Abstract
Predictive approaches to assessing the toxicity of contaminant mixtures have been largely limited to chemicals that exert effects through the same biological molecular initiating event (MIE). However, by understanding specific pathways through which chemicals exert effects it may be possible to identify shared “downstream” nodes as the basis for forecasting interactive effects of chemicals with different MIEs. Adverse outcome pathway (AOP) networks conceptually support this type of analysis. Herein we assess the utility of a simple AOP network for predicting the effects of mixtures of an aromatase inhibitor (fadrozole; FAD) and an androgen receptor (AR) agonist (17β-trenbolone; TRB) on aspects of reproductive endocrine function in female fathead minnows. The fish were exposed to multiple concentrations of FAD and TRB individually or in combination for 48- or 96-h. Effects on two shared nodes in the AOP network, plasma 17β-estradiol (E2) concentration and vitellogenin (VTG) production (measured as hepatic vtg transcripts) responded as anticipated to FAD alone but were minimally impacted by TRB alone. Overall, there were indications that TRB enhanced decreases in E2 and vtg in FAD-exposed fish but results often were not statistically-significant. Failure to consistently observe hypothesized interactions between FAD and TRB could be due to several factors, including lack of impact of TRB, inherent biological variability in the endpoints assessed, and/or an incomplete understanding of interactions (including feedback) between different pathways within the hypothalamic-pituitary-gonadal axis.
Keywords: Fish, Endocrine, Toxicity, Mechanism, Adverse Outcome Pathway
Introduction
Predicting the potential effects of chemical mixtures can be a significant uncertainty in both prospective and diagnostic risk assessments. For mixtures comprised of multiple chemicals that act via the same molecular target, approaches employing additive models based on potency and concentrations of individual mixture components have been successfully employed (Fox et al. 2017). However, the more realistic assessment scenario involves complex mixtures of chemicals that do not necessarily act via the same molecular target. In these instances, mechanism-based prediction of possible interactive effects is more challenging.
The adverse outcome pathway (AOP) framework is designed to help scientists organize and communicate existing knowledge about toxicological pathways, generally starting with the interaction of a chemical with a molecular target (molecular initiating event; MIE), and proceeding through subsequent key events (KEs) at progressively higher levels of biological organization, culminating in adverse outcomes in individuals or populations. The AOP concept was originally described in terms of distinct sequences of KEs without cross-pathway interactions (Ankley et al. 2010). However, as familiarity with the description and development of more sophisticated AOPs increased, the practical utility of assembling individual pathways into AOP networks for certain types of applications became apparent (Villeneuve et al. 2014; Knapen et al. 2015; LaLone et al. 2017a). An AOP network is comprised of multiple AOPs that share at least one KE, which may either diverge or converge with one another in producing downstream-effects (Knapen et al. 2018; Villeneuve et al. 2018). Depicting AOPs in a network context allows consideration of different types of interactions among pathways of interest (LaLone et al. 2017b; Knapen et al. 2018; Villeneuve et al. 2018). One example is evaluation of potential effects of mixtures of chemicals that do not share a common MIE, but affect pathways with shared downstream nodes (i.e., defined KE(s)). An elegant example of this type of network-based approach to predicting mixture toxicity comes from Conley et al. (2018), who evaluated the interactive effects of several chemicals known to produce anti-androgenic responses in rats via different mechanisms. Using a network-based conceptual model they were able to successfully predict the additive effects of a mixture of 18 drugs and pesticides acting via five different MIEs on a variety of apical responses in neonatal and adult animals.
Our research team has, for several years, used graphical systems models as a basis for predictive toxicology research with endocrine-active chemicals in fish (Villeneuve et al. 2007a; 2012). This systems-based emphasis contributed to the development of a series of three related AOPs that could be assembled into a convergent network (Knapen et al. 2015). The three AOPs in the network all involve the production of vitellogenin (VTG, egg yolk protein precursor) in female teleost fish, a function critical to producing viable oocytes and, subsequently, maintenance of stable populations (Miller et al. 2007). Production of VTG occurs in the liver of the females through activation of estrogen receptor(s) (ER) by 17β-estradiol (E2) and can be perturbed by chemicals in multiple ways. The open-access AOP Wiki (2020) contains entries describing in detail inhibition of vitellogenesis through (a) direct ER antagonism (AOP 30); (b) depression of steroid synthesis through inhibition of aromatase, the enzyme that converts testosterone (T) to E2 (AOP 25); and (c) activation of the androgen receptor (AR), which indirectly decreases steroid synthesis through feedback inhibition (AOP 23) (Figure 1a). Although the AOPs operate via different MIEs, they converge at the shared KE of decreased VTG production (Figure 1b). This convergent motif suggests the possibility of additive effects if an organism were concurrently exposed to chemicals that triggered more than one of the MIEs (Villeneuve et al. 2018).
Figure 1.
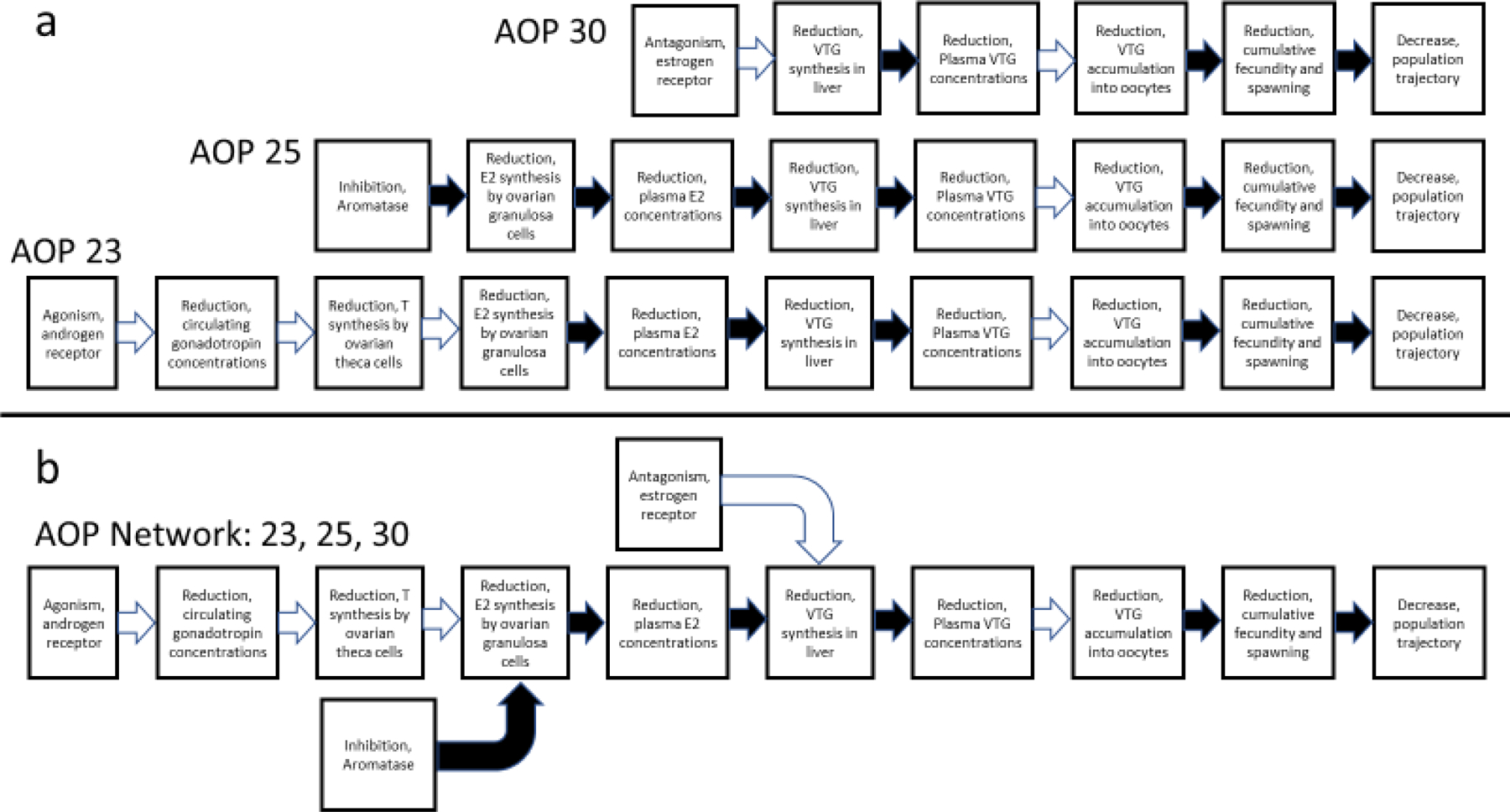
Graphical depiction of (a) three related linear adverse outcome pathways (AOPs) affecting reproduction in fish (AOPS 23, 25 and 30; https://aopwiki.org), and (b) the three AOPs arranged as an AOP network. Solid arrows indicate a key event relationship (KER) with a moderate or high degree of confidence based on a weight-of-evidence analysis, whereas open arrows indicate KERs with low support (Becker et al. 2015).
