Abstract
RPGR-associated retinitis pigmentosa (RP) is a progressive disease with retina degeneration. Optical coherence tomography angiography (OCTA) is an imaging technique that provides novel insights. The authors report two affected male siblings who underwent OCTA imaging. The area of the foveal avascular zone (FAZ) was measured. Although the younger sibling exhibited more advanced clinical disease, his visual acuity was superior to his older sibling. OCTA imaging revealed a better preserved FAZ in the younger sibling as the reason for this. It also highlighted attenuation of choriocapillaris / choroid layers as biomarkers for disease severity. This provides new insights into retinal degeneration in RPGR-associated RP.
INTRODUCTION
Retinitis pigmentosa (RP) is a group of diseases with progressive retinal degeneration, and 15% to 30% of cases demonstrate an X-linked inheritance.1 Mutations in the gene encoding RP GTPase regulator (RPGR, OMIM #312610) accounts for the majority of X-linked RP.2 This protein is localized to the photoreceptor connecting cilia and is involved in protein trafficking.3 The human phenotype has been previously described in detail.4,5,6,7
Optical coherence tomography angiography (OCTA) is a noninvasive imaging modality that provides visualization of the retinal and choroidal vasculature.8 It is based upon spectral-domain OCT (SDOCT) to indirectly highlight vasculature. Although OCTA has been used to characterize other retinal diseases, RPGR-associated RP has yet to be evaluated.9,10
We previously reported on two male siblings with RPGR-associated RP masquerading as Stargardt disease.4 They exhibited a novel mutation in the RPGR gene (c.3070G>T, pGlu1024X). Although the older sibling (Patient 1) displayed a milder clinical appearance compared to his younger sibling (Patient 2), his best-corrected visual acuity (BCVA) was worse. In the current report, OCTA provides important insight into this confounding finding.
