Abstract
Background and Objectives:
Patients undergoing cement fixation for hip arthroplasty are at increased risk of developing bone cement implantation syndrome (BCIS). We sought to determine: What is the occurrence of BCIS in cancer patients after hip arthroplasty? What are the risk factors in cancer patients for the development of this syndrome? What is the outcome for cancer patients with BCIS?
Methods:
We identified 374 cancer patients who underwent cemented hip arthroplasty between 2010-2014. Patient characteristics, operative variables and outcomes were collected.
Results:
BCIS occurred in 279 (75%) patients. 353 (94%) patients had bone metastases and 179 (48%) patients had lung metastases at the time of surgery. Age greater than 60 (HR 2.09, p=0.02) and the presence of lung metastases (HR 1.77, p= 0.019) were associated with increased risk of BCIS. Increased perioperative use of vasopressors (HR 1.72, p= 0.023) and increased hospital stay beyond 10 days (HR 2.67, p=0.003) was associated with BCIS.
Conclusions:
BCIS is a frequent clinical event in cancer patients undergoing femoral cemented arthroplasty with increased risk for patients over age 60 and those with compromised lung function due to lung metastases and lung cancer. Patients who develop BCIS are more likely to require longer postoperative hospitalization. Careful preoperative assessment and intraoperative communication are crucial steps to reduce the consequences of BCIS.
Keywords: bone cement, bone cement implantation syndrome, embolism, cancer patients, orthopedic surgery, hypoxia
Introduction
Bone cement implantation syndrome (BCIS) is a poorly understood and potentially fatal complication associated with orthopedic procedures, especially cemented arthroplasty of the hip[1],[2]. Due to the wide range and vague nature of the symptoms of this syndrome, the true incidence of BCIS is unknown[3]. Authors assume that the lack of precise criteria for defining BCIS may have led to under reporting of cases[1] until 2009, when Donaldson et al. defined BCIS as “hypoxia, hypotension or both and/or unexpected loss of consciousness occurring around the time of cementation, prosthesis insertion, reduction of the joint or, occasionally, limb tourniquet deflation in a patient undergoing cemented bone surgery”[3]. Donaldson et al proposed a classification schema for BCIS, based on severity: grade 1 is characterized by moderate hypoxia or hypotension, grade 2 is defined as severe hypoxia or hypotension, and grade 3 is characterized by cardiovascular collapse requiring cardiopulmonary resuscitation (CPR)[3]. Using this classification, Olsen et al. reported an overall rate of any grade of BCIS of 28% in a retrospective study of 1016 patients undergoing cemented hip arthroplasty; the rates of BCIS grade 1, 2, and 3 were reported at 21.0%, 5.1 %, and 1.7%, respectively[4].
Reported risk factors for BCIS include male gender, ASA grade 3-4, age greater than 85 years, congestive heart failure or other underlying cardiovascular disease, chronic obstructive pulmonary disease (COPD) or other preexisting lung disease, medication with diuretics or warfarin[4], osteoporosis[2, 5], and cancer[3]. While several pathophysiological mechanisms for BCIS have been proposed, including the histamine release hypothesis[6], monomer-mediated[7] and embolus-mediated models[8], the embolic model is the most widely accepted[9],[10]. Transesophageal echocardiography (TEE) during cemented arthroplasty visualizes venous embolization through the heart[11]. The embolus-mediated hypothesis suggests that cementation causes a rapid increase in intramedullary pressure leading to the expulsion of intramedullary contents into the blood stream that embolize through the heart to lungs. Emboli to the lungs can cause hypoxia and lead to right ventriculation dysfunction via increased pulmonary vascular resistance (= PVR) with subsequent hypotension. Increased PVR causes a reduced right-ventricular ejection fraction, the right ventricle distends and causes left ventricle (= LV) dysfunction, reducing LV filling and therefore cardiac output [3, 12].
BCIS is a concern throughout the perioperative period. Patterson et al reported that seven patients in a cohort of 400 patients had intra-operative cardiac arrest while undergoing long-stem cemented arthroplasty, and three of those seven patients had metastatic disease[2]. In a retrospective study of 55 patients receiving long-stem femoral prosthesis (40 for metastatic disease and 15 revisions), three patients experienced cement-associated hypotension or desaturation, with two patients remaining comatose and one dying[13]. The same study showed that patients receiving long-stem prosthesis for metastatic disease had statistically significant higher rates of desaturation than those receiving it for revisions[13].
While the evidence is sparse, cancer and bone metastases have been considered risk factors for BCIS[13]. Malignancy creates a hypercoagulable state, and cancer cells release pro-coagulants like fibrinopeptide A, both of which independently increase the risk of thromboembolism[14, 15]. There is a gap in understanding of BCIS in cancer patients. No study has specifically examined the incidence in cancer patients, thus the risk of BCIS in this patient population is incompletely understood, which limits the capacity to prevent or treat. Therefore, we sought to determine: What is the frequency of BCIS in patients with cancer after hip arthroplasty? What is the outcome for cancer patients with BCIS? What are the risk factors in cancer patients for the development of this condition?
