Abstract
Mild Cognitive Impairment (MCI) is a syndrome characterized by cognitive decline greater than expected for an individual's age and education level. This study aims to determine whether voice quality and speech fluency distinguish patients with MCI from healthy individuals to improve diagnosis of patients with MCI. We analyzed recordings of the Cookie Theft picture description task produced by 26 patients with MCI and 29 healthy controls from Sweden and calculated measures of voice quality and speech fluency. The results show that patients with MCI differ significantly from HC with respect to acoustic aspects of voice quality, namely H1-A3, cepstral peak prominence, center of gravity, and shimmer; and speech fluency, namely articulation rate and averaged speaking time. The method proposed along with the obtainability of connected speech productions can enable quick and easy analysis of speech fluency and voice quality, providing accessible and objective diagnostic markers of patients with MCI.
Introduction
Mild Cognitive Impairment (MCI) is a syndrome characterized by cognitive decline greater than expected for an individual's age and education level. Patients with MCI remain functional in their daily activities [1]. Progression rates vary across studies depending on the diagnostic criteria and methods being employed, although there are indications that about 50% of patients with MCI progress to Alzheimer’s Disease (AD) within five years, yet many patients remain stable for several years [1–3]. Currently, there is no cure for AD, but identifying patients with MCI early and applying therapy in a timely manner can delay the progression of the MCI to AD [4]. It is of utmost importance, to develop straightforward, not intrusive, and reliable objective diagnostic measurements of cognitive impairment that can be conducted at primary care centers and memory clinics to determine whether an individual should seek further professional advice.
Speech can provide such objective measures for the identification of patients with MCI. As language impairment is a common symptom of AD, affecting most language domains and functions including phonetics [5, 6], phonology [7], morphosyntactic structure (e.g., mean length of utterances, proportions of nouns and verbs, and syntactic complexity measures), semantics [8, 9], discourse and conversation [7, 10, 11], it can be employed to provide objective diagnostic markers. One of the less understood and studied aspects of language is speech production in patients with MCI [12, 13] and more research is required on the speech of patients with MCI as speech can convey information about the underlying language system and how it interacts with other language domains [14–16]. For instance, the slow recall of words can affect speech fluency, especially durational and frequency measures, tonal modulation, and pauses [14–18]. Speech can convey information about motoric and cognitive abilities of patients with MCI that relate to articulation, voice quality, and fluency. König et al. [12] employed automated acoustic measures and classified patients with MCI and healthy controls (HC) with 79% classification accuracy, patients with MCI and AD with 80% accuracy, and patients with AD from HC with 89% classification accuracy. In our previous work [19], we analyzed segmental and prosodic features of speech production and showed that vowel formants (F1 to F5), the fundamental frequency, and vowel duration can distinguish patients with MCI from HC with 83% mean cross-validated accuracy.
This study aims to identify a few selected features from voice quality and speech fluency that can function as objective markers distinguishing patients with MCI from HC. An advantage of this study over other studies of language in MCI is that our approach does not require preprocessing, such as transcription and segmentation of the acoustic signal into vowels and consonants. Specifically, we tested two main questions: i. Does voice quality (as estimated by differences between speech harmonics and amplitudes, the cepstral peak prominence (CPP), mean energy concentration of spectral or first spectral moment, the Hammarberg index, jittering, and shimmer) distinguish patients with MCI from HC? And ii. Do measures of speech fluency (namely, the averaged speaking time, articulation rate, and speech rate) distinguish patients with MCI from HC? To answer these questions, we are providing an acoustic analysis of speech productions from the Cookie Theft picture description task from the Boston Diagnostic Aphasia Examination (BDAE) produced by Swedish patients with MCI and HC [20]. This study shows that voice quality and speech fluency provide information that can identify patients with MCI from HC.
Patients and methods
Participants
The 55 participants were recruited as part of the Gothenburg MCI study, which is a large clinically based longitudinal study on MCI [21]. Details about the participants are provided in Table 1. The Gothenburg MCI study provides an in-depth phenotyping of patients with different forms and degrees of cognitive impairment using imaging/physiologic methods, psychometrics, and biochemical methods, namely cerebrospinal fluid characterization of substances in the brain. Participants were selected based on specific inclusion and exclusion criteria: (i) no dyslexia and other reading deficiencies; (ii) no current history of major depression, and recent substance abuse; (iii) no history of serious psychiatric, neurological and other brain-related conditions; (iv) to be native Swedish speakers; (v) to be able to read and understand information about the study; and (vi) to be able to provide written consent. HC had a significantly higher Mini-Mental State Exam score (M = 29.6). The MMSE score is a scale of 0–30 and represents the cognitive status of an individual. Mean MMSE score for the MCI participants was 28.2, which is close to normal [22–24]. Ethic approvals, the consent procedure, and data acquisition were approved by the Swedish Ethical Review Authority, <http://www.epn.se/> (ref. nr: 206–16, 2016) and the ethics amendment was approved by the same institution (ref. nr: T021-18, 2018). Subjects were prospectively recruited from one center: the Memory Clinic at the Sahlgrenska University Hospital, Sweden. All patients provided written informed consent for use of data before the data collection.