CASE REPORT
Both siblings previously presented for evaluation of decreased vision that was mistakenly attributed to Stargardt disease due to the presence of a heterozygous encoding mutation in the ABCA4 gene.4 Whole exome and Sanger sequencing revealed a novel mutation in the RPGR gene indicating RPGR-associated RP,4 a diagnosis that was more consistent with the phenotypic presentation and inheritance pattern. Both siblings returned for follow up examinations.
Patient 1 was a middle-aged male with a current BCVA measured to be 20/200 and 20/250 in the right (OD) and left (OS) eyes, respectively, by Snellen. His previous complaints of central blurry vision, nyctalopia, and photophobia remained stable. Fundus examination and autofluorescence (AF) imaging of both eyes (OU) demonstrated a bull’s-eye maculopathy and peripapillary atrophy (Figure 1). SD-OCT imaging showed significant bilateral retinal atrophy, and OCTA imaging indicated attenuation of the vasculature within the deep retinal layers as well as choriocapillaris / choroid.
Figure 1.
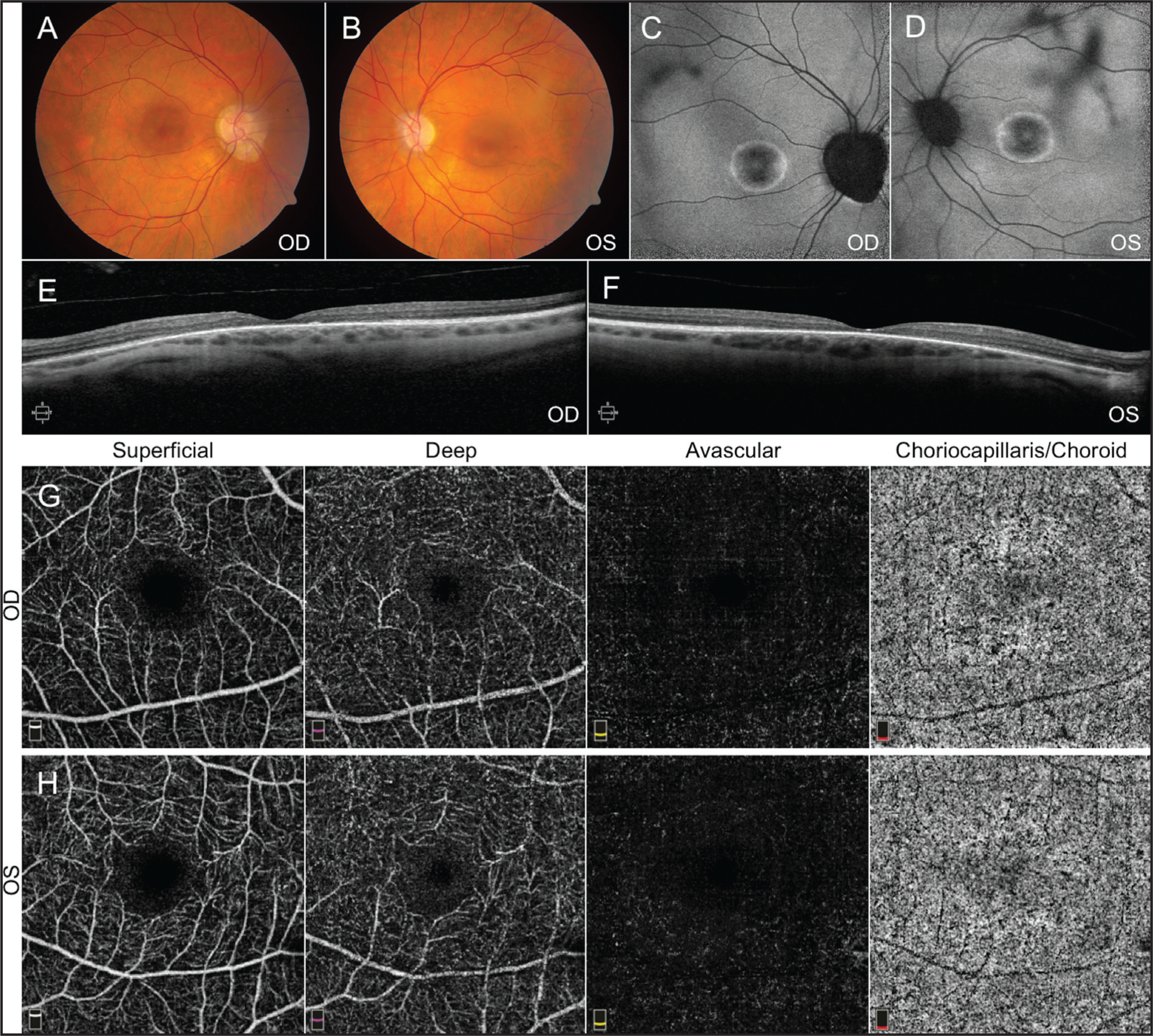
Fundus imaging from Patient 1. Fundus photos (A, B) and autofluorescence (C, D) demonstrate pigmentary changes with bull’s-eye maculopathy. Spectral-domain optical coherence tomography (OCT) of the macula (E, F) is consistent with outer retinal degeneration. OCT angiography imaging (G, H) showed mild attenuation of vasculature in the choriocapillaris / choroid layer.
Patient 2 was a younger, middle-aged male sibling of Patient 1 with more pronounced pigmentary changes and bull’s-eye maculopathy OU. Ophthalmic tests including AF, SD-OCT, and OCTA imaging confirmed advanced atrophy of the retina and retinal pigment epithelium, as well as vascular attenuation of the retina, choriocapillaris, and choroid layers (Figure 2). However, the BCVA of Patient 2 was measured to be 20/50 OU. Measurement of the area of the foveal avascular zone (FAZ) from the superficial retina capillary plexus using OCTA revealed that Patient 1 had a much larger FAZ OU than Patient 2, which may account for the discrepancies in BCVA (Figure 3).11