The current study was designed to test a simple network-based prediction of interactive physiological responses in this AOP network. Fathead minnows (Pimephales promelas) were exposed to a prototypical aromatase inhibitor, fadrozole (FAD), a model AR agonist, 17β-trenbolone (TRB), or various mixtures of the two to test a series of AOP network-based predictions. Briefly, we hypothesized that simultaneous exposure to both an aromatase inhibitor and AR agonist would result in a greater reduction of plasma E2 and VTG than either chemical initiator alone. Additionally, based on previous studies that examined compensatory gene expression responses to these two MIEs, we hypothesized that the typical increases in expression of two steroidogenic cytochrome P450s (CYPs; CYP19A1 [aromatase] and CYP11A [cholesterol side chain cleavage]) following exposure to FAD (Villeneuve et al. 2009; 2013) and decreases observed in these same CYPs following exposure to TRB (Ekman et al. 2011) might off-set one-another, resulting in lesser overall effects on expression of the genes.
Materials and Methods
Evaluating Hypothesized Interactions: Overall Experimental Design
Both FAD and TRB have been tested extensively as model chemicals to define the performance of assays designed to detect endocrine-active compounds and to assess the mechanistic basis of perturbation of specific endocrine pathways of concern. Relatively extensive studies have assessed effects of the chemicals on a variety of molecular, biochemical, histological, and apical endpoints in 21-d reproduction studies as well as time-course experiments with sampling intervals ranging from hours to days (Ankley et al. 2002; 2003; Villeneuve et al. 2009; Ekman et al. 2011; Villeneuve et al. 2013; Schroeder et al. 2017). Data from this work provided the basis for the selection of test concentrations and exposure durations in the context of endpoints of interest for a FAD-TRB mixture experiment.
Effects measures focused on physiological responses indicative of the status of reproductive function in the female fathead minnow. Two of these endpoints, plasma E2 and hepatic VTG mRNA concentrations, reflect shared KEs in the AR activation and aromatase inhibition AOPs (Figures 1a, b). Plasma T concentration also was measured in the current study. Although it is not represented as a shared KE in the AOP network, plasma T can be affected by both FAD and TRB. In prior studies we have noted that exposure to FAD can increase T in females, presumably due to its reduced aromatization to E2 (Villeneuve et al. 2009), while TRB decreases plasma T concentrations in females (Ankley et al. 2003). Finally, ovarian expression of mRNA coding for two key steroidogenic enzymes, CYP19A1 and CYP11A, was measured. Changes in expression of these genes in female fathead minnows following exposure to FAD or TRB are thought to be indirect compensatory responses associated with endocrine feedback along the hypothalamic-pituitary-gonadal axis, not direct effects of the chemicals (Ankley and Villeneuve 2015). Specifically, exposure to FAD tends to increase CYP19A1 and CYP11A transcripts while exposure to TRB tends to decrease them (Villeneuve et al. 2009; Ekman et al. 2011).
Fish were exposed for 48- or 96-h to water-borne FAD (0.5, 5 µg/L) and TRB (5, 15, 50 ng/L) singly or in combination using a complete matrix design (Supplemental Figure 1). The test concentrations were selected to attempt to optimize the likelihood for low to moderate responses in each of the endpoints based on prior FAD or TRB studies. Similarly, based on past work, we believed that the 48-h samples should capture initial direct effects of the chemicals on the various endpoints, while the 96-h sampling should enhance detection of compensatory responses in the fish (Ankley and Villeneuve 2015). In terms of actual test logistics, due to the large number of samples it was necessary to stagger initiation/sampling of the treatment matrix shown in Supplemental Figure 1. Consequently, the entire experiment consisted of 6 d of tank dosing (see Supplemental Table 1 for a detailed description of sample timing).
Test Procedures, Sample Collection and Exposure Characterization
Solvent-free aqueous solutions of FAD (a gift from Novartis in 2001) and TRB (Sigma, >98% purity) were prepared as described elsewhere (Ankley et al. 2002; 2003) and diluted to the target concentrations with Lake Superior (control) water prior to delivery to the exposure tanks. The diluted stock solutions were administered under constant flow conditions (ca. 45 mL/min) to glass tanks containing 10 L of water, separated into two chambers with a mesh divider. Four sexually-mature (6–7 months old) fathead minnow females from an on-site culture were placed in each half of the tank after 24-h of preconditioning the system with the various test solutions. Treatment combinations were tested in triplicate (i.e., a total of 12 fish for each). Tests were conducted at 25±1°C with a 16:8 L:D photoperiod. The fish were fed brine shrimp to satiation twice daily. All procedures involving the fish conformed with an approved, externally-reviewed Animal Care and Use Plan for the Duluth USEPA facility.
Four fish were sampled from each tank after 48 h, and the remaining four were sampled at 96 h. Fish were anesthetized with a buffered solution of tricaine methane sulfonate (MS-222; Argent, Redmond, WA). Blood was collected from the caudal vasculature with a heparinized microhematocrit tube. Plasma was separated by centrifugation and stored at −80°C until determination of steroid concentrations. Liver and ovaries were removed and stored at −80°C until used for gene expression analysis.
Water samples were collected daily for the duration of the experiment for determination of both FAD and TRB. Briefly, FAD was measured by direct injection of 20 µL of exposure water onto an Agilent Technologies 1100 high performance liquid chromatography (HPLC; Santa Clara, CA, USA) system equipped with a Poroshell 120 EC-C18 column (3.0 × 50 mm, 2.7 µm; Agilent Technologies). Fadrozole was detected using an Agilent 1946D single-quadrupole mass spectrometer (MS) with an electrospray ionization source, monitoring m/z 224. An external method of calibration was used with a six-point standard curve.
Water samples for TRB analysis were extracted using Strata-X solid-phase extraction (SPE) cartridges (200 mg sorbent; Phenomenex, Torrance, CA, USA) and concentrated 500-fold prior to analysis by HPLC-MS/MS. Briefly, 50 mL water samples were spiked with 0.25 ng of internal standard (TRB-d3) and loaded onto SPE cartridges that had been previously conditioned with methanol and water. After loading, cartridges were rinsed with 3 mL of 1:1 methanol:water and aspirated to near dryness. Cartridges were eluted with 3 mL of methanol and evaporated to dryness under a gentle stream of nitrogen, then reconstituted in 100 µL of 25:75 of methanol:water. The entire reconstituted sample was injected onto an Agilent 1200 series HPLC coupled to an Agilent 6410 MS with an atmospheric pressure photoionization source. The analytes were separated on a Zorbax RRHD StableBond C18 column (2.1 × 50 mm, 1.8µm; Agilent Technologies) and the TRB detected by monitoring the 271.0 m/z to 199.0 m/z (quantifier ion) and 107.0 m/z (qualifier ion) ion transition. An internal standard method of calibration was used with a six-point standard curve and TRB-d3 as the internal standard.
Lake Superior water blanks, duplicate tank water samples, and FAD- or TRB-spiked samples were analyzed with each sample set. Mean accuracy of FAD spikes (standard deviation [SD], n = 9) and TRB spikes (SD, n = 14) was 91.4 (7.5) and 97.8 (5.6)%, respectively. Mean (SD) agreement between duplicate FAD (n = 24) and TRB (n = 12) tank samples was 95.2 (3.8) and 94.6 (3.2)%, respectively. No FAD or TRB was detected in blank samples (detection limits 0.05 µg/L and 0.3 ng/L, respectively).