Table 1. Demographic information and scores for memory & learning, language, attention, and executive function, by group (mean and standard deviation).
| HC (n = 29) | MCI (n = 26) | Sig. | ||
|---|---|---|---|---|
| Demographic | Age (years) | 67.8 (7.7) | 70.6 (5.8) | * |
| Education (years) | 13.3 (3.7) | 14.3 (3.6) | n.s. | |
| Sex (F/M) | 21/8 | 14/12 | n.s. | |
| MMSE (/30) | 29.6 (0.6) | 28.2 (1.4) | *** | |
| Memory/Learning | RAVLT (total) | 45.5 (11.1) | 37.6 (10.7) | * |
| RAVLT (immediate) | 9.5 (3.5) | 6.1 (3.1) | *** | |
| RAVLT (delayed) | 9.2 (3.6) | 5.8 (3.5) | *** | |
| RCF (3 min) | 18.8 (5.1) | 15.8 (6.8) | n.s. | |
| RCF (20 min) | 18.6 (4.4) | 14.3 (7.0) | * | |
| WLM (immediate) | 25.8 (6.3) | 21.3 (7.6) | * | |
| WLM (delayed) | 21.9 (8.1) | 16.0 (10.5) | * | |
| Language | BNT | 53.3 (4.6) | 50.2 (7.6) | n.s. |
| Verbal Fluency (F-A-S) | 47.2 (11.5) | 43.6 (11.1) | n.s. | |
| Similarities (WAIS-III) | 24.6 (4.7) | 24.0 (5.2) | n.s. | |
| Token Test (Part 5) | 20.9 (1.4) | 20.0 (1.8) | n.s. | |
| Attention | Digit Span (WAIS-III) | 13.1 (3.5) | 12.4 (2.8) | n.s. |
| Digit-Symbol (WAIS-R) | 62.9 (12.3) | 54.2 (10.8) | ** | |
| TMT A | 34.1 (11.9) | 39.5 (13.3) | n.s. | |
| TMT B | 79.8 (32.9) | 97.8 (49.4) | n.s. | |
| Block design (WAIS-III) | 40.6 (9.5) | 35.5 (12.2) | n.s. | |
| RCF (copy) | 33.6 (2.4) | 32.4 (3.4) | n.s. | |
| Silhouettes (VOSP battery) | 22.4 (4.2) | 19.3 (3.3) | *** | |
| Executive Function | Letter-number sequencing (WAIS-III) | 9.5 (2.3) | 8.7 (2.6) | n.s. |
| PaSMO | 68.2 (21.5) | 86.8 (29.1) | * | |
| Stroop (Victoria version) (trial 1) | 13.2(2.4) | 14.6 (3.1) | n.s. | |
| Stroop (trial 2) | 17.6(3.4) | 19.4 (5.4) | n.s. | |
| Stroop (trial 3) | 24.1(6.6) | 27.6 (6.6) | * | |
| Stroop Effect | 1.8(0.4) | 1.9 (0.5) | n.s. |
BNT Boston Naming Test [25]; PaSMO Parallel Serial Mental Operation (a measure of mental control and working memory where the subject is asked to recite the alphabet, stating the number after each letter, i.e., A-1-B-2-C-3…) [26]; RAVLT Rey Auditory Verbal Learning Test [27]; RFC Rey–Osterrieth complex figure (RCF) [28]; TMT A, TMT B Trail Making Test A and B [29]; VOSP Visual object and space perception battery [30]; WAIS Wechsler adult intelligence scale [31]; WLM: Wechsler logical memory [31]
‘*’ p < .05
‘**’ p < .01
‘***’ p < .001.
Procedure and acoustic measurements
MCI diagnosis was based on staging of cognitive and functional abilities using the Geriatric deterioration scale (GDS) (GDS stage 3 = MCI) [32]. Specific operationalization of the GDS scoring in the Gothenburg MCI study has been described previously in detail [21]. The MCI group was mixed—we did not categorize the patients into MCI subgroups (such as amnestic MCI and non-amnestic MCI). A physician and/or registered nurse conducted the GDS assessment procedure, and the neuropsychological tests were administered by licensed psychologists alternatively health care professionals supervised by a licensed psychologist. Neuropsychological tests were selected by specialized psychologists, comprising tests within the cognitive domains speed and attention, learning and episodic memory, visuospatial, language, and executive functions. Testing was performed during clinical visits.
The picture description task was part of additional assessment tests conducted as part of “Linguistic and extra-linguistic parameters for early detection of cognitive impairment” research project funded by Riksbankens Jubileumsfond–The Swedish Foundation for Humanities & Social Sciences (NHS 14–1761:1). This picture shows two children trying to remove cookies from a jar placed on top of a cupboard as their mother is washing the dishes. A speech and language pathologist presented the picture to participants and prompted them to tell everything they see on the picture following the standard BDAE version 3 instructions. The picture description task was audio recorded using a Zoom H4N audio recorder, located at a fixed distance (1ft) in front of the participant. The audio was subsequently converted to 16000 Hz mono format [19, 33] and analyzed acoustically using the open source software for acoustic analysis Praat [34]. Specifically, we analyzed speech sounds and measured acoustic properties related to voice quality and speech fluency. Measurements of voice quality and syllable structure were calculated.
1. Voice quality / phonation
Phonation and voice quality account for the fine control of the sublaryngeal and laryngeal systems. To determine the phonation and voice quality differences of patients with MCI and HC, we have calculated the following measurements.
H1-H2, H1-A1, H1-A3: Difference between the amplitude of the first and second harmonics (H1-H2), the amplitude of the first harmonic and the amplitude of strongest harmonic of the first formant frequency (H1-A1), and the amplitude of the first harmonic and the amplitude of the third formant (H1-A3) demarcate voice quality. Harmonics are estimated by considering the fundamental frequency, and amplitudes from the spectra. H1-H2 indicates breathy (strong H1) and creaky voice (weaker H2) [35].
Cepstral Peak Prominence (CPP): CPP is a reliable measure of dysphonia [36]. It accounts for the periodicity in the voice signal: higher values of CPP correspond to greater periodicity. It stands as the relative amplitude of the CPP in relation to the expected amplitude as derived via linear regression.