Figure 2.
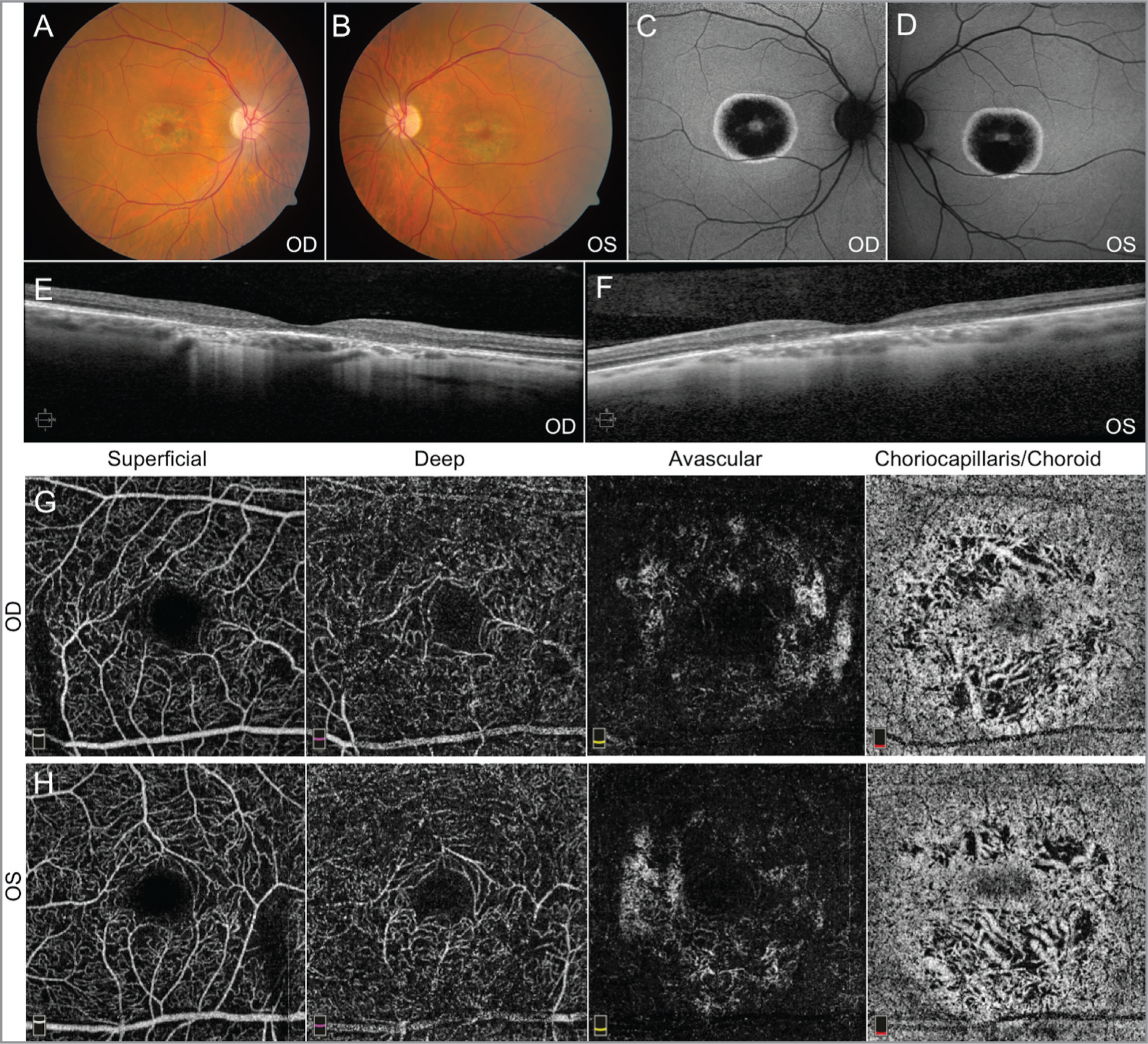
Fundus imaging from Patient 2. Fundus photos (A, B) and autofluorescence (C, D) demonstrate pigmentary changes with bull’s-eye maculopathy. Spectral-domain optical coherence tomography (OCT) of the macula (E, F) is consistent with outer retinal degeneration. OCT angiography imaging (G, H) showed moderate attenuation of vasculature in the choriocapillaris/choroid layer. These findings appear more pronounced than those found in Patient 1.
Figure 3.
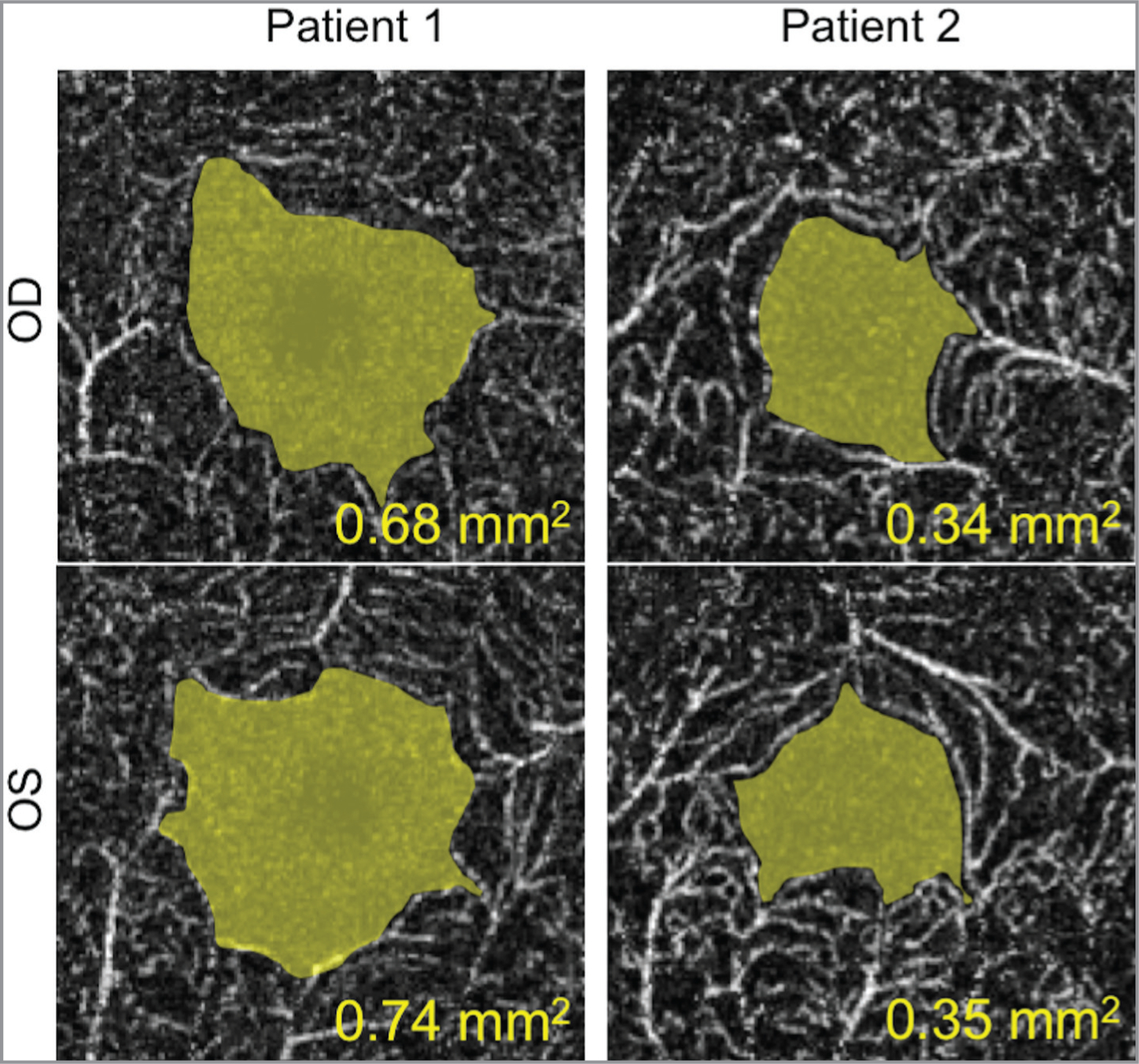
Measuring the foveal avascular zone (FAZ) from the superficial retinal circulation using optical coherence tomography angiography. The area of the FAZ is highlighted in yellow, indicating that Patient 1 had a larger FAZ than Patient 2.
DISCUSSION
RP is a heterogeneous collection of diseases with various degrees of genetic penetrance and phenotype. Although the severity of clinical findings can often be correlated with the level of BCVA decline, cases that do not follow this pattern require further investigation.
In this report, we characterized the clinical findings of two siblings with the same genetic variant in the RPGR gene leading to the development of X-linked RP. The younger sibling exhibited more advanced retinal degenerative findings based on fundus examination, AF, and SD-OCT imaging compared to the older sibling. Further investigation with OCTA imaging highlighted the advanced attenuation of vascular flow within the choriocapillaris / choroid layers as an important biomarker for this disease. These findings have been observed in other forms of RP, as well.12,13