Plasma Steroid and Gene Expression Measurements
Plasma steroid concentrations were determined using HPLC-MS/MS. Plasma from one or two individuals within a single treatment replicate was pooled as needed to obtain an adequate volume for analysis (between 8–10 µL). Briefly, plasma was spiked with an internal standard (13,14,15,16,17,18-13C-E2; 2,3,4-13C-T) then extracted by diluting with 0.1% formic acid and loading onto Novum (Phenomenex) supported liquid extraction cartridges. Cartridges were rinsed with hexane, then eluted with dichloromethane. Extracts were evaporated to dryness and reconstituted in 50 µL of 15:85 methanol:water. A 20 µL aliquot was injected onto an Agilent 1200 series HPLC coupled to an Agilent 6490 MS with an atmospheric pressure photoionization source. The analytes were separated on a Zorbax RRHD StableBond C18 column (2.1 × 50 mm, 1.8µm; Agilent Technologies). Concentrations of E2 and T were determined using an internal standard method of calibration with a 7-point standard curve. Matched isotopically labeled standards were used as the internal standard for quantification of each of the compounds. Matrix spikes, a pooled plasma replicate, and extraction blanks were analyzed with each plasma sample batch. Mean (SD) accuracy of matrix spikes (n = 3) was 92.8 (15.9)% and 118 (22.6)% for E2 and T, respectively. Agreement between replicate pooled plasma (n = 2) ranged 78.7–96.0%. No target compounds were detected in blank extracts.
Expression of mRNA transcripts of hepatic vtg and ovarian cyp19a1a and cyp11a were measured using quantitative real-time polymerase chain reaction (QPCR). Briefly, total RNA from livers and ovaries was extracted using RNeasy Mini kits (Qiagen), including a DNase treatment, according to the manufacturer’s protocol. Quality and concentration of each RNA sample were evaluated using a Nanodrop ND-1000 Spectrophotometer (Nanodrop Technologies). A260 nm / A280 nm ratios generally ranged from 1.8–2.0 for liver samples and 2.0–2.2 for ovary samples. Samples were diluted to 10 ng total RNA/µL prior to analysis. The QPCR assays were conducted using Taqman RNA-to-Ct 1-step kits (Applied Biosystems), with each 20 µL reaction containing 20 ng total RNA, 300 nM forward primer, 300 nM reverse primer, and 150 nM probe (see Supplemental Table 2 for primer and probe sequences). The thermocycling program was set to an initial hold at 48°C for 15 min, followed by 95°C for 10 min, and 40 cycles of 95°C for 15 s and 60°C for 1 min using a 7500 Real-Time PCR System (Applied Biosystems). Relative transcript abundance was quantified using gene-specific mRNA standard curves with six concentrations (10-fold dilution series ranging from 200 to 2 × 107 copies/µL). The standards were prepared through a series of cDNA amplifications and in vitro transcription reactions (MEGAscript, Ambion Inc) as previously described by Villeneuve et al. (2007b). Amplification efficiencies ranged from 74.0 to 97.6%. At least 11 samples were analyzed in duplicate for each gene, and the mean (SD) coefficient of variation between the duplicates was 8.85 (4.67)%. Due to the number of samples, transcript abundance was measured across multiple assays (separate plates). Equal numbers of samples from each treatment group were represented on each plate and every assay included a set of control samples (n=8) that were run with every analysis. Data from each assay were normalized to the mean quantity of transcripts estimated for these eight control samples and expressed as normalized relative abundance.
Statistical Analysis
Data for 48 h and 96 h exposure durations were handled separately. The data were first tested for normality using a Kolmogorov-Smirnov test and homogeneity of variance with Levene’s test. In most cases data were log transformed to meet the assumptions of parametric statistics. One-way analysis of variance (ANOVA) was used to assess the five biological responses measured in the females: plasma concentrations of E2 and T, hepatic expression of vtg, and ovarian expression of cyp19a1a and cyp11a. For each endpoint a series of four ANOVAs’ was conducted focused on examining whether: (a) FAD alone caused an effect; (b) TRB alone caused an effect; (c) TRB enhanced responses seen in the low-dose FAD treatment; or (d) TRB enhanced responses seen in the high-dose FAD treatment. Duncans Multiple Range Test was used to identify differences across the FAD- and TRB-only treatments. Dunnett’s Test was used to identify differences in the various FAD-TRB combinations relative to the corresponding FAD-only control. Differences were considered significant at p<0.05.
Results
There was 100% survival of fish in the experiment. Daily determination of FAD and TRB concentrations in the test tanks showed that, with one exception, measured concentrations were very close (typically within 20%) of the target concentrations (Table 1). On day 6 of the experiment, water flow to one of the replicate tanks in the 0 µg FAD/L:5 ng TRB/L 96-h treatment was partially blocked, resulting in relatively low TRB concentrations for at least some amount of time (< 24 h).
Table 1.
Target and measured water concentrations of fadrozole (FAD; µg/L) and 17β-trenbolone (TRB; ng/L) in a 4-d mixture study with fathead minnows
| Test Day | |||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | Mean | |
| Target Treatment (FAD, TRB) | FAD, TRB | FAD, TRB | FAD, TRB | FAD, TRB | FAD, TRB | FAD, TRB | FAD, TRB |
| 0, 0 | 0, 0 (0, 0)a | 0, 0 (0, 0) | 0, 0 (0, 0) | 0, 0 (0, 0) | 0, 0 (0, 0) | 0, 0 (0, 0) | 0, 0 (0, 0) |
| 0.5, 0 | 0.51, 0 (0.02, NA) | 0.51, 0 (0.01, 0) | 0.48, 0 (0, 0) | 0.53, 0 (0, 0) | 0.60, 0 (0.03, 0) | 0.54, 0 (0.02, 0) | 0.53, 0 (0.04, 0) |
| 5, 0 | 5.01, 0 (0.28, NA) | 5.68, 0 (0.46, 0) | 5.32, 0 (0.38, 0) | 5.71, 0 (0.45, 0) | 5.81, 0 (0.44, 0) | 4.98, 0 (0.34, 0) | 5.43, 0 (0.33, 0) |
| 0, 5 | 0, 4.24 (0, NA) | 0, 3.31 (0, 0.64) | 0, 3.90 (0, 0.35) | 0, 3.51 (0, 0.28) | 0, 3.81 (0, 0.19) | 0, 2.99 (0, 3.21b) | 0, 3.82 (0, 0.58) |
| 0, 15 | 0, 11.15 (0, NA) | 0, 12.47 (0, 0.28) | 0, 11.84 (0, 0.44) | 0, 9.21 (0, 0.10) | 0, 9.95 (0, 1.69) | 0, 13.60 (0, 0.76) | 0, 11.21 (0, 1.37) |
| 0, 50 | 0, 44.67 (0, NA) | 0, 53.93 (0, 3.37) | 0, 42.38 (0, 0.320 | 0, 35.95 (0, 1.43) | 0, 36.83 (0, 2.60) | 0, 50.89 (0, 2.09) | 0, 43.30 (0, 6.42) |
| 0.5, 5 | 0.55, 5.45 (0, NA) | 0.52, 4.43 (0.01, 0.26) | 0.50, 4.20 (0, 0.27) | 0.55, 3.84 (0.02, 0.42) | 0.61, 3.84 (0.01, 0.15) | 0.57, 5.46 (0.01, 0.34) | 0.55, 4.37 (0.03, 0.55) |
| 0.5, 15 | 0.50, 14.10 (0.04, NA) | 0.51, 10.89 (0.01, 0.08) | 0.46, 10.97 (0.01, 0.60) | 0.50, 9.46 (0.01, 0.12) | 0.56, 10.89 (0.01, 0.42) | 0.52, 13.06 (0.01, 0.14) | 0.51, 11.27 (0.03, 1.37) |
| 0.5, 50 | 0.55, 46.15 (0.02, NA) | 0.52, 45.31 (0.02, 1.58) | 0.49, 51.17 (0.02, 2.33) | 0.53, 38.18 (0.03, 1.07) | 0.59, 42.26 (0.03, 1.15) | 0.54, 50.45 (0.03, 0.22) | 0.54, 45.05 (0.03, 4.76) |
| 5, 5 | 4.91, 5.14 (0.11, NA) | 5.46, 4.11 (0.11, 0.05) | 5.27, 4.15 (0.06, 0.07) | 5.65, 3.65 (0.14, 0.08) | 5.74, 3.96 (0.14, 0.21) | 4.97, 5.57 (0.11, 0.13) | 5.33, 4.45 (0.31, 0.67) |
| 5, 15 | 4.49, 12.54 (0.23, NA) | 5.01, 11.81 (0.08, 1.08) | 4.79, 11.98 (0.08, 0.23) | 5.01, 9.95 (0.21, 0.05) | 5.11, 10.51 (0.32, 0.56) | 4.39, 13.82 (0.27, 0.17) | 4.78, 11.87 (0.31, 1.26) |
| 5, 50 | 4.84, 45.11 (0.13, NA) | 5.35, 48.30 (0.17, 6.40) | 5.28, 52.97 (0.32, 1.45) | 5.65, 37.64 (0.25, 2.02) | 5.83, 39.96 (0.24, 0.69) | 5.14, 53.65 (0.26, 1.50) | 5.35, 47.34 (0.34, 6.20) |
Control; detection limits = 0.05 µg/L (FAD) and 0.3 ng/L (TRB). Values indicate mean (± SD) where n=3 tanks for controls and FAD and n=1–2 tanks for TRB.