Mean Energy Concentration: or first spectral moment is the average spectral frequency [37, 38].
-
Hammarberg Index: The Hammarberg index is the difference between the maximum energy in the 0…2kHz energy band and the energy in the 2…5kHz band. The Hammarberg index is considered an indicator of articulatory effort [39].
Finally, we provide measures of shimmering, jittering, and harmonicity elicited using Praat [34].
-
Jitter (Hz): it is the cycle-to-cycle variation of the fundamental frequency (F0) (1), expressed as:
where Ti are the extracted period lengths and N is the number of extracted F0 periods. The F0 is the basic frequency produced during the vibration of the vocal folds and it is one of the primary acoustic correlates of intonation, which manifests linguistic (e.g., different melodic patterns for questions, and statements) and extralinguistic functions (e.g., emotional prosody) [40]. Reduced control on vocal-fold vibration results in higher percentage of jitter [41].(1) -
Shimmer (dB): it is the variability of the amplitude from peak-to-peak (local maxima). Eq (2) shows shimmer as the mean absolute base-10 logarithm (multiplied by 20) of the difference between the amplitudes of successive periods (2):
where Ai are the extracted peak-to-peak measurements of amplitude and N is the number of F0 periods. Shimmer indicates noisy productions and breathiness and it is a correlate of glottal resistance and mass lesions on the vocal folds [41].(2)
2. Speech fluency
Speech rate and articulation rate. These are measures of fluency as described in the introduction. We calculated the following measures: average syllable duration, the articulation rate, and speech rate.
- Average Syllable Duration: Is the mean syllable duration estimated as a measure of the overall speaking time divided by the number of syllables (3).
(3) - Articulation Rate: Articulation rate considers phonation time, which is a measure of phonation times and excludes pauses and silences (4).
(4) - Speech Rate: Is a measure of the number of syllables divided by the overall duration, which includes pauses and silences (5):
(5)
For the statistical analysis, we employed linear mixed effects models using condition (MCI vs. HC) and gender as fixed factors on voice quality and phonation measurements (dependent variables) and condition on speech fluency measurements dependent variables. We included gender in the statistics of voice quality and phonation, as these measures depend on physiological differences between men and women, e.g., lower pitch in men than in women. The R package emmeans was employed to obtain estimated marginal means (EMMs, also known as least-squares means) for factor combinations in the linear mixed effects models and compute the contrasts or linear combinations of these marginal means.
Results
Voice quality / phonation
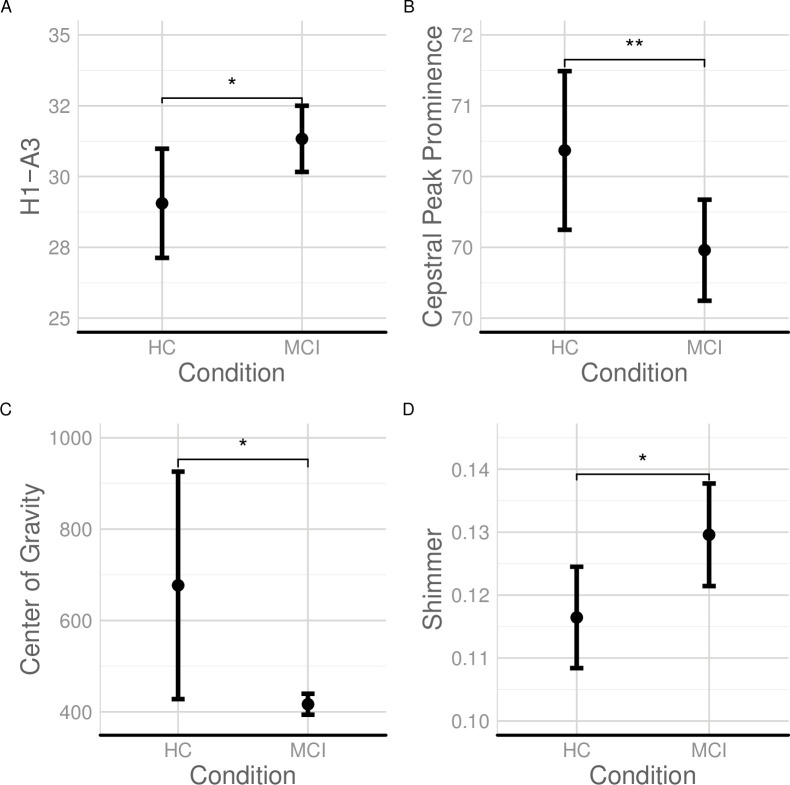
Voice quality measures demonstrate significant differences between patients with MCI and HC as shown in Fig 1. Patients with MCI produce speech that differs from HC in phonation and voice quality, which is measured using objective markers presented in this section and determine differences in the fine-control of the sublaryngeal and laryngeal systems. We found significant differences of patients with MCI from HC with respect to the difference of the first harmonic and third amplitude (H1-A3), shown in Table 2. Patients with MCI differed significantly from HC with respect to their CPP (see Fig 1, Panel B). There is an overall lower CPP in patients with MCI compared to HC, suggesting weaker voice. Also, patients with MCI differed significantly from HC with respect to shimmer and center of gravity. However, patients with MCI and HC did not differ significantly with respect to the Hammarberg Index measurement (F(1:278) = 0.137, p = 0.711). Also, there were no significant differences between patients with MCI and HC in jitter (F (1, 254) = 2.73, p = 0.1).
Fig 1. Voice quality and phonation elicited from audio recordings produced by patients with MCI and HC.
(A) H1-A3 (dB); (B) Cepstral Peak Prominence (dB); (C) Center of Gravity (Hz); and (D) Shimmer (dB) in HC and patients with MCI; ‘.’ indicates p < 0.1; ‘*’ indicates p < .05; ‘**’ indicates p < .01.