It was notable that the younger sibling with the appearance of more-advanced retinal degenerative findings had a better-measured BCVA compared to his older sibling. Further investigation with OCTA imaging revealed the younger sibling had a comparatively smaller FAZ area, closer to the values of normal controls that have been reported in other studies. This finding could account for the discrepancy between clinical severity and visual performance. The FAZ region of the macula is critical for visual performance as it contains the highest density of cone photoreceptors.14 Nourishment and oxygenation is provided by the choroidal circulation to meet the high metabolic demands of this retinal region, whereas the inner retina is supplied by intraretinal arterial circulation. Enlargement of the FAZ indicates attenuation of the surrounding intraretinal arteries that support the downstream cell types responsible for transmitting and modulating neuronal signals that are generated from cones. Although Patient 2 had more attenuation of the choroidal layers, adequate circulation may have remained to maintain cone function. Thus, although cones within the enlarged FAZ may be as drastically affected, inner retinal cells such as bipolar and ganglion cells are unable to function adequately, therefore leading to a decline in measured BCVA of the patient. Previous studies have shown that an increased FAZ area is significantly correlated with decreased BCVA in patients with retinal vascular diseases such as diabetic retinopathy and retinal vein occlusion.15
Although highly variable, the FAZ area of healthy adults has been measured by OCTA to be approximately 0.32 mm.11, 16 Compared to our measurements, the FAZ area OU from the older sibling was approximately twice as large, whereas that from younger sibling was only mildly increased. These findings may account the discrepancies in BCVA between the two siblings. Our report highlights the utility of OCTA in providing new insights into retinal vascular attenuation within patients with RPGR-associated RP.