Materials and Methods
Data collection
The retrospective cohort study was approved by the institutional review board. We reviewed medical records of all adult cancer patients who underwent hip arthroplasty for impending or actual proximal femur fracture between January 1st 2010 and December 31st 2014 at our tertiary cancer center. Patients who did not have cancer at the time of surgery and patients with incomplete medical records were excluded. Indications for surgery including metastatic disease, status post tumor resection or severe osteoarthritis after radiation therapy were captured. Patient characteristics such as age, gender, type of cancer, and concomitant medical comorbidities including coronary artery disease, diabetes, hypertension, pre-existing lung disease and Charleston Comorbidity Index (CCI) were retrieved. Preoperative cardiac history, electrocardiograms and echocardiogram were recorded. Echocardiograms were only available for patients with abnormal pre-operative electrocardiograms. Preoperative x-rays were reviewed to confirm indication for surgery, presence of bone metastasis, and status of the pathologic fracture. Relevant perioperative data including type of anesthesia, type of surgery, stem length of the prosthetic implant, blood loss, intraoperative blood pressure and oxygen saturation were obtained. Postoperative hip x-rays were reviewed to confirm the type of surgical reconstruction. Blood pressure and oxygen saturation were noted at three time points as noted on the anesthetic record: (1) prior to anesthesia delivery; (2) at insertion of prosthesis; and (3) in the post-anesthesia care unit after the surgery. The lowest blood pressure and oxygen saturation were recorded at time points 2 and 3. We also recorded whether there was a therapeutic intervention given at the time of cementing as noted in the anesthetic record or surgical operative report such as administering vasopressors. Insertion of prosthesis and subsequent changes in blood pressure and oxygen saturation were clearly noted in the anesthesia record. Based on the changes in blood pressure and oxygen saturation, each patient was classified as having no BCIS (grade 0) or grade 1, 2 or 3 per Donaldson et al[3]:
Grade 1: moderate hypoxia (SpO2 <94%) or hypotension [fall in systolic blood pressure (SBP) >20%].
Grade 2: severe hypoxia (SpO2 <88%) or hypotension (fall in SBP >40%) or unexpected loss of consciousness.
Grade 3: cardiovascular collapse requiring CPR
The grade of BCIS was calculated by comparing the values for oxygenation and blood pressure prior to anesthesia delivery with the lowest value recorded in the second or third time points. Postoperative outcomes collected included length of stay, need for intensive care, and the complications including deep venous thrombosis, pulmonary embolism, pneumonia, wound healing problems requiring surgical intervention, need for reintubation, cardiac problems, need for CPR, and death.
Operative Technique
Four surgeons performed the surgery with a range of 20 to 200 procedures each. All surgeries were performed under general anesthesia, in the lateral decubitus position with a standard posterolateral approach. A cemented hemiarthroplasty was performed in 196 (52%) cases, a Harrington total hip arthroplasty in 73 (20%), a proximal femur replacement (PFR) in 65 (17%), a total hip replacement in 40 (11%) cases. The stem length was divided into three groups: <150mm was used in 137 cases (37%), from 150 to 300mm in 143 (38%) and ≥300 mm in 94 (25%) cases. One company was used for all implants (Orthopaedic Salvage System, Biomet, Warsaw, IN, USA. or Zimmer systems) and antibiotic impregnated Polymethyl methacrylate (PMMA; Cobalt with Gentamycin 40gm or Simplex P with Tobramycin, Biomet, Warsaw, IN, USA). Prior to cementation, a lateral unicortical vent hole was made in each case either in the proximal or distal metaphysis based on surgeon preference. Extruded cement at the vent hole was removed as necessary.
Follow up
Patients were discharged into rehabilitation or home care as appropriate; follow up every three months with radiographs.
Statistical analysis
Univariate logistic regression with BCIS as an outcome was used to identify baseline and intraoperative predictors of BCIS in the patient cohort. BCIS was evaluated as a risk factor for several post-surgical factors using multiple univariate logistic regression models with BCIS as a predictor for each. The Kaplan-Meier method was used to compare postoperative survival between patients with and without BCIS (including various grades of BCIS). A p-value of <0.05 was considered significant.
Patient characteristics
374 patients were identified for statistical analysis. Median age was 62.3 years (range 18-92). 221 (59%) patients were female. 180 (48%) procedures were performed on the left side of the hip. The study included 17 different types of cancer with breast cancer being the most common type (Table 1). All patients had general anesthesia as epidurals were rarely performed at our institution during this time in this cohort of patients. Median duration of surgery was 273 minutes (range: 117-1284 minutes), median blood loss was 800ml (range: 50-11000ml). The median duration of hospital stay preoperatively was 1 day (0-28 days) and postoperatively was 7 days (1-216 days).
Table 1.