Low detection of TRB in one of the 3 tanks; chemical flow cessation for 24 h in that tank.
NA=Not applicable to calculating SD because only one measurement was made.
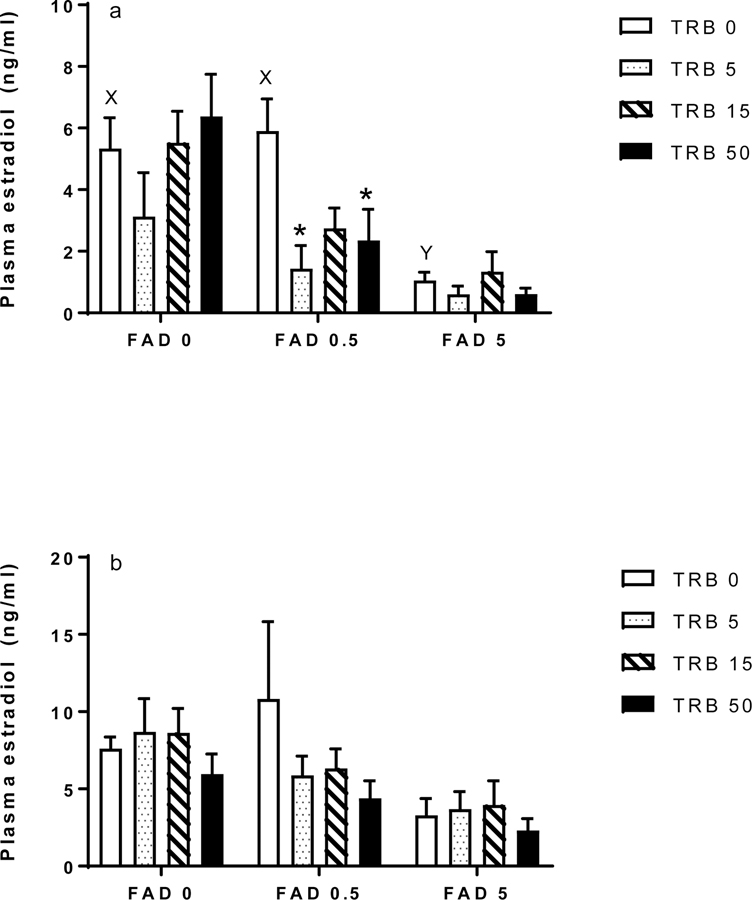
Shared KE nodes for the two AOPs comprising the simple network under investigation are plasma E2 concentrations and hepatic VTG production (estimated herein as hepatic vtg expression; Figure 1b). At 48 h, FAD alone significantly decreased plasma E2 in the 5 but not 0.5 µg/L treatment group (Figure 2a). FAD alone had no significant effect on plasma E2 concentration in the females at 96 h (Figure 2b). TRB alone did not affect plasma E2 at 48 or 96 h (Figure 2a, b). In the 0.5 µg FAD/L treatment, cotreatment with TRB appeared to decrease plasma E2 at 48 and 96 h, but this effect was statistically significant only at the earlier sampling time (in the 5 and 50 ng TRB/L treatments). In the highest FAD treatment (5 µg/L), TRB did not further enhance depressions in plasma E2 compared to FAD alone (Figure 2a, b).
Figure 2.
Plasma concentration of 17β-estradiol (E2) in female fathead minnows exposed to fadrozole (FAD; μg/L) or trenbolone (TRB; ng/L) singly or in combination for (a) 48 h or (b) 96 h. Bars indicate the mean (standard error of the mean, n = 4–7) for each treatment group. Different letters (X, Y) indicate differences across the FAD-only treatments. Asterisks indicate a significant difference for a given FAD-TRB combination compared to the corresponding FAD-only treatment.
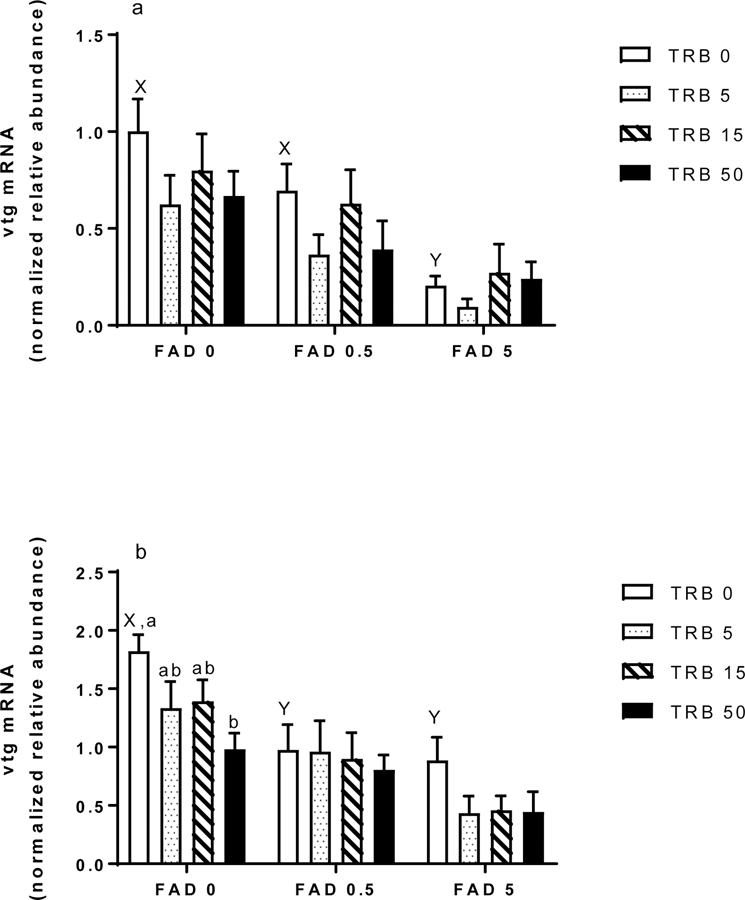
Concentrations of vtg transcripts in the liver were reduced in a dose-dependent manner by FAD alone both at 48 and 96 h, and by the high dose of TRB (50 ng/L) alone at 96 but not 48 h (Figure 3a, b). Visual inspection of the data suggested that TRB enhanced depression of vtg in the 0.5 µg FAD/L treatment at 48 h, and in the 5 µg FAD/L treatment at 96 h, but these effects were not statistically-significant.
Figure 3.
Hepatic expression of vitellogenin (vtg) transcipts in female fathead minnows exposed to fadrozole (FAD; μg/L) or trenbolone (TRB; ng/L) singly or in combination for (a) 48 h or (b) 96 h. Bars indicate the mean (standard error of the mean, n = 11–12) for each treatment group. Different letters indicate differences across the FAD-only treatments (X, Y) or TRB-only treatments (a, b). Asterisks indicate a significant difference for a given FAD-TRB combination compared to the corresponding FAD-only treatment.