Table 2. Regression results for the effect of condition (MCI vs. HC) and gender on H1-A3.
| Predictor | b | b | sr2 | sr2 | Fit | |
|---|---|---|---|---|---|---|
| 95% CI | 95% CI | |||||
| [LL, UL] | [LL, UL] | |||||
| H1-A3 | Intercept | 26.87** | [25.14, 28.59] | R2 = .137** | ||
| MCI | 2.91* | [0.52, 5.29] | .02 | [-.01, .05] | 95% CI[.06,.21] | |
| Male | 9.75** | [6.11, 13.39] | .09 | [.03, .15] | ||
| MCI:Male | -5.52* | [-10.06, -0.97] | .02 | [-.01, .05] | ||
| CPP | Intercept | 71.12** | [70.59, 71.66] | R2 = .057** | ||
| MCI | -1.18** | [-1.92, -0.44] | .03 | [-.01, .07] | 95% CI[.01,.11] | |
| Male | -1.96** | [-3.08, -0.83] | .04 | [-.00, .08] | ||
| MCI:Male | 2.05** | [0.64, 3.46] | .03 | [-.01, .07] | Center of Gravity | |
| Center of Gravity | Intercept | 676.96** | [513.71, 840.22] | R2 = .020* | ||
| MCI | -260.32* | [-476.29, -44.35] | .02 | [.00, .06] | 95% CI[.00,.06] | |
| Shimmer | Intercept | 0.12** | [0.11, 0.13] | R2 = .019* | ||
| MCI | 0.01* | [0.00, 0.02] | .02 | [.00, .06] | 95% CI [.00,.06] |
b unstandardized regression weights, a significant b-weight indicates the semi-partial correlation is also significant.; sr2 semi-partial correlation squared; LL and UL lower and upper limits of a confidence interval
* p < .05
** p < .01.
C2. Speech fluency
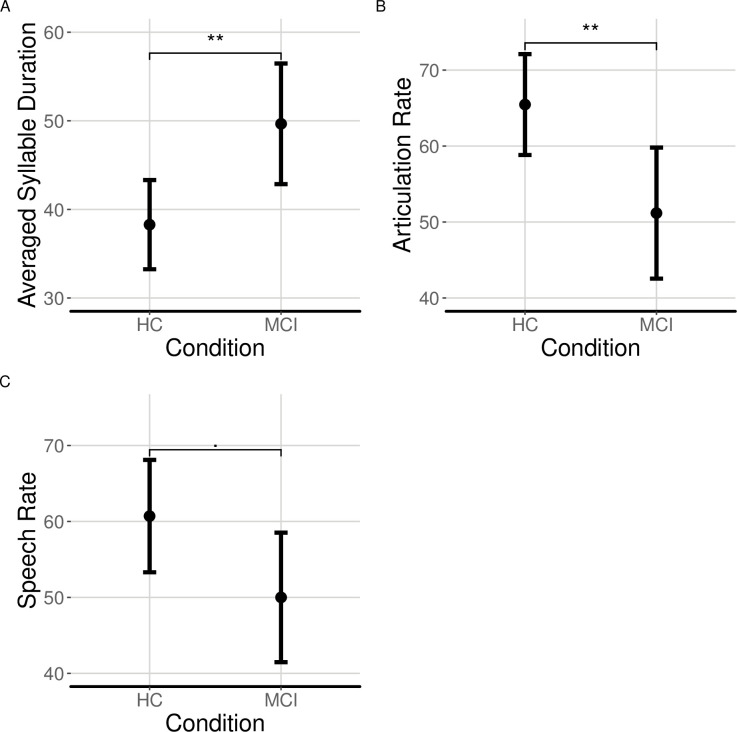
Patients with MCI produced significantly longer syllables from HC, as measured by the average syllable duration and had a slower articulation rate and speech rate but only with respect to average syllable duration and articulation rate we found significant effects (see Fig 2 and Table 3).
Fig 2. Measures of speech fluency elicited from audio recordings produced by patients with MCI and HC.
(A) averaged speaking time; (B) articulation rate; and (C) speech rate in HC and patients with MCI; ‘.’ indicates p < 0.1; ‘*’ indicates p < .05; ‘**’ indicates p < .01.
Table 3. Regression results for the effect of Condition (MCI vs. HC) on averaged syllable duration, articulation rate, and speech rate.
| Predictor | b | b | sr2 | sr2 | Fit | |
|---|---|---|---|---|---|---|
| 95% CI [LL, UL] | 95% CI [LL, UL] | |||||
| Average Syllable Duration | Intercept | 38.28** | [32.86, 43.71] | R2 = .063** | ||
| MCI | 11.38** | [3.19, 19.57] | .06 | [.00, .16] | 95% CI[.00,.16] | |
| Articulation Rate | Intercept | 65.47** | [58.46, 72.48] | R2 = .060** | ||
| MCI | -14.29** | [-24.87, -3.71] | .06 | [.00, .16] | 95% CI[.00,.16] | |
| Speech Rate | Intercept | 60.70** | [53.32, 68.08] | |||
| MCI | -10.70 | [-21.85, 0.44] | .03 | [.00, .12] | ||
| R2 = .031 | ||||||
| 95%CI[.00,.12] |
b unstandardized regression weights, a significant b-weight indicates the semi-partial correlation is also significant; sr2 semi-partial correlation squared; LL and UL lower and upper limits of a confidence interval
‘*’ p < .05
‘**’ p < .01.
Table 4 presents a summary of the main findings with the acoustic measures that differentiate patients with MCI from HC.