Type of Cancer and Incidence of BCIS in all patients.
| Type of cancer | N (%) (N=374) | No BCIS N (%) (N=96) | BCIS N (%) (N=278) |
|---|---|---|---|
| Breast cancer | 63 (17%) | 22 (23%) | 41 (15%) |
| Sarcoma | 55 (15%) | 13 (14%) | 42 (15%) |
| Other types of cancer† | 54 (14%) | 11 (12%) | 43 (15%) |
| Lung cancer | 48 (13%) | 9 (9%) | 39 (14%) |
| Kidney cancer | 45 (12%) | 12 (13%) | 33 (12%) |
| Prostate cancer | 26 (7%) | 6 (6%) | 20 (7%) |
| Multiple myeloma | 15 (4%) | 4 (4%) | 11 (4%) |
| Colorectal cancer | 12 (3%) | 4 (4%) | 8 (3%) |
| Melanoma | 11 (3%) | 3 (3%) | 8 (3%) |
| Lymphoma | 10 (3%) | 3 (3%) | 7 (3%) |
| Endometrial cancer | 9 (2%) | 0 (0%) | 9 (3%) |
| Thyroid cancer | 7 (2%) | 1 (1%) | 6 (2%) |
| Leukemia | 6 (1%) | 3 (3%) | 3 (1%) |
| Bladder cancer | 5 (1%) | 2 (2%) | 3 (1%) |
| Uterine cancer | 4 (1%) | 3 (3%) | 1 (0.3%) |
| Pancreatic cancer | 2 (1%) | 0 (0%) | 2 (0.7%) |
| Ovarian cancer | 2 (1%) | 0 (0%) | 2 (0.7%) |
Other types of cancer included: neuroblastoma, hemangiopericytoma, paraganglioma, glioblastoma, CUP, gastric carcinoma, hepatocellular carcinoma, esophageal carcinoma, chordoma, carcinoid tumor, parotid gland mucoepidermoid carcinoma, squamous cell carcinoma, cholangiocarcinoma, germ cell tumor
Results
Comorbidities and postoperative management
Median value for Charleston Comorbidity Index was 7 (range 0-15). At the time of surgery, 353 (94%) patients had bone metastases, and 179 (48%) had lung metastases. Patient-related factors and perioperative characteristics are presented in Table 2. Nineteen (5%) patients required revision surgery at a median time from initial surgery of 26 months (range: 0.2 −87 months). Reasons for revision surgery were loosening or dislocation of the prosthesis, new fracture or nonunion of the existing fracture, infection or metastases. Six (2%) patients required readmission within 30 days of surgery.
Table 2.
Baseline and Intra-Operative Factors associated with BCIS (Univariate Logistic Regression)
| Baseline | ||||
|---|---|---|---|---|
| No BCIS (N=96) | BCIS (N=278) | Odds Ratio, 95% CI | P-value | |
| Age | 0.002 | |||
| <60 | 53 (55.2%) | 103 (37.1%) | ||
| >=60 | 43 (44.8%) | 175 (62.9%) | 2.09 (1.31-3.36) | |
| BMI | 0.157 | |||
| <25 | 42 (43.8%) | 99 (35.6%) | ||
| >=25 | 54 (56.2%) | 179 (64.4%) | 1.41 (0.87-2.25) | |
| CCI | 0.332 | |||
| <=7 | 60 (62.5%) | 158 (56.8%) | ||
| >7 | 36 (37.5%) | 120 (43.2%) | 1.27 (0.79-2.05) | |
| Bone Metastases | 0.41 | |||
| No | 7 (7.3%) | 14 (5.0%) | ||
| Yes | 89 (92.7%) | 264 (95.0%) | 1.48 (0.55-3.68) | |
| Lung Metastases | 0.019 | |||
| No | 60 (62.5%) | 135 (48.6%) | ||
| Yes | 36 (37.5%) | 143 (51.4%) | 1.77 (1.1-2.86) | |
| Indication | 0.403 | |||
| Impending Fracture | 59 (61.5%) | 184 (66.2%) | ||
| Actual Fracture | 37 (38.5%) | 94 (33.8%) | 0.81 (0.51-1.32) | |
| Stem Length | 0.47 | |||
| <150mm | 39 (40.6%) | 98 (35.3%) | ||
| 150-300mm | 37 (38.5%) | 106 (38.1%) | 1.14 (0.67-1.94) | |
| >=300mm | 20 (20.8%) | 74 (26.6%) | 1.47 (0.8-2.77) | |
| Pre-Op Hospital Stay | 0.907 | |||
| <=2 days | 70 (72.9%) | 201 (72.3%) | ||
| >2 days | 26 (27.1%) | 77 (27.7%) | 1.03 (0.62-1.76) | |
| Intra-Operative | ||||
| Duration of Surgery | 0.32 | |||
| <270 minutes | 42 (43.8%) | 138 (49.6%) | ||
| >=270 minutes | 54 (56.2%) | 140 (50.4%) | 0.79 (0.49-1.26) | |
| Vasopressors | 0.023 | |||
| None | 53 (55.2%) | 116 (41.7%) | ||
| epinephrine/phenylephrine | 43 (44.8%) | 162 (58.3%) | 1.72 (1.08-2.76) | |
| Reconstruction | 0.304 | |||
| THA + Harrington | 33 (34.4%) | 260 (93.5%) | ||
| Hemi + PFR | 63 (65.6%) | 18 (6.5%) | 1.3 (0.79-2.12) | |
| Blood Loss | 0.818 | |||
| <800ml | 46 (47.9%) | 137 (49.3%) | ||
| >=800ml | 50 (52.1%) | 141 (50.7%) | 0.95 (0.59-1.51) |
Complications and survival
No cardiac arrest occurred during surgery. Two patients (0.5%) died within 30 days after surgery of respiratory insufficiency. The all-cause 30-day mortality was 2.9%. Alterations in electrocardiogram occurred in 15 (4%) of patients. Four (1%) patients required reintubation after surgery. Deep venous thrombosis developed in 67 (18%) patients after surgery, and pulmonary embolus developed in 28 (7%) patients. During the postoperative course, 68 (18%) patients suffered from pneumonia, and 15 (4%) patients had infection or wound dehiscence that required surgical intervention.