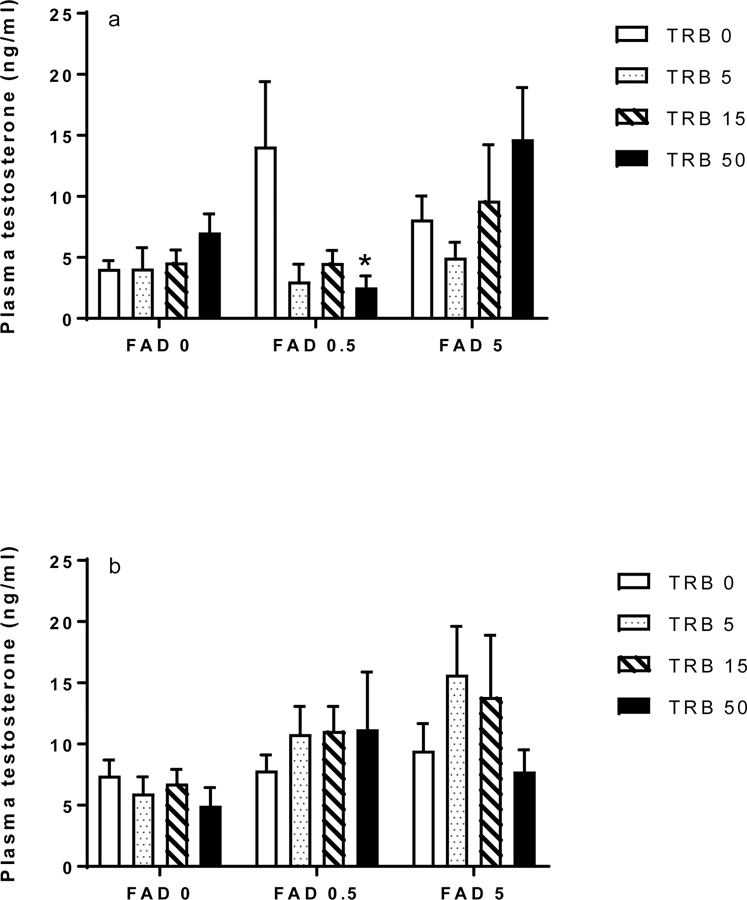
Plasma concentration of T is not a KE in the aromatase inhibition AOP, but T can be increased by chemicals such as FAD due to inhibition of its conversion to E2 (Figure 1). Production of T by ovarian theca cells is a KE in AOP 23, which depicts the effects of AR agonists on VTG and egg production in fish. Specifically, activation of the AR by chemicals such as TRB is thought to elicit negative feedback which decreases expression of CYP11A and reduces production of T by the theca cells. Since androgen production provides the precursor for synthesis of E2 in the granulosa, this is expected to reduce circulating E2 concentrations (Figure 1). In the current study, the only significant effect of the chemicals on T was observed in females exposed for 48 h to the high dose of TRB (50 ng/L) in the 0.5 µg FAD/L treatment (Figure 4a, b).
Figure 4.
Plasma concentration of testosterone (T) in female fathead minnows exposed to fadrozole (FAD; μg/L) or trenbolone (TRB; ng/L) singly or in combination for (a) 48 h or (b) 96 h. Bars indicate the mean (standard error of the mean, n = 4–7) for each treatment group. Asterisks indicate a significant difference for a given FAD-TRB combination compared to the corresponding FAD-only treatment.
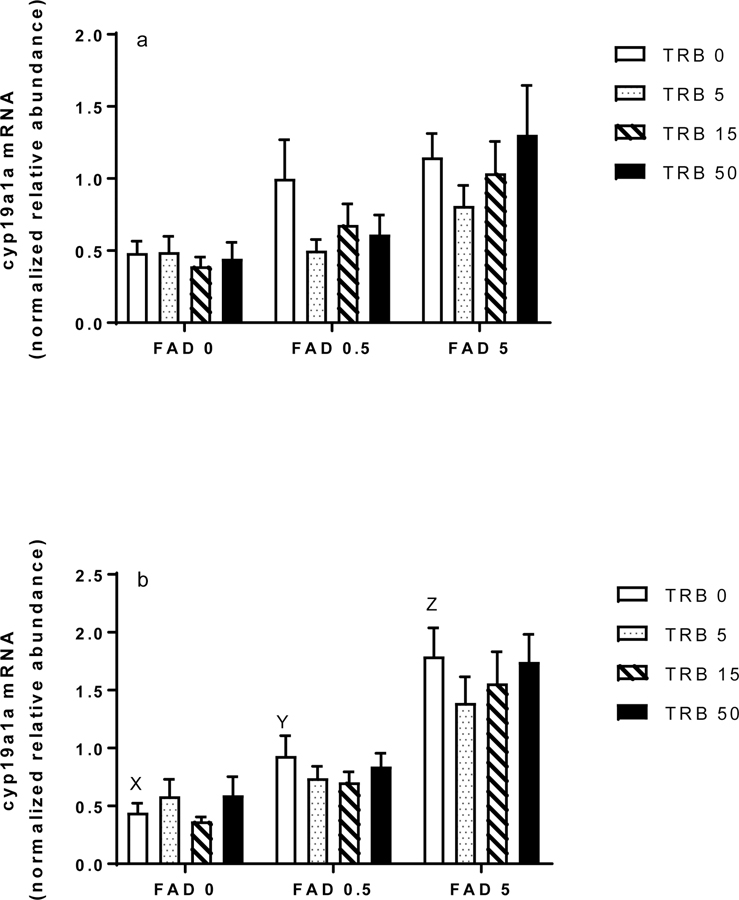
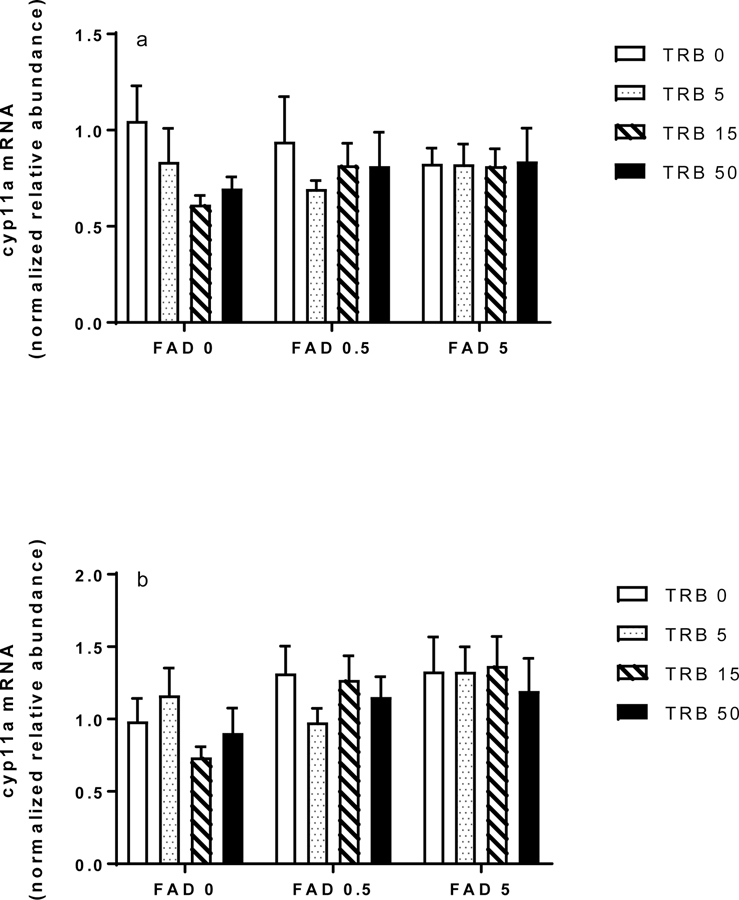
Prior studies with endocrine-active chemicals have shown substantial evidence in female fathead minnows of compensatory responses in the hypothalamic-pituitary-gonadal axis, including changes in ovarian expression of genes involved in steroid synthesis (Ankley and Villeneuve 2015). Of specific interest to the present study were cyp19a1a and cyp11a, because both are upregulated following exposure to FAD but downregulated following exposure to TRB. As expected, ovarian transcripts of cyp19a1a were significantly more abundant in FAD exposed females at both sampling times, with a significant concentration-dependent increase detected at 96 h (Figure 5a, b). Expression of cyp19a1a was not affected by TRB, nor did there seem to be any interaction between the test chemicals relative to expression of the transcript (Figure 5a, b). Neither FAD nor TRB significantly affected expression of ovarian transcripts of cyp11a in fish sampled after 48 h or 96 h of exposure (Figure 6a, b).