Table 4. Summary of the main findings.
| Measure | Result | |
|---|---|---|
| Voice Quality | H1-A3 | Significant differences between patients with MCI vs HC |
| Cepstral Peak Prominence | Significant differences between patients with MCI vs HC | |
| Center of Gravity | Significant differences between patients with MCI vs HC | |
| Shimmer | Significant differences between patients with MCI vs HC | |
| Articulation Rate | Average Syllable Duration | Significant differences between patients with MCI vs HC |
| Articulation Rate | Significant differences between patients with MCI vs HC | |
| Speech Rate | Marginal differences (p < 0.1). |
Discussion
Cognitive decline in patients with Mild Cognitive Impairment (MCI) is manifested as a noticeable memory difficulty in remembering events and situations, impaired language, speech, decision making, planning, interpreting instructions, and orientation [1, 21, 42–48]. Given that MCI patients are a high risk group for developing AD, there is a dire need to elicit objective measures that can enable the early and quick identification of patients with MCI, to provide treatment promptly, facilitate MCI prognosis, and ultimately improve life quality both for patients with MCI and for their family members. This study provides novel findings that show impairment of speech production in patients with MCI with respect to (i) voice quality and (ii) speech fluency and demonstrates that these measures can provide objective diagnostics of patients with MCI.
Voice quality measures of MCI
An unexpected finding is that patients with MCI differed from HC with respect to voice quality. Early cognitive impairment is manifested by disparities in voice breathiness and increased dysphonia. Patients with MCI differed from HC in H1-A3, which suggests that voice breathiness is different in patients with MCI with respect to HC. Our study shows an increased H1-A3 in patients with MCI with respect to HC. Tanaka, Adachi [49] report a similar finding in patients with AD vs. HC. A novel finding was that patients with MCI show lower periodicity in spectra than HC, which corresponds to greater dysphonia, as measured with the CPP. Patients with MCI are characterized by overall lower center of gravity; which can correspond to lower frequency speech productions, that result into a significantly weaker speech than HC of the same age. It also indicates an overall relaxation of articulators during speech production that is manifested by the lowering of the spectral center of gravity. Patients with MCI are characterized by greater shimmer in speech production which indicates greater instability of amplitude. Greater shimmer may indicate less stability and control of the sublaryngeal/pulmonary pressure. Another important finding is that patients with MCI are characterized by differences in breathy voice, greater dysphonia, lower center of gravity and shimmer. These findings may be the result of cognitive and physiological impairment of the fine control and the slowing down of the vocal folds, of pulmonary pressure, respiration, and the co-ordination of phonation with articulatory production [15, 50–53].
Speech fluency measures
Patients with MCI have different speech fluency measures. Our findings show that the overall articulation rate and speech rate are significantly slower in patients with MCI than in elderly HC. The slower articulation rate can be the result of slower cognitive processes due to MCI, affecting attention, memory, and language, including word recall and grammar [1–3]. It can also be the result of impaired motor control as patients with MCI are characterized largely by abnormalities in motor coordination and disinhibition [54], motor preparation [55], and motor planning [56], which can influence motoric functions related to articulation.
Diagnostic utility of speech features
This study brought together speech acoustics and statistical analysis for the study of speech production in MCI. Speech reveals multidimensional information about the speaker (e.g., age, gender, sociolinguistic characteristics, physiological condition) and can function as a fingerprint that identifies patients with MCI from HC. The findings provide objective measures from voice quality that distinguish patients with MCI and HC and at the same time they point to the importance of phonation and speech fluency as a diagnostic measurement [50–53]. Implemented as a computer application, this approach can provide an easy and accessible interface for the automatic quantification of voice quality and speech fluency, utilized by physicians, neuropsychologists, and speech therapists to quantify speech in tasks, such as picture description tasks, scripts, and discourse. By increasing the span of acoustic measurements that can be analyzed and understanding their corresponding speech deficits [57, 58], physicians, neuropsychologists, and speech therapists can tailor therapeutic programs to the specific needs of their clients (e.g., focusing on targeted part of speech productions). Measures of voice quality and fluency from connected speech, discourse, etc. can enable clinicians to assess the overall speech production of patients with MCI and provide information about the differential speech properties of patients with MCI variants and HC and ultimately enable a better understanding of speech symptoms of patients with MCI.
Limitations and future directions
Picture description tasks (e.g., Cookie Theft) constrain the production of speech in that the productions are often narrowed down to labelling rather than on free narration which to a certain degree may constrain fluency measurements. In contrast, storytelling, discourse, and conversation are characterized by expressive variations of fluency. This aspect of fluency cannot be tested using picture description tasks but requires a computational analysis of voice quality and speech fluency in free style conversations and in other conditions affecting language, such as stroke aphasia and primary progressive aphasia [e.g., 58, 59, 60–64]. Another limitation is the relatively small sample size; a larger sample size is expected to increase the effect size of the model. Also, as speakers are recruited at a single recruitment center, the participants may not be representative of the overall population of patients with MCI in Sweden. To address these limitations, we are collecting a variety of linguistic data from a larger population of patients attending different recruitment centers. The acoustic measures proposed in this study along with the obtainability of connected speech productions and the availability of acoustic analysis software can enable the rapid analysis of speech in the primary care centers and memory clinics providing accessible diagnostic methods for MCI.
Data Availability
The data and code are uploaded to GitHub and accessible via the following URL: https://github.com/themistocleous/acoustic_profile-MCI.