The occurrence of BCIS was 278 cases (74%) in total. The occurrence of BCIS grade 1, 2 and 3 was 234 (62.5%), 42 (11%), and 2 (0.5), respectively. Overall survival was 43% as a total of 214 patients died during the follow-up period with a median follow-up of 44 months (range: 0.92-91.3 months). The impact of BCIS on postoperative survival is shown in Figure 1. Patients with BCIS grade 3 were excluded from this analysis since death from this syndrome is part of the definition of BCIS grade 3. Only 2 patients had BCIS grade 3; therefore, this group is too small to be included as an arm in the survival analysis. The p-value for difference in survival is 0.4. Survival probability of patients without BCIS was higher than that of patients with BCIS grade 1 or 2 (Figure 1). Univariate logistic regression of the risk factors for BCIS showed that age over 60 years and the presence of lung metastases are the only significant predictors for development of BCIS (Table 3).
Figure 1: Postoperative survival of patients after cemented total or hemi hip arthroplasty for femoral neck fractures in relation to severity of BCIS.
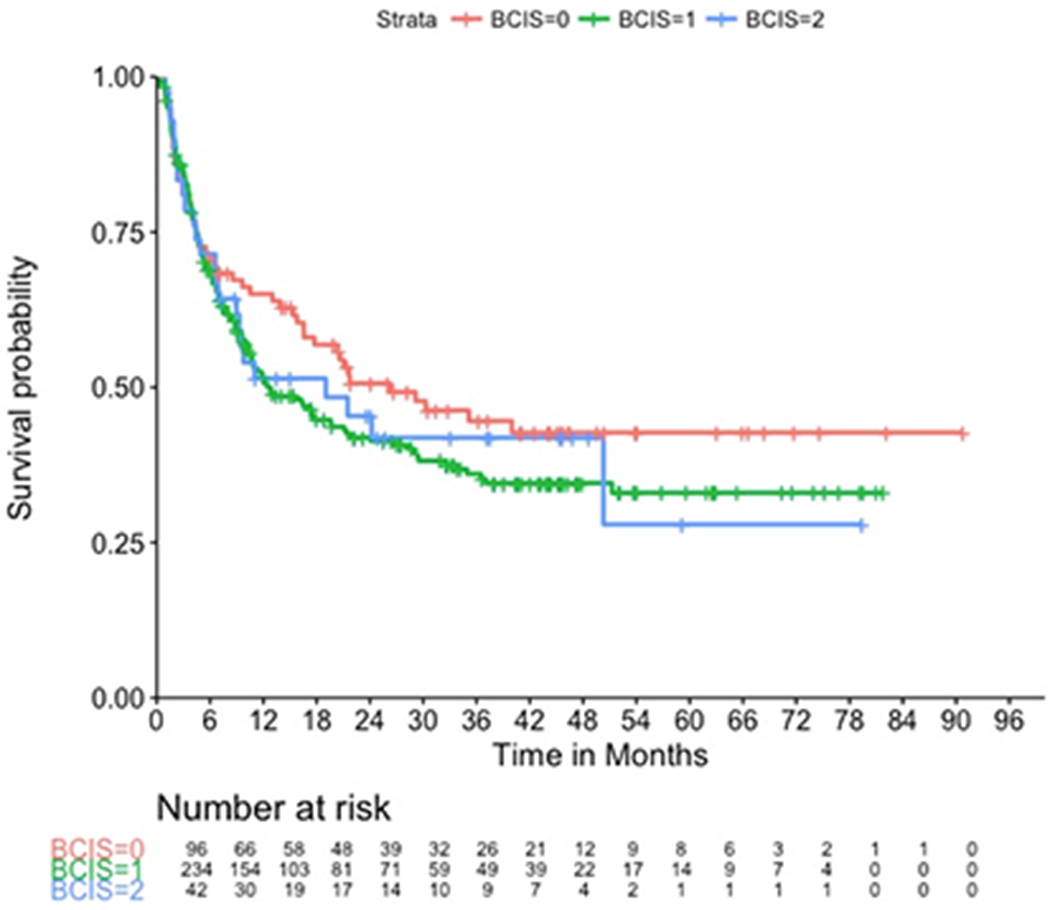
Patients were divided in three groups: red = no BCIS (n=95), green= BCIS grade 1 (n=234), blue= BCIS grade 2 (n=42); patients with BCIS grade 3 (n=2) were excluded from this analysis since death of this syndrome is part of the definition of this grade of BCIS. The survival curves do not separate until six after surgery, which can be explained by the probable presence of lung disease in patients who experienced BCIS. Lung cancer or lung metastases impart a worse prognosis overall, this can be seen in the long-term postoperative period.