Figure 5.
Ovarian expression of cytochrome P450 aromatase (cyp191a1) transcripts in female fathead minnows exposed to fadrozole (FAD; μg/L) or trenbolone (TRB; ng/L) singly or in combination for (a) 48 h or (b) 96 h. Bars indicate the mean (standard error of the mean, n = 11–12) for each treatment group. Different letters (X, Y, Z) indicate differences across the FAD-only treatments.
Figure 6.
Ovarian expression of cytochrome P450 side-chain-cleavage (cyp11a) transcripts in female fathead minnows exposed to fadrozole (FAD; μg/L) or trenbolone (TRB; ng/L) singly or in combination for (a) 48 h or (b) 96 h. Bars indicate the mean (standard error of the mean, n = 11–12) for each treatment group.
Discussion
There is a strong conceptual and empirical foundation underlying the assumption of additive toxicity for chemicals that affect an organism through a common MIE. For example, this serves as the basis for assessing the effects of mixtures of aryl hydrocarbon receptor agonists such as dioxins, furans, and some PCBs (Van den Berg et al. 1998), and ER agonists encompassing a broad range of chemical structures (e.g., Brian et al. 2005). The development and implementation of mechanism-based mixture toxicity models for chemicals that operate via different MIEs is more challenging, requiring explicit knowledge of shared nodes/pathways associated with biological responses of concern. However, as demonstrated by Conley et al. (2018) in rats exposed to mixtures of anti-androgens with different MIEs, this type of approach to predict mixture effects can be successful. In the present study we evaluated the predictive power of a simple AOP network comprised of two MIEs/pathways that influence VTG production in fish, inhibition of aromatase and activation of the AR. These are relatively well-defined AOPs, supported by multiple studies with a variety of fish species (including the fathead minnow) and chemicals (see AOPs 23 and 25, and associated citations in the AOP Wiki [2020]). In fact, the aromatase inhibition AOP is sufficiently well understood to have enabled development of a quantitative AOP relevant to prediction of effects in multiple fish species (Conolly et al. 2017; Doering et al. 2019). Although the AR activation AOP is not as advanced in terms of quantitative understanding, like the aromatase inhibition AOP it has undergone multiple rounds of a weight-of-evidence based technical review as part of assessment and publication through a formal process organized and coordinated through the Organisation for Economic Cooperation and Development (Becker et al. 2015; OECD 2020). Consequently, the two AOPs represent a reasonable basis for constructing a network to test the prediction of the effects of chemical mixtures operating via different MIEs.
For this work, we used two chemicals relatively specific to the MIEs of interest: FAD, a pharmaceutical inhibitor of aromatase designed to treat breast cancer in humans (Miller 1989), and TRB, a non-aromatizable synthetic steroid registered as a veterinary drug, and an active AR agonist in a wide variety of vertebrate species (Ankley et al. 2018). Due to the complexity of the complete matrix mixture design we used comparatively short exposure durations (48, 96 h) to assess the network-based predictions. However, decreases within this time frame in the two shared nodes (E2, VTG) in the hypothesized AOP network (Figure 1b) can be predictive of reduced egg production in longer-term tests with the two chemicals (Ankley et al. 2002; 2003). Both FAD and TRB have been tested on multiple occasions in short-term exposures with female fathead minnows, with FAD being thoroughly characterized in terms of time-course/dose-response relationships (e.g., Villeneuve et al. 2009; 2013; Schroeder et al. 2017). Trenbolone has been less-well studied than FAD in short-term exposures (Garcia-Reyero et al. 2009; Ekman et al. 2011), which is why three test concentrations (as opposed to two for FAD) were chosen for the mixture matrix in the present study. The nominal test concentrations chosen for the two chemicals (0.5, 5 µg FAD/L; 5, 15, 50 ng TRB/L) were based on past work, with an emphasis on selection of a range of treatments that hopefully would produce low to moderate responses in the endpoints of primary interest (E2, vtg), so that one chemical would not cause such substantive effects that responses due to the other could not be detected.
Evaluation of responses of the female fathead minnows to the FAD- and TRB-only treatments indicated that we were not wholly successful in meeting goals relative to test concentration selection. Effects observed with FAD alone were largely as expected, with the aromatase inhibitor decreasing plasma E2 at 48 h, and hepatic vtg at both 48 and 96 h. However, the effects of TRB alone were not as pronounced as hoped for; treatment with the androgen alone did not affect plasma E2 at either sampling time, and only decreased hepatic vtg in one of the treatments with the highest test concentration (50 ng/L) at 96 h. Based on the AOP network, we hypothesized that FAD and TRB would interact in an additive manner in reducing plasma E2 concentration and hepatic vtg in female fathead minnows. The comparative lack of TRB effects complicates interpretation of possible quantitative interactions with FAD from the standpoint of comparing their respective ECx values for endpoints of interest, a commonly-employed approach for assessing mixture additivity (e.g., EsCETOC 2001).
While a formal test of potential additivity of FAD and TRB on responses of the female fathead minnows cannot be achieved with the current dataset, it nonetheless is possible to assess potential interactions between the two chemicals from a more qualitative perspective. Specifically, there is substantial evidence that mixtures of chemicals that are individually below effects concentrations can combine to produce responses consistent with additivity. Some of the most robust work on this topic in the field of ecotoxicology was presented by Broderius et al. (2005) who showed that mixtures of chemicals eliciting toxicity in the fathead minnow via a narcosis mode of action “..act together in a nearly additive fashion to produce effects even when they are present at concentrations below their individual no-observed-effect concentration.” This type of seeming “something from nothing” observation in mixture studies has been verified in experiments with a variety of chemicals and endpoints including, for example, environmentally-relevant estrogen mixtures in vitro (Silva et al. 2002), multiple steroidal chemicals (including estrogen mixtures) in chronic fish reproduction studies (Brian et al. 2005; Thrupp et al. 2018), and in rats exposed to mixtures of anti-androgens operating via different MIE (Conley et al 2018). In examining data from the present study from this perspective, there was a significant effect (decrease) caused by TRB in the lower FAD treatment (0.5 µg/L) on plasma E2 concentration at 48 h, where FAD alone had no discernable effect (Figure 2a). Although the same pattern of TRB/FAD effects on plasma E2 was observed at 96 h, the response was not statistically significant (p=0.17 in the 0.5 µ/g FAD/L treatment). A similar potentiation by TRB was not seen in the high FAD (5 µg/L) treatment, but in this group, FAD alone caused a substantive decrease in plasma E2, which may have obviated detection of any possible additional contribution by TRB. In terms of the second shared node in the AOP network, TRB again seemed to enhance the effects of FAD on hepatic vtg, albeit not in a statistically-significant manner. For example, at 96 h the vtg expression in females exposed to the three TRB treatments in the presence of 5 µg FAD/L was depressed to less than 50% of that measured in the corresponding FAD-only treated fish (p=0.15; Figure 3b). Overall, although statistical support for possible interactions between FAD and TRB was inconsistent, the data are suggestive that the two chemicals were interacting with one another relative to depressing E2 and vtg.
Three other endpoints associated with reproductive endocrine function also were measured in the female fathead minnows. One of these, (decreased) plasma T concentration, is a KE in the AR activation AOP, but is not directly involved in the aromatase inhibition pathway (Figure 1a). Concentrations of T can, however, be elevated by aromatase inhibitors such as FAD due to a decreased conversion of the androgen to E2 (Villeneuve et al. 2009). Potential changes in genes for the two steroidogenic enzymes examined in this study, cyp19a1a and cyp11a, by FAD and TRB would be considered secondary, compensatory responses, not directly related to either AOP. Based on prior work, we hypothesized that FAD and TRB may have interactive (off-setting) effects on plasma concentrations of T, and ovarian expression of cyp19a1a and cyp11a (Ankley and Villeneuve 2015). However, with one notable exception, neither FAD nor TRB alone consistently affected the three endpoints. Expression of cyp19a1a was marginally increased by FAD at 48 h (p=0.07) and significantly elevated in a dose-dependent manner at 96 h (Figure 5a, b). Overall, given the lack of marked changes—or trends in changes—observed in plasma T and ovarian cyp19a1a and cyp11a, assessment of any possible interactive effects on these endpoints between FAD and TRB is not feasible.