Funding Statement
DK, NHS 14-1761:1, Riksbankens Jubileumsfond – The Swedish Foundation for Humanities & Social Sciences, Sweden https://www.rj.se/en. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Archives of neurology. 1999;56(3):303–8. 10.1001/archneur.56.3.303 [DOI] [PubMed] [Google Scholar]
- 2.Gauthier S, Reisberg B, Zaudig M, Petersen RC, Ritchie K, Broich K, et al. Mild cognitive impairment. The Lancet. 2006;367(9518):1262–70. [DOI] [PubMed] [Google Scholar]
- 3.Petersen RC, Thomas RG, Aisen PS, Mohs RC, Carrillo MC, Albert MS. Randomized controlled trials in mild cognitive impairment: Sources of variability. Neurology. 2017. 10.1212/WNL.0000000000003907 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zucchella C, Sinforiani E, Tamburin S, Federico A, Mantovani E, Bernini S, et al. The Multidisciplinary Approach to Alzheimer's Disease and Dementia. A Narrative Review of Non-Pharmacological Treatment. Frontiers in Neurology. 2018;9:1058 10.3389/fneur.2018.01058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Themistocleous C, Kokkinakis D, Marie E, Kathleen F, Kristina Lundholm F. Effects of Cognitive Impairment on vowel duration. Proceedings of the 9th Tutorial & Research Workshop on Experimental Linguistics (ExLing 2018) 28–30 Paris, France. 2018:113–6. cdeff18.
- 6.Lundholm Fors K, Fraser KC, Themistocleous C, Kokkinakis D. Prosodic features as potential markers of linguistic and cognitive deterioration in Mild Cognitive Impairment. Alzheimer's & Dementia. 2018;14(7, Supplement):P1195–P6. 10.1016/j.jalz.2018.06.1658. [DOI] [Google Scholar]
- 7.Sajjadi SA, Patterson K, Tomek M, Nestor PJ. Abnormalities of connected speech in semantic dementia vs Alzheimer's disease. Aphasiology. 2012;26(6):847–66. 10.1080/02687038.2012.654933 WOS:000304530100006. [DOI] [Google Scholar]
- 8.Braaten AJ, Parsons TD, McCue R, Sellers A, Burns WJ. Neurocognitive Differential Diagnosis Of Dementing Diseases: Alzheimer's Dementia, Vascular Dementia, Frontotemporal Dementia, And Major Depressive Disorder. International Journal of Neuroscience. 2006;116(11):1271–93. 10.1080/00207450600920928 [DOI] [PubMed] [Google Scholar]
- 9.Kemper S, LaBarge E, Ferraro FR, Cheung H, Cheung H, Storandt M. On the Preservation of Syntax in Alzheimer's Disease: Evidence From Written Sentences. Archives of Neurology. 1993;50(1):81–6. 10.1001/archneur.1993.00540010075021 [DOI] [PubMed] [Google Scholar]
- 10.Lira JOd Minett TSC, Bertolucci PHF Ortiz KZ. Analysis of word number and content in discourse of patients with mild to moderate Alzheimer's disease. Dementia & neuropsychologia. 2014;8(3):260–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chapman SB, Ulatowska HK, King K, Johnson JK, McIntire DD. Discourse in early Alzheimer's disease versus normal advanced aging. American Journal of Speech-Language Pathology. 1995;4(4):124–9. [Google Scholar]
- 12.König A, Satt A, Sorin A, Hoory R, Toledo-Ronen O, Derreumaux A, et al. Automatic speech analysis for the assessment of patients with predementia and Alzheimer's disease. Alzheimer's and Dementia: Diagnosis, Assessment and Disease Monitoring. 2015;1(1):112–24. 10.1016/j.dadm.2014.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffmann I, Nemeth D, Dye CD, Pákáski M, Irinyi T, Kálmán J. Temporal parameters of spontaneous speech in Alzheimer's disease. International Journal of Speech-Language Pathology. 2010;12(1):29–34. 10.3109/17549500903137256 [DOI] [PubMed] [Google Scholar]
- 14.Martínez-Sánchez F, Meilán JJG, Pérez E, Carro J, Arana JM. Expressive prosodic patterns in individuals with Alzheimer's Disease. Psicothema. 2012;24(1):16–21. [PubMed] [Google Scholar]
- 15.Meilan JJG, Martinez-Sanchez F, Carro J, Carcavilla N, Ivanova O. Voice Markers of Lexical Access in Mild Cognitive Impairment and Alzheimer's Disease. Current Alzheimer Research. 2018;15(2):111–9. 10.2174/1567205014666170829112439 [DOI] [PubMed] [Google Scholar]
- 16.Meilan JJG, Martinez-Sanchez F, Carro J, Lopez DE, Millian-Morell L, Arana JM. Speech in Alzheimer's Disease: Can Temporal and Acoustic Parameters Discriminate Dementia? Dementia and Geriatric Cognitive Disorders. 2014;37(5–6):327–34. 10.1159/000356726 WOS:000337795500008. [DOI] [PubMed] [Google Scholar]
- 17.Horley K, Reid A, Burnham D. Emotional prosody perception and production in Dementia of the Alzheimer's type. Journal of Speech, Language, and Hearing Research. 2010;53(5):1132–46. 10.1044/1092-4388(2010/09-0030) [DOI] [PubMed] [Google Scholar]
- 18.Martínez-Sánchez F, Meilán JJG, García-Sevilla J, Carro J, Arana JM. Oral reading fluency analysis in patients with Alzheimer disease and asymptomatic control subjects. Neurologia. 2013;28(6):325–31. 10.1016/j.nrl.2012.07.012 [DOI] [PubMed] [Google Scholar]
- 19.Themistocleous C, Eckerstrom M, Kokkinakis D. Identification of Mild Cognitive Impairment From Speech in Swedish Using Deep Sequential Neural Networks. Frontiers in Neurology. 2018;9:975 10.3389/fneur.2018.00975 WOS:000450247700002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodglass H, Kaplan E. Boston diagnostic aphasia examination (BDAE) 1983. [Google Scholar]
- 21.Wallin A, Nordlund A, Jonsson M, others. The Gothenburg MCI study: Design and distribution of Alzheimer's disease and subcortical vascular disease diagnoses from baseline to 6-year follow-up. Journal of Cerebral Blood Flow & Metabolism. 2016;36(1):114–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skoog J, Backman K, Ribbe M, Falk H, Gudmundsson P, Thorvaldsson V, et al. A Longitudinal Study of the Mini-Mental State Examination in Late Nonagenarians and Its Relationship with Dementia, Mortality, and Education. Journal of the American Geriatrics Society. 2017;65(6):1296–300. 10.1111/jgs.14871 [DOI] [PubMed] [Google Scholar]
- 23.Fraser KC, Lundholm Fors K, Eckerström M, Themistocleous C, Kokkinakis D. Improving the Sensitivity and Specificity of MCI Screening with Linguistic Information. Proceedings of the LREC 2018 Workshop “Resources and ProcessIng of linguistic, para-linguistic and extra-linguistic Data from people with various forms of cognitive/psychiatric impairments (RaPID-2)”. 2018;(2015):19–26.