Table 3:
Univariate logistic models of post-surgical factors with BCIS as a risk factor
| Post-Surgical | ||
|---|---|---|
| Outcome | Odds Ratio (BCIS vs. No BCIS), 95% CI | P-value |
| Deep venous thrombosis | 1.54 (0.82-3.08) | 0.197 |
| Pulmonary embolism | 1.64 (0.65-5) | 0.33 |
| Pneumonia | 1.28 (0.7-2.46) | 0.44 |
| Revision Surgery | 3.06 (0.86-19.54) | 0.139 |
| Postop. hospital stay (<=10 vs. >10 days) | 2.67 (1.45-5.26) | 0.003 |
Discussion
These results indicate that BCIS is a common, discrete and morbid complication in cancer patients undergoing cemented hip arthroplasties, with a three-fold incidence in our cohort compared to other series. 74% (95% CI: (69.5 – 78.6%) of the patients had symptoms of BCIS, whereas Olsen et al reported an incidence of 25–30% of all grades of BCIS in non-cancer patients[4]. This comparison confirms the previously published finding that cancer patients have a higher rate of BCIS than the general population when undergoing cemented femoral arthroplasty[13]. In the present study, patient-related risk factors for the development of this syndrome were age older than 60 years and the presence of lung cancer or lung metastases. BCIS was also shown as a significant predictor for the use of vasopressors intraoperatively, and to be a risk factor for increased hospital length of stay greater than 10 days. Risk factors previously implicated in the development of BCIS include age[2, 5, 7], impaired cardiopulmonary function[13],[16] and preexisting lung diseases[4]. As a retrospective study, it is limited by selection bias of surgeons indicating patients for femoral arthroplasty as opposed to other reconstructions such as intramedullary nail stabilization. To minimize this, the series consisted of all consecutive patients undergoing this procedure.
Risk factorsGoo
The risk factors identified for BCIS in this study, lung metastasis and increased age, cannot be altered prior to surgery. Review of surgical and anesthetic factors included assessment for “controllable” risk factors and outcomes, with the intent to intervene and minimize the incidence and/or grade and morbidity of BCIS. Acknowledging the high risk of BCIS overall, the authors recommend a combination of surgical techniques and anesthetic management: use of a decompressive vent hole prior to femoral insertion, a careful preoperative assessment including patient functional status and discussion with the anesthesia care team, and intraoperative communication throughout surgery. Our records note consistent and frequent communication between surgical and anesthetic teams prior to cementing and stem insertion, including maximizing FiO2 and optimizing blood pressure. Patients with BCIS had increased use of vasopressors intraoperatively, to treat or prevent hypotension and hypoxia during this portion of the surgery. The study cannot determine causation between vasopressor use and BCIS, however, it is clinically logical that the use of vasopressors decreases the incidence and grade of BCIS and therefore, without this intervention, we propose that the incidence of BCIS may be higher otherwise. Govil et al suggest hemodynamic monitoring during and after surgery to monitor for BCIS and aggressive intervention with vasopressors and resuscitation [17]. Treatment currently focuses on 100% inspired oxygen, control of airway, aggressive volume therapy, and early resuscitation with use of vasopressors as the keys to prevention of catastrophic outcome of BCIS[17]. There is no current evidence that prophylactic use of antihistamines or steroids is preventive for BCIS and were not used routinely in this cohort of patients[17]. Additional prospective studies might identify criteria to further classify BCIS with prognostic or treatment focused significance. The authors support the use of a vent hole as described by others to decompress the intramedullary canal during femoral preparation, cementing and stem insertion[18, 19]. Other surgical strategies to reduce intramedullary pressure is retrograde cementation from distal to proximal via a cement gun with long cannula[19]. Use of a PMMA-vacuum assisted mixing technique reduces the load of volatile vasoactive compounds, as well as low-viscosity cement can reduce the occurrence of BCIS[20].