Overall, success of our network-based predictions concerning the effects of a mixture of FAD and TRB on reproductive endocrine function in female fathead minnows is ambiguous. While there is some suggestion that the two chemicals interacted with one another at two nodes (KEs) within the hypothesized network, due to the lack of effects of TRB alone and inherent data variability, statistical evidence for the hypothesized interactions is weak. Further, we must consider the possibility that that even if the two AOPs comprising the network are independently valid, there are biological interactions between the pathways that may not be adequately represented by the shared KEs in the network. For example, the AR activation AOP (AOP 23) invokes a negative feedback of the exogenous AR agonist on gonadotropin release and subsequent down-regulation of theca cell T synthesis as the cause for reduced E2 synthesis. Assuming all the biology upstream of “reduction, E2 synthesis by ovarian granulosa cells” (Figure 1) is independent of the effects of aromatase inhibition, one could suppose additivity of the two different stressors on that KE and all those downstream. However, there are some potentially important biological interactions between the AOPs that the present network does not account for. For example, E2 is known to play an important role in endocrine feedback in teleosts, including interactions with dopaminergic neurons that regulate gonadotropin releasing hormone release (Zohar et al. 2010; Trudeau 1997) as well as interactions with kisspeptins (e.g., Wang et al. 2013; Alvarado et al. 2016). The exact role that androgens, particularly non-aromatizable androgens, play in neuroendocrine feedback in reproductively mature female fish is not thoroughly understood (Zohar et al. 2010; Trudeau 1997). Nonetheless, it is plausible that the effect of aromatase inhibition, which can impact both the ovarian and brain isoforms of aromatase (Villeneuve et al. 2006), decreasing both circulating E2 and local synthesis of E2 in specific brain regions, could interfere with the second KE in the AR agonism AOP. Prior studies in our lab with endocrine-active chemicals, including FAD and TRB, have shown a consistent pattern of compensatory responses in the system associated with feedback mechanisms/controls (see Ankley and Villeneuve 2015 for review); consequently, there is a reasonable likelihood that both were impacting neuroendocrine signaling along the hypothalamic-pituitary-gonadal axis. However, our current understanding of the mechanisms involved is insufficient to infer whether the effects of aromatase inhibition would effectively block or mask the effect of TRB as an additional stressor.
In summary, determining the potential toxicity of chemical mixtures is, arguably, the greatest uncertainty faced in both human health and ecological risk assessments for chemicals. Conceptual and technical advances in areas such as molecular and network/systems biology theoretically provide a basis for improving predictive approaches to the assessment of mixture effects. But, as the present study clearly demonstrates, these advances are not a panacea. Even for a comparatively simple network based on well-characterized pathways, empirical demonstration of success in predicting biological effects, although promising, was far from definitive. Further research focused on basic attributes and inherent variability of biological systems of concern is needed before network-based approaches to the prediction of the effects of chemical mixtures can be routinely used in risk assessments.
Supplementary Material
Acknowledgment and Disclaimer:
We thank Russ Erickson and Joe Swintek for statistical advice, and Justin Conley for comments on an earlier version of the paper. This paper has been reviewed in accordance with the requirements of the US Environmental Protection Agency (USEPA) Office of Research and Development. However, the recommendations and conclusions made herein do not represent USEPA policy. Mention of products or trade names does not indicate endorsement by the USEPA.
Footnotes
Data Availability: All data from this paper will be publicly available at the EPA Environmental Dataset Gateway (https://edg.epa.gov/metadata/catalog/main/home.page).
References
- Alvarado MV, Servili A, Molés G, Gueguen MM, Carrillo M, Kah), Felip A. 2016. Actions of sex steroids on kisspeptin expression and other reproduction-related genes in the brain of the teleost fish European sea bass. J Exp Biol 219:3353–3365. [DOI] [PubMed] [Google Scholar]
- Ankley GT, Villeneuve DL. 2015. Temporal changes in biological responses and uncertainty in assessing risk of endocrine-disrupting chemicals: Insights from intensive time-course studies with fish. Toxicol Sci 144:259–275. [DOI] [PubMed] [Google Scholar]
- Ankley GT, Kahl MD, Jensen KM, Hornung MW, Korte JJ, Makynen EA, Leino RL. 2002. Evaluation of the aromatase inhibitor fadrozole in a short-term reproduction assay with the fathead minnow (Pimephales promelas). Toxicol Sci 67:121–130. [DOI] [PubMed] [Google Scholar]
- Ankley GT, Jensen KM, Makynen EA, Kahl MD, Korte JJ, Hornung MW, Henry TR, Denny JS, Leino RL, Wilson VS, Cardon MC, Hartig PC, Gray LE. 2003. Effects of the androgenic growth promoter 17β-trenbolone on fecundity and reproductive endocrinology of the fathead minnow (Pimephales promelas). Environ Toxicol Chem 22:1350–1360. [PubMed] [Google Scholar]
- Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, Mount DR, Nichols JW, Russom CL, Schmieder PK, Serrano JA, Tietge JE, Villeneuve DL. 2010. Adverse outcome pathways: A conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem 29:730–741. [DOI] [PubMed] [Google Scholar]
- Ankley GT, Coady KK, Gross M, Holbech H, Levine SL, Maack G, Williams M. 2018. A critical review of the environmental occurrence and potential effects in aquatic vertebrates of the potent androgen receptor agonist 17β-trenbolone. Environ Toxicol Chem 37:2064–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOP (Adverse Outcome Pathway) Wiki. 2020. https://aopwiki.org (Accessed 10 January 2020).