- 24.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(null):189–98. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 25.Kaplan E, Goodglass H, Weintraub S. Boston naming test: Pro-ed; 2001.
- 26.Nordlund A, Rolstad S, Hellström P, Sjögren M, Hansen S, Wallin A. The Goteborg MCI study: mild cognitive impairment is a heterogeneous condition. Journal of Neurology, Neurosurgery & Psychiatry. 2005;76(11):1485–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt M. Rey auditory verbal learning test: A handbook: Western Psychological Services Los Angeles, CA; 1996.
- 28.Meyers JE, Meyers KR. Rey complex figure test and recognition trial (RCFT): Psychological Assessment Resources Odessa, FL; 1995.
- 29.Reitan RWD, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Therapy and Clinical Interpretation (1985). Tucson, AZ: Neuropsychological Press; 1985. [Google Scholar]
- 30.Warrington EK, James M. The visual object and space perception battery. 1991. [Google Scholar]
- 31.Wechsler D, Stone CP. Wechsler memory scale: Psychological Corporation; 1945. [Google Scholar]
- 32.Auer S, Reisberg B. The GDS/FAST Staging System. International Psychogeriatrics. 1997;9(S1):167–71. Epub 2005/01/10. 10.1017/S1041610297004869 [DOI] [PubMed] [Google Scholar]
- 33.Themistocleous C, Kokkinakis D. THEMIS-SV: Automatic classification of language disorders from speech signals. ESOC 2018: European Stroke Organisation Conference Gothenburg2018.
- 34.Boersma P, Weenink D. Praat: doing phonetics by computer (Version 6.0.37). 2018.
- 35.Klatt D, Klatt L. Analysis, synthesis, and perception of voice quality variations among female and male talkers. Time. 1990:820–57. [DOI] [PubMed] [Google Scholar]
- 36.Heman-Ackah YD, Michael DD, Baroody MM, Ostrowski R, Hillenbrand J, Heuer RJ, et al. Cepstral Peak Prominence: A More Reliable Measure of Dysphonia. Annals of Otology, Rhinology & Laryngology. 2003;112(4):324–33. 10.1177/000348940311200406 [DOI] [PubMed] [Google Scholar]
- 37.Boersma P. Praat, a system for doing phonetics by computer. Glot International. 2001;5(9/10):341–5. [Google Scholar]
- 38.Forrest K, Weismer G, Milenkovic P, Dougall RN. Statistical analysis of word-initial voiceless obstruents: preliminary data. Journal of the Acoustical Society of America. 1988;84(1):115–23. 10.1121/1.396977 [DOI] [PubMed] [Google Scholar]
- 39.Hammarberg B, Fritzell B, Gaufin J, Sundberg J, Wedin L. Perceptual and Acoustic Correlates of Abnormal Voice Qualities. Acta Oto-Laryngologica. 1980;90(1–6):441–51. 10.3109/00016488009131746 [DOI] [PubMed] [Google Scholar]
- 40.Themistocleous C. Seeking an Anchorage. Stability and Variability in Tonal Alignment of Rising Prenuclear Pitch Accents in Cypriot Greek. Language and Speech. 2016;59(4). 10.1177/0023830915614602 [DOI] [PubMed] [Google Scholar]
- 41.Teixeira JP, Oliveira C, Lopes C. Vocal Acoustic Analysis–Jitter, Shimmer and HNR Parameters. Procedia Technology. 2013;9:1112–22. 10.1016/j.protcy.2013.12.124. [DOI] [Google Scholar]
- 42.Bidelman GM, Lowther JE, Tak SH, Alain C. Mild Cognitive Impairment Is Characterized by Deficient Brainstem and Cortical Representations of Speech. The Journal Of Neuroscience: The Official Journal Of The Society For Neuroscience. 2017;37(13):3610–20. 10.1523/JNEUROSCI.3700-16.2017 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boccia M, Silveri MC, Sabatini U, Guariglia C, Nemmi F. Neural Underpinnings of the Decline of Topographical Memory in Mild Cognitive Impairment. American Journal Of Alzheimer's Disease And Other Dementias. 2016;31(8):618–30. 10.1177/1533317516654757 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borroni B, Di Luca M, Padovani A. Predicting Alzheimer dementia in mild cognitive impairment patients. Are biomarkers useful? European Journal of Pharmacology. 2006;545(1):73–80. 10.1016/j.ejphar.2006.06.023 [DOI] [PubMed] [Google Scholar]
- 45.Rahman TTA, Mohamed ST, Albanouby MH, Bekhet HF. Central auditory processing in elderly with mild cognitive impairment. Geriatrics and Gerontology International. 2011;11(3):304–8. 10.1111/j.1447-0594.2010.00684.x [DOI] [PubMed] [Google Scholar]
- 46.Ribeiro F, Guerreiro M, de Mendonça A. Verbal learning and memory deficits in Mild Cognitive Impairment. Journal of Clinical and Experimental Neuropsychology. 2007;29. [DOI] [PubMed] [Google Scholar]