Acknowledgments
Dr. Mahajan is supported by NIH grants [R01EY024665, R01EY025225, R01EY024698, R21AG050437, and P30EY026877], and Research to Prevent Blindness (RPB), New York, NY. The Barbara & Donald Jonas Stem Cell & Regenerative Medicine Laborary and Bernard & Shirlee Brown Glaucoma Laboratory is supported by NIH core grants 5P30CA013696 and 5P30EY019007, unrestricted funds from RBP, New York, NY. Dr. Tsang is a member of the RD-CURE Consortium and is supported by Tistou and Charlotte Kerstan Foundation, R01EY018213, New York State C029572, the RPB Physician-Scientist Award, the Joel Hoffman Fund, Charles Culpeper Scholarship, Irma T. Hirschl Charitable Trust, and the Gebroe Family Foundation. The remaining authors report no relevant financial disclosures.
REFERENCES
- 1.Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368(9549):1795–1809. [DOI] [PubMed] [Google Scholar]
- 2.Vervoort R, Lennon A, Bird AC, et al. Mutational hot spot within a new RPGR exon in X-linked retinitis pigmentosa. Nat Genet. 2000;25(4):462–466. [DOI] [PubMed] [Google Scholar]
- 3.Hong DH, Pawlyk BS, Shang J, Sandberg MA, Berson EL, Li T. A retinitis pigmentosa GTPase regulator (RPGR)-deficient mouse model for X-linked retinitis pigmentosa (RP3). Proc Natl Acad Sci U S A. 2000;97(7):3649–3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bassuk AG, Sujirakul T, Tsang SH, Mahajan VB. A novel RPGR mutation masquerading as Stargardt disease. Br J Ophthalmol. 2014;98(5):709–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zito I, Downes SM, Patel RJ, et al. RPGR mutation associated with retinitis pigmentosa, impaired hearing, and sinorespiratory infections. J Med Genet. 2003;40(8):609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tee JJL, Carroll J, Webster AR, Michaelides M. Quantitative analysis of retinal structure using spectral-domain optical coherence tomography in RPGR-associated retinopathy. Am J Ophthalmol. 2017;178:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charng J, Cideciyan AV, Jacobson SG, et al. Variegated yet non-random rod and cone photoreceptor disease patterns in RPGR-ORF15-associated retinal degeneration. Hum Mol Genet. 2016;25(24):5444–5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spaide RF, Fujimoto JG, Waheed NK, Sadda SR, Staurenghi G. Optical coherence tomography angiography. Prog Retin Eye Res. 2018;64:1–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roisman L, Goldhardt R. OCT angiography: An upcoming noninvasive tool for diagnosis of age-related macular degeneration. Curr Ophthalmol Rep. 2017;5(2):136–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coscas G, Lupidi M, Coscas F. Optical coherence tomography angiography in diabetic maculopathy. Dev Ophthalmol. 2017;60:38–49. [DOI] [PubMed] [Google Scholar]
- 11.Fujiwara A, Morizane Y, Hosokawa M, et al. Factors affecting foveal avascular zone in healthy eyes: An examination using swept-source optical coherence tomography angiography. PLoS One. 2017;12(11):e0188572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rezaei KA, Zhang Q, Chen CL, Chao J, Wang RK. Retinal and choroidal vascular features in patients with retinitis pigmentosa imaged by OCT based microangiography. Graefes Arch Clin Exp Ophthalmol. 2017;255(7):1287–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Battaglia-Parodi M, Cicinelli MV, Robiolo A, Pierro L, Bolognesi G, Bandello F. Vascular abnormalities in patients with Stargardt disease assessed with optical coherence tomography angiography. Br J Ophthalmol. 2017;101(6):780–785. [DOI] [PubMed] [Google Scholar]
- 14.Provis JM, Dubis AM, Maddess T, Carroll J. Adaptation of the central retina for high acuity vision: Cones, the fovea and the avascular zone. Prog Retin Eye Res. 2013;35:63–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balaratnasingam C, Inoue M, Ahn S, et al. Visual acuity is correlated with the area of the foveal avascular zone in diabetic retinopathy and retinal vein occlusion. Ophthalmology. 2016;123(11):2352–2367. [DOI] [PubMed] [Google Scholar]
- 16.Iafe NA, Phasukkijwatana N, Chen X, Sarraf D. Retinal capillary density and foveal avascular zone area are age-dependent: Quantitative analysis using optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57(13):5780–5787. [DOI] [PubMed] [Google Scholar]