- Becker RA, Ankley GT, Edwards SW, Kennedy SW, Linkov I, Meek B, Sachana M, Segner H, Van Der Burg B, Villeneuve DL, Watanabe H, Barton-Maclaren TS. 2015. Increasing scientific confidence in adverse outcome pathways: Application of tailored Bradford-Hill considerations for evaluating weight-of-evidence. Regul Toxicol Pharmacol 72:514–537. [DOI] [PubMed] [Google Scholar]
- Brian JV, Harris CA, Scholze M, Backhaus T, Booy P, Lamoree M, Pojana G, Jonkers N, Runnalls T, Bonfa A, Marcomini A, Sumpter JP. 2005. Accurate prediction of responses of freshwater fish to a mixture of estrogenic chemicals. Environ Health Perspect 113:721–7128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broderius SJ, Kahl MD, Hammermeister, Hoglund MD. 2005. A comparison of the lethal and sublethal toxicity of organic chemical mixtures to the fathead minnow (Pimephales promelas). Environ Toxicol Chem 24:3117–3127. [DOI] [PubMed] [Google Scholar]
- Conley JM, Lambright CS, Evans N, Cardon M, Furr J, Wilson VS, Gray LE. 2018. Mixed “antiandrogenic” chemicals at low individual doses produce reproductive tract malformations in the male rat. Toxicol Sci 164:166–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conolly RB, Ankley GT, Cheng W-Y, Mayo ML, Miller DH, Perkins EJ, Villeneuve DL, Watanabe KH. 2017. Quantitative adverse outcome pathways and their application to predictive toxicology. Environ Sci Technol 51:4661–4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doering JA, Villeneuve DL, Poole ST, Blackwell BR, Jensen KM, Kahl MD, Kittelson AR, Feifarek DJ, Tilton CB, LaLone CA, Ankley GT. 2019. Quantitative response-response relationships linking aromatase inhibition to decreased fecundity are conserved across three fishes with asynchronous oocyte development. Environ Sci Technol 53:10470–10578. [DOI] [PubMed] [Google Scholar]
- ECETOC (European Center for Ecotoxicology and Toxicology of Chemicals). 2001. Aquatic Toxicity of Mixtures. Technical Report No. 80. http://www.ecetoc.org/publication/tr-080-aquatic-toxicity-of-mixtures/ (Accessed 30 December 2019)
- Ekman DR, Villeneuve DL, Teng Q, Ralston-Hooper KJ, Martinovic-Weigelt D, Kahl MD, Jensen KM, Durhan EJ, Makynen EA, Ankley GT, Collette TW. 2011. Use of gene expression, biochemical and metabolite profiles to enhance exposure and effects assessment of the model androgen 17β-trenbolone in fish. Environ Toxicol Chem 30:319–329. [DOI] [PubMed] [Google Scholar]
- Fox MA, Brewer LE, Martin L. 2017. An overview of literature topics related to current concepts, methods, tools and applications for cumulative risk assessments (2007–2016). Int J Environ Res Public Health 14, E89, DOI: 3390/ijerph14040389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Reyero N, Villeneuve DL, Kroll KJ, Liu L, Orlando EF, Watanabe KH, Sepúlveda MS, Ankley GT, Denslow ND. 2009. Expression signatures for a model androgen and antiandrogen in the fathead minnow (Pimephales promelas) ovary. Environ Sci Technol 43:2614–2619 [DOI] [PubMed] [Google Scholar]
- Knapen D, Vergauwen L, Villeneuve DL, Ankley GT. 2015. The potential of AOP networks for reproductive and developmental assay development. Repro Toxicol 56:52–55. [DOI] [PubMed] [Google Scholar]
- Knapen D, Angrish MA, Fortin MC, Katsiadaki I, Leonard M, Margiotta-Casaluci L, Munn S, O’Brien JM, Pollesch N, Smith C, Zhang X, Villeneuve DL. 2018. Adverse outcome pathway networks: Development and applications. Environ Toxicol Chem 37:1723–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLone CA, Ankley GT, Belanger SE, Embry MR, Hodges G, Knapen D, Munn S, Perkins EJ, Rudd MA, Villeneuve DL, Whelan M, Willett C, Zhang X, Hecker M. 2017a. Advancing the adverse outcome pathway framework-An international horizon scanning approach. Environ Toxicol Chem 36:1411–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLone CA, Villeneuve DL, Wu-Smart J, Milsk RY, Sappington K, Garber KV, Housenger J, Ankley GT. 2017b. Weight of evidence evaluation of a network of adverse outcome pathways linking activation of the nicotinic acetylcholine receptor in honey bees to colony death. Sci Total Environ 584–585:751–775. [DOI] [PMC free article] [PubMed]
- Miller WR. 1989. Aromatase inhibitors in the treatment of advanced breast cancer. Cancer Treat Rev 16:83–93. [DOI] [PubMed] [Google Scholar]
- Miller DH, Jensen KM, Villeneuve DL, Kahl MD, Makynen EA, Durhan DJ, Ankley GT. 2007. Linkage of biochemical responses to population-level effects: A case study with vitellogenin in the fathead minnow. Environ Toxicol Chem 26:521–527. [DOI] [PubMed] [Google Scholar]
- OECD (Organization for Economic Cooperation and Development). 2020. https://www.oecd-ilibrary.org/environment/oecd-series-on-adverse-outcome-pathways_2415170x (Accessed 10 January 2020).
- Schroeder AL, Ankley GT, Habib T, Garcia-Reyero N, Escalon BL, Jensen KM, Kahl MD, Durhan EJ, Makynen EA, Cavallin JE, Martinovic-Weigelt D, Perkins EJ, Villeneuve DL. 2017. Rapid effects of the aromatase inhibitor fadrozole on steroid production and gene expression in the ovary of female fathead minnows (Pimephales promelas). Gen Comp Endocrinol 252:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva E, Rajapakse N, Kortenkamp A. 2002. Something from “nothing”-eight weak estrogenic chemicals combined at concentrations below NOECs produce significant mixture effects. Environ Sci Technol 36:1751–1756. [DOI] [PubMed] [Google Scholar]
- Thrupp TJ, Runnalls TJ, Scholze M, Kugathas S, Kortenkamp A, Sumpter JP. 2018. The consequences of exposure to mixtures of chemicals: something from “nothing” and a “a lot from a little” when fish are exposed to steroid hormones. Sci Total Environ 619–620:1482–1492. [DOI] [PubMed]
- Trudeau VL. 1997. Neuroendocrine regulation of gonadotrophin II release and gonadal growth in the goldfish, Carassius auratus. Rev Repro 2:55–68. [DOI] [PubMed] [Google Scholar]
- Van den Berg M, Birnbaum L, Bosveld AT, Brunstrom B, Cook P, Feeley M, Giesy JP, Hanberg A, Hasegawa R, Kennedy SW, Kubiak T, Larsen JC, van Leeuwen FX, Liem AK, Nott C, Peterson RE, Poellinger A, Safe S, Schrenk D, Tillitt D, Tysklind M, Younes M, Waern F, Zacharewski T. 1998. Toxic equivalency factors (TEFs) for PCBs, PCDDs, PCDFs for humans and wildlife. Environ Health Perspect 106:775–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve DL, Larkin P, Knoebl I, Miracle AL, Kahl MD, Jensen KM, Makynen EA, Durhan EJ, Carter BJ, Denslow ND, Ankley GT. 2007a. A graphical systems model to facilitate hypothesis-driven ecotoxicogenomics research on the brain-pituitary-gonadal axis. Environ Sci Technol 40:321–330. [DOI] [PubMed] [Google Scholar]
- Villeneuve DL, Miracle AL, Jensen KM, Degitz SJ, Kahl MD, Korte JJ, Greene KJ, Blake LS, Linnum A, Ankley GT. 2007b. Development of quantitative real-time PCR assays for fathead minnow (Pimephales promelas) gonadotrophin β subunit mRNAs to support endocrine disruptor research. Comp Biochem Physiol C 145:171–183. [DOI] [PubMed] [Google Scholar]
- Villeneuve DL, Mueller ND, Martinovic D, Makynen EA, Kahl MD, Jensen KM, Durhan EJ, Cavallin JE, Bencic D, Ankley GT. 2009. Direct effects, compensation, and recovery in female fathead minnows exposed to a model aromatase inhibitor. Environ Health Perspect 117:624–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve DL, Garcia-Reyero N, Martinovic-Weigelt D, Li A, Watanabe KH, Orlando EF, LaLone CA, Edwards SW, Burgoon LD, Denslow ND, Perkins EJ, Ankley GT. 2012. A graphical systems model and tissue-specific gene sets to aid transcriptomic analysis of chemical impacts on the female teleost reproductive axis. Mutat Res 746:151–162. [DOI] [PubMed] [Google Scholar]
- Villeneuve DL, Breen M, Bencic DC, Cavallin JE, Jensen KM, Makynen EA, Thomas LM, Conolly RB, Ankley GT. 2013. Developing predictive approaches to characterize adaptive responses of the reproductive endocrine axis to aromatase inhibition: I. Data generation in a small fish model. Toxicol Sci 133:225–233. [DOI] [PubMed] [Google Scholar]
- Villeneuve DL, Crump D, Garcia-Reyero N, Hecker M, Hutchinson TH, LaLone CA, Landesmann B, Lettieri T, Munn S, Nepelska M, Ottinger MA, Vergauwen L, Whelan M. 2014. Adverse outcome pathway (AOP) development I: Strategies and principles. Toxicol Sci 142:312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve DL, Angrish MM, Fortin MC, Katsiadaki I, Leonard M, Margiotta-Casaluci L, Munn S, O’Brien JM, Pollesch N, Smith LC, Zhang X, Knapen D. 2018. Adverse outcome pathways II: Network analytics. Environ Toxicol Chem 37:1734–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Sham KM, Ogawa S, Li S, Parhar IH, Cheng CH, Liu X, Lin H. 2013. Regulation of the two kiss promoters in goldfish (Carassius auratus) by estrogen via different ERα pathways. Mol Cell Endocrinol 375:130–139. [DOI] [PubMed] [Google Scholar]
- Zohar Y, Muñoz-Cueto JA, Elizur J, Kah O. 2010. Neuroendocrinology of reproduction in teleost fish. Gen Comp Endocrinol 165:438–455. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.