation with type 2 diabetes - A candidate gene for the insulin resistance syndrome. Diabetes 51, 2325–2328 (2002). [DOI] [PubMed] [Google Scholar]
- 62.Kadowaki T, et al. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest 116, 1784–1792 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pajvani UB, et al. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin - Implications for metabolic regulation and bioactivity. Journal of Biological Chemistry 278, 9073–9085 (2003). [DOI] [PubMed] [Google Scholar]
- 64.Bjursell M, et al. Opposing effects of adiponectin receptors 1 and 2 on energy metabolism. Diabetes 56, 583–593 (2007). [DOI] [PubMed] [Google Scholar]
- 65.Holland WL, et al. Inducible overexpression of adiponectin receptors highlight the roles of adiponectin-induced ceramidase signaling in lipid and glucose homeostasis. Molecular metabolism 6, 267–275 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tsuchida A, et al. Insulin/Foxo1 pathway regulates expression levels of adiponectin receptors and adiponectin sensitivity. J Biol Chem (2004). [DOI] [PubMed] [Google Scholar]
- 67.Hug C, et al. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci U S A 101, 10308–10313 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yamauchi T, Iwabu M, Okada-Iwabu M & Kadowaki T Adiponectin receptors: A review of their structure, function and how they work. Best Pract Res Cl En 28, 15–23 (2014). [DOI] [PubMed] [Google Scholar]
- 69.Uhlen M, et al. Proteomics. Tissue-based map of the human proteome. Science 347, 1260419 (2015). [DOI] [PubMed] [Google Scholar]
- 70.Matsuda K, et al. Positive Feedback Regulation Between Adiponectin and T-Cadherin Impacts Adiponectin Levels in Tissue and Plasma of Male Mice. Endocrinology 156, 934–946 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kelesidis I, Kelesidis T & Mantzoros CS Adiponectin and cancer: a systematic review. Br J Cancer 94, 1221–1225 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barb D, Williams CJ, Neuwirth AK & Mantzoros CS Adiponectin in relation to malignancies: a review of existing basic research and clinical evidence. Am J Clin Nutr 86, s858–866 (2007). [DOI] [PubMed] [Google Scholar]
- 73.Taliaferro-Smith L, et al. LKB1 is required for adiponectin-mediated modulation of AMPK-S6K axis and inhibition of migration and invasion of breast cancer cells. Oncogene 28, 2621–2633 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Landskroner-Eiger S, et al. Proangiogenic Contribution of Adiponectin toward Mammary Tumor Growth In vivo. Clinical Cancer Research 15, 3265–3276 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Denzel MS, et al. Adiponectin Deficiency Limits Tumor Vascularization in the MMTV-PyV-mT Mouse Model of Mammary Cancer. Clinical Cancer Research 15, 3256–3264 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Masamoto Y, et al. Adiponectin Enhances Antibacterial Activity of Hematopoietic Cells by Suppressing Bone Marrow Inflammation. Immunity 44, 1422–1433 (2016). [DOI] [PubMed] [Google Scholar]
- 77.Mandal P, Pratt BT, Barnes M, McMullen MR & Nagy LE Molecular Mechanism for Adiponectin-dependent M2 Macrophage Polarization LINK BETWEEN THE METABOLIC AND INNATE IMMUNE ACTIVITY OF FULL-LENGTH ADIPONECTIN. Journal of Biological Chemistry 286, 13460–13469 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ohashi K, et al. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J Biol Chem 285, 6153–6160 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shibata S, et al. Adiponectin regulates psoriasiform skin inflammation by suppressing IL-17 production from gammadelta-T cells. Nat Commun 6, 7687 (2015). [DOI] [PubMed] [Google Scholar]
- 80.Combs TP, et al. A transgenic mouse with a deletion in the collagenous domain of adiponectin displays elevated circulating adiponectin and improved insulin sensitivity. Endocrinology 145, 367–383 (2004). [DOI] [PubMed] [Google Scholar]
- 81.Marangoni RG, et al. Adiponectin is an endogenous anti-fibrotic mediator and therapeutic target. Sci Rep-Uk 7(2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang Y, et al. Adiponectin inhibits tumor necrosis factor-α–induced vascular inflammatory response via caveolin-mediated ceramidase recruitment and activation. Circulation research 114, 792–805 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vasiliauskaite-Brooks I, et al. Structure of a human intramembrane ceramidase explains enzymatic dysfunction found in leukodystrophy. bioRxiv, 348219 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kupchak BR, Garitaonandia I, Villa NY, Smith JL & Lyons TJ Antagonism of human adiponectin receptors and their membrane progesterone receptor paralogs by TNFα and a ceramidase inhibitor. Biochemistry 48, 5504–5506 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sharma AX & Holland WL Adiponectin and its Hydrolase-Activated Receptors. J Nat Sci 3(2017). [PMC free article] [PubMed] [Google Scholar]
- 86.Schmitz-Peiffer C, Craig DL & Biden TJ Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. Journal of Biological Chemistry 274, 24202–24210 (1999). [DOI] [PubMed] [Google Scholar]
- 87.Holland WL & Summers SA Sphingolipids, insulin resistance, and metabolic disease: new insights from in vivo manipulation of sphingolipid metabolism. Endocr Rev 29, 381–402 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hosch SE, Olefsky JM & Kim JJ APPLied mechanics: Uncovering how adiponectin modulates insulin action. Cell Metabolism 4, 5–6 (2006). [DOI] [PubMed] [Google Scholar]
- 89.Mao X, et al. APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat Cell Biol 8, 516–523 (2006). [DOI] [PubMed] [Google Scholar]
- 90.Mitsuuchi Y, et al. Identification of a chromosome 3p14. 3-21.1 gene, APPL, encoding an adaptor molecule that interacts with the oncoprotein-serine/threonine kinase AKT2. Oncogene 18, 4891 (1999). [DOI] [PubMed] [Google Scholar]
- 91.Zhou LJ, et al. Adiponectin Activates AMP-activated Protein Kinase in Muscle Cells via APPL1/LKB1-dependent and Phospholipase C/Ca2+/Ca2+/Calmodulin-dependent Protein Kinase Kinase-dependent Pathways. Journal of Biological Chemistry 284, 22426–22435 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Iwabu M, et al. Adiponectin and AdipoR1 regulate PGC-1 alpha and mitochondria by Ca2+ and AMPK/SIRT1. Nature 464, 1313–1319 (2010). [DOI] [PubMed] [Google Scholar]
- 93.Canto C, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458, 1056–1060 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Awazawa M, et al. Adiponectin suppresses hepatic SREBP1c expression in an AdipoR1/LKB1/AMPK dependent pathway. Biochem Biophys Res Commun 382, 51–56 (2009). [DOI] [PubMed] [Google Scholar]
- 95.Shibata R, et al. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med 11, 1096–1103 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]