- 47.Roark B, Mitchell M, Hosom J-P, Hollingshead K, Kaye J. Spoken Language Derived Measures for Detecting Mild Cognitive Impairment. IEEE Transactions on Audio, Speech, and Language Processing. 2011;19(7):2081–90. 10.1109/TASL.2011.2112351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ryan KA, Weldon A, Persad C, Heidebrink JL, Barbas N, Giordani B. Neuropsychiatric symptoms and executive functioning in patients with mild cognitive impairment: Relationship to caregiver burden. Dementia and Geriatric Cognitive Disorders. 2012;34(3–4):206–15. 10.1159/000339955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tanaka H, Adachi H, Ukita N, Ikeda M, Kazui H, Kudo T, et al. Detecting Dementia Through Interactive Computer Avatars. IEEE journal of translational engineering in health and medicine. 2017;5:2200111–. 10.1109/JTEHM.2017.2752152 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahmed S, Haigh A-MF, de Jager CA, Garrard P. Connected speech as a marker of disease progression in autopsy-proven Alzheimer’s disease. Brain. 2013;136(12):3727–37. 10.1093/brain/awt269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Forbes KE, Venneri A, Shanks MF. Distinct patterns of spontaneous speech deterioration: an early predictor of Alzheimer's disease. Brain and Cognition. 2002;48(2–3):356–61. [PubMed] [Google Scholar]
- 52.Forbes-McKay K, Shanks MF, Venneri A. Profiling spontaneous speech decline in Alzheimer's disease: a longitudinal study. Acta Neuropsychiatrica. 2013;25(6):320–7. 10.1017/neu.2013.16 WOS:000327784100003. [DOI] [PubMed] [Google Scholar]
- 53.Forbes-McKay KE, Venneri A. Detecting subtle spontaneous language decline in early Alzheimer’s disease with a picture description task. Neurological sciences. 2005;26(4):243–54. 10.1007/s10072-005-0467-9 [DOI] [PubMed] [Google Scholar]
- 54.Li H-j, Wang P-y, Jiang Y, Chan RCK, Wang H-l, Li J. Neurological soft signs in persons with amnestic mild cognitive impairment and the relationships to neuropsychological functions. Behavioral and Brain Functions. 2012;8(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ameli M, Kemper F, Sarfeld A-S, Kessler J, Fink GR, Nowak DA. Arbitrary visuo-motor mapping during object manipulation in mild cognitive impairment and Alzheimer's disease: A pilot study. Clinical Neurology and Neurosurgery. 2011;113(6):453–8. 10.1016/j.clineuro.2011.01.011 [DOI] [PubMed] [Google Scholar]
- 56.Salek Y, Anderson ND, Sergio L. Mild cognitive impairment is associated with impaired visual-motor planning when visual stimuli and actions are incongruent. European Neurology. 2011;66(5):283–93. 10.1159/000331049 [DOI] [PubMed] [Google Scholar]
- 57.Themistocleous C, Ficek B, Webster KT, Wendt H, Hillis AE, Den Ouden DB, et al. Acoustic markers of PPA variants using machine learning. Human Neuroscience Archive. 2019. [Google Scholar]
- 58.Themistocleous C, Ficek B, Webster K, den Ouden D-B, Hillis AE, Tsapkini K. Automatic subtyping of individuals with Primary Progressive Aphasia. bioRxiv. 2020:2020.04.04.025593. 10.1101/2020.04.04.025593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fyndanis V, Varlokosta S, Tsapkini K. Agrammatic production: Interpretable features and selective impairment in verb inflection. Lingua. 2012;122(10):1134–47. 10.1016/j.lingua.2012.05.004 WOS:000306869200004. [DOI] [Google Scholar]
- 60.Koukoulioti V, Bastiaanse R. Time reference in aphasia: Evidence from Greek. Journal of Neurolinguistics. 2020;53 10.1016/j.jneuroling.2019.100872 WOS:000500049600004. [DOI] [Google Scholar]
- 61.Dragoy O, Virfel E, Yurchenko A, Bastiaanse R. Aspect and tense attrition in Russian-German bilingual speakers. Int J Bilingual. 2019;23(1):275–95. 10.1177/1367006917728388 WOS:000458862900015. [DOI] [Google Scholar]
- 62.Bastiaanse R, Platonov A. Argument Structure and Time Reference in Agrammatic Aphasia. Cognitive Science Perspectives on Verb Representation and Processing 2015. p. 141–55. [Google Scholar]
- 63.Bastiaanse R. Why reference to the past is difficult for agrammatic speakers. Clinical Linguistics & Phonetics. 2013;27(4):244–63. Epub 2013/01/24. 10.3109/02699206.2012.751626 . [DOI] [PubMed] [Google Scholar]
- 64.Fyndanis V, Themistocleous C. Are there prototypical associations between time frames and aspectual values? Evidence from Greek aphasia and healthy ageing. Clinical Linguistics & Phonetics. 2019;33(1–2):191–217. Epub 2018/06/26. 10.1080/02699206.2018.1480657 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data and code are uploaded to GitHub and accessible via the following URL: https://github.com/themistocleous/acoustic_profile-MCI.