Minimizing risk of BCIS
This study found no differences in BCIS incidence or grade with various stem lengths and extent of concomitant reconstructive surgery with the implication that instrumentation and preparation of the canal are the critical portions of the surgery rather than overall morbidity of surgery, and extent of femoral involvement. Additionally, time of surgery and blood loss are not associated with BCIS, supporting BCIS as a distinct entity from hypovolemia. An alternative surgical strategy to minimize morbidity and mortality of BCIS can be non-cemented femoral fixation as reported by others[18, 21]. Additionally, Thein et al, have shown that uncemented femoral arthroplasty has comparable prosthesis longevity, overall complication rate, postoperative function and survival in cancer patients[22]. However, two methods of femoral fixation have not been compared directly regarding overall risk, survival, revision, and BCIS. Comparison of cemented and non-cemented femoral arthroplasty in this patient cohort may help to elucidate the role of cement as a causative agent of BCIS. Further investigations are necessary prior to creating clinical guidelines and guiding surgical decision making to minimize morbidity and mortality. The use of cement in femoral fixation should be considered on an individual basis. Similar studies have reviewed the increased risk of emboli and other cardiopulmonary complications. Randall et al.[23] retrospectively reviewed 29 long-stem cemented femoral arthroplasties in 27 patients and found no enhanced risk for adverse clinical events including cement-associated desaturation events, cardiac arrests, or intraoperative deaths. They state that increased awareness of cement-related cardiopulmonary pathophysiology, and modifying conventional surgical techniques can minimize cement-associated complications. Herrenbruck et al.[13] analyzed cement-associated adverse clinical events and cement-associated and postoperative hypotension in 55 patients undergoing long-stem femoral arthroplasty identifying metastatic disease, uninstrumented femoral canals, and preexisting medical conditions as increased risk factors. These findings underscore the importance of appropriate patient selection, patient and family education, and anesthesia preparation before long-stem cemented femoral arthroplasty.
Anesthesia
A careful preoperative assessment including patient functional status and discussion with the anesthesia care team, and communication intraoperatively to maximize FiO2 and optimize blood pressure prior to cementing may subsequently decrease the incidence and extent of BCIS in this patient cohort. The use of regional anesthesia may influence the incidence and grade of BCIS, but this study is limited as all patients received general anesthesia, our standard for this operation in the cancer population. Studies in primary total hip arthroplasty found that neuraxial anesthesia is associated with shorter hospital stays, lower hospital costs, and improved long-term overall survival compared to those in the general anesthesia group[24] in mostly non-cancer patient cohorts. The authors could not find a mechanism underlying the association between neuraxial anesthesia and long-term survival[24]. However, the applicability of this to cancer patients is controversial and no study has compared these anesthetic modalities in this cohort.
Pathophysiology
It is well-known that specific surgical steps during hip arthroplasty can increase central venous pressure, pulmonary artery pressure, pulmonary vascular resistance and hypoxemia[25]. COPD and other underlying pulmonary conditions increase the risk of BCIS. Though pathological hip fractures[5], osteoporosis[2] and bone metastases[26],[13] have also been implicated as contributors to the development of this condition. No association was found in this study for impending or actual fractures or other comorbidities. Hemodynamic instability during arthroplasty surgery is caused by medullary fat embolism rather than toxic effects of methylmethacrylate[26–28]. Prosthesis insertion increases intramedullary pressure that display the intramedullary fat into the bloodstream[17]. Cementing can cause hemodynamic and cardiopulmonary disturbances including pulmonary hypertension[25, 26, 28, 29], bronchoconstriction[29], hypoxemia[10, 25, 28–30], cardiogenic shock[30] and sudden death[30]. Comparatively, uncemented arthroplasties have lower intramedullary and fewer emboli are produced[26]; which supports the underlying theory that BCIS is due to fat embolization at the time of cement insertion.
Limitations
The study has several limitations. First, it is retrospective in design and does not account for individual patient factors such as date and type of cancer, thromboprophylaxis protocol or postoperative rehabilitation. Moreover, we did not take neoadjuvant treatments in consideration as preoperative chemo- or radiation therapy and their pathophysiologic side effects likely influences the patient’s overall outcome. Finally, hospital and surgeon-related factors including case volume and surgeon experience were not taken into account.
Conclusions
BCIS is a frequent clinical event in cancer patients undergoing femoral cemented arthroplasty with increased risk for patients over age 60 and those with compromised lung function due to lung metastases and lung cancer. Patients who develop BCIS are more likely to require longer postoperative hospitalization. The authors suggest a careful preoperative assessment including patient functional status and discussion with the anesthesia care team, and communication intraoperatively to maximize FiO2 and optimizing blood pressure may subsequently decrease the incidence and extent of BCIS in this patient cohort. Given the high frequency of BCIS, additional studies comparing non-cemented arthroplasty, regional anesthesia, and the timing of vasopressor use relative to cementation are warranted to assess their potential influence on BCIS in this patient cohort.
Synopsis for Table of Contents.
BCIS is a frequent clinical event in cancer patients undergoing femoral cemented arthroplasty. Patient at increased risk for developing BCIS are older than 60 and those with compromised lung function due to lung metastases and lung cancer. Patients who develop BCIS are more likely to require postoperative hospital stay longer than 10 days. Discussion with the anesthesia care team, communication intraoperatively, and optimizing blood pressure may decrease the incidence and extent of BCIS.
Acknowledgments
The authors certify that they have no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article. This work was performed at Memorial Sloan Kettering Cancer Center. The Institutional Review Board of Memorial Sloan-Kettering Cancer Center approved the study and the Ethics Committee waived written informed consent. Research at Memorial Sloan Kettering Cancer Center is supported in part by a grant from the National Institutes of Health/National Cancer Institute (#P30CA008748).
The manuscript has been read and approved by all the authors, each author believes that the manuscript represents honest work.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Footnotes
Conflict of Interest
The authors certify that they have no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Contributor Information
Eugenia Schwarzkopf, Sloan Kettering Institute, 1275 York Avenue, New York, NY 10065, USA.
Ridhi Sachdev, Montefiore Orthopedic Surgery, 215 East 73rd Street, New York, NY 10021, USA.
Jessica Flynn, Department of Epidemiology-Biostatistics at Memorial Sloan Kettering Cancer Center 485 Lexington Ave, New York, NY 10017, USA.
Venkat Boddapati, New York-Presbyterian/ Columbia University Medical Center, 622 West 168th Street, New York, NY, 10032 USA.
Roger E. Padilla, Department of Anesthesiology & Critical Care Medicine, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA.
Daniel E. Prince, Orthopaedic Surgery Service, Department of Surgery, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA.
References
- [1].Kalra A, Sharma A, Palaniswamy C, El-Oshar S, Desai P, Yazbeck M et al. Diagnosis and management of bone cement implantation syndrome: Case report and brief review. American journal of therapeutics 2013;20(1):121–5. [DOI] [PubMed] [Google Scholar]
- [2].Patterson BM, Healey JH, Cornell CN, Sharrock NE. Cardiac arrest during hip arthroplasty with a cemented long-stem component. A report of seven cases. The Journal of bone and joint surgery. American volume 1991;73(2):271–7. [PubMed] [Google Scholar]
- [3].Donaldson AJ, Thomson HE, Harper NJ, Kenny NW. Bone cement implantation syndrome. British journal of anaesthesia 2009;102(1):12–22. [DOI] [PubMed] [Google Scholar]
- [4].Olsen F, Kotyra M, Houltz E, Ricksten S-E. Bone cement implantation syndrome in cemented hemiarthroplasty for femoral neck fracture: incidence, risk factors, and effect on outcome. British journal of anaesthesia 2014;113(5):800–6. [DOI] [PubMed] [Google Scholar]
- [5].Parvizi J, Holiday AD, Ereth MH, Lewallen DG. The Frank Stinchfield Award. Sudden death during primary hip arthroplasty. Clinical orthopaedics and related research 1999(369):39–48. [DOI] [PubMed] [Google Scholar]
- [6].Tryba M, Linde I, Voshage G, Zenz M. Histaminfreisetzung und kardiovaskuläre Reaktionen nach Implantation von Knochenzement bei totalem Hüftgelenkersatz. Der Anaesthesist 1991;40(1):25–32. [PubMed] [Google Scholar]
- [7].Peebles DJ, Ellis RH, Stride SD, Simpson BR. Cardiovascular effects of methylmethacrylate cement. British medical journal 1972;1(5796):349–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Modig J, Busch C, Olerud S, Saldeen T, Waernbaum G. Arterial hypotension and hypoxaemia during total hip replacement: the importance of thromboplastic products, fat embolism and acrylic monomers. Acta anaesthesiologica Scandinavica 1975;19(1):28–43. [DOI] [PubMed] [Google Scholar]
- [9].Rutter PD, Panesar SS, Darzi A, Donaldson LJ. What is the risk of death or severe harm due to bone cement implantation syndrome among patients undergoing hip hemiarthroplasty for fractured neck of femur? A patient safety surveillance study. BMJ open 2014;4(6):e004853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Orsini EC, Byrick RJ, Mullen JB, Kay JC, Waddell JP. Cardiopulmonary function and pulmonary microemboli during arthroplasty using cemented or non-cemented components. The role of intramedullary pressure. The Journal of bone and joint surgery. American volume 1987;69(6):822–32. [PubMed] [Google Scholar]
- [11].Murphy P, Edelist G, Byrick RJ, Kay JC, Mullen JB. Relationship of fat embolism to haemodynamic and echocardiographic changes during cemented arthroplasty. Canadian journal of anaesthesia = Journal canadien d’anesthesie 1997;44(12):1293–300. [DOI] [PubMed] [Google Scholar]
- [12].Urban MK, Sheppard R, Gordon MA, Urquhart BL. Right ventricular function during revision total hip arthroplasty. Anesthesia and analgesia 1996;82(6):1225–9. [DOI] [PubMed] [Google Scholar]
- [13].Herrenbruck T, Erickson EW, Damron TA, Heiner J. Adverse clinical events during cemented long-stem femoral arthroplasty. Clinical orthopaedics and related research 2002(395):154–63. [DOI] [PubMed] [Google Scholar]
- [14].Rickles FR, Edwards RL, Barb C, Cronlund M. Abnormalities of blood coagulation in patients with cancer. Fibrinopeptide A generation and tumor growth. Cancer 1983;51(2):301–7. [DOI] [PubMed] [Google Scholar]
- [15].Rickles FR, Edwards RL. Activation of blood coagulation in cancer: Trousseau’s syndrome revisited. Blood 1983;62(1):14–31. [PubMed] [Google Scholar]
- [16].Pietak S, Holmes J, Matthews R, Petrasek A, Porter B. Cardiovascular collapse after femoral prosthesis surgery for acute hip fracture. Canadian journal of anaesthesia = Journal canadien d’anesthesie 1997;44(2):198–201. [DOI] [PubMed] [Google Scholar]
- [17].Govil P, Kakar PN, Arora D, Das S, Gupta N, Govil D et al. Bone cement implantation syndrome: a report of four cases. Indian journal of anaesthesia 2009;53(2):214–8. [PMC free article] [PubMed] [Google Scholar]
- [18].Ries MD, Lynch F, Rauscher LA, Richman J, Mick C, Gomez M. Pulmonary function during and after total hip replacement. Findings in patients who have insertion of a femoral component with and without cement. The Journal of bone and joint surgery. American volume 1993;75(4):581–7. [DOI] [PubMed] [Google Scholar]
- [19].Khanna G, Cernovsky J. Bone cement and the implications for anaesthesia. Continuing Education in Anaesthesia Critical Care & Pain 2012;12(4):213–6. [Google Scholar]
- [20].Rothberg DL, Kubiak EN, Peters CL, Randall RL, Aoki SK. Reducing the Risk of Bone Cement Implantation Syndrome During Femoral Arthroplasty. Orthopedics 2013;36(4):e463–e467. [DOI] [PubMed] [Google Scholar]
- [21].Matar WY, Pawasarat IM, Parvizi J. Total joint arthroplasty in patients with a history of cancer. American journal of orthopedics (Belle Mead, N.J.) 2012;41(4):187–91. [PubMed] [Google Scholar]
- [22].Thein R, Herman A, Chechik A, Liberman B. Uncemented arthroplasty for metastatic disease of the hip: Preliminary clinical experience. The Journal of arthroplasty 2012;27(9):1658–62. [DOI] [PubMed] [Google Scholar]
- [23].Randall RL, Aoki SK, Olson PR, Bott SI. Complications of cemented long-stem hip arthroplasties in metastatic bone disease. Clinical orthopaedics and related research 2006;443:287–95. [DOI] [PubMed] [Google Scholar]
- [24].Chen W-H, Hung K-C, Tan P-H, Shi H-Y. Neuraxial anesthesia improves long-term survival after total joint replacement: A retrospective nationwide population-based study in Taiwan. Canadian journal of anaesthesia = Journal canadien d’anesthesie 2015;62(4):369–76. [DOI] [PubMed] [Google Scholar]
- [25].Parry G Sudden deaths during hip hemi-arthroplasty. Anaesthesia 2003;58(9):922–3. [DOI] [PubMed] [Google Scholar]
- [26].Byrick RJ. Cement implantation syndrome: A time limited embolic phenomenon. Canadian journal of anaesthesia = Journal canadien d’anesthesie 1997;44(2):107–11. [DOI] [PubMed] [Google Scholar]
- [27].Clark DI, Ahmed AB, Baxendale BR, Moran CG. Cardiac output during hemiarthroplasty of the hip. A prospective, controlled trial of cemented and uncemented prostheses. The Journal of bone and joint surgery. British volume 2001;83(3):414–8. [DOI] [PubMed] [Google Scholar]
- [28].Jenkins K, Wake PJ. Cement implantation syndrome. Anaesthesia 2002;57(4):416; author reply 416. [DOI] [PubMed] [Google Scholar]
- [29].Byrick RJ, Forbes D, Waddell JP. A monitored cardiovascular collapse during cemented total knee replacement. Anesthesiology 1986;65(2):213–6. [DOI] [PubMed] [Google Scholar]
- [30].Byrick RJ, Bell RS, Kay JC, Waddell JP, Mullen JB. High-volume, high-pressure pulsatile lavage during cemented arthroplasty. The Journal of bone and joint surgery. American volume 1989;71(9):1331–6. [PubMed] [Google Scholar]


